Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The choice of surgical valve replacement or transcatheter valve-in-valve (VIV) implantation for bioprosthetic valve failure depends on careful consideration of patient characteristics and surgical valve and anatomic parameters by a multidisciplinary heart valve team.
Clinical outcomes after a transcatheter VIV procedure differ from those after a native valve transcatheter aortic valve implantation; with VIV, there are fewer mechanical complications and lower rates of mortality, conduction defects, and paravalvular regurgitation.
Adverse effects after VIV transcatheter aortic valve replacement (TAVR) for a failed surgical bioprosthesis include residual stenosis, clinical thrombosis, malpositioning, and coronary obstruction.
Approaches to reduce the risk of stenosis after transcatheter aortic valve implantation include supra-annular positioning of the transcatheter valve, avoidance of underexpansion of the valve, and intentional fracturing of the bioprosthetic valve ring.
Clinical thrombosis after VIV TAVR occurs in about 8% of patients, necessitating careful postimplantation monitoring and appropriate anticoagulation.
A novel approach to prevention of coronary ostial obstruction with a VIV procedure is the bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) procedure.
The evolution of prosthetic heart valves begins with the work of innovative physicians backed by industry support. The first implantation of a homograft in the descending aorta was performed by Murray in 1956. In 1961, Albert Starr and Lowell Edwards introduced clinical orthotopic valve replacement therapy using mechanical valve models. A few years later, Donald Ross and Barratt Boyes introduced aortic homografts and pulmonary autografts into clinical practice, and the work of Carlos Duran and others led to the implantation of porcine aortic and mitral valves. Significant growth of porcine bioprosthetic aortic valve replacements was mainly led by Hancock Laboratory, Edwards Lifesciences (Irvine, CA), Medtronic (Minneapolis, MN), and Shiley.
In 1969, Alain Carpentier introduced the use of glutaraldehyde for prevention of degeneration of porcine aortic valves. In 1971, Marian Ionescu designed the first-generation bovine pericardial valves. In the early 1980s, several types of second-generation bioprosthetic valves were clinically available. Later, transcatheter pulmonary valves were implanted, and in 2002, the first transcatheter aortic valve replacement (TAVR) was performed by Alian Cribier, marking the start of the transcatheter valve replacement era. ,
Tissue bioprosthetic valves are used in almost all surgical implantations and in all clinical transcatheter valve implantations. Bioprosthetic valves are favored over mechanical valves because of lower thrombogenicity and lack of obligatory long-term anticoagulation. Novel anticoagulants do not reduce the risk of thrombogenicity effectively enough in these cases, and the use of mechanical valves is rapidly decreasing. In 1997, more than 50% of valve replacements in the United States used mechanical valves; a decade later, their use had decreased to less than 25%. Currently, bioprosthetic tissue valves are used in more than 90% of valve replacements, with TAVR procedures accounting for a large portion of that group in the aortic position.
Although bioprostheses are much less prone to clinical thrombosis than mechanical valves, tissue valves are associated with structural valve degeneration (SVD), potentially limiting long-term durability. The updated American Heart Association (AHA)/American College of Cardiology (ACC) guidelines recommend that selection of the type of prosthetic heart valve to be implanted should be based on a shared decision-making process that accounts for the patient’s values and preferences. , It should include a discussion about the risks of anticoagulant therapy and the potential risk of associated reintervention.
The age cutoff for considering mechanical valve implantation is defined as less than 50 years in the AHA/ACC guidelines. In the European Society of Cardiology (ESC) guidelines, the cutoffs are less than 60 years for aortic valves and less than 65 years for mitral valves.
Numerous prosthetic heart valve designs and a large variety of proprietary anticalcification treatments are available (see Chapter 26 ). These devices differ in their tissue characteristics, frame designs, and implantation methods. They commonly have unique fluoroscopic appearances, which is essential for optimal VIV deployment. This chapter focuses on the more common heart valve subgroup: bioprosthetic tissue valves (see Fig. 26.1 in Chapter 26 ).
Bioprosthetic valves can be classified according to their method of implantation (i.e., transcatheter vs. surgical). Surgical bioprosthetic valves are commonly stratified according to type of tissue (porcine vs. bovine pericardial) or according to frame design (i.e., stented, stentless, or sutureless). Transcatheter valves are commonly categorized as balloon-expandable versus mechanically expandable or self-expandable devices.
Initially, most surgical bioprosthetic aortic valves were implanted in the plane of the annulus (i.e., intra-annular position); today, many are implanted above the annulus (i.e., supra-annular position), allowing for a larger orifice and possibly decreasing the risk of prosthesis–patient mismatch (PPM).
Bioprosthesis leaflets are conventionally attached to the internal aspect of the stent posts, although several surgical bioprosthetic valves are designed with externally mounted leaflets, including the Mitroflow (Livanova, London, England) and Trifecta (St. Jude Medical, now Abbott Cardiovascular, Santa Clara, CA) valves. This design may enable a better hemodynamic profile and reduce the risk of PPM, but the risk of coronary obstruction after VIV is higher in certain anatomic conditions.
Valve leaflets are commonly fashioned from animal tissue (i.e., xenografts) and sometimes from human valves (i.e., homografts and autografts). Most bioprostheses are made of porcine valve tissue or bovine pericardium, whereas transcatheter valves are occasionally made of porcine pericardial tissue. The tissue is commonly preserved in glutaraldehyde; cross-linking of collagen fibers reduces antigenicity, enzymatic degradation, and remodeling of the extracellular matrix. ,
Stented surgical bioprosthetic valves include a support structure made of Elgiloy, a cobalt-chromium-nickel-molybdenum alloy (Elgiloy Specialty Metals, Sycamore, IL); titanium; or Delrin, an acetal homopolymer (DuPont de Nemours, Inc., Wilmington, DE). The frame is attached to a circular or scallop-shaped basal ring. The semirigid materials of the frame absorb some of the forces acting on the leaflets, with the aim of prolonging leaflet durability. The basal ring is frequently covered by a sewing cuff, which facilitates suturing to the native tissue during cardiac surgery. The sewing ring determines the position of the valve in relation to the patient’s tissue annulus. A supra-annular sewing ring is designed to secure the surgical heart valve fully above the patient’s tissue annulus, whereas an intra-annular ring secures it fully or mostly within the patient’s tissue annulus.
Stentless bioprostheses lack a firm support structure. These valves include homografts and porcine (e.g., Freestyle [Medtronic]) and bovine (e.g., Freedom Solo [Livanova]) pericardial tissue valves, and they offer more natural flow in the aortic root. Homografts can be explanted from recipients of heart transplants or from organ donors. They can be implanted as a full root or with the use of a subcoronary technique or occasionally a modified subcoronary technique. Novel surgical bioprostheses (e.g., rapid-deployment valves) include the Intuity valve (Edwards Lifesciences) and the sutureless Perceval valve (Livanova). They enable reduction in aortic cross-clamp and cardiopulmonary bypass times.
Prosthetic valves are defined in several dimensions. The most common feature is the label size, which traditionally represents the external diameter of the inflow portion. , Inconsistencies and controversies exist regarding the sizing and labeling of bioprosthetic valves. Non-uniform or incomplete reporting of bioprosthesis materials and physical dimensions by the industry was described in a 2019 position statement.
For VIV sizing, it is the inner diameter of the valve that is most relevant. These measures include the manufacturer-defined inner diameter (ID) and the inner diameter of the valve as measured by sizing tools (true ID); the latter takes into consideration the valve leaflets inside the frame. There is significant discrepancy between the manufacturer ID and the true ID. The true ID is commonly 1 to 3 mm smaller than the reported ID. Several applications are used for decision making based on these characteristics.
Other relevant prosthetic valve measures include the height of the frame and leaflets when deflected. Occasionally, the length of the leaflet corresponds to the length of the frame, but not always. Leaflet length and the degree to which the leaflets can be deflected are important measures that can influence the risk for coronary obstruction after VIV.
There are numerous causes of prosthetic heart valve failure ( Box 27.1 ). Mechanical valves are prone to thrombosis, whereas bioprosthetic valves are prone to valve degeneration ( Fig. 27.1 ). SVD is an acquired intrinsic bioprosthetic valve abnormality. It is deterioration of the leaflets or supporting structures that results in thickening, calcification, tearing, or disruption of the prosthetic valve material with eventual associated valve hemodynamic dysfunction, manifested as stenosis and/or regurgitation. ,
Structural valve degeneration
Thrombosis
Endocarditis
Prosthesis–patient mismatch
Pannus formation
Paravalvular leakage
Malposition
Underexpansion or stent creep
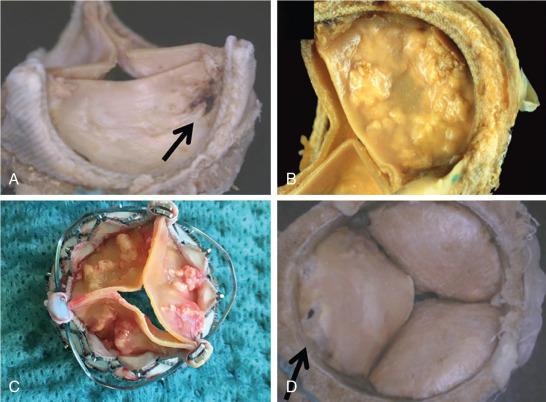
There are two main pathways for SVD: the biochemical pathway, which is mostly related to the interaction between the leaflets and the blood and leads to intrinsic cusp calcification, and the biomechanical pathway, which is related to the stress on the leaflets and can result in leaflet tear. Commonly, the process of SVD is a result of the combined effect of the biochemical and biomechanical pathways manifested by progressive degeneration with periods of accelerated hemodynamic deterioration.
The literature on SVD is extensive and includes numerous definitions for this abnormal condition. Freedom from reoperation, which was considered the primary end point in many studies, is a poor surrogate for SVD because reintervention (surgical or transcatheter) may be performed for causes other than SVD. Conversely, reintervention may not be performed if SVD goes undetected because of a lack of echocardiographic follow-up.
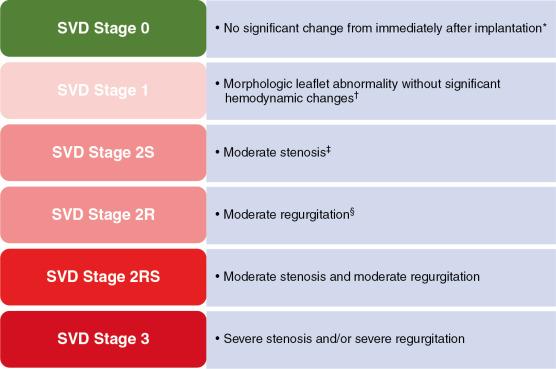
In 2018, a standard definition of SVD of bioprosthetic valves was published, specifying stages according to the hemodynamic severity of the failed valve ( Fig. 27.2 ). The process of valve degeneration typically is gradual, taking place over years. The stages of SVD are based on the state of the implanted valve and not on the patient’s clinical status. Stage 1 includes early morphologic leaflet changes without hemodynamic sequelae. Stage 2 SVD refers to morphologic abnormalities of valve leaflets associated with hemodynamic dysfunction and is divided according to the type of dysfunction (i.e., stenosis and/or regurgitation of moderate degree) after exclusion of PPM, paravalvular leak (PVL), and other non-SVD conditions. Some patients with stage 2 SVD, especially those with mixed failure, may have symptoms and may be considered for reintervention. Stage 3 includes the development of severe stenosis or severe regurgitation, or both.
There are many risk factors for early SVD. Patient age is widely considered to be associated with the risk of SVD: young age at implantation is associated with early valve degeneration. Other correlates for early SVD include the position of the device (i.e., mitral and tricuspid valves degenerate more rapidly than aortic valves), renal dysfunction (e.g., end-stage kidney disease, dialysis therapy), abnormal calcium/phosphate metabolism, very severe dyslipidemia (e.g., patients with homozygous familial hypercholesterolemia), pregnancy, severe PPM, and others.
The type of the device also is related to the risk of SVD. Some devices that were associated with early failures were taken off the market. , Certain valves are more prone to a specific type of failure than others. Bovine pericardial valves tend to fail more often by stenosis, whereas porcine leaflets tend to fail more commonly by regurgitation.
In the current TAVR era, patients with failed bioprosthetic valves can be treated with this less invasive approach, providing a strong argument in favor of a more meticulous echocardiographic surveillance regimen. Consensus documents support yearly echocardiographic surveillance in all patients, but there is a discrepancy about when to begin: from the first year in the ESC guidelines or 10 years after implantation in the AHA/ACC guidelines. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here