Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Historically, the goals of treatment of Crohn disease (CD) and UC have been achievement of symptomatic remission, namely absence of abdominal pain, diarrhea, or rectal bleeding and normalization of primarily symptom-based disease activity indices such as the CD activity index (CDAI) or simple clinical colitis activity index. Achieving these goals in the absence of corticosteroids, termed “steroid-free remission,” was recognized as sufficient to improve the patient’s quality of life (QOL) and short-term outcomes. However, it increasingly has been recognized that in many patients, symptoms demonstrate an imperfect correlation with objective resolution of inflammation, and that the latter rather than the former may be more important to ensure the best long-term outcome. A large Norwegian prospective study of 740 patients with IBD demonstrated that those who achieved mucosal healing within 1 year of diagnosis had a significantly lower likelihood of undergoing colectomy or requiring subsequent courses of corticosteroids up to 5 years after diagnosis. Incorporation of mucosal healing as an endpoint in clinical trials has yielded similar correlation between early objective endoscopic healing on colonoscopy and superior longer-term outcomes, and indeed such an approach may be more cost-effective than one targeting resolution of symptoms. The definition of an appropriate endoscopic treatment target has varied between clinical studies and continues to evolve. In CD, endoscopic healing is frequently defined as the absence of ulceration, corresponding to a cut-off <3 when disease activity is scored using the CD Endoscopic Index of Severity (CDEIS). For UC, a Mayo endoscopic score of 0 (normal, intact mucosa) or 1 (erythema, vascular blunting, but absence of friability, erosions, or ulcerations) is termed “endoscopic remission,” though emerging data suggest that an endoscopic score of 0 may be achievable in many patients and it is associated with better outcomes when compared to a score of 1. Given the transmural nature of inflammation in CD, radiologic resolution of inflammation on cross-sectional imaging is also of interest. There exist several scoring systems for quantifying active inflammation or damage in the small or large bowel. The cut-offs for defining healing using cross-sectional imaging still require validation, however, radiologic improvement of inflammation with initiation of biologic therapy correlates with improved patient outcomes and offers an attractive therapeutic target in many patients. Fecal inflammatory markers such as calprotectin also demonstrate strong correlation with objective inflammation on endoscopy and normalization of such fecal markers can be surrogates for achievement of endoscopic healing. Recognizing the progressive nature of these diseases, disease damage scores like the Lémann Index that incorporate endoscopic and radiologic severity of disease as well as prior history, such as resections, have been developed. The treatment target in CD and UC is not merely resolution of active inflammation but also ensuring normalization of patients’ health-related QOL. Many patients with IBD, despite achieving endoscopic remission, may continue to experience symptoms such as pain, depression, anxiety, and fatigue which can have a negative impact on functioning. A multidisciplinary approach is beneficial and new models of IBD care are emerging to help patients achieve relief from symptoms, restore QOL, and reduce unplanned care. Other treatment targets include achieving fistula healing, maintaining nutrition, preventing therapy-related adverse events, and, in children, normalization of growth and development.
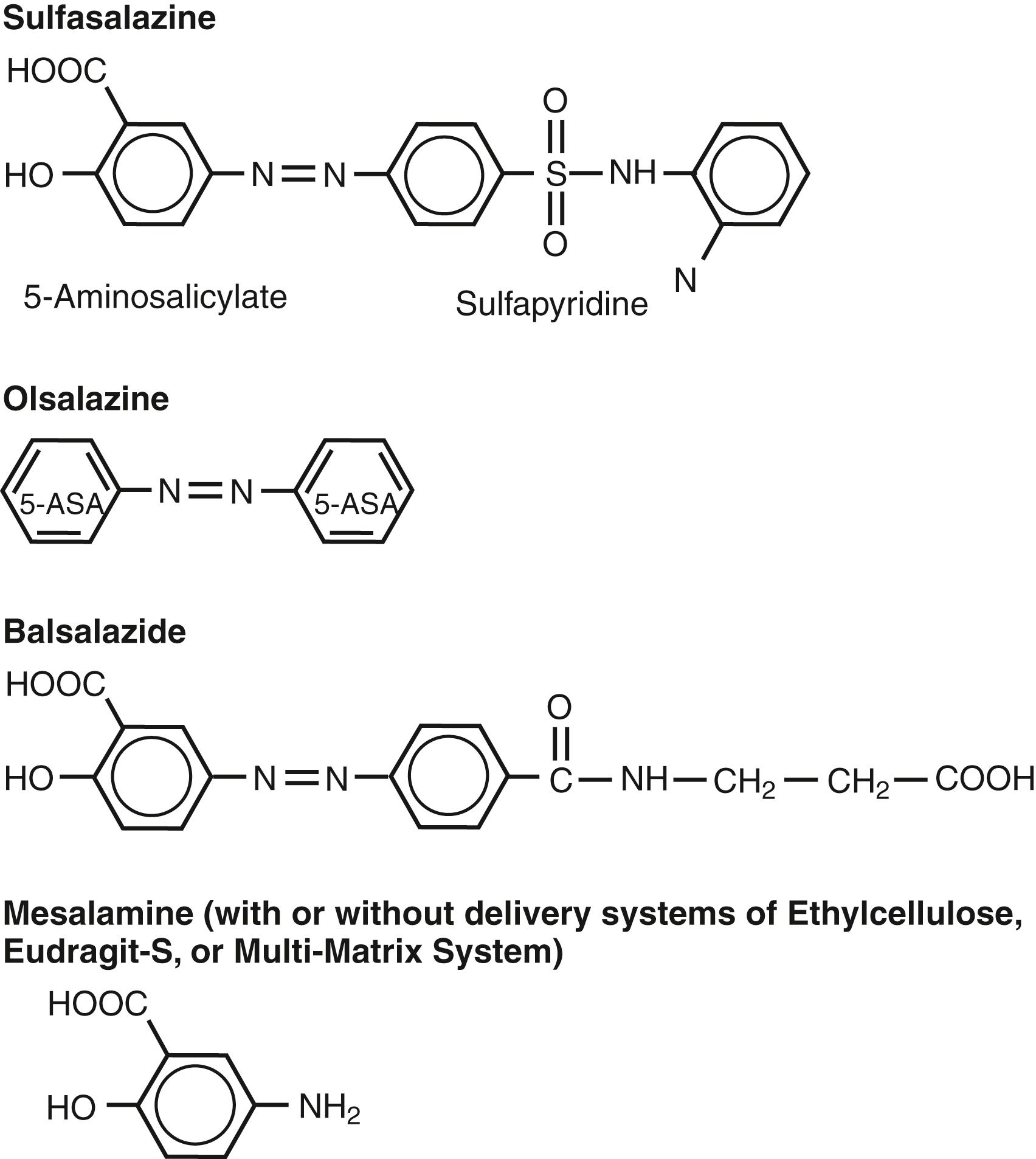
Sulfasalazine, the parent compound of all aminosalicylates (ASAs) used in IBD, was developed by the Swedish physician Nanna Svartz in 1938 to 1939 as a treatment for RA. In 1941 to 1942 it was serendipitously found to improve the intestinal symptoms of patients with colitis who were being treated for associated arthropathy. Most of the adverse effects (AEs) of sulfasalazine are due to its sulfapyridine moiety, which is used to prevent absorption of the 5-ASA portion of the molecule in the small intestine. A classic experiment by Azad Khan and colleagues showed that 5-ASA (mesalamine) rather than sulfapyridine is the therapeutic moiety of sulfasalazine. Approximately 90% of sulfasalazine reaches the colon, and only a small amount is absorbed in the small intestine. On reaching the colon, the enzyme azoreductase, which is elaborated by anaerobic colonic bacteria, cleaves the azo bond to release the active constituent moiety, 5-ASA; this agent is thought to effect its anti-inflammatory activity topically, but possibly also through absorption and via the microenteric circulation. Of the 5-ASA that is absorbed from the colon, 20% undergoes hepatic acetylation, forming N -acetyl 5-ASA, and is excreted in the urine.
Because the sulfapyridine moiety is thought to be responsible for most of the AEs of sulfasalazine therapy, various sulfapyridine-free formulations and controlled-release systems ( Fig. 116.1 ; Table 116.1 ) have been developed to deliver 5-ASA to specific sites of the GI tract. Olsalazine (Dipentum) is a 5-ASA dimer with an azo bond linkage that is formulated in gelatin capsules. Balsalazide (Colazal) consists of a 5-ASA monomer linked to 4-aminobenzoyl-β-alanine, a relatively large biologically inactive carrier molecule. Like sulfasalazine, 5-ASA is released from olsalazine and balsalazide in the colon upon cleavage of the azo bond by bacterial enzyme azoreductase. Approximately 99% of uncleaved drug is delivered intact to the colon, and its metabolites are cleared rapidly in stool and in the urine. Four different commonly used mesalamine preparations allow delivery of 5-ASA before the drug reaches the colon: Pentasa, Apriso, Asacol, and Lialda. Pentasa uses ethyl cellulose-coated microgranules that release mesalamine from the duodenum throughout the small intestine and the colon; about 50% of the 5-ASA is released in the small intestine, and the remainder is released in the colon. Apriso uses a slightly different formulation of mesalamine granules with a delayed-release enteric coating that allows for the gradual release of mesalamine at a pH of ≥6 in the terminal ileum and throughout the colon. Asacol is a Eudragit-S-100–coated mesalamine tablet that is released at a pH >7, usually in the distal ileum and the colon. With Asacol, about 15% to 30% of the mesalamine is released in the small intestine. Lialda (MMX mesalamine) is a novel mesalamine formulation that uses a multimatrix structure composed of an inner lipophilic matrix and an outer hydrophilic matrix. It is coated with a pH-dependent polymethacrylate film to allow the delayed release of mesalamine at a pH >7 in the terminal ileum and colon. This technology also allows mesalamine to be released slowly and in close proximity to the colonic mucosa. Additional delivery systems include mesalamine suppositories or enemas, which provide the drug to the rectum and distal colon.
| Drug | Formulation | Site of delivery |
|---|---|---|
| Prodrugs | ||
| Balsalazide | 4-aminobenzoyl β-alanine + 5-ASA | Colon |
| Olsalazine | 5-ASA dimer | Colon |
| Sulfasalazine | Sulfapyridine + 5-ASA | Colon |
| Mesalamine Preparations | ||
| Asacol, Claversal, Delzicol Salofalk/Apriso | pH sensitive, resin-coated; delayed release | Distal ileum, colon |
| Canasa | Suppository | Rectum |
| Lialda | pH sensitive, multi-matrix and polymethacrylate coated; delayed and slow release | Distal ileum, colon |
| Pentasa | Ethylcellulose-coated microgranules; controlled release | Duodenum to colon |
| Rowasa | Enema | Distal colon |
There are limited data supporting a role for ASAs in the management of CD. Most studies have shown sulfasalazine to be superior to placebo in inducing remission in active CD, when the colon is the primary site affected. Efficacious doses, as used in the National Cooperative Crohn’s Disease Study (NCCDS), are in the range of 4 to 6 g/day (1 g/15 kg body weight). The European Cooperative Crohn’s Disease Study found that sulfasalazine at a dose of 3 g/day provided no significant benefit in achieving remission. Early studies with controlled-release mesalamine (Pentasa) at doses <2 g/day failed to show efficacy in the treatment of mildly to moderately active CD. A large study comprising 466 patients with mildly to moderately active CD compared daily doses of 1, 2, and 4 g with placebo over a period of 16 weeks. The 43% remission rate on 4 g mesalamine was statistically and clinically superior to the placebo response rate of 18%. Subsequent trials of similar design, however, failed to show benefit over placebo; although the treatment effect was of similar magnitude, the placebo response was larger than the originally observed 18%. A meta-analysis failed to demonstrate a clinically significant benefit of Pentasa 4 g/day in patients with mildly to moderately active CD. Numerous studies with a variety of preparations also have failed to demonstrate prevention of relapses of CD with 5-ASA compounds. Therefore, although maintenance therapy with mesalamine commonly is prescribed in CD, little data justify the expense and inconvenience of this practice, and mesalamine-based products have been excluded from recent evidence-based treatment algorithms. In summary, sulfasalazine (4 to 6 g/day) may be useful for inducing remission of mildly to moderately active colonic CD, whereas the role of mesalamine is uncertain. In individual cases, the small margin of benefit and relatively slow onset of effect (4 to 8 weeks) may be weighed against the excellent safety profile of these agents ( Table 116.2 ).
| Agent | Adverse Effects | Pregnancy ∗ | Nursing ∗ |
|---|---|---|---|
| 5- Aminosalicylates (5-ASA ) | |||
| Sulfasalazine | Anorexia, dyspepsia, nausea and vomiting, hemolysis, neutropenia, agranulocytosis, folate deficiency, reversible male infertility, neuropathy; see also sulfa-free 5-ASAs | No evidence of teratogenicity; normal fetal growth; give with folic acid | Negligible amounts found in breast milk; safe for term neonates |
| Sulfa-free 5-ASAs (mesalamine, olsalazine, balsalazide) | Headache, drug fever, rash, paradoxical disease exacerbation, pancreatitis, hepatitis, pericarditis, pneumonitis, nephritis Secretory diarrhea (olsalazine) |
No evidence of teratogenicity in humans, normal fetal growth Branded Asacol and Asacol HD with dibutyl phthalate coating is associated with teratogenicity in animals |
Found in breast milk in low concentrations; rare watery diarrhea in breast-fed infants |
| Antibiotics | |||
| Metronidazole | Anorexia, nausea and vomiting, dysgeusia, disulfiram-like effect, peripheral neuropathy, reversible neutropenia | Questionable teratogenicity, normal fetal growth | Found in breast milk; with rare exception, should not be used |
| Ciprofloxacin | Nausea and vomiting, headache, restlessness, rash, pseudomembranous colitis, elevated serum aminotransferase levels, spontaneous tendon rupture | Theoretical teratogenic potential; insufficient data in humans | Found in breast milk, should not be used |
| Glucocorticoids | |||
| Classic | Sleep and mood disturbance, acne, striae, hirsutism, adrenal suppression, proximal myopathy, glucose intolerance, hypertension, narrow-angle glaucoma, cataracts, pseudotumor cerebri, infection, edema, impaired wound healing, growth retardation, bone loss, aseptic necrosis | No evidence of teratogenicity in humans, more frequent stillbirths and reduced fetal birth weight when used for other diseases; may be used as indicated by severity of disease | Safe for breast-feeding |
| Budesonide (ileal release , multimatrix (MMX) | Adrenal suppression at doses of 9 mg daily, but occurrence of classic glucocorticoid AEs are similar to placebo | Limited human data but probably low risk | No data available, probably safe for breast feeding |
| Immunomodulators | |||
| 6-mercaptopurine, azathioprine | Nausea, drug fever, rash, arthralgias, leukopenia, thrombocytopenia, bone marrow suppression, pancreatitis, hepatitis, infection; lymphoma | Teratogenic in animals, but large series in renal transplantation and other diseases including IBD do not show an increase in birth defects; evidence for fetal growth retardation and prematurity; isolated cases of neonatal immune and bone marrow suppression may be used when indicated because of disease severity | Small amounts excreted in breast milk; not recommended |
| Methotrexate | Anorexia, nausea and vomiting, leukopenia, megaloblastic anemia, alopecia, hepatic fibrosis, interstitial pneumonitis, neuropathy | Highly teratogenic, particularly in the first trimester; abortifacient | Small amounts excreted in breast milk; not recommended |
| Cyclosporine/tacrolimus | Reversible or irreversible decrease in renal function, hypertension, tremor, headache, paresthesias, seizure, gingival hyperplasia, hypertrichosis, hepatotoxicity, infection, lymphoma | Significant levels in fetal circulation; does not appear to be teratogenic; intrauterine growth retardation and premature delivery are increased, especially at higher doses; little reported experience in IBD | Excreted in breast milk; not recommended |
| Tofacitinib | Infections, particularly herpes zoster infection, headache, skin rash, increased serum cholesterol, diarrhea, anemia, increased creatine phosphokinase (CPK) ; possible pulmonary embolism and increased mortality were seen with increased doses in high risk rheumatoid arthritis patients; this has not been seen in UC patients. | Limited information | Limited information; consider avoiding until further information is available |
| Biologic Response Modifiers | |||
| Anti-TNF antibodies (infliximab, adalimumab, golimumab, certolizumab pegol) | Upper respiratory tract and other infections, disseminated TB, increased risk of systemic fungal infection and other intracellular pathogens, acute or delayed hypersensitivity reactions, antinuclear antibodies, anti–double-stranded DNA antibodies, lupus-like reaction, demyelinating disease, lymphoma; contraindicated in heart failure because of increased mortality | Growing amount of evidence supporting safety, but still relatively limited data in humans. Infliximab and adalimumab can freely cross the placenta and lead to high levels in newborns, whereas certolizumab pegol only crosses the placenta in very limited quantities | Minimal levels in breast milk |
| Natalizumab | Headache, flushing, infections, progressive multifocal leukoencephalopathy, jaundice, liver failure | Teratogenic in animals; case series in patients with multiple sclerosis suggest it is safe | Unknown safety in nursing |
| Vedolizumab | Nasopharyngitis, upper respiratory infections, headache | Limited data; likely transplacental transfer occurs | Unknown; likely small amounts are present in breastmilk |
| Ustekinumab | Upper respiratory and other systemic infections, headaches | Limited data; likely transplacental transfer occurs | Unknown; likely small amounts are present in breastmilk |
∗ From Connell WR. Safety of drug therapy for inflammatory bowel disease in pregnant and nursing women. Inflamm Bowel Dis 1996; 2:33-47, with permission. Updated based on Ng S, Mahadevan U. Management of inflammatory bowel disease in pregnancy. Expert Rev Clin Immunol 2013; 9:161-73.
Sulfasalazine and various oral mesalamine derivatives have demonstrated efficacy for induction and maintenance of remission in mildly to moderately active UC ( Fig. 116.2 ). They have not been evaluated in a randomized, controlled fashion in patients with severely active disease. At a dose of 3 to 6 g/day, sulfasalazine induces remission in 39% to 62% of patients with mildly to moderately active UC, about twice the remission rate of placebo-treated patients. Meta-analyses have demonstrated that the mesalamines are as efficacious as sulfasalazine, and the various mesalamine preparations appear to be comparable in efficacy. A Cochrane review of oral 5-ASA for induction of remission included a total of 53 studies comprising 8548 patients. In the induction studies, 29% of patients treated with 5-ASA entered clinical remission compared with 17% of patients given placebo. There was no difference in efficacy between patients who received the conventional dosing in 2 to 4 split doses daily compared with those who received once daily dosing (relative risk [RR] 0.94, 95% confidence interval 0.83 to 1.07) suggesting that the latter may be more effective from the standpoint of patient adherence because of increased convenience.
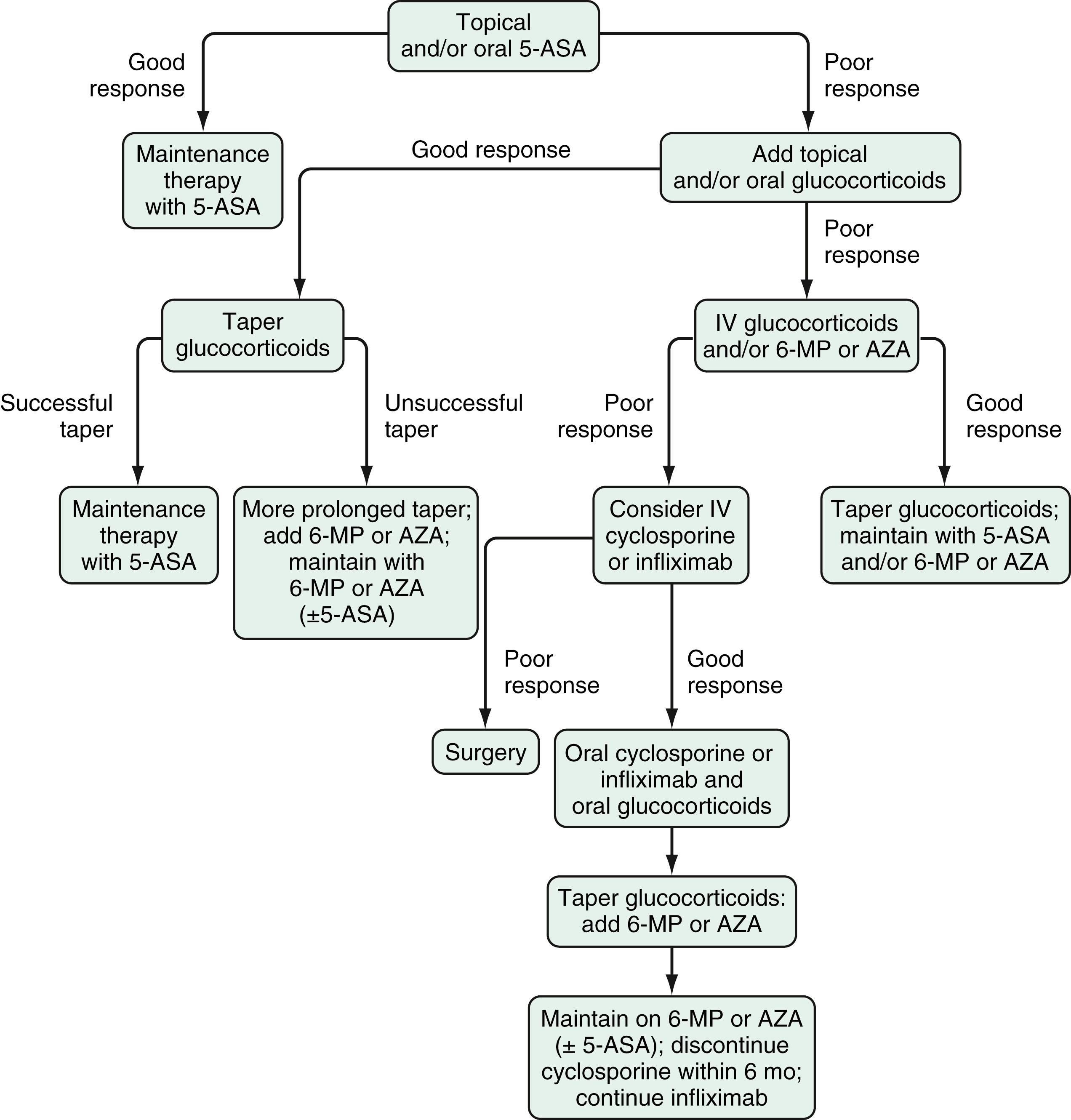
More important than the specific 5-ASA preparation is the dose-dependent response when 5-ASA is used as an induction therapy for active UC. For this indication, mesalamine is not effective at doses less than 2 g daily, and there may be an increased response at doses of 4 to 4.8 g daily for some patients. The ASCEND I and II trials showed that mesalamine at doses of 2.4 and 4.8 g/day had similar efficacy for patients with mildly active disease, but that the higher dose (4.8 g/day) led to significantly higher response rates, but not remission rates, in patients with moderately active disease. The ASCEND III trial reported that patients with moderately active UC treated with Asacol 4.8 g/day had significantly higher remission rates but not response rates than those treated with 2.4 g/day. Also, data from ASCEND III and the Lialda trials showed that the 4.8 g/day dosing was more effective than 2.4 g/day dosing in patients previously treated with mesalamines or for those who did not achieve clinical remission after 8 weeks of therapy. The 4.8 g/day dose of mesalamine is comparable to 12 g/day of sulfasalazine, which is impractical in clinical practice because of the high pill burden and dose-dependent intolerance.
Once remission is achieved, sulfasalazine and other 5-ASAs are effective in maintaining it. This benefit appears to be dose-dependent for sulfasalazine, with a dose of 2 g/day often used to balance efficacy and AEs. Such a dose-dependent response, however, has not been found with the other 5-ASA preparations, and doses of 1.5 to 4.8 g/day maintain remission in more than 50% of patients. A similar review of 41 studies demonstrated that 5-ASAs were superior to placebo for maintaining both clinical and endoscopic remission. Fewer patients (41%) on 5-ASA therapy relapsed when compared with those receiving placebo (58%, RR 0.69, 95% CI 0.62 to 0.77) with a trend toward lower rates of relapse with higher doses of mesalamines. Compared to less than 1 g/day, patients receiving 1 to 1.9 g/day (RR 0.65, 95% CI 0.56 to 0.76) and more than 2 g/day (RR 0.73, 95% CI 0.60 to 0.89) had fewer relapses. Although there was no difference between agents in induction of remission, sulfasalazine was found to be modestly superior to the other 5-ASAs for maintenance of remission (RR 1.14, 95% CI 1.03 to 1.27). Other meta-analyses have suggested that the superiority of sulfasalazine lasts for only 6 months, and when the trial was extended to 12 months, this statistically significant benefit was lost. A double-blind randomized controlled trial (RCT) comparing 2 doses of balsalazide (1.5 g twice daily and 3 g twice daily) with mesalamine 0.5 g 3 times daily for 6 months reported a remission rate of 77.5% with the higher dose of balsalazide compared with remission rates of 56.8% and 43.8% with mesalamine and the lower dose of balsalazide, respectively. The dose of 5-ASA that induces remission is recommended for maintenance, although this has not been formally tested in a randomized placebo-controlled fashion. In the multicenter MOMENTUM trial, patients with mildly to moderately active UC who achieved complete remission after 8 weeks of therapy with 4.8 g daily of MMX mesalazine were more likely to remain in remission after dose reduction to 2.4 g daily for 1 year compared to those who achieved only partial remission, suggesting that complete remission should be the target before considering dose reduction in mildly to moderately active UC.
Common AEs of sulfasalazine include fever, rash, nausea, vomiting, and headache. Other less common but important AEs of sulfasalazine include hypersensitivity reactions, reversible sperm abnormalities, and impairment of folate absorption (by competitively inhibiting the jejunal enzyme, folate conjugase); folate supplementation (≥1 mg/day) should be prescribed to patients receiving sulfasalazine. Approximately 15% of patients taking sulfasalazine develop significant AEs that require discontinuing the medication. Up to 90% of patients who are intolerant to sulfasalazine, however, can tolerate mesalamine. In clinical trials, the newer 5-ASA preparations and balsalazide have been shown to be better tolerated than sulfasalazine. Oral mesalamine preparations do not appear to have significant dose-dependent toxicity. Olsalazine is associated with drug-induced diarrhea in up to 10% of patients, which often limits its use. Oral mesalamine therapy rarely has been associated with reversible acute kidney injury due to interstitial nephritis. Routine measurement of a serum creatinine level every 6 to 12 months is recommended for all patients receiving mesalamines. Up to 5% of patients receiving 5-ASA may develop paradoxical worsening of their colitis symptoms, often within the first few days or weeks of initiating therapy. This may recur with use of other drugs within the same class and even with topical therapy and requires a high index of suspicion to diagnose and appropriately cease the drug.
Rectally administered 5-ASAs can be administered in the form of 5-ASA enemas, 5-ASA suppositories, and 5-ASA foam (not currently available in the USA). Use of enemas allows the medication to be delivered to the level of the splenic flexure in about 95% of patients, and use of suppositories can be used to treat inflammation 15 to 20 cm from the anal verge. Rectally administered mesalamine may be used as monotherapy for patients with ulcerative proctitis or left-sided colitis or as an adjunctive therapy to oral agents in patients with more extensive colitis. They are effective for inducing remission in patients with mildly to moderately active distal UC, without a clear dose-response effect in nonrefractory patients. The standard dosing regimens used to induce remission are 1 to 4 g of 5-ASA in the form of an enema nightly, or mesalamine suppositories (1 to 1.5 g) either nightly or in divided doses throughout the day. Mesalamine enemas have been shown to be comparable to oral sulfasalazine in the treatment of active distal UC, with fewer side effects. Similar efficacies have been demonstrated for mesalamine suppositories and enemas regardless of whether the 1, 2, or 4 g formulation is used for inducing remission in patients with mildly to moderately active left-sided UC not requiring concurrent glucocorticoids or immunomodulators. In fact, mesalamine enemas are more effective than topical glucocorticoid enemas for distal colitis and are, therefore, preferred. A combination of topical and oral mesalamine also is more effective than either agent alone in patients with left-sided colitis or pancolitis, suggesting a dose-response effect. In patients with proctitis, mesalamine suppositories, 500 mg administered twice daily or 1000 mg daily, have been shown to be beneficial for treating active disease. Mesalamine foam has a more uniform distribution and longer persistence in the distal colon compared with mesalamine enemas. The foam preparation has been shown to have better patient acceptance than the enema preparation, but mesalamine foams currently are not available in the USA. Rectally administered mesalamine preparations also are effective for maintaining remission in left-sided UC or proctitis. The effective maintenance dosing interval ranges from nightly to every 3 days. Topical mesalamine is as effective as oral mesalamine, and the combination of topical and oral mesalamine may be more effective than oral mesalamine alone as a maintenance regimen.
Glucocorticoids play a central yet vexing role in the treatment of CD and UC. Although their efficacy and rapid induction of clinical improvement is well established, repeated use or prolonged exposure leads to significant AEs (see Table 116.2 ). The most common AEs are troubling neuropsychiatric symptoms, such as mood disturbance and insomnia, and cosmetic effects, including acne, Cushingoid appearance, hair loss, and hirsutism. More serious AEs associated with glucocorticoids are metabolic consequences, e.g., adrenocortical suppression, glucose intolerance, myopathy, and bone loss. The risk of infectious complications also is increased, particularly at higher doses of prednisone (>40 mg per day). Among patients taking immunomodulators or biologics, the concomitant use of prednisone appears to lead to more frequent serious infections and higher rates of mortality than when these agents are used alone. The lack of maintenance benefit and unfavorable risk profile of glucocorticoids makes their prolonged use hazardous and they are, therefore, not recommended for long-term use. The availability of glucocorticoids, like budesonide, with high hepatic first-pass metabolism, low systemic steroid exposure, and fewer AEs have made these agents an appealing alternative to traditional glucocorticoids. Nevertheless, prolonged use of rapidly metabolized steroids also has systemic AEs and achievement of steroid-free remission remains an important therapeutic target for both diseases.
There are several principles of glucocorticoid use in the management of CD and UC:
Use an effective dose , e.g., a dose equivalent to 40 mg to 60 mg of prednisone. Underdosing at the start of therapy typically leads to dose escalation and prolonged dosing to achieve a response.
Do not overdose. Patients who do not benefit from 40 to 60 mg of prednisone are unlikely to benefit from increased or prolonged oral dosing, and have the potential to develop more significant AEs. Such patients require IV dosing or replacement with a biologic agent (see later).
Do not treat for excessively long periods. Patients in whom disease-related symptoms recur with a glucocorticoid taper should be considered corticosteroid-dependent and transition to glucocorticoid-sparing immune modulators or biologics. Glucocorticoids should not be begun without a strategy in mind for terminating the agent.
Anticipate side effects. Bone loss in particular may be anticipated with even short-term use (see later, “Adjunctive Therapies.”).
Early favorable series of glucocorticoid treatment led to the validation of the short-term efficacy of glucocorticoids shown in the NCCDS (prednisone 0.5 to 0.75 mg/kg/day for initial treatment of active disease, with the dose adjusted according to the CDAI) and the European Cooperative Crohn’s Disease Study (6-methylprednisolone 48 mg/day in the first week, tapered to 12 mg by week 6, and held at 8 mg for remission up to 2 years). In usual practice, patients with mildly to moderately active disease who do not respond to primary therapy, and patients with moderately severe symptoms are treated initially with 40 to 60 mg of prednisone, the dose then being tapered off over 6 to 12 weeks. Response rates are approximately 80% by the end of the first month. When doses are pushed as high as 1 mg/kg/day for up to 7 weeks, 92% of patients can achieve clinical remission. The onset of response is rapid, usually within the first 3 weeks of treatment. A Cochrane review of the use of corticosteroids for the induction of remission of CD supports this efficacy of traditional steroids over 5-ASA therapy and placebo.
Glucocorticoids are not as effective as long-term therapy. A meta-analysis of maintenance glucocorticoid therapy in CD failed to detect benefit in the prevention of relapse at 6, 12, or 24 months. Conversely, once glucocorticoids are introduced, they cannot be discontinued without recurrent symptoms in many patients, even with gradual tapering; this problem is referred to as glucocorticoid dependence . Among patients with CD who received glucocorticoids for the first time, no response (glucocorticoid resistance) was seen in 20% in the first 30 days. Among the 80% who were complete or partial responders, 55% had a prolonged response, and 45% relapsed or could not have treatment tapered off within 1 year ; similar results were seen in both adult and pediatric populations in a cohort from Olmsted County, Minnesota. Clinical factors associated with glucocorticoid-dependence include smoking, younger age at disease onset, colonic location, and non-fibrostenotic disease. Mechanisms that can contribute to glucocorticoid resistance include up-regulation of the multidrug resistance (mdr) gene and increased serum levels of glucocorticoid-binding globulin. Moreover, only 29% of patients who achieve clinical remission on glucocorticoids also achieve endoscopic remission. This finding suggests that the effect of glucocorticoid treatment in most patients is to suppress symptoms when given in doses above a threshold that can vary among patients and even in the same patient over time.
In an attempt to limit the unintended systemic effects of glucocorticoid therapy, novel glucocorticoids have been developed. Budesonide is a glucocorticoid preparation that is structurally different from prednisone. The presence of 16α,17α-acetyl side chains allows enhanced topical anti-inflammatory activity and affinity for glucocorticoid receptors compared with prednisone. In addition, budesonide has an approximately 90% first-pass metabolism in the liver and erythrocytes and is converted to metabolites that have little or no biologic activity. The resultant low systemic bioavailability translates to significantly less toxicity compared with traditional glucocorticoids. Entocort (Cambridge, United Kingdom) is a controlled ileal release oral budesonide preparation consisting of Eudragit L 100 coated microgranules with an internal ethyl cellulose component; it releases budesonide at pH >5.5, and about 50% to 80% of budesonide is absorbed in the ileocecal region. Studies have demonstrated that 9 mg/day of this preparation are superior to placebo and mesalamine and about 15% less effective than prednisolone in achieving remission, but with fewer AEs and less effect on the adrenal axis. Pushing the dose higher results in better efficacy but at the expense of greater adrenocortical suppression and AEs. To evaluate the efficacy of budesonide for maintenance of remission, an RCT compared 6 mg to 9 mg at 12 months. Both doses were associated with relatively low relapse rates (24% and 19%, respectively) that were not significantly different, but a placebo comparison group was not included, which limits the ability to draw clinically relevant conclusions from this study. AEs were not different in these 2 dosage groups, supporting the safety of the 9 mg dose over a 1-year period. A meta-analysis reviewing all studies that evaluated budesonide for the treatment of active luminal CD concluded that budesonide was superior to placebo for inducing remission (RR = 0.73; 95% CI, 0.63 to 0.84) but not in preventing relapse (RR = 0.93; 95% CI, 0.82 to 1.04). Therefore, lack of a maintenance effect is consistent for both novel and traditional glucocorticoids. In light of the superior response in comparison with mesalamine and its relative safety, budesonide may be considered as first-line therapy for patients with active ileal, ileocecal, or right-sided colonic disease. In addition, some patients who are dependent upon conventional glucocorticoids may be switched successfully to budesonide, with the potential benefits of decreased systemic glucocorticoid exposure. Overall, the goal of therapy should be to avoid any glucocorticoid and, if needed, only use for induction as an immunomodulator or biologic agent is being introduced. Glucocorticoids should never be administered for more than 12 weeks and use is ideally limited to 1 course over 4 to 8 weeks.
At doses equivalent to 40 to 60 mg/day of oral prednisone, glucocorticoids are effective first-line therapy for moderately to severely active UC. Use of doses higher than 60 mg/day is associated with increased AEs without appreciable clinical benefit and thus should be avoided. Although no study has directly compared the efficacy of oral and parenteral glucocorticoids, the latter commonly are used in severely active disease. Options for IV glucocorticoid formulation include hydrocortisone (100 mg IV every 8 hours), prednisolone (30 mg IV twice daily), or methylprednisolone (16 to 20 mg IV every 8 hours). Hydrocortisone can cause slightly more salt retention and sodium wasting, but it is likely to be equally effective. In a randomized double-blind trial with UC patients, continuous infusion was no better than divided dosing for efficacy and safety. The use of ACTH has been suggested as an alternative to conventional glucocorticoid therapy of active UC in small studies. One double-blind RCT suggested that IV ACTH was more effective than IV hydrocortisone for the treatment of severely active UC only in steroid-naïve patients ; this observation has not been confirmed. Because most patients with severely active disease have been treated previously with glucocorticoids, ACTH rarely is used in clinical practice. A noteworthy complication of ACTH therapy is bilateral adrenal hemorrhage.
As in CD, glucocorticoids have no maintenance benefits in patients with UC. Steroid-dependent patients, or patients who are unable to taper off glucocorticoids without experiencing disease exacerbation, benefit from the addition of steroid-sparing agents. There has been no trial to date assessing the efficacy of mesalamine therapy and its efficacy in maintaining remission induced with glucocorticoids. The long-term remission rate in patients who require parenteral glucocorticoids for severe UC is approximately 50%. Immunomodulatory or biologic agents should be considered in patients who are steroid-dependent, who require 2 courses of glucocorticoids for induction of clinical response or remission within 1 year, or who require parenteral glucocorticoids to induce remission.
Although a controlled study did not show benefit of conventional oral budesonide for the treatment of active UC, an oral formulation of MMX budesonide (Uceris) that provides optimal release characteristics throughout the length of the colon has been shown to be efficacious for the induction of remission in mildly to moderately active UC. Patients in this study were treated with MMX budesonide at doses of 9 mg or 6 mg once daily, Asacol at 800 mg 3 times daily, or placebo, for 8 weeks; the authors noted remission rates of 18%, 13%, 12%, and 7.4%, respectively (P = 0.014 for MMX budesonide 9 mg vs. placebo) at week 8. For this reason, the use of MMX budesonide at a dose of 9 mg daily for 8 weeks was approved by the FDA for the treatment of mildly to moderately active UC. Just as with other steroid formulations, there are no data supporting a role for budesonide MMX in maintaining remission in UC. Pooled safety analysis from the clinical trials demonstrated fewer AEs (including steroid-related AEs) in patients receiving budesonide MMX than in those receiving systemic steroids. In addition, the mean morning plasma cortisol was similar to that with placebo for the duration of the induction period in the clinical trials.
Topical glucocorticoids in liquid and foam formulations are effective short-term therapy for active UC distal to the splenic flexure. Foam preparations often are tolerated better by patients and may be easier to retain than liquid preparations. Topical glucocorticoids have been found to be less effective than topical mesalamine for inducing remission of distal UC, however, the combination of topical corticosteroids and topical mesalamine has been more efficacious than either alone in the short-term treatment of distal UC. Whereas systemic absorption of glucocorticoids with topical therapy is significantly less than that with oral administration, prolonged treatment with topical glucocorticoids still may be associated with steroid-related AEs and should be avoided. Budesonide foams and enemas have been shown to be effective for the treatment of active distal UC in several controlled trials. In a double-blind RCT of patients with active distal UC, budesonide, 2 mg/100 mL for 6 weeks resulted in a remission rate of 19% compared with 4% in patients receiving placebo therapy (P < 0.05). Subsequent trials have shown budesonide enema to be as efficacious as or even superior to prednisolone enema without resultant depression of endogenous cortisol levels. Budesonide enema perhaps is inferior in efficacy to mesalamine enema, but it clearly presents an alternative topical glucocorticoid for treatment of distal UC. The optimal dose for budesonide enema consistently has been shown to be 2 mg/100 mL once daily. Budesonide foam is available at a similar dose and is effective in inducing clinical remission and complete mucosal healing. Additional studies are needed to determine the effect of longer-term topical budesonide use. As with other glucocorticoids, budesonide enema is not effective for maintaining remission in UC.
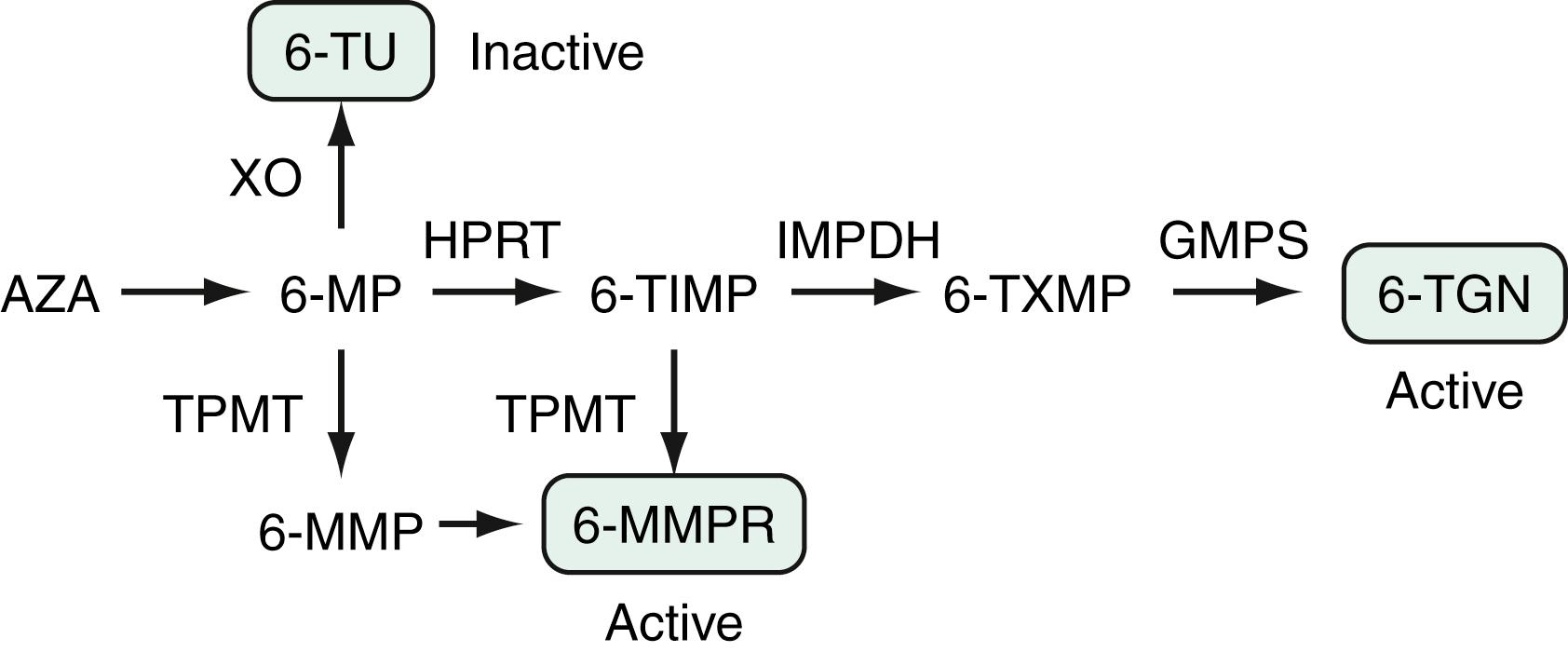
Of the various immunomodulatory agents, the most widely used are the thiopurine therapies azathioprine (AZA) and 6-mercaptopurine (6-MP). These 2 agents are purine analogs that interfere with nucleic acid metabolism and cell growth to exert cytotoxic effects on lymphoid cells. They are inactive pro-drugs with subtle structural differences. AZA is nonenzymatically converted in the peripheral circulation to 6-MP, which is then metabolized through a series of enzymatic pathways to active and inactive metabolites ( Fig. 116.3 ). The 2 primary classes of metabolites of 6-MP are 6-thioguanine nucleotides (6-TGns) and 6-methylmercaptopurine ribonucleotides (6-MMPrs). The 6-TGn metabolites are thought to be responsible for the immunomodulatory action of azathioprine and 6-MP and their bone marrow suppression property, whereas hepatotoxicity is thought to be related to 6-MMP. Xanthine oxidase (XO) converts 6-MP to 6-thiouric acid, in competition with hypoxanthine phosphoribosyltransferase. The former enzymatic pathway accounts for an important drug reaction with allopurinol, an XO inhibitor (see later). Thiopurine methyltransferase (TPMT) also plays a key role in the metabolic pathway. Persons who are homozygous for a recessive mutation that results in inactivation of TPMT (≈1 in 300 persons) produce exceedingly high levels of 6-TGn nucleotides. These persons are unlikely to tolerate thiopurine agents and tend to develop profound leukopenia and other limiting AEs. In contrast, persons who are TPMT heterozygous (≈10% of the population) are likely to have moderately high levels of 6-TGn nucleotides. They usually require lower doses of drug (∼50% of the weight-based optimal dose) but are much more likely to have a therapeutic response. A TPMT activity level should be measured in all patients prior to initiation of AZA or 6-MP. A steady state in the production of erythrocyte 6-TG nucleotides may be reached as early as 14 days after dosing, but has been reported at a median time of 55 days in pediatric patients. Thiopurines are associated with rare but significant AEs, such as nonmelanoma skin cancer (NMSC) and lymphoma (see later). Thiopurines are effective steroid-sparing medications and prevent immunogenicity when combined with monoclonal antibody biologics, however, evolving treatment strategies may replace or limit the use of thiopurines in the future. Recently, a separate genetic polymorphism associated with thiopurine metabolism (NUDT15) has been identified in people of Asian ancestry and is associated with early myelosuppression.
AZA and 6-MP have been used to treat CD since the initial report of Brooke and colleagues describing healing of fistulas with AZA. Another decade would pass, however, before the efficacy of this class of drugs was demonstrated in an RCT by Present and colleagues ; earlier studies were marred by either insufficient power or incomplete understanding of adequate dosing and the slow onset of action of these agents. A Cochrane meta-analysis of studies of AZA and 6-MP in CD has provided the best summary of the effects of these drugs. For active disease, thiopurine treatment produced a remission rate of 47% compared with a 37% placebo rate, but this corresponded to a non-significant Odds Ratio of 1.23 (95% CI, 0.97 to 1.55). The outcome of remission or clinical response similarly yielded a higher but not statistically significant result compared with placebo (48% vs. 36%; RR 1.26; 95% CI, 0.98 to 1.62). The odds ratio for response increases after 17 weeks of therapy, suggesting the minimum duration for a trial of 6-MP or AZA. A steroid-sparing effect was significant (RR, 1.34; 95% CI, 1.02 to 1.77), and the number needed to treat (NNT) was about 6. Only 18 patients with fistulas were included in the original study, but a 54% rate of fistula response was noted, compared with a 29% healing rate on placebo; this, however, was not statistically significant (RR, 2.00; 95% CI, 0.67 to 5.93). There is more convincing evidence of the benefit of thiopurines for maintenance of remission. The OR for maintenance of remission with AZA was 2.32 (95% CI, 1.55 to 3.49; NNT = 6) and with 6-MP 3.32 (95% CI, 1.40 to 7.87; NNT = 4). The OR for AZA maintaining remission increased from 1.20 at 1 mg/kg up to 4.13 (95% CI, 1.59 to 10.71) at 2.5 mg/kg, demonstrating the importance of appropriate dosing.
Overall, approximately one half of patients may respond to thiopurine therapy; once in remission, about half to two thirds of patients will maintain that response. In earlier studies, mucosal healing was seen in approximately half of the patients who received thiopurines, however, in a more recent large study, mucosal healing was seen in only 16.5% of patients at 26 weeks. In children, early administration of 6-MP soon after diagnosis was associated with steroid sparing and maintenance of remission ; however, this was not reproduced in adults in a recently published trial. In clinical practice, AZA and 6-MP are used virtually interchangeably, with the exception of dosing. AZA generally is used in doses of 2 to 2.5 mg/kg/day, and 6-MP is given in doses of 1 to 1.5 mg/kg/day. The introduction of thiopurine medications should be timed with their slow onset of action in mind; many patients require a long, tapering regimen of glucocorticoids to bridge the time period until the thiopurines have taken effect.
When a patient is not responding to thiopurine therapy after 3 to 4 months of therapy, it is useful to measure metabolite levels to identify individuals who are noncompliant, under-dosed, or shunting (see later). Although mixed results have been reported of the correlation between 6-TGn nucleotide levels and response to therapy, a meta-analysis of 6 studies did find an overall significant relationship. A threshold of 230 to 260 pmol/8 × 10 10 red blood cells corresponded to a 62% rate of remission, compared with a rate of 36% in those with lower levels (OR, 3.27; 95% CI, 1.71 to 6.27) of 6-TGn. Correlations between higher levels of 6-TGn nucleotides and leukopenia, and between metabolite levels and response to therapy, might explain the clinical observation that patients who achieve mild leukopenia are more likely to respond to such therapy. Conversely, however, leukopenia is not necessary to achieve a therapeutic response. Further, it is not clear if routine measurement of thiopurine metabolite levels and directed dose adjustment would contribute to improved management of Crohn disease, as opposed to the standard weight-based dose approach. Shunting refers to high TPMT activity that results in low 6-TGn levels and high 6-MMP levels (see Fig. 116.3 ); a 6-MMP:6-TGn ratio of >10 has been suggested as a profile of metabolism that is unlikely to lead to clinical benefit. In these patients, it may be possible to add allopurinol and take advantage of the drug interaction noted earlier. A study testing this hypothesis showed that by decreasing the thiopurine dose to 25% to 50% of the original dose and adding a low (100 mg daily) dose of allopurinol, 6-TGn levels rose significantly, with a coincident drop in 6-MMP levels and an improvement in clinical outcomes. Although this strategy requires careful following of the WBC count, it has been shown to be well tolerated and safe in one study with a median follow-up period of 19 months.
In addition to its role as monotherapy for CD, emerging evidence suggests that thiopurines may have an additional important role in combination therapy with a biologic agent. This hypothesis is supported by analysis of clinical trial data in which individuals on combination therapy with an anti-TNF biologic and a thiopurine have lower rates of anti-drug antibody and higher serum trough levels of the biologic agent. A large clinical trial, the Study of Biologic and Immunomodulator Naïve Patients with Crohn’s Disease (SONIC) study, randomized 508 thiopurine- and biologic agent-naïve adults with moderately to severely active CD to receive either AZA alone, infliximab alone, or a combination of both drugs. At week 26, the combination of AZA with infliximab resulted in superior clinical efficacy and mucosal healing compared with those receiving infliximab alone, or AZA alone. A similar benefit has also been noted in moderately to severely active UC. When used as combination therapy, one may observe a benefit even with lower doses of thiopurine, though this has not been prospectively studied. In a retrospective study, a 6-TGn trough level of 125 pmol/8 × 10 8 RBCs was sufficient to achieve a higher infliximab trough level.
AEs are frequently noted in patients receiving thiopurines. In a Cochrane analysis, AEs severe enough to result in drug withdrawal were seen in 10% of patients. AEs that lead to drug discontinuation typically occur soon after drug initiation, with a median time of 1 month. Nausea within the first few weeks of treatment is reported in approximately 8% of patients, but typically will subside gradually. Allergic reactions consisting of fever, rash, or arthralgias are seen in 1% to 2% of patients, also usually within a few weeks of introducing the drug. Pancreatitis, observed in 3% to 4% of patients, is another idiosyncratic reaction and usually occurs in the first month of therapy. The presentation is typically classic with epigastric pain that radiates to the back, but may be atypical and subtle, with nausea and vague dyspepsia. When symptoms are recognized promptly, discontinuation of the drug leads to resolution of pancreatitis. Rechallenge with either drug, AZA or 6-MP, should not be attempted, because recurrent pancreatitis is certain to occur. Elevated serum aminotransferase levels develop in approximately 3% of patients and have been correlated with the presence of very high levels of 6-MMP. Mild elevations of liver biochemical tests can often revert to normal without any intervention, or with dose reduction. An exception is in the rare occurrence of cholestatic hepatitis or nodular regenerative hyperplasia, in which case thiopurine therapy should be withdrawn. Bone marrow suppression is another concern with thiopurine agents. A 27-year, retrospective, single-center study of 739 IBD patients treated with AZA found 28 patients (3.8%) developed leukopenia (WBC count <3 × 109 cells/L [<3000 cells/mm 3 ]), 9 of whom (1.2%) had severe leukopenia (WBC count <2 × 109 cells/L [<2000 cells/mm 3 ]); 3 of these patients became pancytopenic, and 2 died of sepsis. A review of 66 studies that included more than 8000 patients had a similar rate of myelotoxicity and, fortunately, a low risk (<0.1%) of death attributable to treatment. Although leukopenia occurs early among patients with low TPMT activity, it might not be related solely to TPMT genotype and can occur at any time during therapy. For this reason, it is advisable to continue monitoring the CBC every 1 to 3 months for the duration of therapy and more frequently (every 2 weeks if TPMT activity is normal, weekly if it is heterozygous) in the 8 to 12 weeks after introducing the drug or increasing dosage. Temporary cessation of therapy for a week or 2 and an adjustment in dose usually suffice to bring the leukocyte count back within normal range. Careful monitoring of the leukocyte count also should be performed during a tapering regimen of glucocorticoids. Concurrent treatment with glucocorticoids can raise the leukocyte count, but as the glucocorticoid is discontinued, leukopenia can develop.
Infections can occur in the setting of thiopurine therapy. Serious infections are reported to occur approximately 2% to 6% of the time, not necessarily in the setting of leukopenia. Treatment should be interrupted when serious infections occur, although the effect of the drug will endure for weeks. Systemic viral infections are of particular concern in thiopurine users. Patients treated concurrently with glucocorticoids also are at greater risk of serious infection, including CMV. Case series suggested that thiopurine users who develop primary Epstein-Barr virus (EBV) infection are at a higher risk for aggressive lymphoproliferative diseases including hemophagocytic lymphohistiocytosis leading a few to recommend routine screening for EBV seropositivity, particularly in young men and avoiding thiopurines in seronegative patients. The routine benefit of this, however, has not been established and nearly 100% of IBD patients are seropositive for EBV by age 26.
Malignancy associated with thiopurines has been a longstanding concern of patients and providers. Immunosuppressive regimens given to patients after organ transplantation and for other immune-mediated conditions are associated with an excess risk of malignancy, particularly non-Hodgkin lymphoma (NHL). Such regimens have included AZA, often administered in higher doses than for IBD and in conjunction with other immunosuppressive agents. In IBD, there does appear to be an association between thiopurine exposure and lymphoma, specifically NHL. The rate of NHL is reported to be approximately 4 to 9 per 10,000 patient-years. Data suggest that once stopping thiopurines, the risk of lymphoma returns to the patient’s baseline risk. NMSC appears to have a clear association with thiopurine exposure, but not melanoma. Multiple prospective cohort studies have suggested a 4- to 6-fold increase in risk of NMSC in patients who continue thiopurine therapy; this risk appears lower in those who are past users. Whereas the relative risk is highest in younger individuals, the absolute increase in likelihood of NMSC is significant in older adults and requires close dermatologic follow-up. Thiopurine use has also been linked to increase in risk of cervical neoplasia in some cohort studies. No other solid tumors have been found to be consistently associated with thiopurines when used for the treatment of IBD and use of thiopurines appears to be safe in individuals with prior solid-organ cancer. Continued thiopurine use, however, is associated with increased risk of recurrence of NMSC.
Once treatment with a thiopurine agent has proved to be effective, the question inevitably arises of how long to continue such therapy. One RCT demonstrated a clinical relapse rate of 21% 18 months after withdrawal of AZA in patients who had been in remission for at least 3.5 years on the drug, compared with a relapse rate of only 8% in the group who continued AZA. The authors concluded, and most authorities agree, that AZA maintenance therapy should be continued for more than 3.5 years. The decision to withdraw thiopurine therapy should only be undertaken after discussion between doctor and patient of possible risks and benefits.
There are less robust data supporting the efficacy of AZA in the treatment of UC, although it remains widely used for this indication. Four RCTs have evaluated AZA for inducing remission in active UC ( Table 116.3 ), all of which were small, heterogeneous in design, used different outcome definitions for response, and reached different conclusions. Two of the studies involved steroid-dependent patients, one study used steroids for induction, and 2 studies used 5-ASAs as a comparator group rather than placebo. Only one study showed a significant benefit with AZA compared with 5-ASA for induction therapy in steroid-dependent disease. With respect to the use of AZA for maintenance of remission in UC, 4 RCTs have been performed. Just as with studies of induction therapy, these 4 studies also had small sample sizes, used heterogeneous designs with different outcome definitions of response, allowed for various co-therapies, and again reached different conclusions. One of the studies was in steroid-dependent disease, another allowed the use of steroids for relapse, one study used 5-ASA as a comparator group rather than placebo, and another included patients who were mostly taking 5-ASAs and was actually a study of AZA withdrawal. Only this withdrawal study showed a benefit with continued AZA. Thus, for the purpose of induction or maintenance therapy for UC, the use of AZA is largely based on its established efficacy in CD rather than any proved benefit in UC. One subset of patients, however, has been shown to obtain benefit with the use of AZA: patients who have severely active UC and who are able to attain induction of remission with cyclosporine. In these patients, maintenance therapy with AZA has been reported to decrease colectomy rates (see later). A single RCT has also demonstrated benefit of AZA when used in combination with infliximab. Among 239 patients with moderately to severely active UC, corticosteroid-free remission was achieved at week 16 more frequently in the combination therapy group (40%) than in those receiving infliximab monotherapy (22%, P = 0.017). Mucosal healing was also more common with combination therapy than with each agent alone and no additional safety concerns were noted with combination therapy.
| Drug and Trial | Trial Type | Dosing Schedule | Number of Participants | Trial Duration | Primary Outcome |
|---|---|---|---|---|---|
| A dalimumab (ADA) | |||||
| CLASSIC-I (16472588) | Induction | ADA 160 mg/80 mg or 80 mg/40 mg at wks 0 and 2 or placebo | 299 | Week 4 | Clinical remission: 36% ADA 160/80 vs. 12% placebo |
| GAIN (17470824) | Induction | ADA 160/80 mg at wks 0 and 2 or placebo | 325 | Week 4 | Clinical remission: 21% ADA vs. 7% placebo |
| Watanabe (22325170) | Induction | ADA 160/80 mg or 80/40 mg at wks 0 and 2 or placebo | 90 | Week 4 | Clinical remission: 33% ADA 160/80 vs. 13% placebo |
| CHARM (17241859) | Maintenance | ADA 40 mg SQ EOW, weekly, or placebo | 778 | Week 56 | Clinical remission: 36% ADA EOW vs. 12% placebo |
| CLASSIC II (17299059) | Maintenance | ADA 40 mg SQ EOW, weekly or placebo | 55 | Week 56 | Clinical remission 79% ADA EOW vs. 44% placebo |
| Watanabe (22325170) | Maintenance | ADA 40 mg SQ EOW or placebo | 43 | Week 52 | Clinical remission: 38% ADA vs. 9% placebo |
| C ertolizumab pegol (CZP) | |||||
| Schreiber (16143120) | Induction | CZP 100, 200 or 400 mg at wks 0, 4, and 8 or placebo | 292 | Week 12 | Clinical response: CZP 400 mg 33% vs. 15% placebo |
| PRECISE 1 (17634458) | Induction | CZP 400 mg SQ at wks 0, 2, 4 | 662 | Week 6 | Clinical response: CZP 35% vs. 27% placebo |
| PRECISE 1 (17634458) | Maintenance | CZP 400 mg at wks 0, 2, 4 and then every 4 wks | 662 | Week 26 | Clinical response: 23% CZP vs. 16% placebo |
| PRECISE 2 (17634459) | Maintenance | CZP 400 mg every 4 wks or placebo | 428 | Week 26 | Clinical response (CRP >10 mg/L): 62% CZP vs. 34% placebo |
| I nfliximab (IFX) | |||||
| Targan (9321530) | Induction | IFX 5, 10 or 20 mg/kg at wk 0 | 108 | Week 4 | Clinical response: 81% IFX 5 mg/kg vs. 17% placebo |
| ACCENT 1 (12047962) | Maintenance | IFX 5 or 10 mg/kg every 8 wks or placebo | 335 | Weeks 54 | Clinical remission: 39% IFX 5 mg/kg vs. 21% placebo |
| Rutgeerts (10500056) | Maintenance | IFX 10 mg/kg every 8 wks or placebo | 73 | Week 44 | Clinical response: 62% IFX vs. 37% placebo |
| N atalizumab (NAT) | |||||
| ENACT 1 (16267322) | Induction | NAT 300 mg IV at wks 0, 4, 8 or placebo | 905 | Week 10 | Clinical response: 56% NAT vs. 49% placebo |
| ENACT 2 (16267322) | Maintenance | NAT 300 mg IV every 4 wks or placebo | 339 | Week 56 | Sustatined response: 61% NAT vs. 28% placebo |
| ENCORE (17484865) | Induction | NAT 300 mg IV at wks 0, 4, and 8 or placebo | 509 | Week 12 | Response through week 12: 48% NAT vs. 32% placebo |
| U stekinumab (UST) | |||||
| UNITI-1 (27959607) | Induction | UST 130 mg or 6 mg/kg IV or placebo | 741 | Week 6 | Clinical response: 34% UST 6 mg/kg vs. 22% placebo |
| UNITI-2 (27959607) | Induction | UST 130 mg or 6 mg/kg IV or placebo | 628 | Week 6 | Clinical response: 56% UST 6 mg/kg vs. 29% placebo |
| IM-UNITI (27959607) | Maintenance | UST 90 mg SC every 8 wk or every 12 wk or placebo | 397 | Week 44 | Clinical remission: 53% UST every 8 wks vs. 36% placebo |
| V edolizumab (VDZ) | |||||
| GEMINI 2 (23964933) | Induction | VDZ 300 mg IV at wks 0 and 2 or placebo | 368 | Week 6 | Clinical remission: 15% VDZ vs. 7% placebo |
| GEMINI 3 (24859203) | Induction | VDZ 300 mg IV at wks 0, 2, and 6 or placebo | 416 | Week 6 | Clinical remission: VDZ 15% vs. 12% placebo |
| GEMINI 2 (23964933) | Maintenance | VDZ 300 mg IV every 4 or every 8 wks or placebo | 461 | Week 52 | Clinical remission: 39% VDZ every 8 wks vs. 22% placebo |
| EOW, every other week; SC, subcutaneously. | |||||
Methotrexate (MTX) has long been used to treat psoriasis and rheumatoid arthritis. Although the cytotoxic effect of MTX is attributed to inhibition of DNA and RNA synthesis through inhibition of dihydrofolate reductase, its efficacy in immune-mediated diseases may be due to inhibition of other folate-dependent enzymes, inhibition of pro-inflammatory cytokines, and increased production of regulatory cytokines. In an RCT, patients were studied who had chronically active CD despite at least 3 months of prednisone (at least 12.5 mg/day) and with at least one failed attempt to taper off treatment. All patients were brought to a 20 mg/day dose of prednisone to standardize therapy, with separate stratification for patients in whom the dose of prednisone was increased and for those in whom the dose had been reduced to 20 mg before entry. Subjects then received either weekly injections of MTX 25 mg intramuscularly or placebo while executing a tapering prednisone regimen over 16 weeks. Overall, 39.4% of patients assigned to MTX achieved remission off prednisone compared with 19.1% of placebo-treated patients. Most patients responded by the eighth week of treatment. A follow-up study randomized patients who achieved remission by week 16 on MTX 25 mg intramuscularly once weekly to receive either weekly injections of placebo or MTX at a dose of 15 mg. At week 40, 65% of patients treated with MTX were still in remission, compared with 39% of placebo-treated patients (P = 0.04). Thus, MTX has been shown to be beneficial at inducing and maintaining remission
Among patients who relapsed on the maintenance dose, more than half were able to achieve remission again by resuming a 25 mg dose. If the 16 weeks of induction therapy were included, the combined duration of therapy was nearly 1 year, with some patients in selected practices treated successfully for more than 4 years. Although 15 mg intramuscular dosing was studied for maintenance, many patients continue on 25 mg weekly without dose reduction. Pharmacokinetic studies in RA have shown equivalency for subcutaneous and intramuscular dosing, and therefore most gastroenterologists administer MTX subcutaneously. Although oral dosing would be more convenient for long-term administration, a Cochrane review did not find evidence of efficacy when oral dosing was compared to placebo. This may be explained by the variable intestinal absorption of MTX, particularly in the presence of more extensive small intestinal disease.
In addition to its role in monotherapy, like thiopurines, the role of MTX as co-therapy with a biologic agent has been examined under the hypothesis that it suppresses immunogenicity. A 50-week RCT compared the combination of MTX and infliximab to infliximab alone in 126 patients initiated on prednisone within 6 weeks prior to the study. At the end of the study, there was no difference in steroid-free remission between the 2 groups. However, patients on combination therapy had higher trough infliximab levels than those receiving biologic monotherapy. There are fewer studies, particularly in IBD, on whether dosing of MTX influences its benefit when used in combination with an anti-TNF biologic agent. A single-center study examining dosing in patients on remission with combination anti-TNF therapy and MTX found that MTX dose greater than 12.5 mg weekly was associated with higher rates of clinical remission. A prospective 24-week trial in patients with RA who had previously failed MTX suggested slightly higher adalimumab trough levels in patients on high-dose MTX (20 mg/week) compared with low-dose MTX (7.5 mg/week), although there was no statistically significant difference in the rate of anti-drug antibody positivity.
MTX is given with folic acid (1 to 2 mg/day) to prevent folate deficiency (through inhibition of dihydrofolate reductase) as well as symptoms of nausea and stomatitis; diarrhea, hair loss, and mild leukopenia also can occur with MTX. Elevation of serum aminotransferase levels sometimes occurs, but correlate poorly with the complication of hepatic fibrosis. Liver biopsy is performed routinely in patients with psoriasis after cumulative doses of 1.5, 3, and 5 g have been administered, but these guidelines have not been widely adopted in patients with RA or IBD, in whom the risk of hepatic fibrosis appears to be lower. In one series of IBD patients who received a mean cumulative dose of MTX >2.5 g and had liver biopsy, only minimal hepatic toxicity was evident; obesity, diabetes, and alcohol intake, however, correlate with hepatic fibrosis. In clinical practice, liver biopsy is usually performed in those with persistently elevated liver enzymes or likelihood of an alternate cause of liver abnormality. MTX interacts with sulfa medications and with AZA and 6-MP to cause severe leukopenia. Rarely but potentially life-threatening, interstitial pneumonitis can manifest as cough and dyspnea of insidious onset. Early detection, cessation of MTX, and prompt treatment with glucocorticoids is essential. MTX is a potent abortifacient and is strongly teratogenic. Women of childbearing capacity must use MTX only with highly effective contraception and must cease therapy 3 to 6 months prior to planned conception. There are less data on the safety of MTX in men contemplating conceiving, although emerging data in patients with RA and IBD suggest that paternal exposure to MTX before conception is not associated with increased risk of congenital malformation, still births, or preterm birth. However, many physicians still consider cessation 3 months before planned conception in men.
MTX long lacked data supporting its use in UC with the first randomized, placebo-controlled trial failing to demonstrate efficacy for the treatment of active UC. In one study of 67 patients with chronic active UC, oral MTX at 12.5 mg/wk for 9 months was comparable to placebo therapy in the rate of achieving first remission, time to first remission, relapse following remission, and the mean glucocorticoid dose. Given the difference in dose and route of administration from the more successful trial in CD, however, it was unknown if a higher dose of parenteral administration would be of benefit in UC. Two recent RCTs provide more answers to the question. The METEOR study randomized 111 patients with steroid-dependent UC to receiving either parenteral MTX 25 mg weekly or placebo for 24 weeks. At the end of the trial, there was no statistically significant difference in steroid-free remission or endoscopic healing between the 2 groups. However, patients were more likely to be in clinical remission with MTX when compared to placebo (42% compared to 24%, P = 0.04). The subsequently completed multi-center MERIT-UC study did not show benefit of parenteral MTX 25 mg weekly for induction and maintenance of remission. The study design included a 16-week open label treatment with MTX, followed by a 32-week double blind, placebo-controlled maintenance period. Although 51% of patients responded in the open-label portion of the study, there was no difference between active treatment and placebo in maintenance, with 60% of patients in the placebo group and 66% in the MTX group relapsing (P = .75).
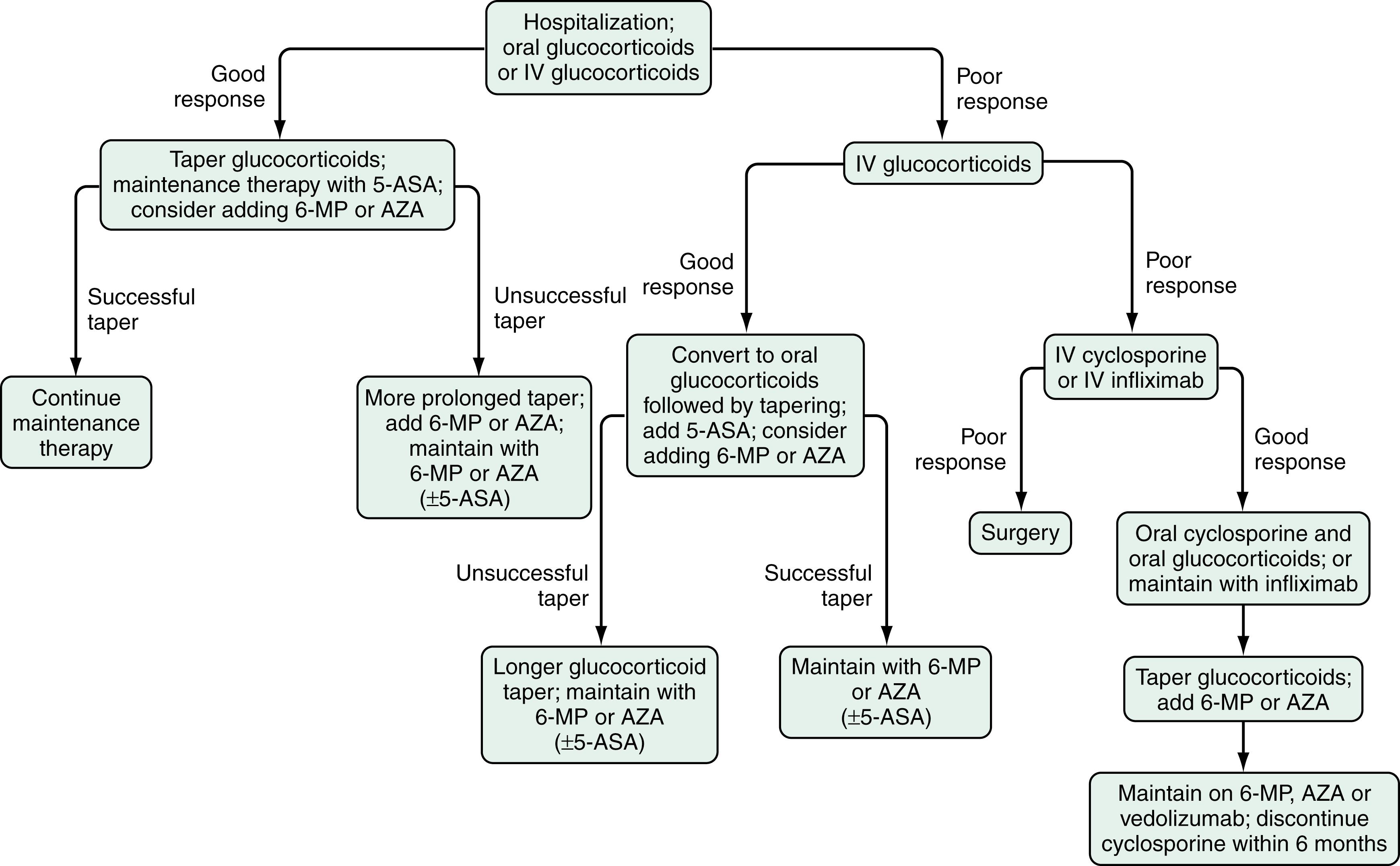
Cyclosporine A (CSA) is a potent inhibitor of cell-mediated immunity through a similar calcineurin inhibition pathway as tacrolimus. Its use in IBD is primarily in patients with acute severe, steroid-refractory UC. The initial RCT of CSA randomized 20 patients who did not respond to at least 7 days of IV hydrocortisone to CSA or placebo. Nine (82%) of the 11 patients receiving continuous IV infusion of CSA at 4 mg/kg/day responded, compared with none of the 9 patients receiving placebo therapy. The time to clinical response was rapid, at a mean of 7 days. After the IV route of therapy was converted to oral CSA, only 44% of those patients who responded initially required colectomy during the 6-month follow-up period. IV CSA monotherapy may be as effective as IV glucocorticoids in patients with severely active UC; its use thus potentially minimizes the toxicities of combination therapy. The addition of AZA or 6-MP in patients who have responded to IV CSA has been shown in other studies to reduce the rate of relapse or colectomy. Thus, CSA can be considered a bridge therapy to control active disease in patients with steroid-refractory UC while waiting for elective surgery or the onset of action of azathioprine or 6-MP ( Fig. 116.4 ). With the addition of AZA, long-term remission at 1 year may be more likely in patients who initially respond to IV CSA monotherapy than in those who respond to IV glucocorticoids. A European retrospective cohort study of 142 patients who were treated with CSA, 118 of whom responded initially, reported the probability of avoiding colectomy to be 63% at 1 year, 41% at 4 years, and 12% at 7 years; overall, 54% of patients required colectomy at some point. Patients who were already taking AZA or 6-MP at the time CSA was initiated had a higher rate of colectomy (59%) than patients naïve to these drugs (31%, P < 0.05). With intent to minimizing potential for AEs related to the dose of CSA, another randomized trial showed than a dose of 2 mg/kg is as effective as 4 mg/kg given IV in patients with severely active UC. The mean plasma CSA levels were 237 ng/mL in patients receiving the 2 mg/kg dose and 332 ng/mL in patients receiving the 4 mg/kg dose. If patients respond to IV CSA, the route of administration can be changed to oral therapy with 2 mg of oral agent for each 1 mg of IV CSA. The drug can be administered in 2 divided doses daily. Oral CSA should be continued while waiting for surgery or for an alternate maintenance therapy to take effect.
Two recent RCTs compared CSA with infliximab for the management of acute severe UC. The CYSIF trial randomized 115 patients with steroid-refractory severe UC to either CSA or placebo. Treatment failure, defined as lack of clinical response at day 7, was similar in the 2 groups (60% and 54%, P = 0.52). The median time to respond also was similar in both groups of patients and a comparable proportion of patients underwent colectomy (17% with CSA and 21% with infliximab, P = 0.60). The CONSTRUCT trial was an open-label pragmatic randomized trial in the United Kingdom which randomized 270 patients with severe UC who were refractory to IV steroid therapy by day 5 to receiving either CSA or infliximab. The primary outcome, quality-adjusted survival, was similar between both groups. The overall rates of colectomy in both groups were high (41% with infliximab, 48% with CSA) but were similar in both treatment arms. Together, both trials demonstrated that infliximab and CSA are similarly effective as rescue therapy in acute severe UC. CSA also has been used as a third-line salvage therapy in patients refractory to both IV steroids and infliximab, although this population remains at high risk of needing a colectomy or developing serious infectious complications and even mortality. Availability of an alternate treatment for maintenance in responders is important for patients in whom CSA has been initiated. This role traditionally has been played by thiopurines, though early reports suggest that vedolizumab, a bowel-selective anti-integrin may also be a safe and effective option for maintenance.
CSA has been associated with many AEs, including paresthesias, tremors, headache, hypertrichosis, and gingival hyperplasia. Other potentially serious AEs include hypertension, seizures, electrolyte and liver biochemistry abnormalities, nephrotoxicity, anaphylaxis, and opportunistic infections. These complications are mostly dose-dependent. Severe complications have been reported with CSA in up to 12% of patients with UC and 2 large series have reported death rates of 1.8% to 2.8% with CSA, more than half of which resulted from infections acquired while taking the drug. Careful monitoring for AEs is critical during CSA therapy. Baseline serum electrolytes, creatinine, cholesterol, and liver biochemical values should be measured. CSA therapy should be avoided in patients with an impaired creatinine clearance to minimize the risk of nephrotoxicity. Patients with serum cholesterol levels <120 mg/dL should receive nutritional support to improve the level before initiating CSA therapy, because a low cholesterol level is associated with an increased risk of seizures. During IV therapy, CSA levels should be monitored daily, and the dose should be adjusted to achieve a trough concentration (measured 1 hour before dosing) between 200 and 400 ng/mL, determined by high-pressure liquid chromatography. Serum electrolytes and serum creatinine levels should be monitored daily or every other day and the dose of CSA decreased when the serum creatinine increases by 20% to 30% over baseline. Drug monitoring during oral CSA therapy includes weekly trough CSA levels and weekly to biweekly electrolyte and creatinine levels. Patients on long-term CSA therapy should receive Pneumocystis jiroveci pneumonia prophylaxis with trimethoprim/sulfamethoxazole.
There appears to be little role for CSA in CD. Series of uncontrolled RCTs have shown high doses of CSA to be efficacious in treating IBD and fistulas, but at an unacceptably high cost of AEs. Moreover, lower doses, although somewhat safer, are not effective in maintaining remission. One uncontrolled study suggested a benefit for hospitalized patients with CD of the colon, many of whom had been previously exposed to anti-TNF agents, but further data are required to understand its benefit to risk profile.
Tacrolimus is absorbed more reliably from the intestine than is CSA and has a similar mode of action via inhibition of calcineurin, thereby diminishing T-cell activation. A number of small uncontrolled studies have suggested benefit of oral or IV tacrolimus for the treatment of patients with refractory UC. The only RCT of tacrolimus in UC involved 63 Japanese patients with either steroid-dependent or steroid-refractory disease who were randomized to receive either initial oral tacrolimus at 0.05 mg/kg or placebo twice daily. Patients in the high trough concentration (10 to 15 ng/mL) tacrolimus group had a significantly higher rate of response and nonsignificantly higher rate of remission than those in the placebo group at week 2, and a number of patients demonstrated response or remission (or both) after an additional 10 weeks of open-label therapy. Another RCT demonstrated efficacy of tacrolimus in healing perianal fistulas in CD. The drug also may be effective as a topical agent for oral and perianal ulcerating disease ; long-term application of 0.1% tacrolimus applied to broken skin and mucosa was safe and serum levels were undetectable. A role has recently emerged for tacrolimus enemas dosed between 2 to 4 mg daily for management of refractory distal colitis. As with CSA, tacrolimus can result in a number of toxicities including nephrotoxicity, electrolyte abnormalities, nausea, diarrhea, headache, tremors, paresthesias, insomnia, alopecia, hirsutism, and gingival hyperplasia.
Alternative immunomodulators have been explored for patients who do not tolerate or have not responded to the previously mentioned immunosuppressants. Mycophenolate mofetil has pharmacodynamic properties similar to those of AZA and 6-MP but a more rapid onset of action. A pilot study of patients with chronic active UC receiving concomitant prednisolone found AZA to be superior to mycophenolate mofetil throughout the 1-year study period, with remission rates at 1 year of 100% and 88%, respectively. Uncontrolled studies reported less than 50% remission rates with mycophenolate mofetil therapy in patients with steroid-dependent UC, and the intolerance rate was high. A substantial number of patients developed AEs necessitating drug withdrawal, including recurrent upper respiratory tract infection, bacterial meningitis, depression, and migraine headache.
Thalidomide may have a role for some patients with CD through the mechanism of down-regulation of TNF-α and inhibition of NFκB activity. It has been shown to be effective in small studies of patients who are naïve to biologics, and also those in whom thiopurine, MTX, and anti-TNF therapy have failed. The most frequent long-term toxicity is peripheral neuropathy, which is typically reversible, although not in all cases. Careful contraceptive measures are critical owing to the recognized teratogenicity, particularly phocomelia. Lenalidomide, an analog of thalidomide with similar immunomodulatory properties but with less toxicity (specifically neuropathy), was studied in an RCT, but did not show a response significantly different from placebo.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here