Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The malignant glioma microenvironment is infiltrated by multiple types of immune cells, including myeloid cells and microglia, which are a significant component of these tumors. These cells contribute to immunosuppression and facilitate tumor cell invasion of normal brain. Targeting these cells has become an important therapeutic concept.
Extracellular matrix (ECM) in malignant gliomas has a unique composition that enables tumor growth and survival. Interactions of glioma cells with the brain ECM play a critical role in tumor invasion of normal brain.
In malignant gliomas, the presence of neurons, astrocytes, oligodendrocyte precursors, and a distinct type of ECM results in microenvironmental components that are unique compared with other solid tumors.
Interactions of malignant glioma cells with other cells of the normal brain including vasculature, neurons, and astrocytes form a cellular ecosystem that is geared toward driving rapid tumor growth and invasion. Studies in this area are uncovering therapeutic opportunities.
Single-cell sequencing is beginning to revolutionize our understanding of the molecular features of individual cell types in the tumor microenvironment and promises to bring many new therapeutic concepts forward in the near future.
The microenvironment of a tumor is the set of cellular and molecular components that form the context in which the tumor originates, grows, and eventually disperses through normal tissue. These cells and molecules are in intimate contact with tumor cells and participate in a two-way communication that ultimately supports tumor progression. The tumor microenvironment includes normal epithelial cells; fibroblasts that form the supporting structure—or stroma—of the tissue; blood vessels that grow in response to tumor signals; resident and infiltrating immune cells; signaling molecules provided by both tumor and normal cells; and the extracellular matrix (ECM) that is remodeled by the growing tumor. Owing to the rapid growth of tumor cells and their high metabolic demands, the tumor microenvironment also involves alterations and temporal fluctuations in the biochemical conditions within the tissue, such as hypoxia, low pH, and nutrient deprivation, which create additional demands on cells and impose a highly selective milieu. In malignant gliomas, the presence of neurons, astrocytes, oligodendrocyte precursors, and a distinct type of ECM results in microenvironmental components that are unique compared with other solid tumors. The past decade of research has seen a momentous increase in the study of the interactions of malignant glioma cells with normal components of the neural tissue. This has generated considerable interest in strategies to identify and target key elements of the tumor microenvironment that could disrupt glioma growth and have an impact on the final outcome of the disease. This chapter presents an overview of the interactions between glioma cells and the major components of the neural microenvironment, with particular emphasis on the distinctive phenomenon of glioma invasion. In addition, we review current knowledge on the interactions of tumor and immune cells, which are of great therapeutic interest and considered one of the most promising approaches to improve the outcome of this deadly disease.
Nowhere is the interaction between glioma cells and their microenvironment better illustrated than during the process of tumor cell dispersion throughout the CNS. While the molecular and cellular mechanisms of tumor cell proliferation can be largely studied with isolated tumor cells in vitro, understanding tumor invasion requires a faithful replication of the interactions between tumor cells and normal cell types and ECM molecules that form the natural barriers to movement in neural tissue (as illustrated in Fig. 138.1 ).
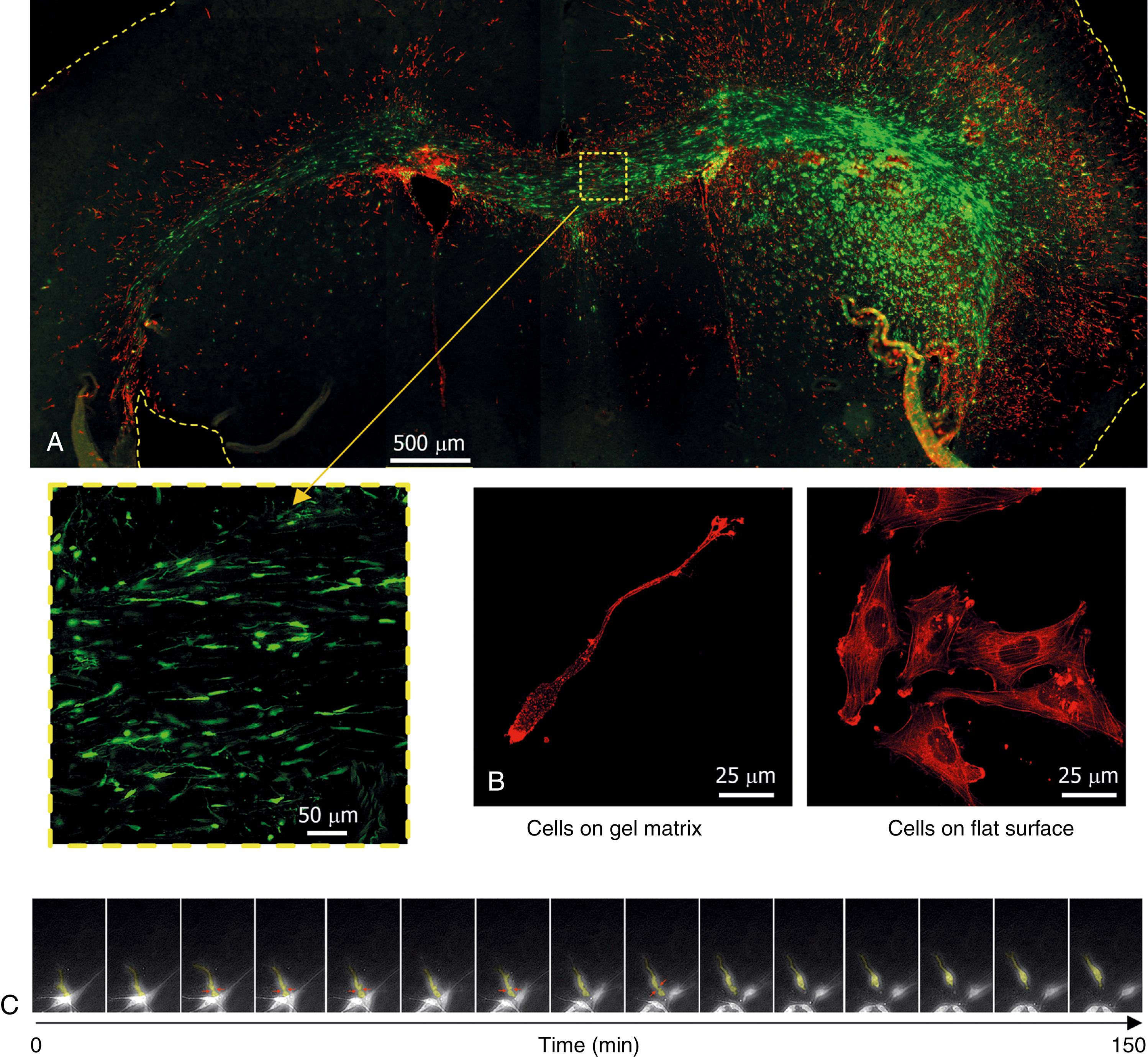
The infiltration of malignant gliomas through the neural parenchyma is a hallmark of these tumors and a major factor that contributes to tumor recurrence and the eventual failure of current treatments. Radiographic and histologic analysis of glioma invasion reveals a pattern of contiguous infiltration that results in poorly defined tumor margins followed by a scattering of malignant cells beyond the observable boundaries of the tumor. Motile cells at the periphery of glioma aggregates in vitro have been compared with the cells from the core and found to be less proliferative. , A differential gene expression profile associated with the invasive phenotype has also been confirmed by analyzing glioblastoma cells recovered by laser-capture microdissection from tumor cores and their paired surrounding white matter containing infiltrated tumor cells. Several differentially expressed genes in invasive glioma cells are associated with their ability to resist cytotoxic therapies, and therefore become the source of tumor recurrence following therapy. , Indeed, effective control of local glioma recurrence (i.e., at the original tumor site) has been positively correlated with increased incidence of distant recurrence (at distances farther than 2 cm from the original tumor site), suggesting that distant tumor foci result from invasive tumor cells that escaped the initial treatments. Data from single-cell RNA sequencing (scRNAseq) are beginning to uncover invasion programs at the level of the single tumor cell. However, isolation of bona fide invading cells remains challenging. To overcome this, bulk sequencing of 5-aminolevulinic acid–labeled tumor cells isolated from the surgical resection margins was used to identify specific gene expression signatures.
In stark contrast to other solid tumors, glioma invasion of normal tissue is not a late feature acquired during malignant progression but a defining feature of both low- and high-grade tumors, suggesting that motility could be a constitutive ability of the neural cell type(s) that give rise to gliomas. , Indeed, neural progenitor cells have the ability to migrate through brain tissue, and a number of studies have suggested that specific types of progenitors (such as oligodendrocyte precursors , ) are candidate cells of origin for malignant gliomas.
Malignant gliomas continuously infiltrate neural tissue as they grow (as illustrated in ), which results in a radiographic nonenhancing penumbra around the tumor core observed with MRI. Prior histologic studies of tumor infiltration have described increased frequency and distance of dispersed cells in grade II and III astrocytomas compared with the more aggressive glioblastomas. The increased time for dispersion of malignant cells in lower grade gliomas, before the bulk of the tumor is detectable, could explain the reported higher frequency of distant recurrence for grade III astrocytomas compared with glioblastomas. Tumor dispersion and distant recurrence have also been observed in low-grade gliomas, including ependymomas, even several years after the original treatment. It is important to remark that these studies have used classical histologic classification of gliomas, and tumor dispersion patterns have not yet been evaluated systematically in gliomas classified by molecular phenotyping. To address this matter, a study analyzed the radiographic pattern of invasion in gliomas classified by their expression of the mutant gene for the enzyme isocitrate dehydrogenase 1 (IDH1), which is a predominant mutation in grade II and III gliomas. This study found that tumors with IDH1 mutation were more invasive than those with wild-type IDH1. Because IDH1 -mutant status is an excellent biomarker of improved prognosis, this appears a paradoxical result, but it is possible that a protracted distant recurrence of those gliomas, as opposed to faster local recurrence in IDH1 wild-type tumors, may contribute to their extended overall survival.
Despite their insidious infiltrative behavior, malignant gliomas very rarely metastasize outside the CNS, , and they grow as contained masses if implanted peripherally in animal models. On the other hand, a majority of invasive extracranial tumors that metastasize to the brain show little to no diffuse infiltration of neural tissue. The marked differences in invasive behavior between malignant gliomas and other solid tumors in the brain underscore the presence of unique interactions between the glial neoplasms and their microenvironment, despite the fact that many proteases, cytokines, and ECM molecules are expressed in a similar fashion in gliomas and other solid tumors that metastasize to the CNS. In the next sections we review the interactions of glioma cells with the neural ECM, with vascular cells that support the tumor and can also help dispersion, with reactive astrocytes, and with cells of the immune system.
The neural ECM is the molecular scaffold that fills the extracellular space (ECS) in the CNS and forms the immediate physical support of all cell types. The presence of a significant neural extracellular volume was disputed until the early 1980s owing to artifacts in the preparation of neural tissue for microscopy and deficiencies in the histologic detection of proteoglycans and mucopolysaccharides. , However, nonmorphologic methods applied to fresh tissues, based on the diffusion of charged or fluorescent molecules, demonstrated that the ECS occupies up to 20% of total CNS volume, which was later confirmed with improved fixation techniques and immunohistochemistry. , Measurements in live animals put the CNS among the tissues with largest extracellular volume when compared with other highly vascularized tissues such as kidney or skeletal muscle. Measurements of the ECS have been performed in freshly resected gliomas, revealing changes in the ECS volume and its tortuosity compared with normal brain tissue. Both low- and high-grade astrocytomas have larger ECS volume than normal brain, with volume increases that correlate with grade. Even in highly cell-dense glioblastomas, this acellular volume can reach up to 40% of the total tumor volume (and 58% of total volume in the necrotic regions of these tumors).
Extracellular tortuosity (not to be confounded with the tortuosity of blood vessels) is a measurement of how much the extracellular diffusion of small molecules is limited in real tissues compared with a perfect homogeneous medium. Changes in the amount and composition of the ECM can increase the extracellular tortuosity, revealing important barriers to molecule diffusion. Extracellular tortuosity is similar between low-grade astrocytomas and normal brain, but it increases significantly in anaplastic astrocytomas and glioblastomas. This increase does not correlate with tumor cell density, but with the accumulation of ECM forming fine “molecular nets” in high-grade gliomas. Interesting to note, neither the ECS volume nor the extracellular tortuosity are different in grade II oligodendrogliomas compared with normal brain; this is thought to be due to the fact that these tumors are able to intermix with brain tissue without causing significant disruption of the neural architecture. ,
The normal stromal ECM of the CNS, found in the white matter and gray matter neuropil, is a soft gel rich in proteoglycans, glycoproteins, and hyaluronic acid, but notoriously devoid of large fibrillar proteins (e.g., collagens, elastins, fibronectin). , , A different type of ECM, forming a well-defined basal lamina (BL) rich in fibrillar proteins (in particular collagen type IV, laminin type I, and fibronectin), is tightly restricted to the choroid plexus and the perivascular and subpial spaces in the CNS. The major components of the typical neural ECM and the perivascular BL in the CNS, as well as their modifications by malignant gliomas, are listed in Tables 138.1 and 138.2 , respectively.
| ECM Component (References) | Characteristics | Normal Neural Tissue | Malignant Glioma |
|---|---|---|---|
| Hyaluronic acid (HA) | An extremely large (>10 6 Da) acidic polysaccharide not attached to proteins. Generates a large hydrophilic mesh with elastic spaces that facilitate cell growth and motility. | Highly expressed during early neural development but decreases in adult brain and associates noncovalently with proteoglycans, forming insoluble matrix aggregates that inhibit cell motility. | Increases up to fourfold higher than in normal brain. Tumor cells proliferate and migrate through the HA mesh. HA is degraded by tumor hyaluronidases and the fragments stimulate synthesis of proteases and ECM proteins by tumor cells. |
| Chondroitin sulfate proteoglycans (CSPG, lectican family) | Large, secreted glycoproteins (aggrecan, versican, neurocan, and brevican) that bind HA and cell membrane receptors, tethering cells to the ECM. CSPGs exhibit many variants generated by alternative splicing, cleavage, and posttranslational modifications. | Although each molecule has a distinctive expression profile, CSPG expression generally increases in the adult CNS. CSPGs are considered inhibitory molecules that prevent cell motility and axonal extension. They are major components of the glial scar. | The CSPGs brevican and versican are highly increased in gliomas and paradoxically promote tumor invasion (brevican) and proliferation (versican). Specific isoforms upregulated in gliomas are credited for these protumoral mechanisms. |
| Phosphacan | Soluble form of the membrane tyrosine phosphatase RPTP-β (a proteoglycan carrying chondroitin- and keratan-sulfate that does not bind HA). Binds the neurite growth-promoting factor pleiotrophin ( PTN gene). | Highly expressed by neurons in the developing CNS and associated with axonal extension. Decreases in the adult CNS but is reexpressed by astrocytes following injury. | RPTP-β is increased in grades II and III gliomas, whereas the soluble form phosphacan is increased in GBM. It is postulated to increase cell migration through interaction with PTN. |
| Link proteins (LPs) | Small soluble glycoproteins that bind HA and CSPG, forming multimolecular complexes considered the basis of the neural ECM scaffold. | LPs follow an expression profile similar to CSPGs, increasing in the adult CNS and contributing to the axon-inhibitory ECM around neurons. | Expression is strongly reduced in GBM tissue, but they may be found in cultures of GBM-initiating cells. They promote glioma cell migration in vitro. |
| Tenascin-R (TNR) and tenascin-C (TNC) , | Multimeric proteins that bind CSPG, fibronectin, and cell surface receptors, contributing to the structure of the neural ECM. | Highly expressed in neural development but decrease and become restricted to white matter in adult CNS. They form boundaries within the ECM scaffold to regulate neural precursor migration and axonal extension. | TNC is highly expressed in the perivascular space and may promote angiogenesis, tumor cell proliferation, and glioma invasion in combination with PDGF signaling. |
| SPARC, hevin, testicans | A family of phosphorylated glycoproteins that bind growth factors and fibrillar proteins. | Expressed in the developing CNS and involved in neural circuit formation and tissue remodeling. Expressed by astrocytes in adult CNS. | Highly expressed by malignant glioma cells, regulate expression of metalloproteases, and promote adhesion and migration of glioma cells on perivascular basal lamina |
| Fibulins (FIBs) | A family of secreted proteins that associate with proteoglycans and fibrillar proteins (collagens, elastins, and fibrillins) to form large fibrillar structures in elastic matrices, | Large FIBs (FIB1 and FIB2) are expressed in early neural development and can replace tenascins to form ECM scaffolds with CSPG. Their expression is much reduced in adult CNS. Small fibulins (FIB3, FIB4, and FIB5) are virtually absent in normal CNS. | FIB3 and FIB4 are highly upregulated in malignant glioma cells and promote tumor invasion and resistance to apoptosis. FIB3 is also a proangiogenic signal in the tumor vasculature. |
| ECM Component | Characteristics | Normal Neural Tissue | Malignant Glioma |
|---|---|---|---|
| Fibronectin , , | A multidomain-secreted glycoprotein and major component of the basal lamina. Binds multiple ECM proteins and integrin receptors. | Expressed during fetal development by neurons and astrocytes, potentially involved in synapse formation. It is absent in the adult CNS stroma and restricted to the blood vessels (expressed by endothelial cells) and the glia limitans (expressed by meningeal epithelial cells). | Expressed by most glioma cell lines in vitro but less frequently in cultures of glioma-initiating cells. Although it is listed as one of the top upregulated genes in glioblastomas, expression in vivo is largely restricted to the tumor perivascular space. Fibronectin strongly stimulates integrin-dependent tumor cell adhesion and motility. |
| Laminin , , | A secreted protein with multiple isoforms derived from the combination of three different chains. Laminin-1 is a major component of the basal lamina and the Matrigel a mixture used to study glioma invasion in vitro. | Expressed mostly around adult brain blood vessels (laminin-1) or by reactive astrocytes during development and in the adult CNS (laminin-2). | Laminins are major components of the perivascular niche where glioma-initiating cells arise and proliferate. They activate α 6 integrins and enable tumor cell growth. |
| Collagens , | The most abundant proteins in mammals; form a superfamily with 28 known members. Characterized by three chains forming a triple-helix structure; collagens form supramolecular, stretch-resistant fibers or participate in other ECM networks in which they provide resistance to tension forces. | Fibrillar collagens (COL-I, COL-II, COL-III, COL-V, and COL-XI) are expressed at very low levels in the CNS. Basal lamina collagens (COL-IV and COL-VI) are abundant during development and in the adult CNS around blood vessels. They are not expressed by astroglia and do not participate in the structure of the amorphous neural ECM. | Malignant gliomas express several types of fibrillar and sheet-forming collagens. They bind integrins and discoidin receptors and facilitate tumor cell adhesion and invasion. Collagens secreted by glioma cells (in particular, COL-IV) introduce major changes in stiffness, tortuosity, and adhesive properties of the ECM. Glioma cells further modify the collagen-rich ECM by degradation (via metalloproteases) and enzymatic cross-linking (via lysyl oxidases). |
| Heparan sulfate proteoglycans (HSPGs) , | Cell surface glycoproteins with a transmembrane domain (syndecans) or lipid anchor (glypicans). Some HSPGs are secreted into the ECM (agrin, perlecan) and form the scaffold of the basal lamina. HSPGs act as coreceptors of cell adhesion molecules and receptor tyrosine kinases. | Regulate proliferation and differentiation of neural cells by binding to and forming gradients of cytokines, growth factors, and morphogens. Interactions of HSPG with NCAM and integrins are required for migration of neural cell precursors. | Expression of HSPG and sulfation of HS chains are increased in gliomas and correlate with tumor grade. HSPGs promote activation of receptor tyrosine kinases and contribute to glioma malignancy. |
| Elastin , | Secreted fibrillar protein and major component of elastic fibers in connective tissue and arteries. Fibers are formed by covalent polymerization and cross-linking of tropoelastin monomers. | Elastin transcription is active in the developing neuroepithelium, but elastin fibers are only found in adult meninges and the brain vasculature, where they contribute to the basal lamina structure. | Glioma cells express and degrade elastin but do not form elastic fibers. Glioma cells also express proteins that can bind elastin (including elastin-binding protein and fibulins) and lysyl oxidases that can cross-link it. Elastin fragments increase glioma cell proliferation and invasion. |
Malignant gliomas show very different interactions with both types of ECM: tumor cells usually adhere well to fibrillar proteins of the BL in vitro and migrate along the BL of perivascular and subpial surfaces, which form major anatomic routes for tumor dispersion. , However, glioma cells do not seem to be able to cross this BL in vivo, which would lead to intravasation or intrameningeal spread, both of which are extremely rare in these tumors. , On the other hand, adhesion of isolated glioma cells in vitro can be attenuated by stromal chondroitin sulfate, hyaluronic acid, or myelin-associated glycoprotein. However, individual glioma cells readily traverse the ECM of the neural parenchyma, dispersing along white matter tracts and through the gray matter neuropil. It is worth noting that the interaction of glioma cells with the BL and stromal neural ECM seem to be essentially reversed in nonneural tumors that metastasize to the CNS. Metastatic cells cross the BL and readily extravasate into the brain perivascular space, but they rarely invade the inhibitory neural ECM and instead grow close to their extravasation sites as contained masses.
The motility of individual glioma cells appears to involve locally restricted degradation of the ECM , and may be largely facilitated by “squeezing” of the cell body through ECM spaces (see Fig. 138.1B–C ), which is driven by myosin II–dependent cell contraction , and rapid changes in total cellular volume , ( ). The resulting amoeboid movement has been largely compared with similar migratory behavior observed in neural cell precursors, reinforcing the concept that glioma cells may derive from a motile neural precursor. Cell motility is, in addition, facilitated by additional mechanisms of ECM remodeling, such as incorporation of glioma-secreted matrix molecules into the neural ECM and covalent modification of the ECM scaffold. The resulting changes open intercellular spaces and increase the stiffening of the ECM, facilitating tumor cell adhesion and migration.
Most of the glioma-secreted proteases that degrade and remodel the ECM belong to the large families of matrix metalloproteases (MMPs) and ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs). MMPs have been classically implicated in glioma invasion owing to their ability to cleave and degrade fibrillar proteins such as collagens and fibronectin. , The collagenases MMP-2 and MMP-9 have been identified numerous times as typical MMPs upregulated in gliomas compared with normal brain tissue, and have been shown to be required to promote tumor invasion. Although these metalloproteases can also degrade chondroitin sulfate proteoglycans in vitro, , cleavage of these proteoglycans by MMPs in vivo is less common and possibly less functionally relevant. , Instead, neural proteoglycans are primarily cleaved by ADAMTS enzymes, in particular the aggrecanases ADAMTS-4 and ADAMTS-5, with minor contribution of ADAMTS-1. Of these, ADAMTS-5 is particularly elevated in malignant gliomas and correlates with tumor grade. , Finally, it is worth noting that although studies of MMPs and ADAMTS have dominated the protease-oriented research for malignant glioma, other proteases such as the cysteine cathepsins also play a significant role in degrading the neuropil and BL ECM. , Although these proteases are mostly active in the acidic lysosomal environment, their secretion into the acidified tumor stroma makes them a relevant factor in ECM remodeling that may contribute to glioma invasion and angiogenesis. , ,
As indicated earlier, the dispersion of single glioma cells through the neural ECM is largely dependent on their ability to open intercellular spaces and squeeze through them in an amoeboid manner. , This process may also be facilitated by the secretion of proteoglycans and hyaluronic acid by glioma cells. These high-molecular-weight, hygroscopic molecules retain water and increase the hydrated space around the tumor cell, facilitating cell growth and movement. Brevican and versican are the two major chondroitin sulfate proteoglycans secreted by glioma cells and have been shown to promote tumor growth and invasion. , , Both proteoglycans exhibit specific isoforms upregulated in glioma , and are, in addition, substrates for the ADAMTS enzymes. Work with glioblastoma cells in culture has shown that specific domains of these proteoglycans may be released from the ECM scaffold by ADAMTS cleavage, acting as signals that activate integrin and epidermal growth factor receptor–mediated signaling to promote tumor cell proliferation (versican) , and invasion (brevican). ,
Finally, the ECM of the tumor microenvironment can change not only by the addition or degradation of ECM molecules but also by the chemical (covalent) modification of these molecules. For example, glioma cells secrete enzymes of the lysyl oxidase family, which are copper-dependent enzymes that cross-link neighboring lysine residues in collagen and elastin. In normal tissues, this crosslinking is critical to provide strength and stability to major fibrillar proteins, and absence or inhibition of lysyl oxidases results in overall weakness of bone, ligaments, and skin. In solid tumors, lysyl oxidase increases the firmness of the ECM, which facilitates cell migration and contributes to tumor invasion and metastasis. The same role has been postulated for lysyl oxidases in gliomas, although their specific molecular targets in the glioma ECM have not yet been identified.
Glioma-induced changes in the composition and structure of the ECM not only promote the growth and invasion of these tumors but also limit the efficacy of current therapeutic strategies. For example, the increased extracellular tortuosity in gliomas, coupled with increased interstitial pressure, results in reduced solute diffusion. This limits the ability of therapeutic agents to spread in the tumor and reduces their efficacy, even when they are delivered intraoperatively.
The critical role of the ECM as a scaffold that supports tumor cell division and dispersion makes it also a particularly relevant molecular target. Strategies targeting the glioma ECM have largely focused on approaches to inhibit ECM-degrading metalloproteases and ECM-binding integrins, with the goal of limiting tumor invasion. The MMP-inhibitor marimastat has been tested in clinical trials for high-grade glioma following radiotherapy, both as a single agent and in combination with temozolomide. Results have been disappointing, with no significant advantages of marimastat as a single agent and minimal increase in progression-free survival for the marimastat-temozolomide combination, but with considerable toxicity. The cyclopeptide cilengitide, which inhibits the binding of α V integrins to ECM substrates has also been tested as an antiinvasive and antiangiogenic agent and is described later (see section “Therapeutic Relevance of the Glioma-Associated Vasculature”). An alternative approach has consisted of direct targeting of ECM molecules to deliver a toxic payload. The most relevant example of this approach has been the iodine 131 ( 131 I)–conjugated anti–tenascin-C monoclonal antibody 81C6 (Neuradiab). A phase 2 clinical trial in recurrent glioblastoma in which 81C6 was added to standard chemoradiation found increased progression-free survival. At the time of writing, there is no ongoing clinical activity for this agent.
Significant advances have been made in recent years in uncovering the ways in which malignant glioma cells integrate with the major CNS cell types—astrocytes and neurons. These interactions are revealing important potential therapeutic developments, as well as the concept that malignant glioma cells integrate with functional neuronal networks, and indeed that these interactions play an important role in tumor cell survival and growth.
Glial cells of the astrocytic lineage are the most abundant cell type in the adult neural parenchyma and the major source of molecules that form the stromal ECM of the CNS. Astrocytes are, in addition, a key cell type in the perivascular niche where most gliomas arise by transformation of neural precursors of the glial lineage into glioma initiating cells. Astrocytes are therefore in intimate contact with neoplastic cells and contribute to the metabolic and functional support of nascent gliomas through the delivery of nutrients and cytokines and removal of metabolic byproducts. It has been hypothesized that newly formed glioma cells can functionally “co-opt” surrounding astrocytes, which would help in the initial stages of tumor dispersion. , A recent experimental model has confirmed this co-optation by showing that glioma cells secrete molecules of the tumor necrosis factor (TNF) superfamily that do not promote their own invasion but activate astrocytes instead, which in turn respond with their own proinvasive factors in vivo.
Histologic analysis of the tissue surrounding human gliomas reveals a broad band of astrocytes with the characteristic reactive phenotype that is observed in other neural injuries: hypertrophic and highly ramified bodies, high expression of intermediate filament proteins such as glial fibrillary acidic protein (GFAP) and vimentin (see Fig. 138.1A ), and formation of a glial scar rich in chondroitin sulfate proteoglycans, which extends several hundreds of microns around the diffuse borders of the tumor. , The glial scar isolates damaged neural tissue and restricts cell motility and axonal extension into or through the injury epicenter. However, current evidence suggests that reactive astrogliosis around malignant gliomas does not limit the dispersion of the tumor cells and may even contribute to tumor invasion through the accumulation of astrocyte-secreted trophic factors in the periphery of the tumor. , ,
The communication between glioma cells and the reactive astrocytes found within and around the tumor is complex and bidirectional. Soluble factors secreted by glioma cells promote astrocyte proliferation and likely contribute to exacerbating the peritumoral gliosis. These soluble factors have not been completely elucidated but are known to include the transforming growth factors α and β (TGF-α and TGF-β), platelet-derived growth factor (PDGF), TNF molecules (mentioned earlier), and cytokines such as CXCL12. , Astrocytes and astrocyte precursors not only proliferate and increase their GFAP immunoreactivity in the presence of glioma cells but also exhibit changes in their expression of molecules that regulate ECM structure. For example, astrocytes co-cultured with glioblastoma cells show increased expression of MMP-2 , and decreased expression of the MMP inhibitor TIMP-2, both of which may contribute to increased peritumoral ECM degradation and therefore to tumor expansion and invasion. Similarly, GFAP-reactive astrocytes recovered from PDGF-induced glioblastomas have been shown to upregulate MMP-10 (a protease from the stromelysin family), tenascin-C (an ECM protein in the perivascular niche), and basal lamina–associated collagen type VI, all of which can also contribute to increased tumor cell adhesion and migration. Indeed, glioma-initiating cells co-cultured with astrocytes or their conditioned medium exhibit increased expression of promitotic and proinvasive genes, as well as increased migration toward glial cells.
Interesting to note, glioma cells and astrocytes interact not only through a two-way paracrine communication mediated by soluble factors, but also by direct physical association through the formation of gap junctions between normal and malignant cells. These gap junctions, identified by expression of connexin-43, have been demonstrated as functional connections that permit coordinated propagation of intercellular calcium signaling, and may increase the invasive potential of glioblastoma cells through the neural parenchyma. , More recently, co-culture experiments have shown that astrocytes increase the resistance of glioma cells to the standard chemotherapeutics temozolomide, doxorubicin, and vincristine. The increased apoptotic resistance is, at least in part, mediated by intercellular communication through gap junctions between glioma cells and astrocytes.
It is known that neuronal signaling can produce factors that promote glioma growth—for example, via the secretion of neuroligin 3 (NLGN3) —and data show that direct synaptic interactions between glioma cells and normal neurons may play an important role in tumor biology, providing a fundamental new concept in the field. Glioma cells in mouse models can integrate into neuronal networks via synapses, and postsynaptic currents mediated by glutamate receptors of the AMPA subtype can drive tumor progression. ,
Although astroglia seem to play an important role in supporting glioma proliferation and invasion, there are currently no therapeutic strategies formulated to disrupt this support or to revert the reactive status of tumor-associated astrocytes. However, experimental work has demonstrated that astrocytes can be used as an associated target to induce apoptosis of glioma cells. For example, the adenosine reuptake inhibitor propentofylline does not affect glioma cells directly but can increase glutamate uptake in co-cultured astrocytes, reducing the availability of glutamate/glutamine for tumor cells and increasing their apoptosis. Similarly, it has been proposed that gap junctions between astrocytes and glioma cells could be inhibited with gap-channel blockers to reduce astrocyte support and potentially increase glioma chemosensitivity. Alternatively, these gap junctions could be exploited to improve the efficacy of suicide-gene therapy, whereby cytotoxic products generated in one cell can induce apoptosis in neighboring cells (“bystander effect”). Cytotoxic products that do not diffuse between cells and must cross intercellular junctions (such as nucleoside analogues that are generated by the suicide gene thymidine kinase ) could show increased efficacy if the suicide gene were locally delivered not only to glioma cells but also to neighboring reactive astrocytes.
NLGN3 has been shown to be a potential target released by neurons that promotes glioma growth. Because the formation of functional NLGN3 is dependent on proteolytic cleavage, inhibition of ADAMTS may be a useful approach to target this pathway. Studies on glioma/neuronal connectivity in glioma models also showed that targeting AMPA signaling reduced glioma growth in mouse models. Electrochemical signaling in glioblastoma therefore has emerged as a rational therapeutic target. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here