Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mechanical circulatory support (MCS) is a means of imparting energy for forward flow of blood in the body by manmade devices. Its intent is to remove some or all work of cardiac output from either or both left and right ventricles. Mechanical circulatory support devices (MCSDs) can be used to provide temporary ventricular assistance after cardiac surgery, with the assumption that ventricular function will recover rather quickly (days). Durable mechanical devices can be used for prolonged (months to years) circulatory support with the intent of bridge-to-transplant support, bridge to recovery (with the expectation of sufficient ventricular recovery to allow device explantation), or permanent (destination) therapy.
Mechanical pumping mechanisms can be placed internally (implantable) or external to the body (paracorporeal). Their power source can be electric or pneumatic, located outside the body or completely within it, with electric power conducted transcutaneously. Their pump flow characteristic can be pulsatile or continuous flow .
This chapter does not include use of biological means for MCS nor passive (non–energy imparting) devices used primarily to address ventricular remodeling (see Chapter 20 ). This chapter also does not include short term temporary devices.
Early descriptions of mechanically supporting human circulation date back at least to the early 19th century in the writings of LeGallois. Carrel and Lindberg as well as Demikhov reported experimental application of mechanical support systems in animal models in the 1930s. However, major interest in mechanical support of human circulation would await the dawn of open heart surgery in the 1950s.
With successful application of cardiopulmonary bypass (CPB) for a cardiac surgical operation by Gibbon in 1953 and the subsequent first successful series of cardiac operations using CPB by Kirklin and colleagues at the Mayo Clinic, the stage was set for rapid proliferation of the technology of temporary MCS (in this case the heart-lung machine) for repair of cardiac malformations. Failure to successfully wean some patients from CPB stimulated surgeons to seek additional methods of mechanical support while awaiting myocardial recovery. Roller-pump technology was complicated by trauma to blood elements and difficulty in modulating pump speed in response to fluctuations in atrial filling pressures. The first application of a true ventricular assist device (VAD) was attributed to Michael DeBakey, who in 1966 reported the successful application of a pneumatically driven diaphragm pump for 10 days in a 37-year-old woman unable to be weaned from CPB following aortic and mitral valve replacements. Cooley subsequently reported the first successful bridge to transplantation in a 47-year-old man for 64 hours while awaiting heart transplantation with a pneumatically driven artificial heart, the Liotta Heart, developed by the DeBakey-Baylor-Rice research team.
MCS research focused on pneumatic, electric, and even nuclear-powered designs through the 1980s. The experimental work of Kolff, Olsen, Jarvik, and others paved the way for the first permanent total artificial heart implant in Dr. Barney Clark by DeVries and his team in 1982. Five patients received permanent total artificial hearts under a U.S. Food and Drug Administration (FDA) protocol, with a maximum reported survival of 620 days. The close scrutiny of this initial trial of the Jarvik-7 total artificial heart stimulated intense and sometimes acrimonious debate among ethicists, economists, and healthcare experts about the application of expensive and human-intensive technologies in end-of-life situations.
Just as open heart surgery using CPB paved the way for early application of MCS, cardiac transplantation provided the stimulus for proliferation of ventricular assist systems as a bridging therapy to transplantation. With nearly 30% of patients dying while awaiting cardiac transplantation in the early 1980s, a clear need developed for effective and durable MCSDs that could safely support patients until suitable donor hearts could be identified. The improving outcomes following cardiac transplantation and the scarcity of available organs provided the impetus for a major collaborative effort among the heart transplantation community, the National Institutes of Health (NIH), and scientists and clinicians dedicated to the development of durable MCS systems. In 1984, Oyer, Portner, and colleagues reported the first successful cardiac transplant following bridging with a Novacor (WorldHeart Corp., Oakland, Calif.) left ventricular assist device (LVAD). Hill and colleagues subsequently published successful transplantation following support with a Pierce-Donachy pneumatic LVAD. About the same time (1985), Copeland and colleagues performed the first planned total artificial heart implant as a bridge to transplantation. Continuing advancements in mechanical support over the ensuing decade led to the first FDA-approved implantable device as a bridge to transplantation in 1994.
Despite the early focus on MCS as a bridging therapy to transplantation, the clear intent of the scientific and engineering community was the development of devices capable of long-term safe circulatory support. The landmark feasibility study of long-term mechanical support was the Randomized Evaluation of Mechanical Assistance for Treatment of Congestive Heart Failure (REMATCH) trial. As reported by Rose and colleagues in 2001, the HeartMate VE VAD provided significant survival benefit at 1 and 2 years compared with medical therapy for patients with very advanced heart failure who were not suitable for cardiac transplantation. This NIH-sponsored multi-institutional trial provided the impetus for FDA approval of this device for so-called destination therapy in 2002. This set the stage for multiple clinical trials in the application of long-term mechanical support.
In recent years, device technology has increasingly focused on smaller, simpler, and likely more durable continuous flow (rotary) pumps that lack the pulsatile characteristics of earlier “first-generation” pumps. Following the earlier experimental work of Saxton, Andrews, Wampler, DeBakey, and others, recent clinical applications have focused on axial flow and centrifugal pumps.
A biomaterial is a natural or artificial material that remains in contact with one or more internal components of the human body for the purpose of replacing organ function or treating an abnormal condition. Biocompatibility refers to the effect of a specific biomaterial on exposed host tissues, whereas hemocompatibility refers to the specific effects of a biomaterial or circulatory support system on blood components, coagulation cascade, and the tendency for thrombus formation. Successful blood pump design requires special knowledge of microlevel interactions between blood elements and the contact surface as well as macrolevel considerations that include choice of prosthetic valves (if required), inflow and outflow port design, and blood flow pathways within the pump. The ideal biocompatible surface for blood is functioning endothelium, but the creation of a functioning endothelial layer on a bioprosthetic surface remains elusive. Minimizing thrombogenicity requires avoidance of highly thrombogenic biomaterials, specific features of pump design, and pharmacologic inhibition of the coagulation cascade. The requirement for blood-exposed pump components that minimize thrombogenicity has limited compatible materials for blood-exposed surfaces to titanium, polymers (primarily polyurethanes), silicone, graphite, and pyrolytic carbon.
A fundamental concept for the understanding of blood–pump surface interaction is the process of protein adsorption to biomaterial surfaces. Following exposure of pump surfaces to circulating blood in vivo, a protein layer develops that covers the biomaterial surface. The make-up of this protein layer is determined by the protein composition of the patient's blood, the chemical composition of the biomaterial surface (more specifically, surface charge and hydrophobicity), and surface topography (rough vs. smooth surface, porous vs. nonporous). Concentration of proteins in blood, net protein charge relative to the biomaterial surface, distribution of charges on the protein surface, and ability of the protein to undergo conformational changes all contribute to the propensity for a given protein to adsorb to the pump surface. Protein interactions with the biomaterial vary over time and are therefore dynamic. The change in composition of proteins that adsorb to the pump surface over time is termed the Vroman effect . The specific details of these protein-surface interactions contribute directly to the likelihood of pump thrombogenicity, because these proteins are biologically active and can initiate platelet adhesion and activation and trigger coagulation cascades.
Both smooth and rough surface designs have been used successfully in pump design. The textured titanium surface of the HeartMate pulsatile LVAD stimulates the formation of a thin, stable coagulum that, although counterintuitive, has proven effective in minimizing development of pump thrombus.
Application of computer simulations called computational fluid dynamics (CFD) analyses has greatly facilitated the ability to predict the effects of shear stresses in the pump flow pathway and areas of relative stasis on platelet activation and thrombus formation.
Contact between the pump surfaces and specific plasma proteins, including factor XII (Hageman factor), prekallikrein, and factor XI, can initiate the coagulation cascade via the intrinsic clotting system (also called the contact system ) particularly in areas of relative blood stagnation ( Fig. 22-1 ).
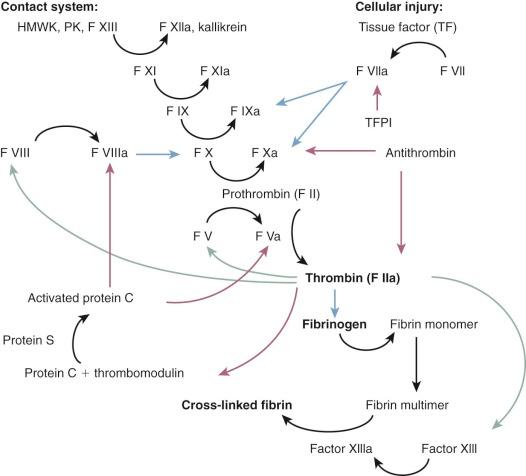
The additional critical component of thrombus formation is platelet adhesion, aggregation, and activation . Normal endothelium is antithrombogenic, in part related to active biochemical reactions involving nitric oxide and prostacyclins. In areas of low shear rates, fibronectin functions as the adhesive system for platelets, whereas von Willebrand factor (factor VIII) is the active adhesive protein for platelets at high shear rates, particularly in combination with fibrinogen.
Adhesion of platelets to vascular subendothelium is facilitated by von Willebrand factor, which forms a bridge between collagen fibrils in the vessel wall and platelet receptors. Following contact activation, platelets undergo changes in shape in which they display spreading pseudopods and release the contents of their α granules (which contain fibrinogen, fibronectin, thrombospondin, von Willebrand factor, β-thromboglobulin, platelet factor IV, and platelet-derived growth factor), which stimulate thrombin generation. Platelet aggregation is stimulated by the release of adenosine diphosphate (ADP). Under laminar flow conditions, the released granule contents cannot accumulate, but when flow is nonlaminar, platelets are more likely to aggregate and accumulate. As newly recruited platelets release their granular contents, thrombin formation is facilitated by the generation of fibrinogen/fibrin bridges. Increasing thrombin generation accelerates the formation of thromboxane A 2 and the release of ADP, which further promotes conversion of fibrinogen to fibrin and platelet activation and aggregation. Damaged red cells from shear stress–induced hemolysis also release ADP, further perpetuating platelet activation.
Preserved vascular endothelial cell function is critical for prevention of intravascular thrombus. Synthesis and release of prostacyclins inhibits platelet activation. Thrombomodulin, an endothelial cell product, neutralizes the procoagulant properties of thrombin and activates protein C, a potent anticoagulant that destroys factors Va and VIIIa. Antithrombin III binds to the endothelial cell plasma membrane and inactivates thrombin.
Specific discussion on anticoagulation therapy for pumps can be found in Special Features of Postoperative Management.
It remains controversial whether VADs differ in their propensity to induce an immunologic response. In vitro studies of the textured titanium surface of the HeartMate XVE indicate that T lymphocytes (probably activated helper T cells) in the neointimal surface lining express strong immunoreactivity for CD3, CD4, and CD25 (interleukin [IL]-2 receptors). Demonstrated defects in T-cell function post implant induce T-cell apoptosis and possibly decreased resistance to infection.
A major disadvantage of MCS as a bridge to transplantation is the frequency of patient sensitization against foreign HLA antigens, which increases the likelihood of developing anti-HLA antibodies against a potential donor (positive cross-match). Although the pump surface has been implicated in this process, it is more likely that transfused blood products, particularly platelets, account for sensitization. Platelets exhibit high concentrations of major histocompatibility complex (MHC) class I and class II HLA antigens (in contrast to red blood cells), and patients receiving more than 6 platelet units are more likely to develop immunoglobulin (Ig)G antibodies against MHC class I antigens.
Specific technologic barriers challenging successful MCS include development of corrosion-resistant materials with minimal toxicity and a high level of structural integrity, management of specific blood-contacting surfaces to minimize thrombogenicity and damage to blood elements, blood pump design, and methods to store energy.
Energy to generate flow from circulatory assist pumps requires conversion of either electrical or pneumatic energy (compressed gas) into kinetic energy (energy of motion). Pulsatile or volume-displacement pumps are usually driven by electric motors that either transfer power directly to a pusher plate mechanism or compress gas or liquid in order to transfer energy to the blood sack or pusher plate. Continuous flow (rotary) pumps use electric motors to transmit kinetic energy to the blood.
The principles of Starling's law also apply to circulatory pumps, in that the pump must respond to higher inflow into the pump by increasing output. As in the natural heart, this balance is maintained in pulsatile pumps by variations in stroke volume or pump rate.
The major cause of hemolysis in blood pumps is rapid acceleration or deceleration of red cells through the pump, which can induce red cell membrane fracture. In general, pump-induced hemolysis is considered acceptable if the plasma free hemoglobin is maintained at less than 19 mg/dL. The rate of pressure increase and flow-channel velocities are maintained at levels designed to avoid high shear stress.
Proper application of fluid dynamics is critical to minimize thrombus formation. Because blood stasis—particularly flow cessation—promotes clot formation, stationary vortex flow must be avoided because the central stagnant portion of the vortex can become a nidus for thrombus formation.
Power sources and alarms must provide reliability and durability backed up by software programs designed to activate appropriate alarm systems when deviations from normal function occur. Approximately 1.6 watts of power are needed to pump 6 L/min at 120 mmHg. Power in excess of 1.6 watts is both wasted and converted to heat that must safely dissipate within the body.
Major pump designs in clinical use today are either pulsatile (volume displacement) or continuous flow (rotary) pumps, which include axial flow and centrifugal pump design. The main features of these basic pump designs are summarized in Table 22-1 . Pulsatile pumps cyclically change the internal volume of a pumping chamber, displacing a specific volume of blood with each ejection. Such pumps require one-way valves to generate forward flow, typically utilizing valves in the inflow and outflow portions of the pump. In hermetically sealed (not vented to the atmosphere) pulsatile pumps, cyclic displacement of blood volume within the pumping chamber must be accompanied by an equal increase of volume elsewhere within the casing. This usually occurs via a compliance sack placed outside the device but within the patient.
| Feature | Positive Displacement | Rotary |
|---|---|---|
| Means of producing flow and pressure | Cyclically changing volume chamber (plus check valves) | Rotating impeller(s) |
| Source of energy (typical) | Air pressure or electricity | Electricity |
| Power requirements | Roughly equivalent, but rotary may be less efficient at high flow | |
| Exterior size | Rotary typically much smaller, also requiring smaller cannula (with continuous flow) | |
| Priming volume | Rotary typically much smaller | |
| Flow range and hemocompatibility | Both types of pumps are plagued by risk of thrombosis at low flow and hemolysis at high flow. | |
| Afterload response | Typically unaffected by afterload | Flow typically drops with increasing systemic vascular resistance (unless speed is regulated). |
| Preload response | Typically passive filling; output typically follows venous return | Flow will increase with venous return, but not capable of active suction (unless speed is regulated) |
| Failsafe (pump stoppage) | Provides effective valved shunt from ventricle to aorta | Creates effective aortic insufficiency, but nominal forward flow is still possible |
| Cost to manufacture | Differences are debatable, although rotary pumps have offered lower cost owing to (ostensibly) less complexity. | |
| Effect on physiology | Influence of pressure and flow patterns (e.g., pulsatility) on end-organ function, arterial remodeling, valvular fusion, lymphatic stasis, and so on, is still inconclusive. | |
Continuous flow pumps consist of a rotating component that has one or more impellers (usually a disk or cylinder with vanes that propel blood forward). One or more bearings support the impeller. The assembly comprising all rotating elements is termed the pump rotor . As the impeller rotates, it imparts rotational velocity to the blood, and this rotational energy must be converted into pressure energy to achieve forward blood flow. To facilitate this process, additional stationary blades or other structures redirect the swirling blood to create pressure and forward blood flow. In axial flow pumps, the stator typically consists of stationary blades, whereas the stator of a centrifugal pump typically consists of a scroll-shaped passage or volute.
The forward flow of blood through an axial flow pump is determined primarily by the speed of the rotor (pump speed) and the pressure difference across the inlet and outlet orifices of the pump. In the absence of obstruction to pump inflow, pressure at the outlet orifices (aorta) always exceeds inlet pressure (left ventricle). At any given pump speed, blood flow through the pump increases as the pressure difference across the inlet and outlet orifices decreases . At any pressure difference across inlet and outlet orifices, blood flow will increase with increasing pump speed. The relationship among flow, pressure, and pump speed is predicted by a series of flow pressure curves called HQ curves ( Figs. 22-2 and 22-3 ). Under physiologic conditions, inlet pressure to the axial flow pump changes in a cyclic fashion during systolic and diastolic phases of the left ventricle. In most situations, even in the presence of severe left ventricular dysfunction and absence of opening of the aortic valve, continuous flow devices contribute some degree of pulsatility to the aortic pressure waveform secondary to the changing differential pressure across the inlet and outlet orifices. Nonpulsatile blood flow occurs in situations of ventricular fibrillation, operation of the pump at too high a pump speed, or with negative inflow pressure causing left ventricular collapse around the inflow orifice.
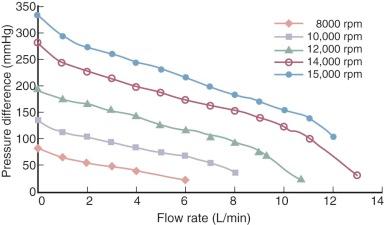
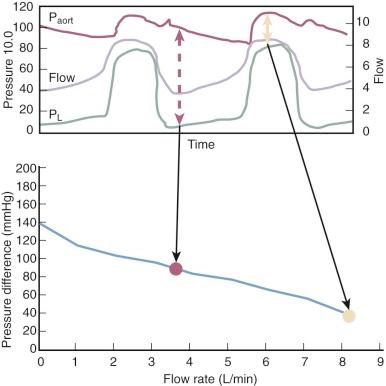
Assessment of left ventricular volume status during axial flow pump support is facilitated by echocardiography, in which ventricular filling, presence or absence of aortic valve opening, and any tendency toward left ventricular chamber collapse around the inflow cannula (suction event) can be assessed. Ideally, pump speed should be adjusted to permit intermittent aortic valve opening, which minimizes the risk of a suction event and may promote more effective washout of the sinuses of Valsalva, decreasing the likelihood of thrombus formation along the aortic valve. However, optimal operating conditions for exercise reserve and promotion of reverse remodeling remain controversial.
Proper design of bearings and seals within devices has provided a major challenge in the progress of MCS. Bearings are devices that provide support, guide movement, and reduce friction of motion between fixed and moving parts. A moving part may be a bladder or pusher plate in a pulsatile pump, or a rotary impeller in a rotary pump. Bearings pose a risk of wear, and therefore failure, secondary to continuous physical contact between solid components. Bearings that remain dry (without direct contact to blood) require special seals that are themselves subject to wear and failure. More recent second-generation pumps avoid seals by using blood itself as the lubricant fluid, with so-called blood-immersed bearings. The third generation of rotary pumps incorporates electromagnetic levitation; these magnetic bearings provide support through magnetic force fields.
Paracorporeal assist devices have a pump positioned outside the body cavity, with inflow and outflow cannulae that traverse the skin and subcutaneous tissues. The most commonly employed paracorporeal pump suitable for outpatient use is the Thoratec (Thoratec Corp., Pleasanton, Calif.) VAD.
The Thoratec paracorporeal ventricular assist device (PVAD) can be used for support of the left ventricle (LVAD), right ventricle (RVAD), or both ventricles (BVAD) ( Fig. 22-4 ). The Thoratec PVAD received FDA approval for bridge-to–cardiac transplantation therapy in 1995 and has been implanted in more than 3000 patients. The PVAD has a 65-mL stroke volume polyurethane chamber with two mechanical valves. The pneumatic driver applies alternating positive and negative air pressure to achieve a clinical beat rate of 40 to 110 beats/min. The PVAD is positioned on the anterior abdominal wall, with cannulas traversing the skin and mediastinum to provide connections to the heart and great vessels ( Fig. 22-5 ). The device can be pneumatically actuated in an ambulatory setting with a portable TLC-II (Thoratec) driver. The TLC-II was approved for home discharge by the FDA in 2003.


Chronic anticoagulation with warfarin is required, with a target international normalized ratio (INR) of 2.5 to 3.5. Aspirin or other antiplatelet therapy is usually added to the anticoagulant regimen. Anticoagulation usually begins with heparin following cessation of bleeding, but some protocols recommend avoidance of heparin completely in the early anticoagulation phase.
The Thoratec implantable ventricular assist device (IVAD) is designed to provide all the same features as the PVAD, with pump placement in the intracorporeal position ( Fig. 22-6 ). The IVAD has the same pumping chamber, mechanical valves, and stroke volume as the PVAD. The major differences in the IVAD include a smooth, polished titanium housing for implantability, lighter weight (339 g vs. 417 g), and a narrower 9-mm percutaneous lead, compared with the 20-mm paracorporeal driveline. An optical sensor detects when the pump is full or empty. Internal implantation is appropriate for patients with a body surface area (BSA) greater than about 1.6 m 2 . The same anticoagulation regimen is used as for the PVAD.

The largest experience with durable pulsatile VADs stems from the HeartMate XVE LVAD, a variant of which was first introduced in 1986 with pneumatic power. More than 5000 patients have been treated with this device over the past 20 years, and it was used in the REMATCH trial, which led to its approval in the United States for permanent MCS therapy.
The HeartMate LVAD ( Fig. 22-7 ) is a positive-displacement pump made of titanium with a polyurethane diaphragm and a pusher-plate actuator that converts electrical energy to mechanical energy. It may be powered pneumatically or electrically, but only the vented electric version (XVE) is currently used. Cannulation involves a left ventricular apical cannula and an outflow graft to the ascending aorta. Two porcine valves provide directional flow.

The vented electric system is powered by an electric motor that rotates and displaces a pusher-plate that compresses the blood sack to initiate ejection. Air displaced by the diaphragm is vented to the atmosphere by a venting apparatus incorporated into the percutaneous driveline. The vent site provides a portal for pneumatic activation in the event of electrical failure. Power is supplied by two external batteries and an external controller.
Maximum stroke volume is 83 mL, and pumping may be performed in a fixed-rate or “automatic” mode, in which the stroke volume is maintained at a level of 97% full by varying the rate in response to preload. If the rate is adjusted manually, stroke volume should be maintained between 70 and 80 mL, because slower stroke volumes increase the potential for thrombogenesis.
The most unique feature of the HeartMate XVE device is the blood pumping surface, which consists of titanium microspheres and a fibrillar texture that promotes formation of a “pseudointima” that is resistant to thrombogenesis. The neointimal surface that develops is composed of collagen as well as cells derived from circulating progenitors of fibroblasts, myofibroblasts, monocytes, macrophages, and endothelial cells. Anticoagulation requirements for the XVE pump are unique among current MCS systems. The textured titanium surface creates a thrombo-resistant pump that requires only aspirin for effective anticoagulation. This creates a special indication for this pump in the setting of clinical comorbidities likely to produce bleeding complications during VAD support.
Unfortunately, the major advantage of minimal anticoagulation requirements is currently outweighed by the poor long-term durability of this pump. With the current iteration of the XVE, bearing wear is a major source of device malfunction. Typically, the HeartMate XVE develops signs of bearing wear within 18 to 24 months ( Box 22-1 ). Pump stoppage can be expected within days to weeks of the onset of signs of important bearing wear. When bearing wear is accompanied by transient pump stoppage or repetitive alarms, the patient can be switched to the pneumatic drive mode in the hospital setting while plans are made for surgical pump exchange. In the event of pump stoppage at home, emergent hand pumping can safely support the patient during transport to the hospital.
Excessive pump motor dust in the air vent filter
Excessive current alarm indicating increased current use
Changes in pump motor sounds or rhythm
The Novacor LVAD (WorldHeart Corp., Oakland, Calif.; Fig. 22-8 ) holds a special place in the history of MCS as one of the first devices designed for permanent device support. In 1984, the Novacor was the first durable pump used as a successful bridge to transplantation. Implanted in over 1600 patients worldwide, the Novacor has demonstrated high system reliability in the outpatient setting, with a low incidence of device malfunction.
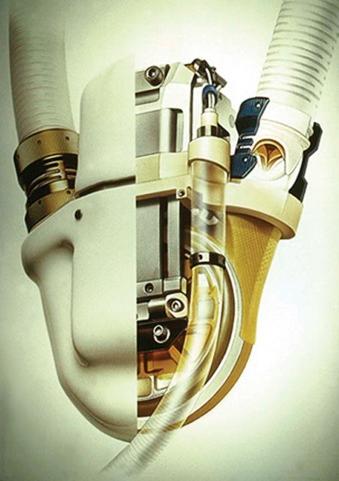
The pump drive unit incorporates a dual pusher-plate pump mechanism coupled to a pulsed solenoid energy converter driver. The percutaneous drive line contains the vent tube. Two porcine valves are incorporated in the inflow and outflow conduits.
Thromboembolic complications have been the major weakness of the Novacor device, despite standard anticoagulation with warfarin and antiplatelet agents. The primary source of particulate embolization was likely a friable pannus that often developed in the inflow conduit. Subsequent design modifications to include expanded polytetrafluoroethylene (PTFE) have importantly reduced embolic events. In a multi-institutional analysis, the incidence of embolic stroke was 5.3% during an average support duration of 162 days using the modified inflow conduit.
Rotary (continuous flow) pumps differ from volume displacement pumps in that instead of employing a chamber that changes volume and ejects blood via unidirectional valves, rotary pumps impart forward flow by rotating impellers with no valves. (See also discussion of continuous flow technology in Engineering Concepts in Pump Design .) These pumps are electrically driven and have the distinct advantages of small size and smaller drive lines. In contrast to volume displacement pumps, rotary devices are more afterload sensitive; at constant pump speed, the flow drops with increased systemic vascular resistance (see Fig. 22-2 ). The absence of inflow and outflow valves in rotary pumps improves overall pump durability and simplicity but removes a key failsafe feature of one-way valves: pulsatile pumps typically have a mechanism for hand pumping in the event of an electrical failure, but no such hand pumping device is available for rotary pumps. Specifically, pump stoppage with rotary pumps induces varying degrees of “aortic insufficiency,” with nothing to prevent reversal of flow from aorta into ventricle during diastole. If not promptly corrected, this rare event is frequently fatal in the setting of advanced heart failure, which invariably accompanies pump implantation.
Although continuous flow pumps are usually not pulsatile to a degree that produces a palpable pulse (unless the native heart is rejecting), these rotary pumps typically produce some pulsatility in blood pressure (as measured with an indwelling arterial line) due to changing pump flows throughout the cardiac cycle.
Rotary pumps currently in clinical use are of two major types: axial flow and centrifugal flow. The designations axial and centrifugal refer specifically to the design of the impeller, without reference to the orientation of inlet or outlet ports. It is the rotation of the impeller that imparts rotational velocity to the blood, creating forward flow. Pure axial flow pumps act like a fan, adding energy by deflecting flow in the circumferential direction. Centrifugal pumps typically contain disk-shaped impellers, with blood entering at the center of the impeller and exiting radially at the periphery. Typically, axial pumps feature inlet and outlet ports that are opposed 180 degrees from each another, and centrifugal pumps have ports oriented perpendicular to each other. Within this spectrum of pumps lies a continuum of mixed flow pumps, incorporating aspects of axial and centrifugal design.
Axial flow pumps in current use in clinical practice contain many features represented in the HeartMate II (Thoratec Corp., Pleasanton, Calif.), the only rotary pump currently approved (as of March 2011) by the FDA ( Fig. 22-9 ). The blood pump component of the HeartMate II is composed of a straight titanium tube that houses the inlet stator, the fixed component that forms the pivotal housing for the rotor; the rotor, the rotational component that includes the impeller blades; the rotor magnet, which generates the magnetic field to induce impeller movement; and the outlet stator, which converts the radial velocity of blood flow to an axial direction ( Fig. 22-10 ). The other major pump components include the inlet cannula, implanted into the left ventricular apex, and the outlet cannula, which returns blood from the pump outlet to the ascending aorta.

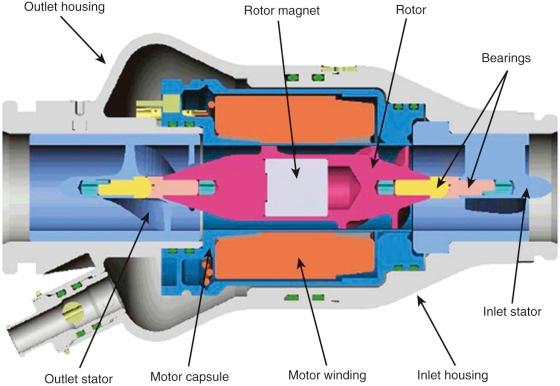
The system driver sends electric power and operating signals to the pump and receives pump information. The wearable driver is powered by either a power base unit or two 12-volt rechargeable batteries that generally provide 2 to 4 hours of power. Power transmitted to the electric motor within the hub of the rotor creates a spinning magnetic field. The pump rotor spins on two bearings located at the inlet and outlet stators. The rotor that spins within the magnetic field is the only moving component of the device. Ceramic bearings are washed by the flow of blood. Operating pump speed ranges from 6000 to 15,000 rpm and is capable of generating blood flow up to about 8 L/min. The approximate pump weight is 350 g, with a length of 7 cm and diameter of 4 cm.
Special features of the HeartMate II pump include a flexible joint between the intraventricular portion of the cannula and its connection to a titanium elbow that joins the pump. The flexible portion of the inlet cannula reduces the risk of malalignment of the intraventricular portion of the inlet cannula with respect to the ventricular cavity. The inlet cannula design reduces torque on the intraventricular portion of the inlet cannula. An extension in the length of the intraventricular rigid cannula improves reliability of continuous flow throughout the cardiac cycle and at various levels of left ventricular filling. The pump rotor, blood tube within the pump housing, and inlet and outlet stators are smooth titanium surfaces. The inlet and outlet elbows and the intraventricular cannula are textured with titanium microsphere coatings similar to the design of the HeartMate XVE.
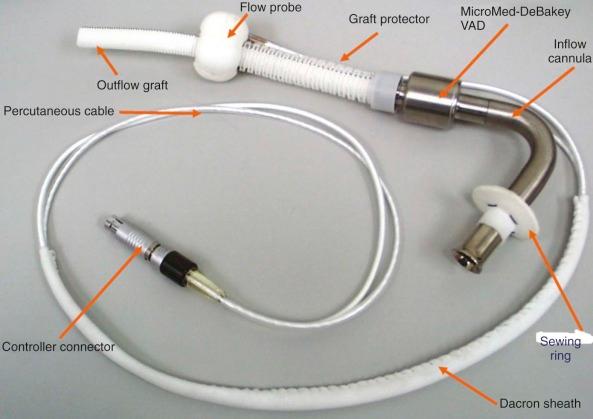
The MicroMed-DeBakey LVAD (MicroMed Cardiovascular Inc., Houston, Tex.) is an axial flow pump which consists of an elbow-shaped inflow cannula that inserts into the left ventricular apex, a pump housing unit, a Dacron outflow graft which connects to the ascending aorta, and an ultrasonic flow probe that encircles the outflow grafts and provides direct measurement of pump flow ( Fig. 22-11 ). The pump impeller is actuated by an electromagnet and is the only moving part of the pump system. Wiring from the pump and the flow probe are contained in a flexible driveline that connects to the portable controller. External components include a controller system that operates the pump and a clinical data acquisition system. A potential advantage of this pump is the presence of a flow probe that allows accurate rather than estimated pump flow. Only the pediatric version of this pump (DeBakey Child) is FDA approved, but the adult version has been extensively used in Europe.
This and the remaining continuous flow pumps discussed are not yet FDA approved in the U.S. The Jarvik 2000 (Jarvik Heart Inc., New York, N.Y.) is an electrically powered, axial-flow blood pump that provides continuous flow from the left ventricle to the ascending or descending thoracic aorta ( Fig. 22-12 ). A unique feature of this device is the placement of the axial flow pump within the left ventricular cavity. The overall system consists of the blood pump, a Dacron outflow graft, percutaneous power cable, pump speed controller, and direct-current power supply. The single moving part is the impeller located in the center of the titanium housing. Electromagnetic forces rotate the impeller, which consists of an electromagnet suspended by two ceramic bearings. All blood-contacting surfaces are made of smooth titanium. The pump operates at 8000 to 12,000 rpm and can generate flows up to 8 L/min. This pump is particularly suited to “off-pump” implantation as described in Techniques of Operation.
The Arrow CorAide LVAD (Cleveland Clinic Lerner Research Institute, Cleveland, Ohio) is a centrifugal-flow electrically powered third-generation LVAD ( Fig. 22-13 ). The pump can be operated in a fixed-speed mode or an automatic-control mode in which the pump speed varies according to heart rate and systemic blood pressure. The pump and electrical cable weighs about 300 g and operates at speeds between 2000 and 3000 rpm. Pump flow rates are up to 8 L/min. Blood contact surfaces are fabricated from titanium. The rotating assembly uses a combination of magnetic forces and hydrodynamic forces for suspension, in which there is no surface contact with the bearings. A unique apical cuff clamp allows positioning and fixation of the apical cannula to optimize pump flow.

The Terumo DuraHeart (Terumo Heart Inc., Ann Arbor, Mich.) is a magnetically levitated centrifugal pump ( Fig. 22-14 ). Contact-free rotation of the impeller contributes to minimal material wear, making the pump design favor a high level of durability. The blood-contacting surfaces of the pump and the inlet conduit are made of titanium and modified with a heparin coating. The pump weighs 540 g and has a diameter of 7.2 cm and a height of 5 cm, suitable for patients with a 1.3 m 2 or greater BSA.

The EVAHEART (Sun Medical Technology Research Corp., Nagano, Japan) is a centrifugal pump with a weight of 420 g ( Fig. 22-15 ). The pump contains a thromboresistant coating over its blood contact surfaces. The pump contains a unique system whereby the impeller rotates on a shaft and is cooled with fluid (Cool-Seal) pumped by way of a percutaneous channel from an external device that circulates behind the mechanical seal and removes debris. The 16-mm inflow and outflow conduits are made of PTFE.

The HeartWare LVAD (HeartWare Ltd., Sydney, Australia) is a miniaturized centrifugal flow pump that sits entirely within the pericardial cavity ( Fig. 22-16 ). A unique feature is the left ventricular apical inflow cannula, which is an integral part of the pump. The device weighs only 145 g but can generate flows up to 10 L/min. An apical sewing ring attaches to the left ventricular apex and allows adjustment of the inflow cannula orientation. Impeller blades are held in place by a hybrid magnetic and hydrodynamic bearing system.

The major advantage of the total artificial heart (TAH) over VADs is the provision for biventricular support with an implantable device. (The only currently available implantable separate right and left VAD system is the Thoratec IVAD system [see previous section].) The major disadvantage of the TAH is the necessity of removing the native heart, which removes the option of recovery. The second major disadvantage of current iterations is the space requirement for the device, such that only patients with a BSA of about 1.7 m 2 or greater are suitable.
The two TAH devices currently available for clinical use are the SynCardia CardioWest C-70 TAH and the AbioCor TAH. Both pumps replace the native ventricles and all four valves in the orthotopic position.
The SynCardia TAH (SynCardia Systems Inc., Tucson, Ariz.) is a pneumatic pulsatile pump in which a rigid spherical outer housing around each artificial ventricle supports a seamless blood-contacting segmented polyurethane diaphragm, two intermediate diaphragms, and an air diaphragm ( Fig. 22-17 ). Two Medtronic 27-mm inflow valves and two Medtronic 25-mm outflow valves provide unidirectional flow. The full ejection volume of each ventricle is 70 mL per beat, and the TAH typically generates a cardiac output of 7 to 8 L/min. The atrial components are sewn to the native atrial cuffs, and the atrioventricular and ventricular outflow graft connections are via “quick snap” connectors. The external console consists of one primary and one secondary pneumatic driver, air tanks, transport batteries, an alarm, and a computer monitoring system. Current portable pneumatic drivers and consoles allow out-of-hospital living with full ambulation.
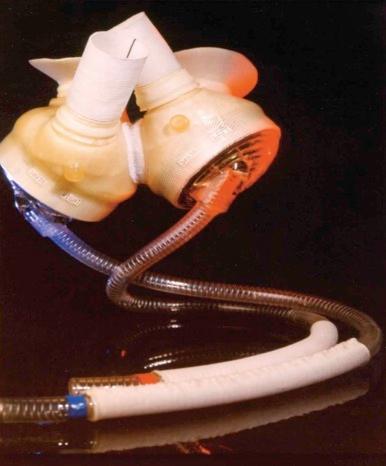
The AbioCor (Abiomed, Danvers, Mass.) is the first totally implantable heart replacement system that does not require percutaneous lines ( Fig. 22-18 ). The four internal components are the thoracic unit, battery, controller, and transcutaneous energy transfer (TET) coil. Situated between the two artificial ventricles is an energy converter that contains a miniature centrifugal pump that moves hydraulic fluid alternately between left and right ventricles, producing alternate left and right systole. A balance chamber allows for adjustment of right-sided stroke volume to compensate for bronchial blood flow. All blood contact surfaces, including the trileaflet valves (24-mm internal diameter) are polyurethane. An internal controller drives the energy converter in the thoracic unit, monitors the implanted components, and transmits device performance data to an external console via radiofrequency telemetry. An internal TET coil receives high-frequency power across the skin from an external TET coil that powers the thoracic unit and charges an internal battery. Current software directs changes in pump rate in response to venous return to the heart.

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here