Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Malignant tumors of the sinonasal tract (SNT) are rare, comprising less than 1% of all neoplasms and about 3% of those of the upper aerodigestive tract. Squamous cell carcinoma (SCC) and adenocarcinoma are strongly associated with environmental factors, including the use of tobacco and alcohol and occupational exposures, such as heavy metal particles (nickel, chromium) and the leather, textile, furniture, and wood industries.
SNT malignancies most commonly affect the maxillary sinus (~60%), followed by the nasal cavity (~22%), ethmoid sinus (~15%), and frontal and sphenoid sinuses (~3%). SNT tumors are diverse, with the majority composed of SCC and its variants (55%), followed by nonepithelial neoplasms (20%), glandular tumors (15%), undifferentiated carcinoma (7%), and miscellaneous tumors (3%).
Carcinoma of the nasopharynx differs in many aspects from that of the nasal cavity and paranasal sinuses and is discussed as a distinct entity in this chapter.
The clinical presentations, radiologic features, and pattern of tumor spread for SCC, adenocarcinoma, and most of the other malignant neoplasms of the SNT are similar. These features are discussed in detail under the section on SCC and not repeated elsewhere. The gross appearance of SNT and nasopharyngeal malignancies has limited value in aiding diagnosis because the initial diagnosis depends on tissue obtained by endoscopy or polypectomy. With the exception of nasopharyngeal carcinoma (NPC), malignant lymphoma, and rhabdomyosarcoma (RMS), the treatment of choice for most SNT malignancies is surgical resection with clear margins. Here treatment for SCC is used as a model.
SCC is a malignant epithelial neoplasm arising from surface epithelium with squamous cell differentiation. There are two major histologic subtypes: keratinizing and nonkeratinizing (also known as transitional cell or cylindrical cell carcinoma). All SCC variants can be encountered, although less frequently in the SNT than other head and neck sites. Up to 20% to 30% of SNT SCCs harbor high-risk human papillomavirus (HPV), particularly the nonkeratinizing subtype. Sinonasal papilloma, inverted type specifically, is a precursor lesion in a subset of SCC.
Malignant neoplasm of squamous epithelial cells
Most common malignant neoplasm of sinonasal tract representing ~60%-70% of sinonasal tract carcinomas
Paranasal sinuses: maxillary sinus > ethmoid sinus
Nasal cavity: lateral nasal wall and nasal septum
Mortality is 30%-40% at 5 years
Males > females (2 : 1)
6th to 7th decade
Nasal obstruction, nasal discharge, epistaxis, pain
Orbital and eye symptoms and signs
Cranial nerve involvement
Mass in the nasal cavity
CT and MRI complement each other
MRI separates inflammatory from neoplastic areas and shows relationship to soft tissue
CT is best for highlighting bony destruction and yielding extent, location, and size of tumor
Prognosis if confined: 80% 5-year survival
Surgery, with postoperative radiotherapy (T3 and T4)
Prognosis less favorable than nasal cavity, with common recurrences extending into cranial vault
Cervical lymph node metastasis in ~20%
Radical en bloc resection, if possible, with postoperative radiotherapy and/or chemotherapy (preoperatively or postoperatively)
SCC represents approximately 3% of all head and neck malignancies but is the most common malignant epithelial tumor of the SNT. SCC has a male predilection (2 : 1), with a peak incidence in the 6th to 7th decade. The location, in order of decreasing frequency, is the maxillary sinus, nasal cavity (usually lateral wall), ethmoid sinus, frontal sinus, and sphenoid sinus. Early diagnosis is difficult because symptoms and signs are nonspecific and closely resemble those of chronic sinusitis, allergic reactions, and nasal polyposis. Initial symptoms are related to the effects of the mass causing unilateral nasal obstruction. Secondary infection is common, giving rise to a mucoid or purulent rhinorrhea. Epistaxis develops when the mucosa is ulcerated or tumor extends into the sinus wall. Tumors involving the ethmoid, maxillary, or frontal sinuses may cause proptosis, restriction of eye movements, diplopia, or loss of vision. Epiphora results from lacrimal sac or duct obstruction by the tumor. Compression of the nerve at the primary site or perineural space invasion can compromise the function of cranial nerves, resulting in numbness, paresthesia, or pain. Teeth loosening or fistula may be identified within the oral cavity. A mass or discolored lesion may be visualized endoscopically and biopsied.
Late manifestations include facial swelling and cheek paresthesia resulting from anterior maxillary extension into the soft tissue and infraorbital nerve involvement, respectively. Inferior extension into the oral cavity forms a visible mass in the palate or alveolar ridge. Posterior extension can cause trismus from pterygoid muscle invasion. Ear symptoms suggest possible involvement of the nasopharynx, eustachian tube, and pterygoid plates. Upward extension into the skull base may lead to cranial nerve involvement and invasion of the dura. In the initial workup, it is rare to find cervical lymph node metastasis.
Computed tomography (CT) and magnetic resonance imaging (MRI) have largely replaced conventional radiographs in imaging SNT disease and are indispensable in evaluating the extent of disease. CT and MRI complement each other, helping to separate inflammatory disorders and benign and malignant neoplasms and to provide pretreatment information, including location, size, extent, local invasion, and regional and distant metastasis. Of particular interest is tumor extension into the pterygopalatine and infratemporal fossae and tumor relationship to the blood vessels (especially the internal carotid artery and cavernous sinus), nerves, and cranial cavity. CT best highlights bony structures, with bony destruction and soft tissue invasion usually indicative of an aggressive lesion. MRI is superior to CT in its ability to delineate sinus tumors from inflammatory disease, and it can better delineate tumor from the adjacent soft tissues. Using MRI, inflamed mucosa, polyps, and noninspissated secretions with a high water content have high signal intensity on T2-weighted images. In contrast, cellular paranasal neoplasms have lesser amounts of water and demonstrate intermediate signal intensities on T2-weighted images. Perineural spread is best demonstrated by a gadolinium-contrasted MRI and T1-weighted images with fat suppression. CT helps detect cervical lymph node metastasis, especially when there are multiple clustered lymph nodes exceeding 1.0 cm. The status of cervical lymph nodes is best determined by positron emission tomography (PET) using 18 fluorodeoxyglucose ( 18 FDG-PET) to detect tissue with increased metabolism (i.e., nodal metastasis).
Exophytic, friable, necrotic, ulcerated mass
Local soft tissue infiltration and bony destruction
Usually keratinizing (80%-85%)
Squamous pearls, intercellular bridges, hyperchromatic nuclei
Usually nonkeratinizing
Spindle cell, papillary, endophytic, verrucous types recognized
Positive: p63, p40, CK5/6, CK8, CK13
Negative: CK10
Pseudoepitheliomatous hyperplasia, sinonasal papilloma, squamous papilloma, nasopharyngeal carcinoma, oropharyngeal carcinoma, NUT carcinoma
Nasal tumors are usually exophytic/fungating and prone to become friable, necrotic, and ulcerated with increasing tumor size. Sinus tumors may be well circumscribed, filling the sinus cavity in an expansile fashion with erosion of the bone wall, while others are more destructive, inverted, necrotic, and hemorrhagic.
In general, SCC of the nasal cavity is well differentiated and keratinizing, whereas sinus counterparts are nonkeratinizing and moderately or poorly differentiated.
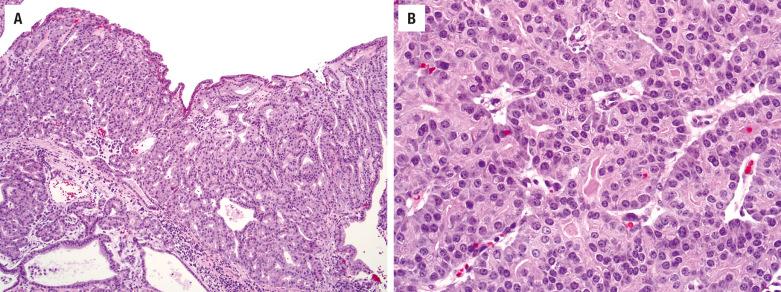
As previously mentioned, SCC of the SNT is subclassified by cell type into keratinizing (80% to 85%) and nonkeratinizing (15% to 20%) subtypes. Keratinizing SCCs of the SNT are histologically indistinguishable from their counterparts of other head and neck sites, such as larynx and oral cavity. Dysplasia of the adjacent or overlying surface epithelium may be encountered. Tumor cells exhibit keratinization, intercellular bridges, dyskeratosis, and squamous “pearls” and usually have enlarged, hyperchromatic nuclei, with a variable degree of nuclear anaplasia ( Fig. 3.1 ). Mitotic figures are usually easy to find and include atypical forms. Stromal invasion by irregular nests and cords of cells in a desmoplastic stroma is often associated with a chronic inflammatory response. However, in superficial biopsies, the only sign of stromal invasion may be in the form of single cells becoming isolated from the base of rete pegs or the tip of tongue-like protrusions. As in other sites, keratinizing SCCs of the SNT are graded as well, moderately, or poorly differentiated.
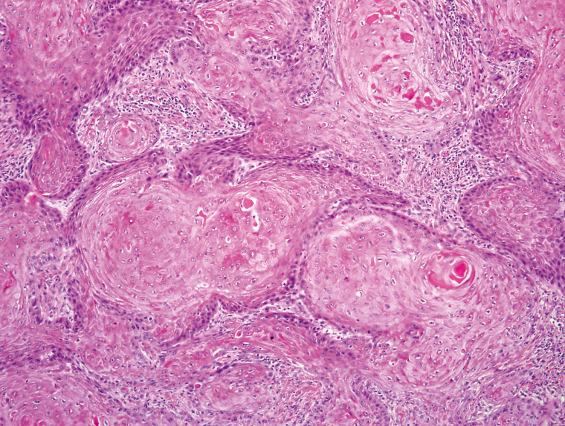
The nonkeratinizing subtype of SCC forms anastomosing ribbons and nests of variable sizes, frequently with a smooth stromal interface and a pushing border with minimal desmoplasia ( Fig. 3.2 ). There is a loss of polarity. Individual tumor cells reveal uniform large, round, or oval nuclei with prominent nucleoli. The cytoplasm varies from pale acidophilic to amphophilic to vacuolated. The cells may have distinct borders. Occasionally individual cell keratinization may be identified. Papillary features (malignant epithelium lining fibrovascular cores) are often encountered within the invasive or surface components ( Fig. 3.2 ). Swirling or spindled tumor cells may be seen; when predominant, they are diagnosed as spindle cell SCC. Because of the smooth stromal interface and lack of significant desmoplasia, it is often difficult to recognize nonkeratinizing SCC as invasive, especially in superficial or small biopsies ( Fig. 3.2 ). In these cases, correlation with radiologic evidence of local destruction confirms the invasive nature of the malignancy. Similarly, biopsies of verrucous squamous carcinoma and papillary squamous carcinoma are prone to be under diagnosed without including the base of the lesion. Variants of SCC, such as verrucous carcinoma and basaloid type, are rare in the SNT.
HPV-related multiphenotypic sinonasal carcinoma (formerly HPV-related carcinoma with adenoid cystic-like features) is a newly described form of SNT carcinoma that is provisionally listed in the 2017 World Health Organization (WHO) classification of head and neck tumors as a subtype of nonkeratinizing SCC. HPV-related multiphenotypic sinonasal carcinoma closely resembles salivary-type carcinomas, especially solid adenoid cystic carcinoma (ACC), with predominantly solid nests but also foci of cribriform growth and a biphasic population of ducts and basaloid myoepithelial cells. Unlike true ACC, however, HPV-related multiphenotypic sinonasal carcinoma usually demonstrates foci of squamous dysplasia of the overlying surface epithelium ( Fig. 3.3 ).
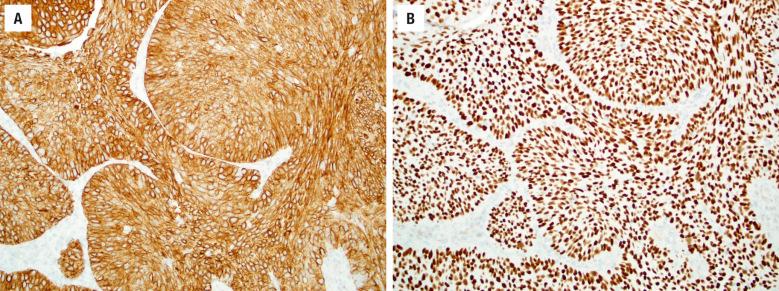
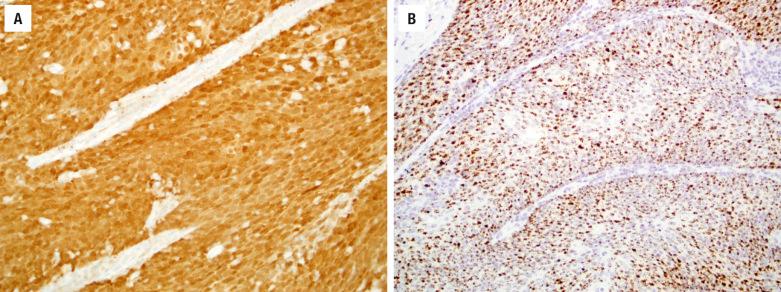
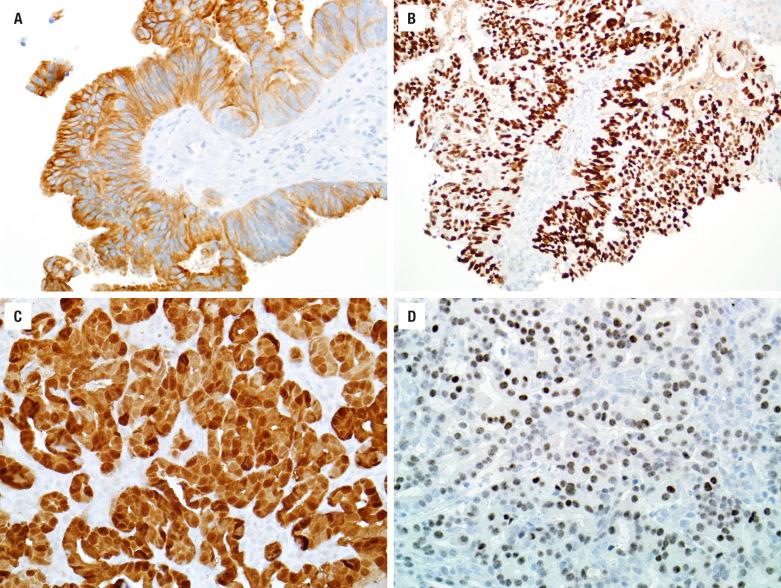
The keratinizing and nonkeratinizing subtypes have similar immunohistochemical staining patterns: cells are diffusely immunoreactive with CK5/6 ( Fig. 3.4 ), p63, p40 ( Fig. 3.4 ), CK8, and CK13 but negative with CK10. Approximately 30% to 50% of nonkeratinizing SCCs (but only rare keratinizing SCCs) of the SNT harbor high-risk HPV ( Fig. 3.5 ). HPV-related multiphenotypic sinonasal carcinoma has two cell populations: myoepithelial cells that are positive for p40, p63, actin, calponin, and S100 and ducts that are positive for c-kit ( Fig. 3.6 ). HPV-related multiphenotypic sinonasal carcinoma is p16- and HPV-positive (usually the rare type 33) ( Fig. 3.6 ).
Pseudoepitheliomatous hyperplasia in the SNT region is most commonly associated with mucosal ulcer with or without prior medical intervention and may be associated with rhinoscleroma, fungal infection, and neoplastic disease (granular cell tumor, lymphoma, and fibrohistiocytic tumors). The elongated and thickened rete pegs extend into the underlying connective tissue and have smooth, sharp, and sometimes pointed borders. There is no desmoplastic stroma. The cells resemble each other and have uniform nuclei without nuclear atypia and rare mitotic figures. Sinonasal (Schneiderian) papillomas and squamous papillomas may occasionally have malignant transformation, with areas of squamous dysplasia and carcinoma in situ. Sinonasal papillomas have inverted epithelial growth, with admixed mucocytes and intraepithelial inflammatory cysts. They lack desmoplasia, single-cell invasion, and atypical mitoses. When broad sheets of cytologically malignant squamous cells are seen in biopsies, the diagnosis of SCC should be considered even though stromal invasion is not demonstrable. Clinical and radiographic features should be requested to aid decision making. Oropharyngeal carcinoma is histologically indistinguishable from some forms of nonkeratinizing SCC, and both tumors are frequently p16- and HPV-positive. Fortunately spread from the oropharynx to the SNT is very rare. Sinonasal undifferentiated carcinoma (SNUC) shows more of a syncytial arrangement, lacks keratinization, and shows vesicular chromatin with prominent nucleoli, while it is negative or focal with p40, p63, and CK5/6. Neuroendocrine carcinoma should show cytologic neuroendocrine features in addition to immunohistochemistry features before the diagnosis is employed. NUT carcinoma , defined by the balanced chromosomal translocation t(15;19) resulting in the BRD4 - NUTM1 oncogene, tends to be a poorly differentiated carcinoma with areas of abrupt squamous differentiation; immunostaining for NUT protein or molecular studies for NUT rearrangement are diagnostic. ACC closely resembles HPV-related multiphenotypic sinonasal carcinoma; this differential is further discussed in the adenocarcinoma section of this chapter.
If SCC is confined to the nasal cavity, the 5-year survival rate is in the range of 80%, depending on keratinization, tumor grade, and stage. Clinical stage is most important, while nonkeratinizing tumors have a better prognosis than keratinizing carcinomas. Involvement of the paranasal sinuses affects the prognosis adversely. Most treatment failures are related to locally advanced disease and tumor recurrence in areas inaccessible to surgical resection, such as the skull base, dura, and brain. Local recurrence is seen in up to 45% of patients, with spread to adjacent structures. Cervical lymph node metastasis develops in up to 20%, with uncommon distant metastases. These patients are at higher risk for the development of a second primary.
Treatment depends on the location and extent of the tumor. T1 and T2 nasal tumors are treated by surgical resection, while T3 and T4 tumors receive postoperative radiotherapy. Various surgical approaches are employed, matched to the complex anatomy of the region, with cosmetic reconstruction as permitted. Adjuvant radiotherapy is usually employed, with variable protocols based on tumor type. Chemotherapy may be used neoadjuvantly or postoperatively.
Unlike the oropharynx where high-risk HPV-related carcinomas have a good prognosis, the prognostic significance of high-risk HPV in sinonasal SCC is currently unclear and still under investigation.
Malignant glandular neoplasms of the SNT can originate from the respiratory epithelium or the underlying mucoserous glands, with the majority (60%) arising from the mucoserous glands. The respiratory epithelial-derived tumors tend to develop high in the nasal cavity and ethmoid sinus, while salivary gland neoplasms develop more frequently in the lower nasal cavity and maxillary sinus. SNT adenocarcinomas are separated into salivary gland, intestinal, and nonintestinal types. By definition the intestinal-type adenocarcinomas are malignant epithelial glandular tumors of the SNT that histologically resemble intestinal adenocarcinoma.
Salivary gland–type adenocarcinoma arising from mucoserous glands (60%)
Non–salivary gland–type adenocarcinoma arising from respiratory mucosa
Second most common carcinoma of sinonasal tract
15% of sinonasal tract carcinomas
Paranasal sinuses > nasal cavity
Salivary gland–type adenocarcinomas have 40%-60% mortality
Non–salivary gland–type adenocarcinomas have ~60% mortality, depending on grade
Equal sex distribution
3rd to 8th decade, mean: 55 years
Males > > > females (specifically with industrial exposure)
5th to 7th decade (low grade: 6th decade; high grade: 7th decade)
Unilateral nasal obstruction
Epistaxis
Purulent or clear rhinorrhea
Pain or visual disturbances if large
Intestinal type has very strong association with woodworkers and leather workers (500× increased incidence)
CT and MRI define extent of the tumor and identify invasion
Prognosis depends on stage and tumor type (~50% 10-year survival for adenoid cystic carcinoma)
Recurrences common (60%)
Complete surgical resection with optional radiation
80% 5-year survival for papillary intestinal-type adenocarcinoma, but 40% overall survival for poorly differentiated carcinoma (depending on histologic grade)
Recurrence in 50% of patients
Complete surgical resection with radiation
The sexes are equally affected with a wide age range, although most are older patients (mean, 55 years). The majority of these tumors develop in the maxillary sinus (~60%) or a combination of sinuses and nasal cavity. Symptoms are nonspecific and include obstruction, epistaxis, and pain. Palatal swelling may be seen.
These are divided into two major categories: intestinal-type adenocarcinoma and nonintestinal-type adenocarcinoma (also nonenteric or seromucinous adenocarcinoma). The intestinal type has a strong male predominance (about 90%) and tends to affect older males (mean, 5th to 7th decade). There is a well-known occupational exposure, specifically in wood workers and leather workers. Although the carcinogenic substance is unknown, it is thought to be particulate in nature, as spouses of these workers also have an increased risk. Prolonged occupational exposure, frequently over decades, is necessary for development. These tumors tend to occur in the ethmoid sinus and nasal cavity, specifically the lower and middle turbinate in the latter. Unilateral obstruction, rhinorrhea, and epistaxis are the most common symptoms.
The nonintestinal-type adenocarcinomas are separated into low- and high-grade adenocarcinoma. Tumors tend to develop in men slightly more commonly, although high-grade tumors are much more common in men than in women. There is a wide age range, although low-grade tumors tend to occur in patients about a decade younger than those with high-grade tumors (54 years vs. 63 years, respectively). The nasal cavity and ethmoid sinus tend to be affected more commonly than other sites.
Large, firm, solid, submucosal mass
Fungating, ulcerated, and friable mass, often mucoid to translucent
Adenoid cystic carcinoma most common, with cribriform and cystic pattern, small basaloid cells with hyperchromatic nuclei
Intestinal and nonintestinal types
Papillary, colonic, solid, mucinous, and mixed types
Usually tall, nonciliated, columnar cells, mucin producing
Tumor grade determines nuclear features
Frequently have a background of necrosis and inflammation
Rarely, clear cell tumor is similar to metastatic renal cell carcinoma
Intestinal types are positive for CK7, CK20, CDX-2, villin, mCEA, MUC2, MUC5, BRST-1, and B72.3
Nonintestinal types are positive for CK7 and S100 but negative for CK20, CDX-2, villin, and MUCs
Sinonasal renal cell-like carcinoma is positive for CAIX and CD10; negative for PAX8 and RCC
p53, EGFR, c-erbB-2 expression, and RAS mutation provide prognostic value
Sinonasal papilloma, hamartoma, metastatic colon carcinoma, metastatic renal cell carcinoma, lymphoma, olfactory neuroblastoma
Salivary gland–type adenocarcinomas tend to be large, firm, solid masses, extensively infiltrative at the time of diagnosis. Intestinal-type adenocarcinomas tend to be fungating, with an ulcerated, friable surface. Cut surface reveals gray, translucent, mucoid parenchyma.
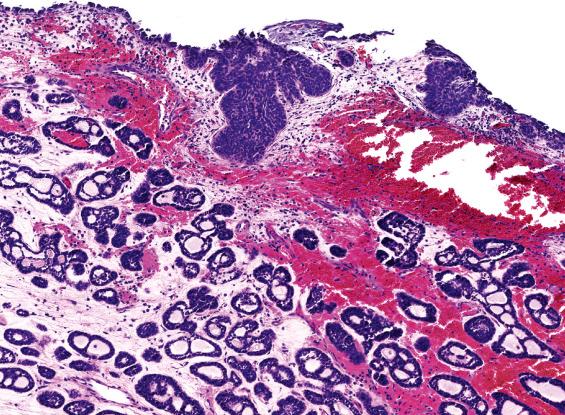
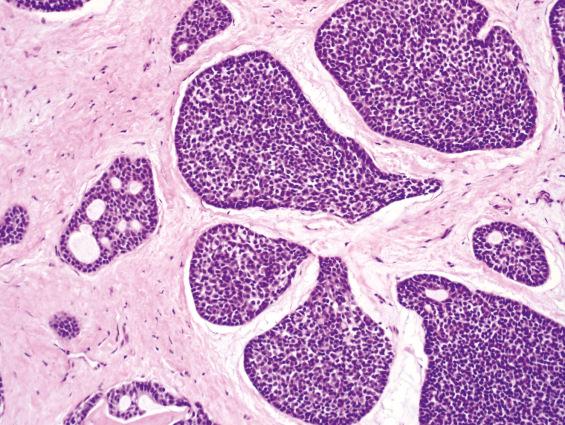
ACC is the most common salivary gland–type adenocarcinoma to occur in the SNT. Other salivary gland–type tumors—such as mucoepidermoid carcinoma, secretory carcinoma, and low-grade papillary adenocarcinoma—rarely involve this region. ACC is invasive, with perineural and bone invasion; it is composed of small basaloid cells with hyperchromatic nuclei and scant cytoplasm arranged in tubules, cribriform glands, and solid sheets ( Fig. 3.7 ). Reduplicated basement membrane material and bluish glycosaminoglycan material within the spaces is common. Predominantly solid ACC can be distinguished from undifferentiated small cell carcinomas and basaloid SCC by its lower mitotic activity and the presence of myoepithelial cell differentiation by immunohistochemistry.
This is a heterogeneous group of tumors that is divided into intestinal and nonintestinal types, with the intestinal type further separated into papillary (~25%), colonic (~45%), solid (~18%), mucinous/colloid (5%), and mixed (7%) types (Barnes classification).
Intestinal-type adenocarcinoma is made up of absorptive cells and goblet cells forming glands, nests, and mucin. The degree of differentiation varies. Some are extremely well differentiated, having the appearance of colonic tubular and villous adenomas, with nuclear stratification and mild nuclear atypia ( Fig. 3.8 ). Some tumors contain small intestinal-type cells, such as Paneth cells and enterochromaffin cells. Occurring at the bases of glands are a few layers of smooth muscle cells simulating muscularis mucosae. Other tumors resemble moderately differentiated colonic adenocarcinoma with confluent glands, nuclear pleomorphism, prominent nucleoli, and increased mitotic activity ( Fig. 3.8 ). Some tumor cells produce abundant mucinous material, while others have a signet-ring formation ( Fig. 3.8 ). Necrosis is common, while multinucleated giant cells may be seen reacting to extravasated mucin. Papillary and solid patterns are recognized. In biopsies, the presence of mucous pools and necrotic debris dissecting between the stroma, creating an alveolar pattern, should raise a suspicion of malignancy. In all cases, the patient should be examined for evidence of intestinal tumor before the neoplasm is accepted as a primary lesion of the upper respiratory tract.
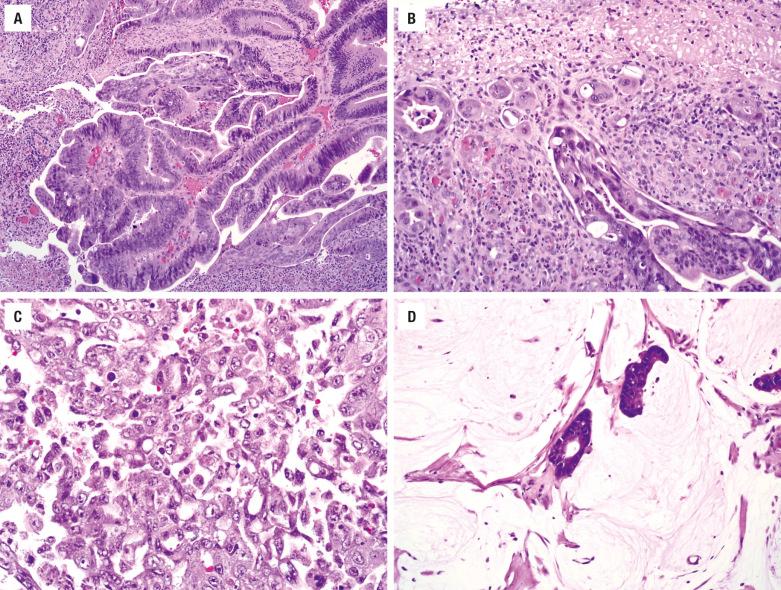
The nonintestinal-type adenocarcinomas are also divided into low- and high-grade types on the basis of architecture, nuclear features, and mitotic activity. Low-grade adenocarcinomas are submucosal, unencapsulated proliferations of uniform cells arranged in compact acini, back-to-back confluent glands, cystic spaces, and papillae ( Fig. 3.9 ). They maintain nonciliated tall columnar to cuboidal configurations and are arranged in a single layer with basal nuclei lacking nuclear stratification. The cytoplasm is abundant but variable in appearance, eosinophilic, basophilic, granular, or clear mucinous. The nuclear atypia is mild to moderate and nucleoli may be prominent ( Fig. 3.9 ). The mitotic activity is generally low. High-grade adenocarcinomas are usually invasive, with angioinvasion, neurotropism, and bone destruction. They are often solid, demonstrating necrosis, nuclear pleomorphism, prominent nucleoli, and high mitotic activity. Some contain abundant signet-ring cells. Special stains are helpful to identify mucus secretion. The sinonasal renal cell-like adenocarcinoma morphologically resembles metastatic renal cell carcinoma but shows more of a follicular to glandular pattern as well as peculiar intranuclear holes.
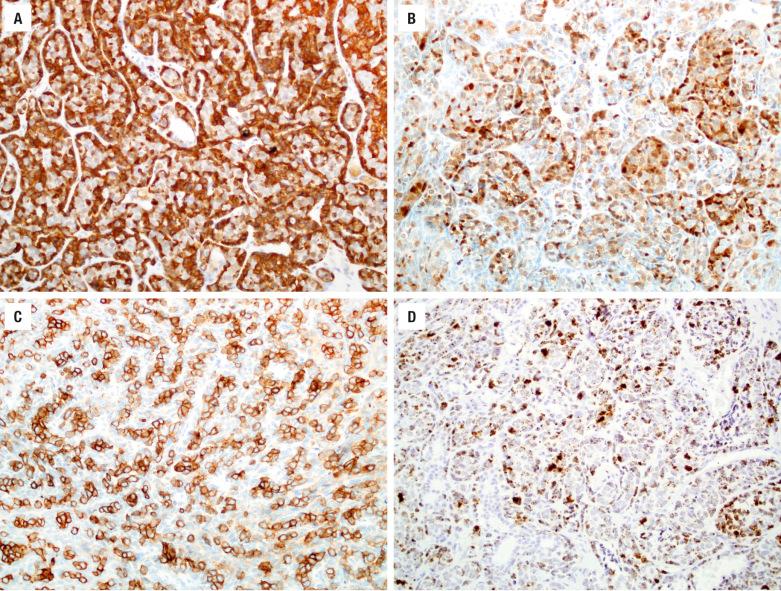
Mucicarmine-positive material is usually easily identified both intracytoplasmically and intraluminally. Most adenocarcinomas do not require immunohistochemical stains. ACC is positive for keratin, CK7, S100 protein, calponin, and p63. Intestinal-type adenocarcinomas show keratin, CK20 (up to 86%) ( Fig. 3.10 ), CDX-2, SATB2 ( Fig. 3.10 ) , villin, CK7, EMA, B72.3, BRST-1, mCEA, and MUC2 and MUC5 immunoreactivity. Various neuroendocrine markers may also be present (chromogranin, synaptophysin, CD56). Prognostic information may be provided by p53, epidermal growth factor receptor (EGFR), and c-erbB-2. There may be an increased proliferation rate with Ki-67. K - or H-RAS point mutations tend to be associated with a poor prognosis. The nonintestinal-type adenocarcinomas are positive with keratins, including CK7 and usually S100 protein ( Fig. 3.10 ) , but are negative with CK20, CDX-2, villin, and MUCs. New data suggest that a subset of nonintestinal sinonasal adenocarcinomas is positive for SOX10 ( Fig. 3.10 ) and DOG1, suggesting a seromucinous rather than surface origin. Also recently, a subset of low-grade sinonasal nonintestinal adenocarciomas has been shown to harbor the ETV6-NTRK3 fusion seen in salivary secretory carcinoma and other tumors. Myoepithelial markers (p63, calponin) are negative, as are neuroendocrine markers. The renal cell-like adenocarcinoma is positive with CAIX and CD10 while negative with PAX8 and RCC.
ACC must be separated from high-grade carcinomas, lymphoma, and olfactory neuroblastoma (ONB). There is a newly reported SNT carcinoma that resembles solid ACC: HPV-related multiphenotypic sinonasal carcinoma . Unlike true ACC, this peculiar SNT carcinoma harbors high-risk HPV (usually type 33), often demonstrates squamous dysplasia of the overlying surface epithelium, and consistently lacks the MYB translocations seen in about half of ACCs. Sinonasal papilloma (oncocytic variant specifically) with their complex back-to-back, confluent glands and papillary architecture may be overdiagnosed as low-grade adenocarcinoma or mucoepidermoid carcinoma, but the cells are cytologically benign. Papillary sinusitis lacks dysplasia and infiltration. Hamartomas have widely separated glands with ciliated, stratified nuclei and thick basement membranes. Metastatic adenocarcinoma of gastrointestinal origin and clear cell tumors of any site must be excluded, especially when considering the renal cell-like adenocarcinoma category.
The overall 10-year survival rate for ACC is 40% to 60%. Aggressive local therapy is warranted even in patients with distant metastases, since a significant number of patients will live for many years with their disease. Recurrences are common (up to 60%). The treatment of choice for ACC is surgical resection with clear margins often accompanied by postoperative radiotherapy to improve local control, especially in cases with positive or close margins. The propensity for perineural spread makes it difficult to obtain clear margins.
Among patients with non–salivary gland–type adenocarcinoma, histologic grade affects outcome. Well-differentiated tumors with predominantly papillary and tubular structures have a better prognosis (80% 5-year survival) than their poorly differentiated counterparts (40% 5-year survival). Patients with industrial exposure have a better outcome than the sporadic cases, perhaps because they are detected earlier owing to regular surveillance. Recurrences develop in approximately 50% of patients, with distant metastasis in about 15%. Overall survival is around 40%, with death in about 3 years. Treatment is radical surgical resection with postoperative radiotherapy.
SNUC is a rare and highly aggressive undifferentiated carcinoma, showing extensive local destruction, histologic pleomorphism, and necrosis, separate from ONB. The taxonomy is not well developed or accepted, but it is considered a distinctive carcinoma of the SNT of uncertain histogenesis lacking squamous or glandular differentiation. There is no known etiology, although isolated Epstein-Barr virus (EBV)-positive cases are reported in Asians.
High-grade aggressive undifferentiated carcinoma with extensive local disease, lacking squamous and glandular differentiation
Rare
Nasal cavity, maxillary sinus, ethmoid sinus, often combined
Mortality of 80% in 5 years
Males > females (2 to 3 : 1)
Wide age range (20-76 years); mean: 6th decade
Nasal obstruction and/or epistaxis of short duration
Proptosis, periorbital swelling, and facial pain
Destructive lesion
May have cranial nerve involvement
Median survival is <18 months
About 30% have cervical lymph node metastasis
Combination multimodality therapy (surgery, chemotherapy, radiation)
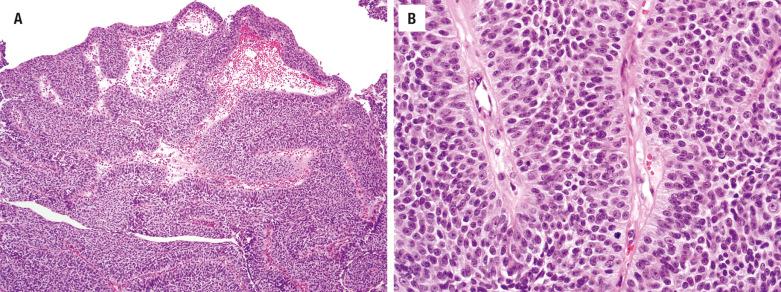
This type of undifferentiated carcinoma is a distinct clinicopathologic entity. The majority of patients present with locally advanced disease with frequent bony, cranial, or orbital involvement at diagnosis. The median age is in the 6th decade with a male predominance (2 to 3 : 1). Previous radiation may be an etiologic factor. Patients have nonspecific symptoms indistinguishable from those of other SNT tumors, but the symptoms are usually of short duration (rapid onset of symptoms). The nasal cavity, maxillary sinus, and ethmoid sinus are usually involved, frequently showing spread into directly contiguous sites ( Fig. 3.11 ).
Large fungating mass (>4 cm) with bone destruction/invasion
Hypercellular tumor arranged in nests, lobules, and sheets of undifferentiated cells (no squamous or glandular differentiation)
Polygonal cells with high nuclear/cytoplasmic ratio, with medium to large nuclei and single, variable nucleoli
High mitotic activity
Tumor necrosis, lymphovascular invasion, and perineural invasion are common
Usually positive for pan-keratin, CK7, CK8, CK19
Occasionally positive for EMA, NSE, p53, chromogranin/synaptophysin
Negative or only focally positive for squamous markers p63, p40, CK5/6
Rarely may be positive for S100 protein and CD99
Olfactory neuroblastoma, neuroendocrine carcinoma, nonkeratinizing squamous cell carcinoma, nasopharyngeal carcinoma, lymphoma, melanoma, rhabdomyosarcoma, rhabdomyosarcoma, SMARCB1 -deficient carcinoma, Ewing sarcoma/primitive neuroectodermal tumor, NUT carcinoma
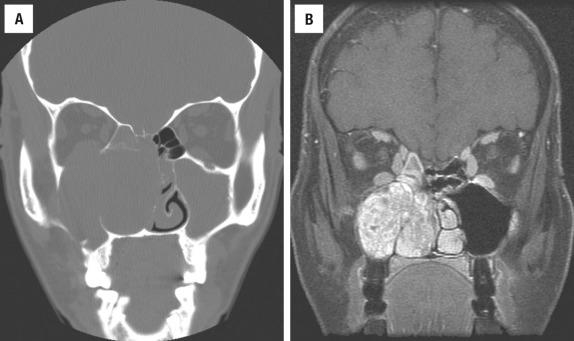
Tumors are usually large (>4 cm) and fungating with poorly defined margins and bony destruction.
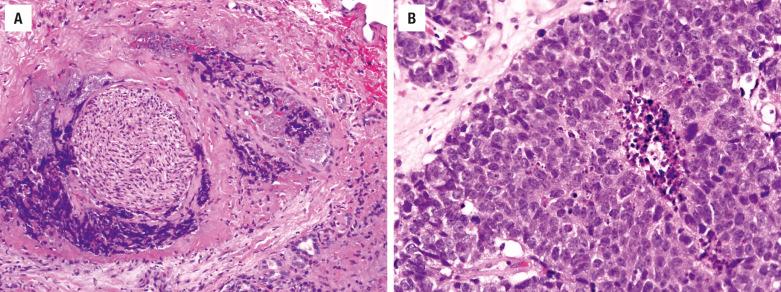
The tumor is hypercellular; it is arranged in nests, lobules, trabeculae, and sheets without any squamous or glandular differentiation. Surface involvement is rare, but ulceration may preclude such a determination. The large polygonal cells have high nuclear/cytoplasmic ratios with medium- to large-size nuclei surrounded by scant eosinophilic cytoplasm ( Fig. 3.12 ). The chromatin may be hyperchromatic to hypochromatic, with inconspicuous to prominent single nucleoli. Necrosis, including comedonecrosis, is common ( Fig. 3.12 ). Mitotic figures are increased. Lymphovascular invasion and neurotropism are common findings ( Fig. 3.12 ).
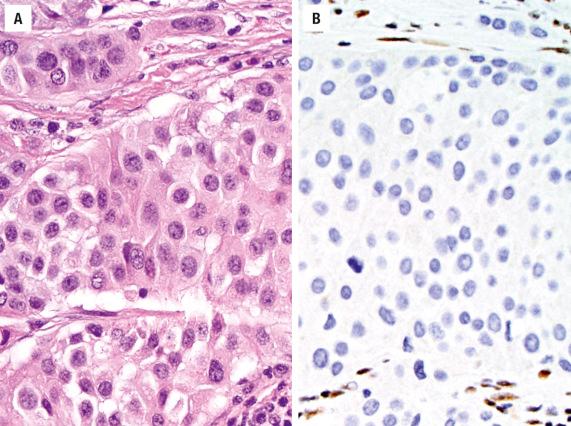
The majority of tumors react with keratins (simple keratins, especially CK7, CK8, and CK19). Epithelial membrane antigen (EMA), neuron-specific enolase (NSE), and p53 may be positive. Chromogranin and synaptophysin are occasionally focally positive. The squamous markers p63, p40, and CK5/6 are negative or, at most, focal. EBER is negative (in Western patients). p16 is commonly positive, but only rarely does SNUC harbor high-risk HPV.
Separation of SNUC from high-grade ONB and neuroendocrine carcinoma remains controversial. ONB has a specific anatomic site of involvement, lobular architecture, and neural features and is typically keratin-negative. Neuroendocrine carcinomas may overlap with SNUC, a separation that at present does not have clinical implications. Nonkeratinizing SCC may resemble SNUC but is diffusely positive for squamous markers like p40. Immunohistochemical stains are valuable to diagnose NPC, lymphoma, melanoma, RMS, and Ewing sarcoma/primitive neuroectodermal tumor (ES/PNET) ( Table 3.1 ). A NUT carcinoma may appear undifferentiated, showing abrupt keratinization and NUT immunoreactivity, along with t(15;19) fusion gene BRD4-NUTM1 . A recently described undifferentiated carcinoma known as SMARCB1-deficient sinonasal carcinoma is characterized by loss of SMARCB1 (INI1) immunoexpression and some degree of rhabdoid cytomorphology ( Fig. 3.13 ). It is not yet clear whether SMARCB1 -deficient sinonasal carcinoma is a distinct tumor entity or a group of entities.
| Feature | Squamous Cell Carcinoma | Sinonasal Undifferentiated Carcinoma | Malignant Melanoma | Olfactory Neuroblastoma | Extranodal NK/T-Cell Lymphoma, Nasal | Rhabdomyosarcoma | Ewing Sarcoma/PNET |
|---|---|---|---|---|---|---|---|
| Mean age | 55-65 years | 55-60 years | 40-70 years | 40-45 years | 50-60 years | <20 years | <30 years |
| Site | Nasal cavity and/or sinuses | Multiple sites usually | Anterior nasal septum > maxillary sinus | Roof of nasal cavity | Nasal cavity > paranasal sinuses > nasopharynx | Nasopharynx > sinonasal tract | Maxillary sinus > nasal cavity |
| Radiographic studies | Little destruction or spread | Marked destruction or spread | Central destructive mass | “Dumbbell-shaped” cribriform plate mass | Early changes nonspecific; midline destruction later | Size, extent of tumor | Mass lesion, with bone erosion |
| Prognosis | 60% 5-year (stage and tumor type dependent) | <20% 5-year survival | 17%-47% 5-year survival | 60%-80% 5-year survival | 30%-50% 5-year survival (stage-dependent) | 44%-69% (age, stage- and subtype-dependent) | 60%-70% 5-year (stage, size, FLI1 ) |
| Cranial nerve involvement | Uncommon | Common | Uncommon | Sometimes | Sometimes | Uncommon | Sometimes |
| Pattern | Syncytial | Sheets and nests | Protean | Lobular | Diffuse | Sheets, alveolar | Sheets, nests |
| Cytology | Squamous differentiation, keratinization, opaque cytoplasm | Medium cells, inconspicuous nucleoli | Large, polygonal, epithelioid, rhabdoid, plasmacytoid, spindle; pigment | Salt-and-pepper chromatin, small nucleoli (grade-dependent) | Polymorphous, small to large, folded, cleaved and grooved nuclei | Round, strap, spindled, rhabdomyoblasts, primitive | Medium, round cells, vacuolated cytoplasm, fine chromatin |
| Anaplasia | Present | Common | Common | Occasionally and focally | Common | Common | Minimal |
| Mitotic figures | Present | High | High | Variable | High | Variable | Common |
| Necrosis | Limited | Prominent | Limited | Occasionally | Prominent | Limited | Frequent |
| Vascular invasion | Rare | Prominent | Rare | Occasionally | Prominent | Rare | Rare |
| Neurofibrillary stroma | Absent | Absent | Absent | Common | Absent | Absent | Absent |
| Pseudorosettes | Absent | Absent | Rare | Common | Absent | Absent | Present |
| Keratin | Positive | >90% | Negative | Focal, weak | Negative | Negative | Rare |
| CK5/6 | Present | Negative | Negative | Negative | Negative | Negative | n/a |
| EMA | Present | 50% | Rare | Negative | Negative | Negative | n/a |
| NSE | Negative | 50% | Negative | >90% | Negative | Negative | Positive |
| S100 protein | Negative | <15% | Positive | + (sustentacular) | Negative | Negative | Rare |
| Chromogranin/ synaptophysin | Negative | <15% | Negative | >90% (can be weak) | Negative | Negative | Positive |
| HMB45 | Negative | Negative | Positive | Negative | Negative | Negative | Negative |
| CD45RB | Negative | Negative | Negative | Negative | Positive a | Negative | Negative |
| CD56 | Negative | Negative | Negative | Positive | Positive | Negative | Rare |
| CD99 | Negative | <10% | Negative | Negative | Negative | Rare | >99% |
| Vimentin | Negative | Negative | Positive | Negative | Positive | Positive | Positive |
| Desmin | Negative | Negative | Negative | Negative | Negative | Positive b | Negative |
| In situ EBER | Absent | Absent | Absent | Absent | Nearly 100% | Negative | Negative |
| Electron microscopy | Epithelial junctions | Junctions, rare neurosecretory granules | Premelanosomes, melanosomes | Neurite-like processes, neurofilaments, neurosecretory granules | n/a | Thick and thin filaments, sarcomeres, Z-bands, glycogen | Glycogen; primitive cells |
a Lymphoma is positive with CD3e, CD2, CD56, perforin, TIA1, and granzyme B.
b Rhabdomyosarcoma is positive with desmin, actin, myoglobin, fast myosin, MYOD1, and myogenin.
Prognosis is usually poor, with a median survival of less than 18 months, and no reported disease-free survival. There is frequent recurrence, with metastasis to lymph nodes and distant sites. A combination of chemotherapy, radiotherapy, and radical surgery provides the best chance of survival, although the exact sequence of modalities and specific agents is controversial and undecided.
Nuclear protein in testis (NUT) carcinoma (previously known as NUT midline carcinoma) is a poorly differentiated carcinoma defined by a genetic rearrangement of the NUTM1 gene. NUT carcinoma can arise from virtually any anatomic site, but among head and neck sites it affects the SNT most commonly. NUT carcinoma is known for its highly aggressive clinical behavior.
Highly aggressive carcinoma, often demonstrating abrupt squamous differentiation defined by the presence of NUTM1 gene rearrangement
Rare
Nasal cavity, maxillary sinus, ethmoid sinus, often combined
Mortality of >90% in 5 years
Females slightly > males
Wide age range (0.1-82 years) but most often children and young adults; median 22 years
Nasal obstruction and/or epistaxis of short duration
Proptosis, periorbital swelling, and facial pain
Destructive lesion
May have cranial nerve involvement
Median survival is 9.8 months
About 50% have regional or distant metastasis at presentation
Combination multimodality therapy (surgery, chemotherapy, radiation). Experimental targeted therapies (e.g., bromodomain inhibitors) under investigation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here