Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The author wishes to acknowledge the contribution of John A. Herring for his work in the previous edition version of this chapter.
Osteosarcoma (osteogenic sarcoma) is the most common malignant bone tumor in children and adolescents. The most prevalent neoplasm is composed of high-grade malignant osteoblasts that directly form tumor osteoid or bone, although fibrous and cartilaginous elements may coexist or even predominate and there are low grade variants. The classic osteosarcoma develops in the medullary cavity of a bone, usually in the metaphysis of a long bone. There are several variants of the classic high-grade osteosarcoma. Osteosarcomas may also arise from the surface of bones in relation to the periosteum and immediate periosteal connective tissue. These are termed juxtacortical osteosarcomas and are less common than central lesions. They may be low-grade fibroblastic osteosarcomas, termed parosteal osteosarcomas, , , or intermediate-grade chondroblastic osteosarcomas, termed periosteal osteosarcomas. , , , Rarely, a low-grade endosteal osteosarcoma variant that arises within bone from the endosteum is encountered. These lower grade lesions grow slowly and may metastasize later in the course of the disease, less frequently than high-grade osteosarcoma. Thus the names of the lesions vary with their location in relation to the bone, but the histologic grade of the sarcoma determines its biologic aggressiveness. Telangiectatic osteosarcoma is a high-grade malignant lesion that shows little evidence of ossification but undergoes cystic and necrotic changes because of its rapid growth. , Because the bone is weakened by the rapid destructive osteolytic process, pathologic fracture may occur as the tumor progresses. a
a References , , , , , .
Approximately 400 new cases of osteosarcoma are diagnosed in patients younger than 20 years in the United States annually. The second most common bone sarcoma (Ewing sarcoma, or primitive neuroectodermal tumor) is more common than classic osteosarcoma in those younger than 10 years. , Generally, osteosarcoma occurs between the ages of 10 and 25 years, although it has been found in children as young as 5 years and in older adults. When osteosarcoma develops in an older person, the possibility of malignant transformation of a preexisting benign bone disease, such as Paget disease of bone, fibrous dysplasia, or a bone infarct, should be considered. b
b References , , , , , , , , .
Osteosarcomas may also arise in bones that have been irradiated for other reasons. c
c References , , , , , , , , , , .
The incidence is almost equal in boys and girls.
The cause of osteosarcoma is unknown. Current thinking is that the development of osteosarcoma is likely because of an aberration in skeletal growth and remodeling, although no consistent pattern has been identified. Viral causes have been proposed in a number of studies, but most have been disproven in follow-up investigations. , , , Trauma has also been proposed, but because of the frequency with which children injure themselves, this is probably only an association. Irradiation is known to cause osteosarcoma in patients irradiated for malignant diseases. Alkylating agents are also associated.
The genetics of osteosarcoma has received much attention. It is known to be a component tumor in familial cancer syndromes such as Li-Fraumeni syndrome, and alterations in genes such as Rb, p53, and others are common in these sarcomas. Patients with hereditary retinoblastoma have a high incidence of osteosarcoma, as do those with autosomal recessive Rothmund-Thomson syndrome. , Patients with Rothmund-Thomson syndrome have been noted to have a mutation in the RECQL4 gene. More recent evidence suggest a role for micro-RNAs in the pathogenesis and prognosis for osteosarcoma and other bone sarcomas. , , ,
The tumor is usually situated near the metaphyseal region of a long bone, but on occasion it may be diaphyseal in location. The most common sites, accounting for more than 50% of cases, are the lower end of the femur and the upper end of the tibia. , , The upper ends of the humerus and the femur are next in frequency. Less commonly, a classic osteosarcoma is encountered in the fibula, pelvic bones, , , , , or vertebral column. , , Occurrence in the distal part of a limb (hand or foot) is rare. , However, the tumor has been described in every bone in the body. There are also numerous reports of multiple or multicentric osteosarcomas. d
d References , , , , , , , , , , , .
Classic osteosarcoma ordinarily begins developing in the medullary cavity of a long bone near the metaphysis, but by the time it is recognized it has already penetrated and extended through the cortex, raising the periosteum ( Fig. 26.1 ). e
e References , , , , , , , .
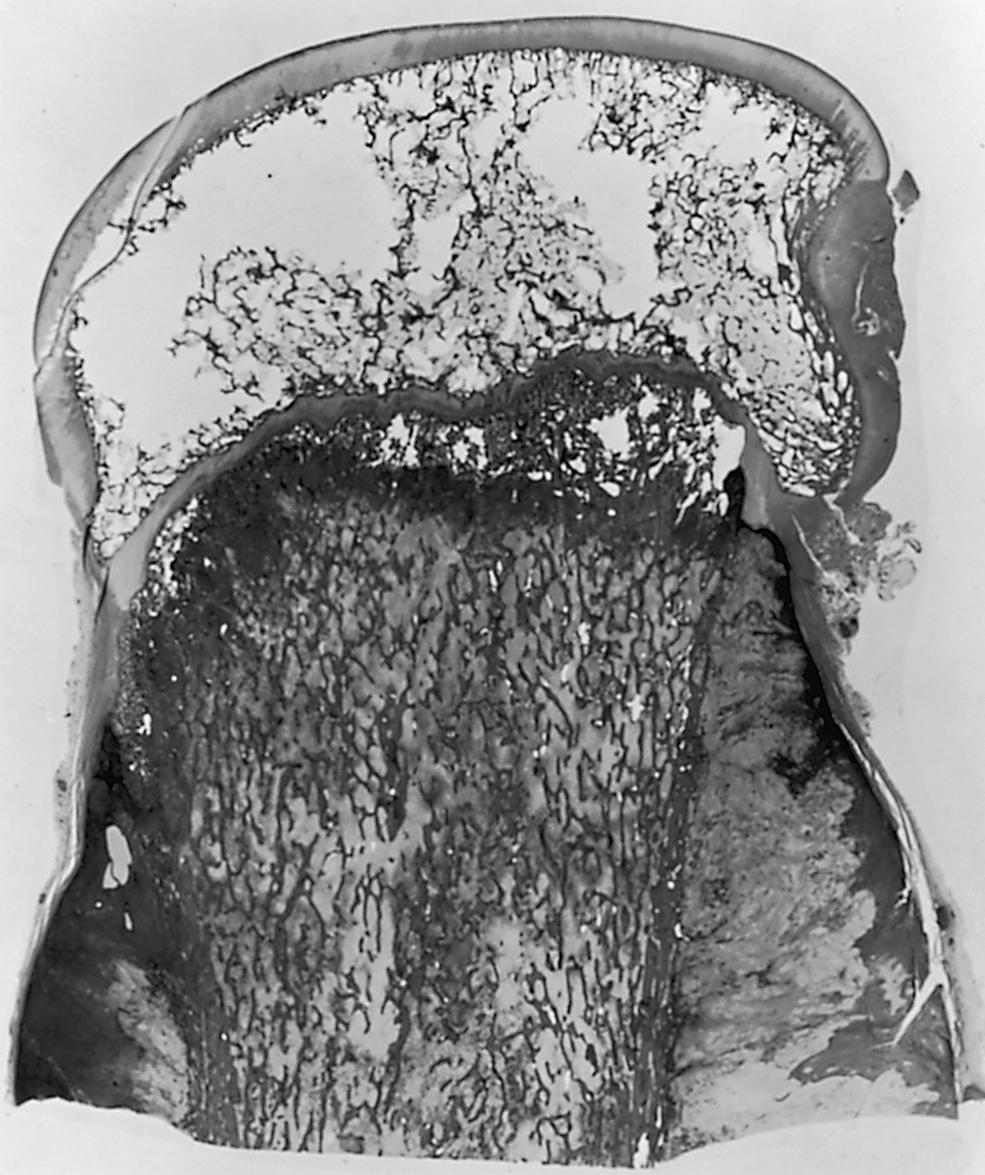
In more advanced cases, the periosteal barrier may be broken, and a soft tissue tumor mass may be seen invading the adjacent muscle tissue. In general, the central portions of the neoplasm are more heavily ossified than the peripheral areas. The ossified portions are of a gritty consistency and have a yellowish appearance; the more cellular areas are softer and tan to whitish in color. In a sagittal section of an amputated specimen, the boundaries of the endosteal portions of the tumor are not clearly distinguishable. The physis is less readily violated than the cortical wall and remains unpenetrated until later in the course of the disease. The articular hyaline cartilage serves to block the extension of the neoplasm into the joint due to its lack of a blood supply. Transepiphyseal extension has been reported, , , , but extension across the articular cartilage typically does not occur unless there has been a fracture. However, the tumor may enter the joint by extending along ligamentous and capsular structures (e.g., the cruciate ligaments). , Toward the diaphyseal end, the advancing tumor presents as a conical plug that marks the limit of growth of the lesion lengthwise along the shaft. Skip metastases (isolated foci of tumor in the same or adjacent bone, but separated from the main tumor mass by normal marrow or the adjacent joint) occasionally occur; this is significant when determining the optimal level for resection. , Skip metastases are usually detectable by bone scans and magnetic resonance imaging (MRI) , and portend a poorer prognosis, similar to that of a patient with lung metastases.
The histologic findings of osteosarcoma usually show a frankly sarcomatous tumor composed of malignant osteoblasts, rich vasculature, and the direct formation of neoplastic osteoid and woven bone ( Fig. 26.2 ). f
f References , , , , , , , , , .
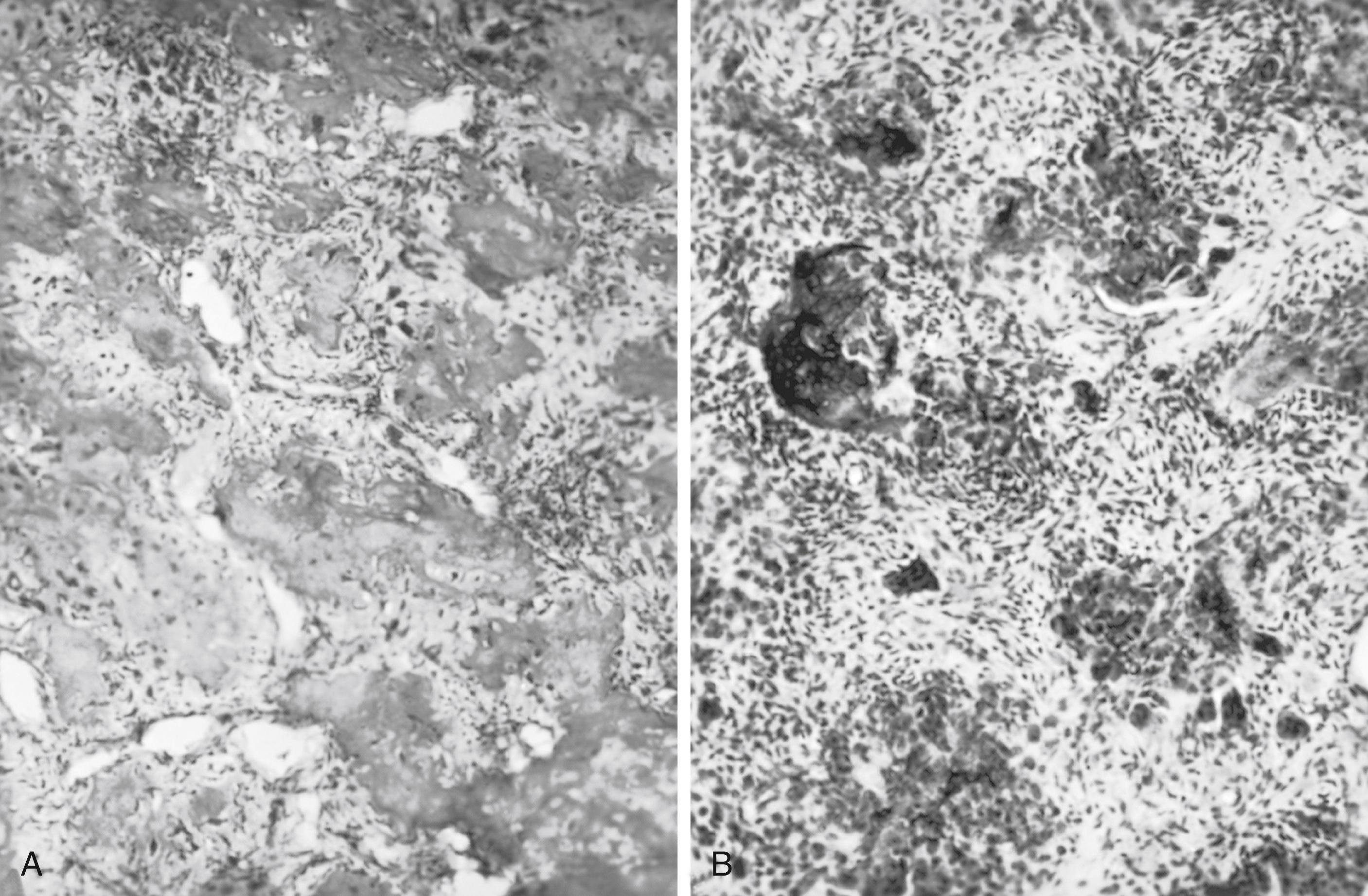
However, in some pathologic specimens, tumor woven bone cannot be demonstrated; only collagen strands interwoven with the tumor cells are seen. In anaplastic areas, the neoplasm consists of pleomorphic cells, with little intercellular substance. In other tumors, neoplastic cartilage and atypical spindle-shaped cells may be the predominant feature. Aegerter and Kirkpatrick have divided the microscopic picture of osteosarcoma into four types. In the first type, osteoid production is the predominant finding; in the second type, both osteoid and cartilage are formed. In the third type, neither osteoid nor cartilage is produced, but collagen is formed. In the fourth type, there is little or no indication of the presence of these intercellular substances. Attempts to correlate the four histologic types with the clinical manifestations of osteosarcoma have been futile. On the basis of histologic findings alone, other than tumor grade, one cannot predict the rate of growth, the advent of metastasis, or the duration of survival. g
g References , , , , , .
It is important to remember that osteosarcoma may have large areas with little or no bone formation, but if any neoplastic bone is present, it is called osteosarcoma and treated as such. In an adolescent, the diagnosis of chondrosarcoma should be viewed with suspicion, despite the demonstration of only high-grade chondrosarcoma in a biopsy specimen. It is highly likely that examination of the entire specimen of a so-called chondrosarcoma in an adolescent will reveal neoplastic bone formation, indicating that it is chondroblastic osteosarcoma.
The pathologist determines the histologic grade of the tumor based on cellularity, atypia, pleomorphism, degree of tumor necrosis, and number of mitoses. A three- or four-grade system is used, depending on the pathologist. The prognostic significance of the number of mitotic figures is uncertain; at best, it is an index of the rate of growth. The histologic grade of the tumor is important in that a low-grade surface or central osteosarcoma , , , has a much better prognosis than a high-grade (grade 2 or 3) osteosarcoma. h
h References , , , , , .
Local pain in the affected part is the presenting complaint. Initially the pain is intermittent, but within a matter of weeks it becomes severe and constant. There may be a history of trauma that has precipitated discomfort from the tumor. It is often presumed that the trauma caused the tumor, but it is more likely that the injury merely called attention to the affected site. When a lower limb is affected, an antalgic limp may develop. As the condition progresses, a local mass that is hard and fixed to the underlying bone may be palpated ( Fig. 26.3A and B ). Also, there may be increased local heat and sensitivity to pressure. The firmness of the tumor varies, depending on the extent of ossification. The tumor may become visible as it enlarges. Limitation of joint motion and disuse atrophy of the muscles are other findings. It is important to recognize that the great majority of patients with osteosarcoma are not sick. They do not have fever, weight loss, or cachexia, and except for disease at the primary site, they appear to be healthy. This is one reason the diagnosis may be delayed. However, on rare occasions, in a patient with a rapidly growing neoplasm with pulmonary metastases, the patient may exhibit systemic symptoms. At other times, a pathologic fracture through the lesion may be the presenting condition. , , , ,
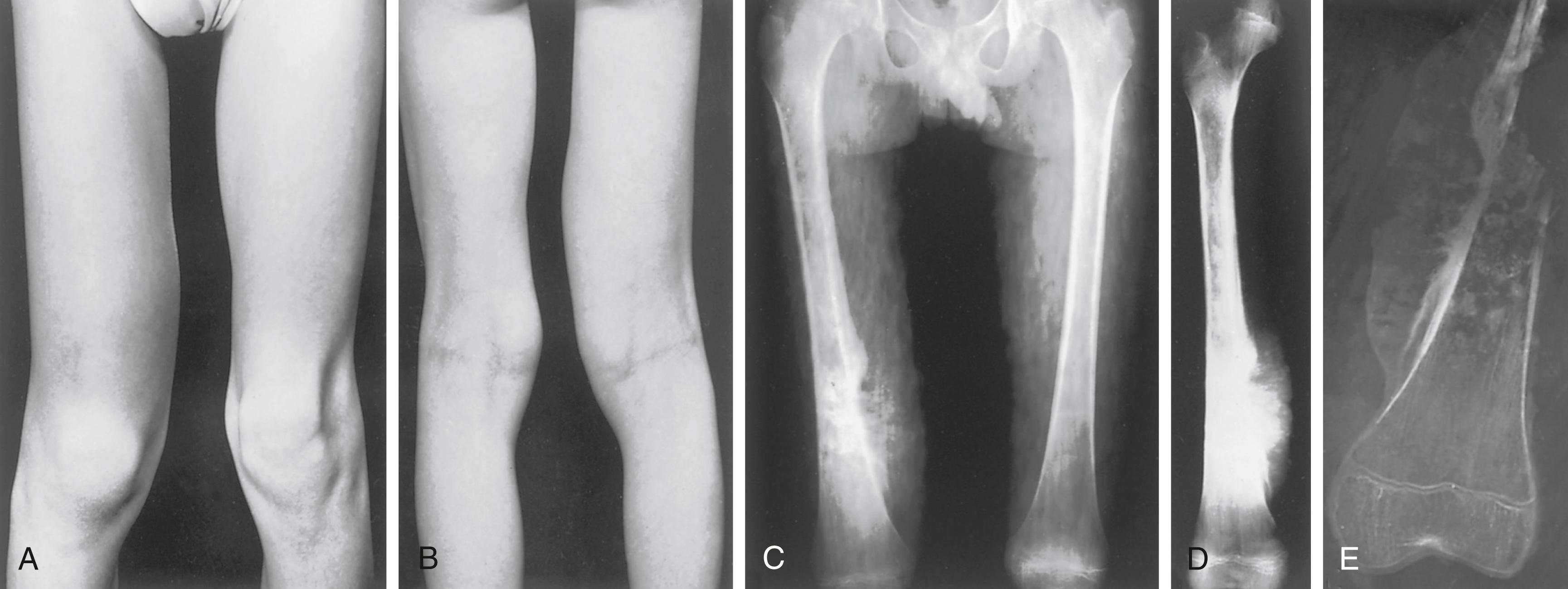
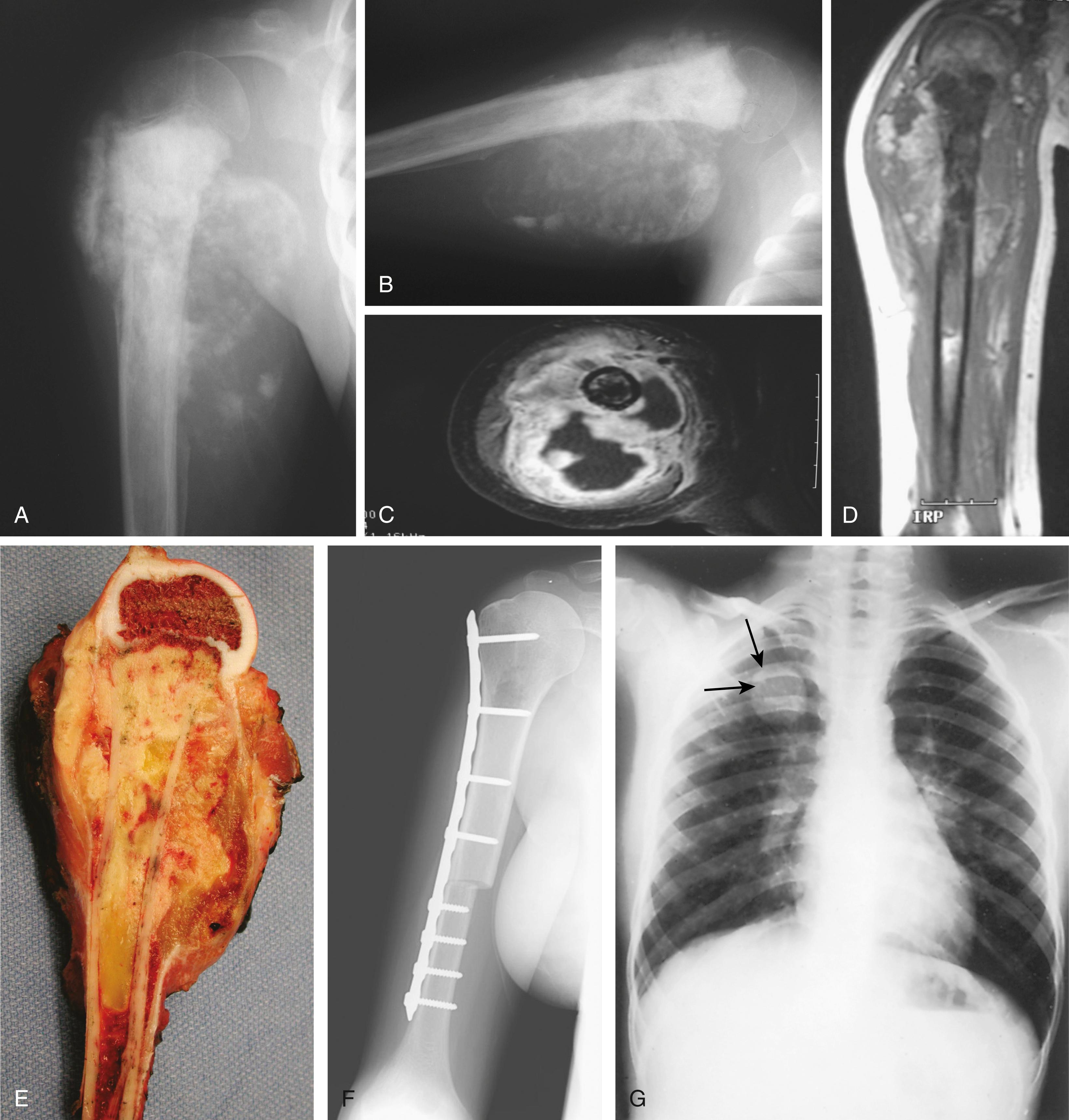
Osteosarcoma has a typical radiographic picture characterized by destructive and osteoblastic changes ( Figs. 26.4 to 26.6 ; see Fig. 26.3C to E ). i
i References , , , , , .
It may be purely radiodense or purely radiolucent, but commonly it is a mixture of both. The neoplasm usually begins eccentrically in the metaphyseal region of a long bone. Bone destruction is evident, with loss of the normal trabecular pattern and the appearance of irregular, ill-defined, poorly marginated, ragged radiolucent defects. New bone formation may be neoplastic or reactive and appears as areas of increased radiopacity. The cortex is invaded by the growing tumor, as evidenced by destruction of the cortical wall and raising of the periosteum. There is an incomplete attempt to contain the tumor by periosteum, forming Codman’s triangle. The base of Codman’s triangle is perpendicular to the shaft and is created by the subperiosteal reactive new bone; it is not diagnostic of osteosarcoma because it is also seen in osteomyelitis and Ewing sarcoma. The sunburst appearance is produced by the formation of spicules of new bone laid down perpendicular to the shaft along the vessels passing from the periosteum to the cortex. A soft tissue mass is discernible on the radiographs as the tumor advances and transgresses the cortex. Pathologic fracture may occur.
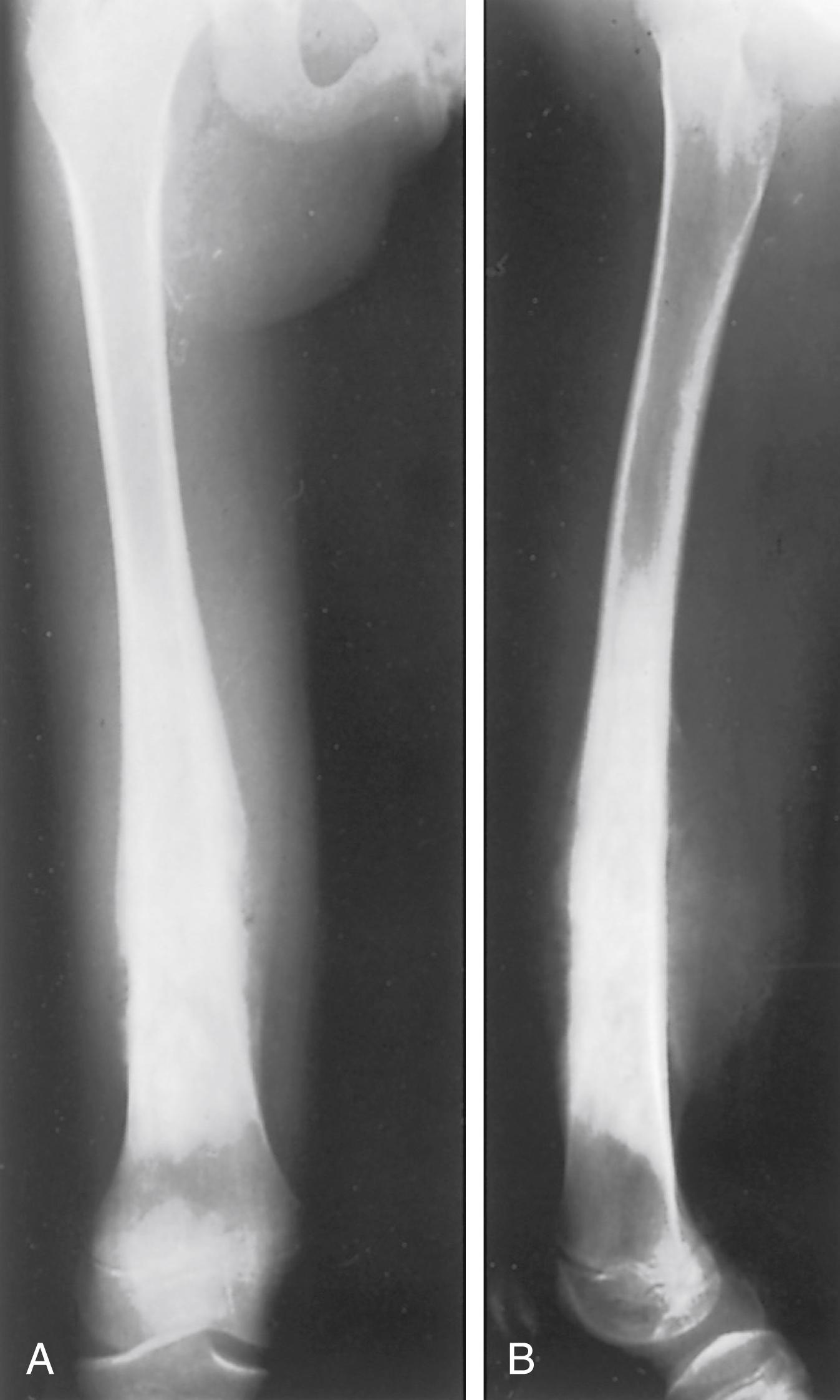
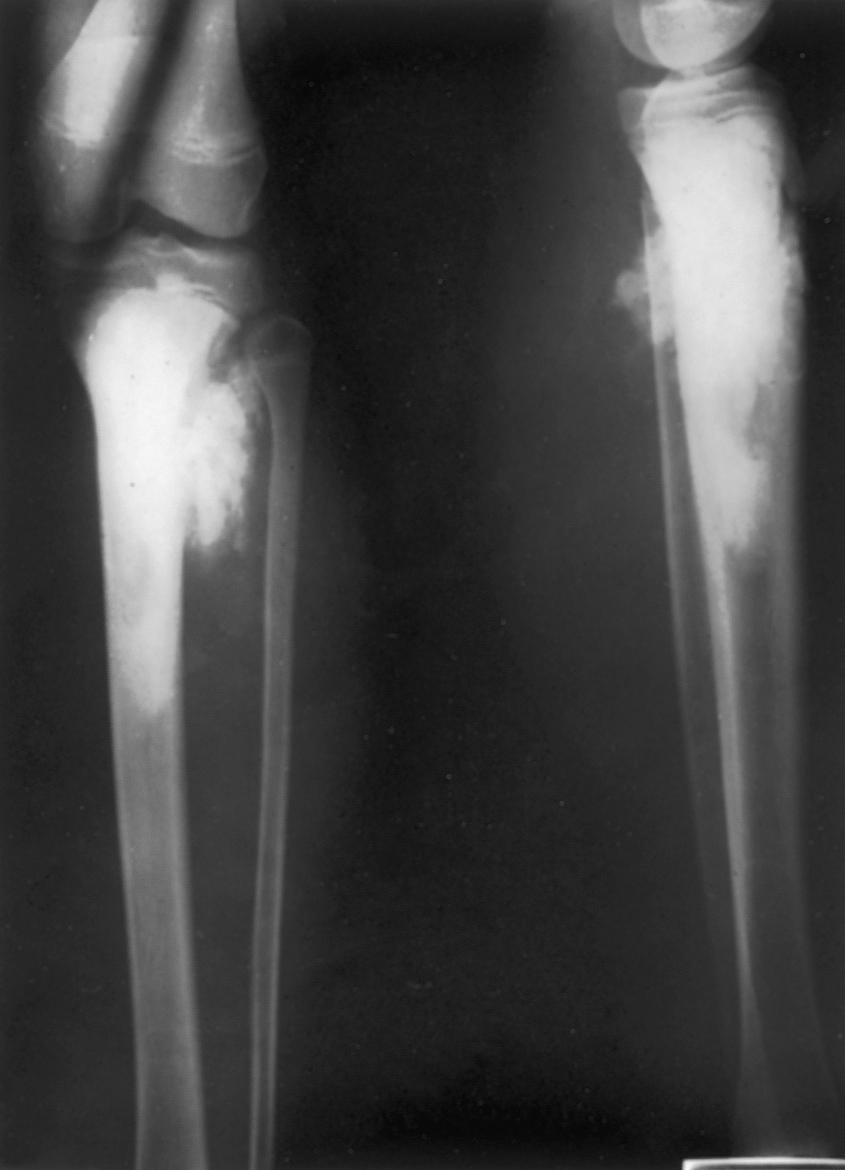
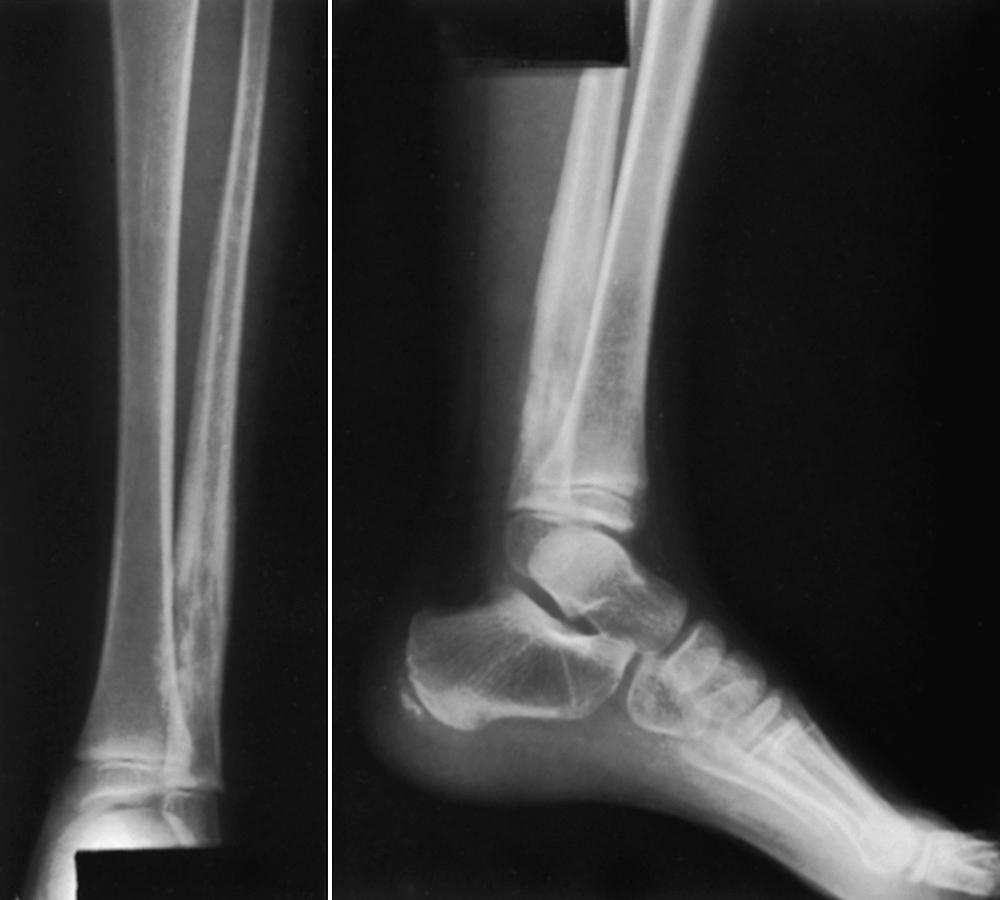
Osteosarcomas do not always exhibit the classic radiographic pattern. They may be subtle in the early stages and may be radiolucent and diaphyseal, leading one to assume that they are Ewing sarcoma. We have seen one case detected serendipitously on a comparison radiograph obtained for a suspected fracture. Pathologic fracture ( Fig. 26.7 ) may make the diagnosis difficult, and it is not uncommon for patients to be treated for long bone fractures only to have an underlying neoplasm discovered weeks later. Aneurysmal bone cysts can mimic osteosarcomas, and osteosarcomas may have fluid-fluid levels on MRI scans, adding to the confusion. Clinical suspicion should be raised if a teenager presents with unexplained pain about the knee or shoulder, especially if the pain does not resolve quickly or is present at rest or at night. Radiographs in such cases should be analyzed critically and if there is any doubt, the patient should be further evaluated by MRI.
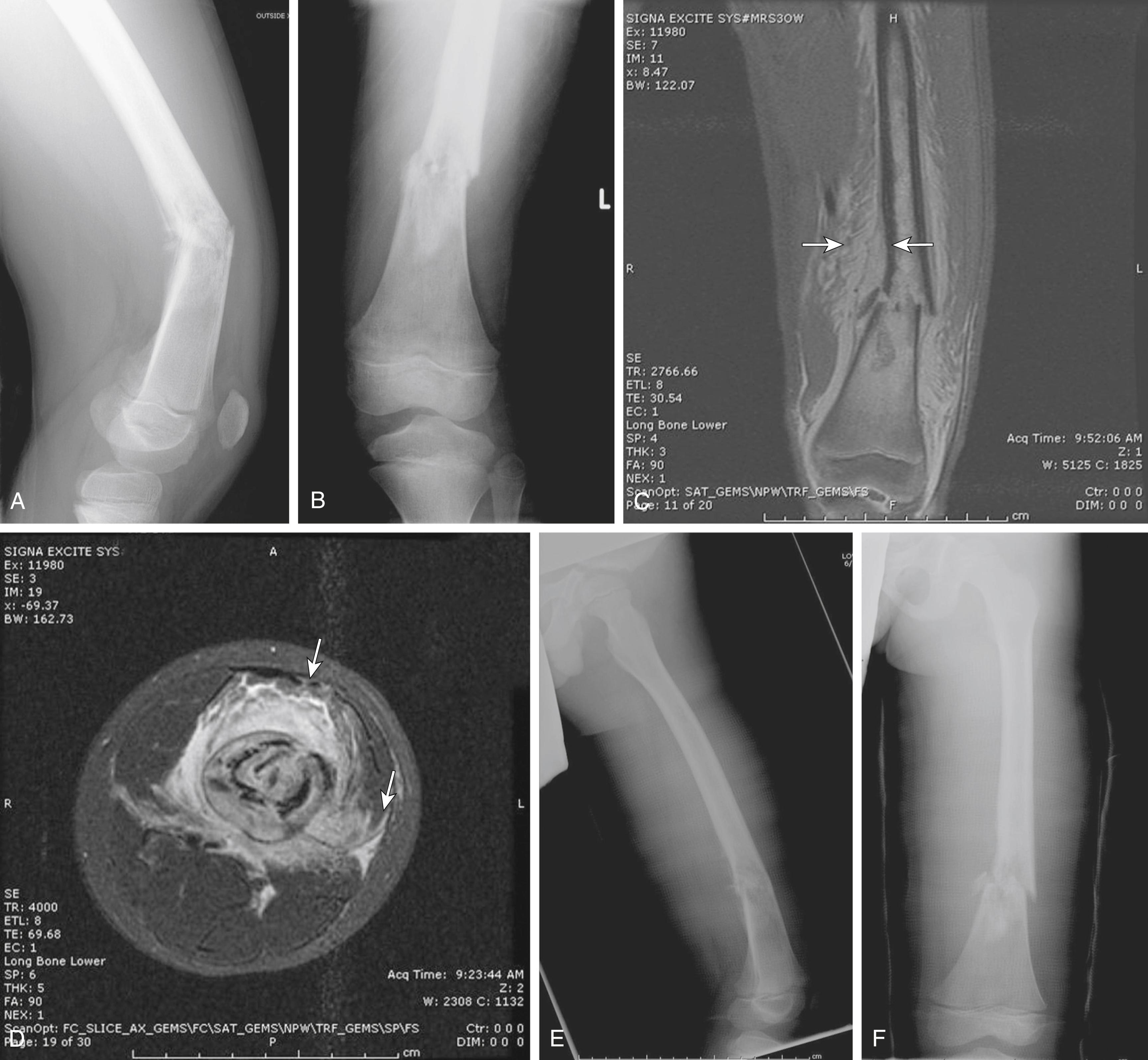
MRI and computed tomography (CT) are of great value in depicting the details of bone destruction and tumor bone production within the lesion. MRI has largely replaced CT as the optimal modality for imaging the primary tumor, and CT is used to evaluate the chest for pulmonary metastases. j
j References , , , , , .
On CT, the neoplastic bone appears amorphous and not stress oriented ( Fig. 26.8 ). The areas of cortical erosion by the tumor tissue are well delineated. MRI optimally demonstrates the degree of soft tissue extension and relationship of the extracompartmental tumor to fascial planes and neurovascular structures. Perhaps the best feature of MRI is its ability to evaluate the extent of tumor in the medullary cavity precisely. Coronal T1-weighted images of the entire involved bone should be included. This is useful when planning limb-sparing resections. The radiologist can measure the extent of the tumor from fixed palpable landmarks to help the surgeon plan osteotomies. Occult skip metastases of 2 mm or more in long bones are well seen on MRI. MRI is also useful for evaluating the adjacent joint for tumor spread although at times it may be difficult to determine if the joint is actually contaminated by tumor extension.
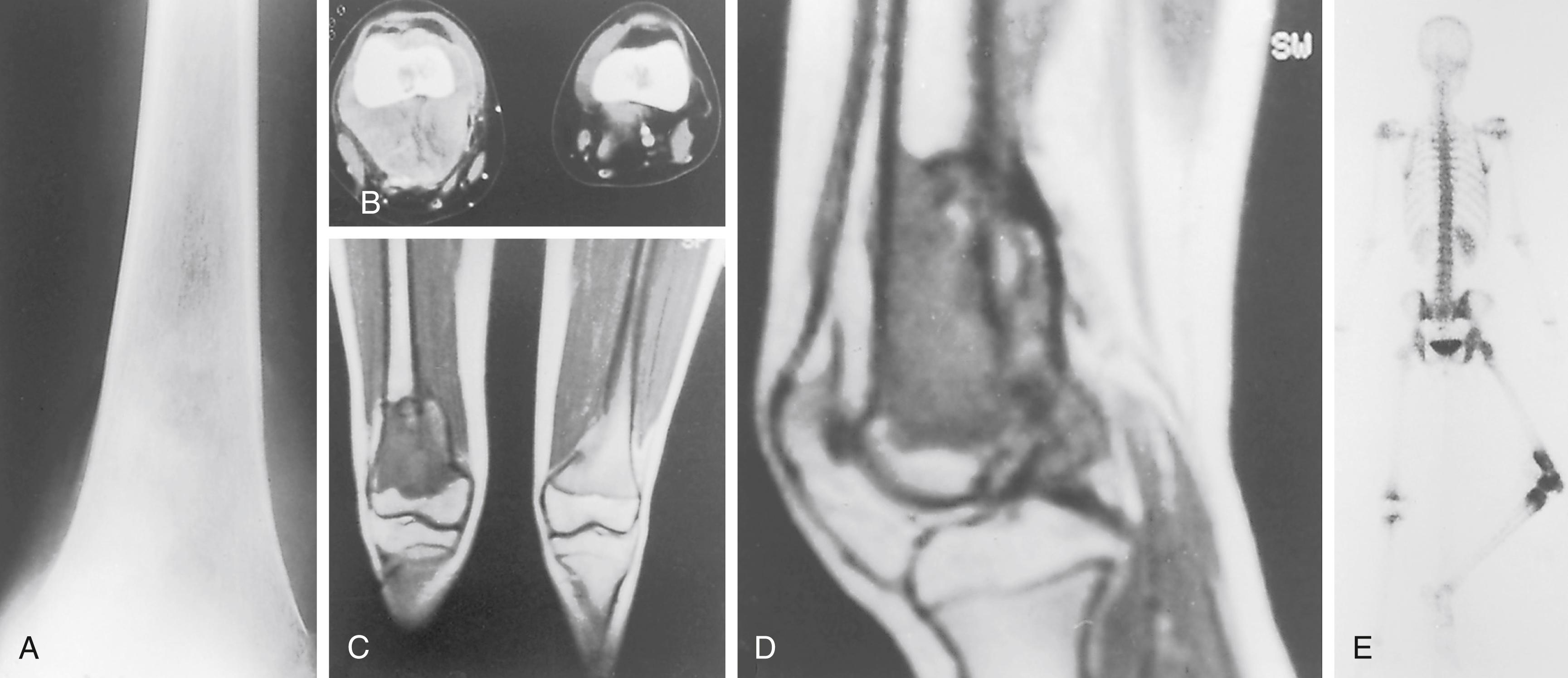
Pulmonary metastases 3 to 7 mm or more in diameter can be identified with CT. Conventional radiographs of the chest (dual inspiration and expiration views) show metastatic nodules 10 mm or more in diameter. The importance of pulmonary CT in the staging of osteosarcoma cannot be overemphasized. , , , , Approximately 10% to 20% of patients with osteosarcoma present with radiographically detectable metastases at diagnosis. Most of these are in the lungs. Chest CT is superior to plain radiography in demonstrating these metastases, and spiral CT is superior to conventional CT for this purpose. , , , ,
However, chest CT will not detect all pulmonary metastases, and some advocate thoracotomy with manual palpations of the lung to more precisely assess pulmonary lung nodules. , ,
Bone scanning with technetium-99m shows a marked increase in the uptake of the radionuclide in the primary tumor. The increased uptake is caused by active formation of new tumor and host bone, as well as the vascularity of the lesion ( Fig. 26.9 ). Radionuclide bone scintigraphy is used to look for bony metastases in the involved bone (skip metastases) , , , , and at other skeletal sites. k
k References , , , , , , , , .
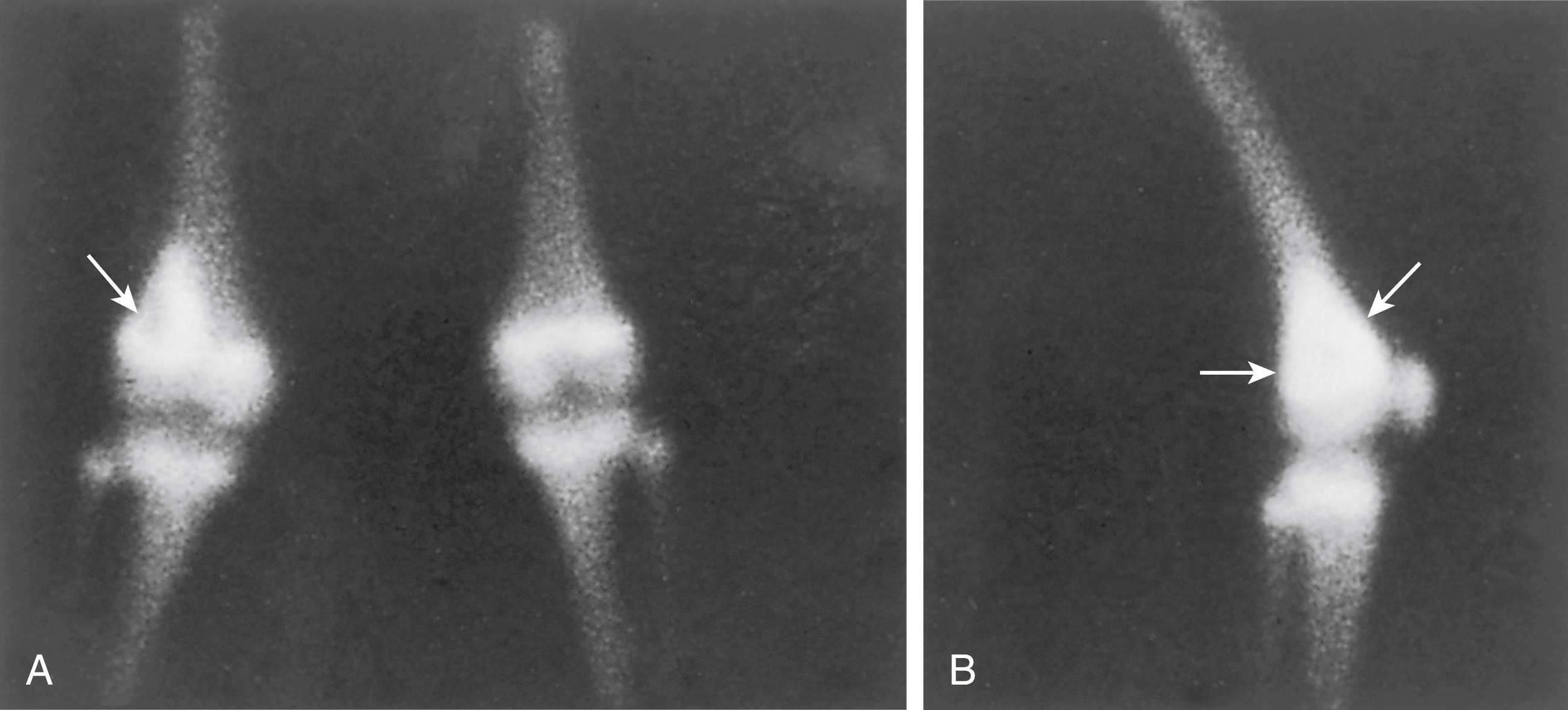
Mineralized metastases are more likely to be detected by bone scanning than nonmineralized metastases at extrapulmonary sites. The intensity of the uptake increases with the vascularity of the lesion. Ordinarily, the margins of the increased isotope activity mark the extent of the osteosarcoma; however, this is not absolute because the tumor may extend beyond the margin of increased radioisotope uptake.
Angiography is of great value in delineating the extent of soft tissue extension and its relationship to adjacent neurovascular structures, but it is seldom used now because MRI can display this information more easily and less invasively. Angiography is also useful for demonstrating the response to preoperative chemotherapy, but dynamic MRI has replaced it for this purpose as well. , , ,
Recently fluorodeoxyglucose-positron emission tomography (FDG-PET) has been employed to stage patients with bone tumors. The role in osteosarcoma is at this point unclear with some studies showing improved ability to detect occult bony metastases compared to bone scans and others showing that a bone scan is superior. PET-CT may be useful in detecting recurrent osteosarcoma. ,
There are no specific laboratory tests for osteosarcoma. The complete blood cell (CBC) count is usually normal and although the erythrocyte sedimentation rate (ESR) may be elevated, it is not specific. The serum alkaline phosphatase (ALP) level is usually increased in osteosarcoma, reflecting osteogenesis in the neoplastic tissue. , , The degree of elevation of this enzyme level is commensurate with the activity of the neoplastic osteoblasts within the lesion and the size of the tumor. In some studies, an elevated ALP level has been associated with a worse prognosis. , , The course of osteosarcoma can be monitored by serial determination of serum ALP levels. Following ablation of the tumor, the enzyme level falls to near normal; it rises with the development of metastases and with recurrence. Clinically, sequential serum ALP levels are used to assess response to chemotherapy. In some studies, the lactate dehydrogenase (LDH) level has been shown to be of prognostic importance. An elevated LDH level is associated with a worse prognosis. ,
The primary entity from which osteosarcoma must be differentiated is Ewing sarcoma, but benign conditions may also mimic osteosarcoma. Exuberant callus of a fatigue fracture, subacute osteomyelitis, active myositis ossificans, aneurysmal bone cyst, and Langerhans cell histiocytosis (eosinophilic granuloma) are some of the benign conditions that may be mistaken for osteosarcoma. Ewing sarcoma, fibrosarcoma, lymphoma, and metastatic carcinoma are some of the malignant lesions that must be excluded. Age is a major factor in sorting out the various diagnostic possibilities. In a child younger than 5 years, histiocytosis, metastatic Wilms tumor, and neuroblastoma should be considered. In an adolescent, osteosarcoma and Ewing sarcoma are the most common bone malignancies. Chondrosarcoma is very uncommon in children and adolescents, and most lesions considered to be chondrosarcoma by biopsy are actually chondroblastic osteosarcoma. Leukemias and lymphomas should also be considered in an adolescent with an aggressive bone neoplasm.
Once the diagnosis of osteosarcoma has been made, the disease should be staged. The objectives of the staging workup are to establish the final tissue diagnosis, delineate the local extent of the tumor, and discover any distant metastases. Radiologic staging and open biopsy should be done by the surgeon who will perform the definitive operation. l
l References , , , , , .
The following questions are to be answered:
Is it a low- or high-grade tumor?
Is the tumor limited to the bone (intracompartmental), or has it spread to the adjacent soft tissues (extracompartmental)?
Is there evidence of metastatic spread to the lungs or other bones?
Carefully planned imaging of the lesion should precede open biopsy. If a needle biopsy is chosen, the surgeon should direct the placement of the needle in careful discussion with the interventional radiologist. Determining the local extent of disease after biopsy performed elsewhere is difficult and inaccurate because of the disruption of tissue planes, hematoma formation, edema, and wound healing. In choosing the proper surgical procedure, it is vital to know whether there are natural barriers to tumor extension. , , Is the lesion intracompartmental (bounded by natural barriers to tumor extension) or extracompartmental (with no proximal, distal, or peripheral barriers to tumor extension)? The vast majority of high-grade osteosarcomas are extracompartmental. During staging, the surgeon should meticulously assess the muscle compartment and the tumor’s proximity to neurovascular structures to determine whether limb salvage is feasible. Usually the final decision is based on postchemotherapy MRI.
In the preoperative staging of osteosarcoma, the following diagnostic tests are performed :
Complete history and physical examination
CBC with differential
ESR determination
Serum levels of calcium, phosphorus, ALP, and LDH
Conventional radiography of the tumor site, ideally encompassing the entire bone involved
Scintigraphy with technetium-99m and in some cases FDG-PET
MRI to assess the intraosseous extent of the tumor, joint involvement, and relationship of the soft tissue mass to adjacent neurovascular structures
CT of the chest to rule out metastases
A pediatric oncologist, radiologist, and pathologist should be part of the treatment team from the beginning, taking part in the staging and subsequent decision making. The management of osteosarcoma requires a multidisciplinary approach, and patients should be treated in medical centers specializing in pediatric oncology.
Before performing an open biopsy, the surgeon should be knowledgeable in the differential diagnosis and local extent of the lesion; before placing the incision, he or she should be cognizant of the principles of limb salvage surgery and amputation flaps. The surgeon who will perform the definitive operation should perform the biopsy or work closely with an interventional radiologist if a needle biopsy is chosen. The technical details of performing a biopsy are presented elsewhere (see Chapter 24 ). Adequate hemostasis must be obtained. The use of a tourniquet is at the discretion of the surgeon. If a drain is used, it should be placed near and along the direction of the biopsy tract because it will be excised at the time of primary resection. It is crucial to verify the biopsy site with C-arm imaging in the operating room. Frozen sections should be used to ensure that diagnostic tissue has been obtained, and cultures of the tissue specimen should be performed. The pathologist should have the radiographic studies available to review before or during the biopsy. Special stains, cytology, electron microscopy, and immunocytochemistry may be important for establishing the correct diagnosis.
Image-guided core-needle biopsies are more commonly performed for diagnosis of osteosarcoma than open biopsies and have a variety of benefits. There is always the danger of local tumor spread as a result of an open biopsy. Core-needle biopsies or fine-needle aspirations (FNAs) are used at institutions with experience in these techniques, but not all pathologists are comfortable making the diagnosis from limited tissue. m
m References , , , , , .
It is crucial that there is close communication between the surgeon and the radiologist when planning a needle biopsy in order that the needle biopsy tract can be excised at the time of resection of the tumor, although some surgeons believe that resecting of the needle tract is not essential if neoadjuvant chemotherapy is used.
Although an experienced pathologist might make a correct diagnosis on the basis of a frozen section, immediate, definitive, wide excision of osteosarcoma is seldom performed at the time of biopsy because most patients receive preoperative (neoadjuvant) chemotherapy. Thus it is always best to rely on permanent sections for the final diagnosis. If there is uncertainty about the diagnosis, an experienced bone pathologist should be asked to review the slides.
The treatment of high-grade osteosarcoma occurs in two phases: administration of adjuvant chemotherapy and surgical resection of the tumor.
It is important to recognize that osteosarcoma is, in most cases, a systemic disease. Following amputation alone, metastatic disease, usually in the lungs, occurs in 80% to 90% of patients within the first 2 years. n
n References , , , , , , .
This implies that micrometastatic disease is present from the time that osteosarcoma is detected clinically. Because micrometastatic disease is often controlled by adjuvant chemotherapy, it was hypothesized in the 1960s and 1970s that the administration of chemotherapy might prevent the appearance of metastatic disease. , This hypothesis proved to be true, although the premise was challenged initially. Randomized and nonrandomized studies have shown a disease-free and overall survival advantage in patients who receive adjuvant chemotherapy. , , , Before the chemotherapy era, the probability of remaining disease-free after amputation for osteosarcoma was less than 20%. , , , , Currently, it is between 65% and 80%, or perhaps higher, although unfortunately this survival rate has not improved in the past 30 years. o
o References , , , , , , , , , , , , .
The standard agents used include high-dose methotrexate, doxorubicin (Adriamycin), and cisplatin. These agents have been tested in large series of patients in national trials of the Pediatric Oncology Group and Children’s Cancer Group (now combined as the Children’s Oncology Group), providing a good example of how cooperative groups can carry out trials to study the outcomes of therapy for a rare disease. Initially there was doubt about the effectiveness of chemotherapy. A randomized study definitively addressed this issue and conclusively demonstrated that adjuvant chemotherapy improves the disease-free and overall survival rates of osteosarcoma patients. , However, the dramatic improvement in the ability to cure patients with osteosarcoma has come at a price. The drugs used are highly toxic; adverse effects include infection from neutropenia, cardiotoxicity, renal toxicity, and hearing loss. p
p References , , , , , , , , , , .
The next advance in treatment was the use of preoperative, or neoadjuvant, chemotherapy. By administering chemotherapy before resection, one can treat the micrometastatic disease earlier, perhaps shrink the tumor to make resection easier, and study the histologic response to the drugs. , , , However, there was concern that if the patient’s tumor did not respond, it might progress during the preoperative period. This also was studied in a randomized trial, and it appears that the outcome is similar regardless of whether chemotherapy is administered both pre- and postoperatively, or only postoperatively. However, the study was difficult to complete because by the time it was opened, surgeons already had a bias toward preoperative chemotherapy. Because of poor patient accrual, the power to detect a 15% difference in the two groups was only 80%. A recent study showed no difference in survival in patients with pelvic osteosarcoma treated with immediate surgery compared to those treated with neoadjuvant chemotherapy. Nevertheless, preoperative chemotherapy is now standard despite limited evidence that it offers superior survival. , ,
One of the main advantages of preoperative chemotherapy is that it provides prognostic information. The pathologist can examine the specimen for the percentage of histologic necrosis following resection. q
q References , , , , , .
Patients with a higher degree of necrosis (>90%) have a better outcome than those with less necrosis. It seems logical that giving alternative chemotherapy to patients with less tumor necrosis would improve outcome, but to date this has not been the case in studies addressing the issue. A large, prospective, international multi-institutional trial of the addition of ifosfamide and etoposide to postoperative regimens for poor responders, EURAMOS1, has been completed, but failed to show an advantage to changing chemotherapy in poor responders. Similarly, this same study looked at good responders to see if the addition of interferon would improve the survival further, but the two arms were equivalent. Another cooperative trial in the United States studied the results of the addition of ifosfamide and an immunostimulant, muramyl tripeptide (MTP), in a randomized trial to determine whether the addition of either or both of these agents improved the survival of patients with osteosarcoma. It concluded that the addition of ifosfamide in the adjuvant setting did not improve event-free survival compared with the standard combination of cisplatin, doxorubicin, and high-dose methotrexate, but it was confounded by the use of MTP, which appeared to have a beneficial effect in the ifosfamide arm of the study. Further investigation is needed to determine whether this is a reproducible effect, although the agent is not currently approved for use by the U.S. Food and Drug Administration in the United States.
Many advances have been made in the treatment of osteosarcoma, but 20% to 40% of patients do not respond to treatment despite similar histology, staging, and other patient characteristics. Just as there are some patients who could benefit from more aggressive chemotherapy, there are others who may need very little or no chemotherapy. It is hoped that more information about the molecular makeup of these tumors will provide insight in this regard and allow us to target therapy more precisely. , , , This has led to research efforts in drug-resistance mechanisms, genetic alterations in these sarcomas, and novel radiographic approaches to detect nonresponders at diagnosis. Multidrug resistance has been demonstrated in osteosarcoma and is a powerful prognostic indicator. , The P-glycoprotein membrane pump actively exports agents, such as doxorubicin, out of the cell and can be detected by various immunohistochemical methods. , The exciting aspect of these findings is that the resistance pump can be blocked by other agents, offering a potential means of overcoming resistance in these patients. Unfortunately, this has not been translated into clinically relevant treatment strategies, and the results of these studies have been mixed, probably because there are multiple resistance mechanisms available to the cancer cell and we are only beginning to understand them.
Genetic alterations in tumor suppressor genes also have been demonstrated in osteosarcomas, and there is some indication that, in addition to providing clues to the cause of the tumor, they may be of prognostic and possibly therapeutic import. r
r References , , , , , , , , , , , , .
Human epidermal growth factor receptor 2 (HER2/erbB-2) appears to be overexpressed in patients with advanced disease (greater expression in metastatic osteosarcomas) in some studies , but not in others. , , A monoclonal antibody to HER2/erbB-2, trastuzumab (Herceptin), has been studied to determine its therapeutic value in advanced metastatic disease, but to date it has not been shown to be of benefit. Determination of expression is difficult, and it is not clear that immunohistochemical techniques are sufficiently accurate.
Finally, more aggressive or intensified administration of chemotherapy and novel agents may further improve outcome. Some of these avenues are currently being investigated in cooperative trials.
In addition to advances in the medical management of osteosarcoma, surgical treatment has improved. Amputation was once the standard of care and remains an important part of the armamentarium of the tumor surgeon, especially in children. Currently, however, most patients who present with osteosarcoma are treated with limb-sparing procedures. There was initial concern about the effect of limb salvage on survival rates, and no randomized studies have been carried out that compare limb salvage and amputation. s
s References , , , , , , , .
However, nonrandomized studies do not show a survival advantage for patients treated with amputation, and the local recurrence rate after limb salvage procedures is similar to that after cross-bone amputation. t
t References , , , , , , , , .
Some studies have actually shown a worse prognosis for patients treated by amputation, but it is likely that this is the result of selection bias—amputations being performed in those with larger, more aggressive tumors or those with pathologic fractures. One large retrospective study of distal femoral osteosarcomas showed a higher local recurrence rate after limb salvage procedures and cross-bone amputation than after hip disarticulation, but the three groups did not differ in overall or disease-free survival. , It is apparent that achieving a wide margin is important and doing so, coupled with a good response to chemotherapy, is associated with a low incidence of local recurrence. A less than wide margin or a less than good histologic response dramatically increased the recurrence rate in one study.
Irrespective of the method chosen to treat osteosarcoma, the local tumor must be completely excised with negative margins. Although amputation is performed less frequently than in the past, it remains the gold standard of local control, and in the lower extremity it may be the most functional reconstruction in young athletic patients. The primary indications for amputation are as follows: very young age, when limb length inequality would be a major problem (lower extremity); displaced pathologic fractures; large soft tissue masses involving neurovascular structures; disease progression during chemotherapy; and local recurrence following limb salvage procedures. In the upper extremity, one usually tries to preserve at least hand function, because prosthetic limbs are not nearly as good as a functional hand. However, in the lower extremity, modern prosthetics are very functional ( Fig. 26.10 ).
The level of amputation is determined by close scrutiny of conventional radiographs, bone scans, and MRI scans. These surgical staging studies should be performed immediately before definitive surgery is undertaken and after completion of preoperative chemotherapy. The entire involved bone should be carefully evaluated by MRI for skip metastases. Usually, a wide cross-bone amputation is performed rather than a radical (whole-bone) amputation. Exceptions might be a young child with a tibial osteosarcoma, in whom knee disarticulation or above-knee amputation is performed, or a hindfoot osteosarcoma requiring a below-knee amputation. For distal femoral lesions, a hip disarticulation is seldom performed and is not routinely necessary, as shown by a study from the Musculoskeletal Tumor Society. , The operative techniques of amputation and disarticulation at various levels in the upper and lower limbs are described and illustrated in Plates 26.1 to 26.9 on pages 1079 to 1128.
In very young children, residual limb overgrowth may be a problem. For below-knee amputations, this can be addressed by placing a metacarpal plug in the distal tibial canal if the ipsilateral foot is uninvolved by tumor. Furthermore, in very young children, the predicted length of the residual limb at maturity may be very short if a growth plate is resected. For foot tumors, this can be addressed with a Syme-type amputation rather than a below-knee amputation ; for proximal tibial lesions, a knee disarticulation may be preferable to an above-knee amputation. , , These can be revised at maturity, if necessary, for prosthetic fitting.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here