Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Magnetic resonance imaging (MRI) is more sensitive for breast cancer than standard x-ray mammography or ultrasound, when using dynamic contrast-enhancement (DCE) methods after administration of intravenous (IV) gadolinium-based contrast agents. Breast MRI both detects breast cancer and distinguishes it from benign breast with high-resolution scanning using breast lesion morphology and enhancement characteristics. Noncontrast MRI methods are in development, but currently they are not as sensitive for cancer detection. Both the National Comprehensive Cancer Network (NCCN) and the American Cancer Society recommend breast MRI screening for women if they have a ≥20% lifetime risk for breast cancer based on family history of breast cancer or if they have a genetic predisposition for breast cancer. This chapter reviews MRI techniques, how to interpret breast MRI, and MRI indications and MRI-guided procedures. Evaluation of breast implants by MRI is discussed in Chapter 9 .
MRI uses repeated radiofrequency pulses in concert with precise spatial modulation of a strong magnetic field to image the distribution and nuclear magnetic resonance characteristics of hydrogen atoms within human tissue. MRI provides either two-dimensional (2D) thin slices or three-dimensional (3D) volumetric tomographic images without ionizing radiation. Like mammography, MRI is comprehensive, reproducible, and operator independent. Like sonography, MRI is not limited by dense breast tissue in detecting breast cancer.
Various MRI pulse sequences create images that reflect different tissue properties, such as T1, T2, or T2 ∗ relaxation times; proton density; apparent diffusion coefficient (ADC); and others. Pulse sequences can also be made to specifically image particular tissues, such as fat, water, or silicone, by a variety of techniques. Because MRI is exquisitely sensitive to paramagnetic substances, such as intravenously injected gadolinium chelate contrast agents, even minimal concentrations of these agents in tissues substantially shorten the T1 relaxation time, resulting in a high signal for enhancing breast lesions on T1-weighted images and improving differentiation of cancers from benign breast entities.
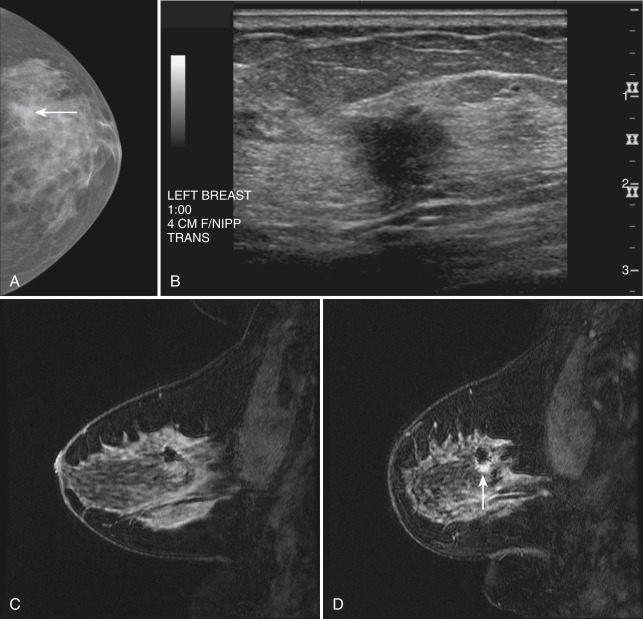
Invasive breast tumors are characterized by an ingrowth of neovascularity at their periphery. Tumor angiogenesis is associated with increased perfusion and abnormal leaky endothelium, leading to preferential tumor enhancement compared with normal breast tissue ( Box 7.1 ). After a bolus administration of an IV contrast agent, the increased vascular flow and the rapid exchange rate of contrast between blood and the extracellular compartments of invasive breast tumors cause a more rapid, avid enhancement of tumors compared with normal fibroglandular tissue (FGT), even in patients with dense breasts. Thus after injection of IV contrast, invasive breast cancers have high signal intensity and are brighter than the surrounding normal tissue on the first postcontrast scan, which ideally should be obtained about 90 seconds after injection. As a result, MRI exquisitely reveals invasive tumors that are occult on mammography ( Fig. 7.1 ). The sensitivity of MRI for invasive breast cancer is extremely high—over 90%. However, as discussed in detail in this chapter, contrast enhancement on MRI is seen in many benign conditions as well; the specificity of MRI is around 80% to 90%. As also detailed in this chapter, morphology, T1 and T2 characteristics, and the time course of contrast enhancement, or kinetics, help differentiate benign from malignant lesion.
Contrast-enhanced magnetic resonance imaging is extremely sensitive for tumor angiogenesis, regardless of radiographic breast density.
Tumor angiogenesis leads to preferential enhancement of cancers with intravenous contrast.
Lesion morphology helps distinguish cancer from benign conditions.
The time course of contrast enhancement helps distinguish invasive cancer from other conditions:
Most cancers initially enhance rapidly ( fast ) in early phase ( fast washin ).
Most cancers have late-phase stable signal intensity ( plateau ) or declining signal intensity ( washout ).
Benign conditions enhance usually gradually in early phase ( slow ) and continuously in late phase ( persistent ).

Benign hormone-related enhancement of normal breast tissue, called background enhancement, occurs before the onset of menses and can lead to false-positive studies. When possible, patients should be imaged 7 to 14 days after the onset of their menstrual cycle, when spurious contrast enhancement of normal breast tissue is at its nadir ( Box 7.2 ). The American College of Radiology (ACR) recommends that breast MRI scans be performed during the second week of the menstrual cycle for women undergoing screening studies (ACR standard amended 2014).
Magnetic resonance imaging (MRI) should be performed 7 to 14 days after onset of menses, which minimizes false-positive enhancement.
Patients should be screened for MRI safety, including pacemakers or other implanted devices.
Patient should be cleared for gadolinium MRI contrast injection.
Patients should document previous and current breast problems on a breast history form.
Before MRI scanning, the patient fills out an MRI safety form to exclude contraindications of entering the strong magnetic field, such as ferromagnetic vascular clips, metallic ocular fragments, pacemakers, and implanted electromechanical devices ( Fig. 7.2 ). The ACR has a document on recommendations for MRI safe practices ( ) and safety of gadolinium contrast injection ( ). Patients are cleared for gadolinium contrast injection to assure that they can excrete the contrast, and most facilities have a protocol and policy for gadolinium contrast injection safety (ie, renal function, age). A qualified person reviews the standardized MRI safety form with the patient before scanning.

As with mammography, an MRI-specific breast history form is helpful to detail patient breast risk factors, family history, breast lumps, scars, or other areas of patient complaints. The technologist places MRI-compatible markers on the patient’s breast to indicate lumps or areas of concern and annotates them on the history form. The patient details the location, date, and results of previous breast biopsies. The patient also documents any use of exogenous hormone therapy and the phase of the menstrual cycle or menopause, because those factors may cause spurious background enhancement of normal tissue, which can produce false-positive results. The patient notes if they have undergone prior MRI or other breast-related imaging studies, so they can be compared with the current examination. The patient is then instructed that, for the MRI study to work well, she must remain still during the examination and that motion artifact can cause spurious results.
The ACR Breast MRI Accreditation Program provides guidance and recommendations on personnel qualifications, equipment, quality control, quality assurance, MR safety policies, and image quality for facilities performing breast MRI and breast MRI-guided procedures ( http://www.acr.org/Quality-Safety/Accreditation/BreastMRI ). This program also provides feedback to facilities on image quality and minimum requirements to obtain high-quality studies and is a good resource for information on current ACR recommendations on equipment and scanning techniques. The following paragraphs will cover the authors’ experience with breast MRI equipment.
Although the ACR does not have a minimum MRI field strength requirement, all MRI equipment is required to meet all state and U.S. Food and Drug Administration (FDA) or a similar regulating agency to meet performance requirements for maximum static magnetic field strength, maximum rate of change of magnetic field strength (dB/dt), maximum radiofrequency power deposition (specific absorption rate [SAR]), and maximum auditory noise levels to pass ACR accreditation.
The authors advise that a high-performance MRI scanner with high-performance gradient systems should be used to ensure adequate rates of dynamic imaging and the spatial resolution; scan quality should be limited by intrinsic signal-to-noise ratio (SNR) and FDA limits on scan parameters, not by the quality of the MRI system. A field strength of at least 1.5 T increases SNR by providing more available magnetization than lower field strengths. To further increase SNR, breast MRI is increasingly performed at 3 T, but is more complicated.
Coverage, spatial resolution, temporal resolution, and SNR are all important for breast MRI, but as with all MRI studies, they are a compromise. For coverage of breast tissue and axillae, most women can be imaged within a 20-cm field of view (FOV) in the superoinferior and anteroposterior directions and a 36-cm FOV in the left–right direction. The reason for high spatial resolution during the first volumetric acquisition (obtained between 1.0 and 1.5 minutes after contrast injection) is to capture the morphologic features of malignancies when they are brightest and most sharply defined against normal breast tissue. The ACR Breast MRI Accreditation Program states the precontrast and postcontrast T1-weighted scan series sequence acquisition may be sagittal, axial, coronal, and/or slightly oblique. To ensure high resolution, they also recommend a slice thickness of ≤3 mm with a 0-mm gap and ≤1 mm maximum in-plane pixel dimension for phase and frequency. After the initial contrast injection, repeated dynamic postcontrast scans throughout the period of contrast uptake are obtained to capture the change in signal intensity of breast tumors by measuring initial washin and subsequent washout of contrast material over at least 5 minutes and up to 10 minutes. The reason for a high SNR is to provide the highest quality and nongrainy images to display the most subtle signs of breast cancer on the MRI images.
Major safety concerns in MRI studies, including breast MRI, are as follows: (1) force and torque on magnetic materials caused by static magnetic field, (2) tissue heating caused by radiofrequency magnetic field used to flip spins, (3) nerve stimulation caused by gradient magnetic field, and (4) implanted medical devices. To estimate temperature increase caused by radiofrequency-induced tissue heating, the SAR, which is defined as power deposited by a radiofrequency field in a given mass of tissue, can be used. A higher SAR indicates higher risk of tissue heating. The doubling of field from 1.5 T to 3 T leads to a quadrupling of SAR; however, most manufacturers have modified pulse sequences to reduce SAR at 3 T.
Before scanning, the technologist places an IV catheter, usually in the patient’s antecubital fossa contralateral to any known, previous, or suspected malignancy. The IV is connected to an MRI-compatible remote power injector to allow standard contrast injection, continuous flushing of the IV by using the keep vein open (KVO) setting, the ability to inject contrast without moving the patient, and the ability to flush the contrast through with a 20-mL saline flush.
The patient is then placed prone on the MRI table with her breasts placed within a dedicated breast coil, which is a box-like structure with two holes through which the breasts hang dependently ( Fig. 7.3 ). Many facilities scan with the patient’s arms up by her head to minimize artifacts from the arm tissue on axial images. The technologist makes sure that all the breast tissue, including the axillary tail, is included in the FOV, and that she is comfortable. The ACR states that all MRI equipment must have a dedicated bilateral breast coil and be capable of simultaneous bilateral imaging. Dedicated commercial breast coils image the breast in the prone position to minimize respiratory motion, contain an array of four or more elements in close proximity to the breasts to maximize SNR, and may or may not allow gentle breast stabilization by external compression paddles or coil elements. Those breast coils used for interventional procedures contain grids or open apertures in the compression plate for biopsies and localizations. In either diagnostic or interventional breast MRI, one avoids firm breast compression commonly used for x-ray mammography because of the theoretical decrease in breast perfusion, which could reduce the enhancement of cancers.

The phased-array breast coils maximize SNR of the image. Patient discomfort is the primary cause of motion; keeping the patient still during scanning is important to ensure high-quality MR images. The technologist spends considerable time discussing the importance of holding still with the patient to obtain the best scan. The patient then works with the technologist to obtain a comfortable position within the breast coil. Optional mild breast stabilization, or compression, may be used to reduce breast motion and decrease the volume of tissue to be scanned so that the whole breast is included. However, marked compression is discouraged so there is free inflow of contrast material into the breast. Scanners with a magnetic field strength of 1.5 T or 3.0 T provide the best SNR. Magnets with high-performance gradients enable the fastest, highest resolution scans ( Box 7.3 ).
Remote-controlled power injector connected to the antecubital IV line on keep vein open setting
Dedicated breast coil
Prone position minimizes respiratory motion
Small volume maximizes signal-to-noise ratio (SNR)
Multiple receiver channels enable parallel imaging acceleration
Mild stabilization or no compression used; firm compression may alter contrast enhancement.
1.5 T magnet with high-performance gradient system
3.0 T magnets may increase SNR but are limited by tissue heating and radiofrequency transmit field inhomogeneity
Contrast dose: 0.1 mmol/kg followed by saline flush ≥10 mL
Bilateral simultaneous breast imaging
Spatial resolution: ≤1 mm in-plane; ≤3 mm slice thickness fat suppression chemical suppression preferred over sole reliance on subtraction.
Scan time: every 4 minutes or less for kinetic information
The ACR Breast MRI Accreditation Program recommends at minimum a T2-weighted/bright fluid series and at least a three multiphase T1-weighted series including precontrast T1-weighted series, early-phase (first) postcontrast T1-weighted scan, and delayed-phase (last) postcontrast T1-weighted scans ( http://www.acr.org/∼/media/ACR/Documents/Accreditation/BreastMRI/Requirements.pdf ; Table 7.1 ). Diffusion-weighted series are optional, and not yet standard of care.
| Series | Requirements | |
|---|---|---|
| 1 | Localizer | |
| 2 | T2-weighted/bright fluid series | Sufficient bright fluid contrast |
| 3 | Multiphase T1-weighted series
|
Slice thickness ≤3 mm Gap 0 mm In-plane resolution ≤1 mm Bilateral breasts must be included Early-phase postcontrast imaging must be completed within 4 minutes after contrast injection Fat suppression or subtraction of precontrast from postcontrast series may be used bright signal from fat |
Conventional breast MRI begins with T1-weighted images, a so-called localizer or scout, to define the position and anatomy of the breast. T1-weighted images using the signal from the “body coil” rather than the breast coil enable basic evaluation of the axillae, anterior mediastinum, chest wall, and supraclavicular fossa for enlarged regional lymph nodes. Thereafter, the dedicated breast coil should be used to perform all subsequent sequences.
T2-weighted noncontrast fast spin-echo (FSE) images, or other sequences that shows fluid as high signal, are then obtained to characterize the breast and any lesions. T2-weighted scans using an FSE, turbo spin-echo (TSE), or rapid acquisition with relaxation inhibition technique, with effective echo time (TE) 80 to 100 ms and repetition time (TR) at least 3000 ms, produce high-quality images within reasonable scan times of 5 to 6 minutes using 3- to 4-mm-thick slices and 256 × 192 matrix or higher for small FOV sagittal images.
High fat signal on T2-weighted FSE images can be prevented with fat suppression. Proper shimming of the magnetic field and choice of center frequency are essential to ensure adequate fat suppression. Bilateral high-spatial resolution imaging is possible on most scanners because of the development of hardware and software required for bilateral shimming and because coverage is possible with reasonable scan times. However, despite optimal shimming, the breast anatomy near the nipple, axilla, chest wall, and interior breast may cause unavoidable variations in B0 field homogeneity and fat suppression failures, resulting in artifacts. Evaluation of T1-weighted scan allows the radiologist to evaluate shimming of the magnetic field and helps determine whether any abnormally bright areas on fat-suppressed T2-weighted images are caused by poor fat signal suppression.
Dixon imaging (also known as two-point Dixon, three-point Dixon, IDEAL, or Flex) is another approach to water-specific imaging that uses the differential evolution of water and fat spin phase on nonfat-suppressed images obtained with different echo times to generate water- and fat-specific images, but may take longer than fat suppression, unless performed with high acceleration factors.
Diffusion-weighted imaging (DWI) is an unenhanced breast MRI image sequence sensitive to the variability of water molecule mobility in vivo. The amount of movement or diffusion of a water molecule depends on the random molecule mobility called Brownian motion. In vivo, it is also affected by structural or biological factors, such as cell density, membrane integrity, vascular structures, and extracellular matrix composition. Higher signal intensity on DWI indicates increased restriction of diffusion. However, high DWI signal is occasionally seen in regions in which diffusion is not restricted; this is known as T2 shine-through phenomenon. B -values have not been standardized yet, but most investigators use 500 to 1000 with good results. Parallel imaging may reduce distortions in echo planar imaging.
ADC is a measure of the magnitude of diffusion, and is calculated from two or more DWI scans obtained at different b -values. A lower ADC value represents increased restriction of diffusion. The ADC map is more accurate in visualizing the diffusion variability than DWI, without misleading artifacts from the T2 shine-through phenomenon. A reliable estimation of the ADC requires perfect registration of high-quality source images at different b -values without motion artifacts.
Significant variation exists worldwide in methods used for the contrast-enhanced portion of the examination. Most investigators agree that both the time course of enhancement provided by dynamic contrast-enhancement (DCE) scanning and lesion morphology on high-spatial resolution scanning provide distinct and useful information about the risk of malignancy in enhancing lesions. However, commercially available MRI pulse sequences necessitate a compromise between the dynamic and high-spatial resolution approaches. Currently, most scans are done with repeated T1-weighted, fat-saturated, 3D spoiled gradient-echo (SPGR) scans (TR ≤6 ms; flip angle [FA] 10°–15° for T1 weighting). SPGRs provide adequate T1-weighted images with faster scanning than spin-echo sequences, but must be used with optimized flip or tip angles, depending on field strength and repetition time, for proper T1 weighting. Three-dimensional imaging is most often used to avoid interslice gaps, to image faster than 2D multislice imaging, to provide improved reformatted images caused by reconstruction of overlapping slice locations, and to avoid SAR limits at 3 T. Slice thickness and resolution are selected to give maximum resolution and bilateral whole-breast coverage within a 60- to 90-second scan duration. Parallel imaging, fractional k -space (1/2 NEX, etc.), and intermittent/partial fat saturation substantially speed up imaging, allowing much higher resolution within the same scan time. This sequence is repeated as rapidly as possible before, during, and for approximately 5 to 7 minutes after administration of a rapid IV bolus of gadolinium contrast agent. Most investigators use axial or coronal images with a rectangular FOV to maximize efficiency.
According to ACR guidelines ( http://www.acr.org/∼/media/ACR/Documents/PGTS/guidelines/MRI_Breast.pdf ), dynamic images should be obtained at intervals separated by 4 minutes or less. In the authors’ experience, a shorter time interval than 4 minutes is advised to capture the features of the dynamic curves. Contrast material should be administered as an IV bolus dose of 0.1 mmol/kg bodyweight, followed by a flush of at least 10 mL of saline, so that the entire bolus rapidly reaches the systemic circulation. A poor bolus may result in poor image quality or false-negative DCE curves. Although the ACR does not specifically recommend a specific rate for contrast injection, many studies achieve satisfactory results with 2 mL/s. All standard low molecular weight gadolinium-based contrast materials (gadoteridol, gadopentetate dimeglumine, gadobutrol, etc.) have been used by the vast majority of studies with very high-quality results, without clear differences in performance. Gadobutrol (Gadavist) is specifically labeled for use for breast MRI in the United States.
Subtraction images describes the images of contrast enhancement derived from a subtraction of precontrast images from postcontrast images so that only brightly enhancing findings are seen, such as cancer. Subtraction processing suppresses signal from bright fat because adipose tissue does not enhance significantly. However, motion artifact may plague subtraction images, resulting in grainy or noisy images with altered shapes or kinetics. Some software packages allow spatial smoothing of adjacent voxels, reducing noise and artifacts in color mapping, but at the risk of obscuring some intrinsic spatial heterogeneity and features within tumors. For this reason fat suppression is more often used so that high spatial resolution scans may reveal subtle features of cancer such as spiculated margins, and fat suppression can also reduce motion artifacts during subtraction processing. Fat suppression pulses applied intermittently can achieve good fat suppression with little effect on scan time; adiabatic spectrally selective inversion recovery fat suppression is the most efficient method that yields robust, rapid, high-quality images regardless of field strength from 1 T to 3 T.
Maximum intensity projections (MIPs) present the entire breast in a 3D display rather than a single slice to show the entire extent of enhancing findings from the first postcontrast scan. recently showed that a rapid 3-minute breast MRI first postcontrast subtracted T1-weighted image (FAST) scan displayed as an MIP showed 11 breast cancers (7 invasive and 4 ductal carcinoma in situ [DCIS]) in 443 women undergoing 603 screening rounds. MIP analysis showed a sensitivity of 98.9% increasing to 100% with the FAST images; this is a very promising technique for breast cancer screening. MIPs may be reconstructed to sagittal and axial projections resembling x-ray mammograms or may be manipulated to show either volume renderings or thin slabs to display extensive multifocal tumors or extent of nonmass enhancement, while limiting overlapping signal from other regions in the breasts. However, cross-sectional thin-slice MRI planes display fine details of lesions, whereas the MIP may obscure some internal features. Thus the MIP alone should not be used to diagnose tumors. On the other hand, multiplanar MIPs may be reformatted to display tumor distances to the nipple, skin, and chest wall; there are software programs that measure these distances automatically or semiautomatically.
A DCE curve (kinetic curve, or a time intensity curve) shows a lesion’s signal intensity as a function of time during the bolus IV administration of contrast material and over the postcontrast scan time periods. This is usually done by using commercially available programs or computer workstations with the user drawing a region of interest (ROI) greater than 3 pixels large over the enhancing finding.
A variety of image-processing techniques have been developed to automate analysis of dynamic images throughout the breast. Most commercially available programs generate color maps and kinetic curves on a pixel-by-pixel basis using the following steps. First, the program calculates the percentage of enhancement or relative enhancement ( RE ) to the baseline/background signal intensity [SI] value at the operator-defined time point of the early postinjection phase using the formula (SI1 − SI0)/SI0 × 100 (%), where SI0 and SI1 are signal intensities at the baseline and at the operator-defined early postinjection time, respectively. The early postinjection time point is usually defined as between 60 and 90 seconds or at least within the first 2 minutes after a bolus injection. The program then identifies voxels that enhance more than a specified threshold, which can be set by the operator (frequently an RE of 50%–100%, but may vary depending on the intrinsic T1-weighting of the scan protocol, which depends on field strength, FA, and scan repetition rate), at the early postinjection time point, and colorizes these voxels according to the subsequent dynamic kinetics during the delayed phase. The late postinjection time point is usually defined at 4 to 5 minutes after the bolus injection or later, because washout is generally seen at 4 to 5 minutes postinjection. Although no specific color has been set by ACR for the color of the lesion, most vendors determine the color using the following thresholds: (1) increase or decrease by 10% of the early-phase enhancement (Breast Imaging Reporting and Data System [BI-RADS] 2013) or (2) signal enhancement ratio (SER) of 0.9 and 1.1 ( ). SER is the ratio of SI at the early postinjection time point relative to SI at the late postinjection time point expressed by (SI1 − SI0)/ (SI2 − SI0), where SI0, SI1, and SI2 are signal intensities at the precontrast, the early postinjection, and the late postinjection time points, respectively ( ). For example, voxels with persistent enhancement, characterized by increase of relative SI by at least 10% or defined as SER <0.9, is colored in blue, and those with washout enhancement, defined as decease of relative SI by at least 10% or SER >1.1, in red. The others, which belong to plateau kinetics, are colored in green or yellow. Suspicious lesions, that usually have plateau or washout kinetics, stand out against normally gradually enhancing background parenchymal enhancement (BPE).
Some software packages automatically calculate and segment breast tumor volumes by detecting suspicious enhancement kinetics (visually these would be based on their color). Automated volume measurements are less accurate in malignancies that are heterogeneous, enhance slowly or poorly, and may produce spurious results in the face of uncorrected motion artifact. Strong spatial variations in signal intensity caused by variations in surface coil sensitivity, and B1 + transmit inhomogeneity may also produce inaccurate breast tumor results.
Pharmacokinetic scans or physiologic scans are scans that superimpose physiologic maps, such as enhancement information, on morphologic images, combining both types of information into one format. This type of scan usually shows the morphologic appearance of a lesion with the physiologic image superimposed in color.
MRI spectroscopy can be performed as an optional study to obtain information about the chemical content of breast lesions. Several studies have shown that choline-containing compounds (tCho) peak measured with hydrogen ( 1 H)-spectroscopy were more often elevated in malignant breast lesions than in benign lesions or normal breast tissues ( ), suggesting the potential utility of MRI spectroscopy in the diagnosis of breast cancer. For single-voxel choline spectroscopy, a minimum voxel size of 1 × 1 × 1 cm is recommended for adequate signal-to-noise. Localized shimming and high-quality spatial saturation pulses, fat suppression, and partial water suppression improve quality of spectra. It has been recommended to avoid negatively charged gadolinium agents if spectroscopy is performed after contrast enhancement study, because they may reduce choline signal ( ).
Common artifacts and pitfalls seen in breast MRI include line(s) of noise, ghosting artifact, blurring, misregistration artifacts, wraparound artifact ( aliasing ), susceptibility artifact, poor fat saturation, and chemical shift artifact ( Table 7.2 ). They can make interpretation of breast MRI challenging and lead to misdiagnosis.
| Artifact | Cause | Solution |
|---|---|---|
| Line(s) of noise | Electronic noise/poor room shielding/scan room door open | Close the door, call MRI service technician |
| Ghosting (eg, ghosting from heart across the breast) | Moving objects that cause wrong frequency-encoding direction, errors in parallel imaging | Change frequency direction to anterior posterior, correct parallel-imaging parameters |
| Blurring | Patient motion | Keep patient still |
| Misregistration (bright and dark edges on subtraction) | Patient motion | Keep patient still |
| Wraparound artifact (eg, wraparound from abdominal fat on sagittal images) | Aliasing from excited tissue outside imaging FOV | Increase the imaging FOV, perform oversampling (eg, change to axial scan plane from sagittal scan plane), decrease parallel imaging acceleration factor |
| Susceptibility artifact | Metallic objects that cause inhomogeneity in the magnetic field | Remove all metallic objects from breast |
| Poor fat suppression | Poor shimming, center frequency, non-MRI compatible skin marker left on breast, some anatomies | Repeat shim, remove all MRI compatible objects from breast, volume shimming over just the breasts |
| Chemical shift artifact | Shift of fat signal in the read-out direction relative to water signal. | |
| Failure of dynamic contrast enhancement study | Slow or failed contrast injection, delayed contrast injection, late MRI scanning, impaired heart function, etc. | Detect by putting ROI over heart and seeing poor contrast injection, use power injector followed by saline flush bolus, correct the protocol |
Lines of noise can be generated when there is electronic noise, room shielding is poor, scan room door opens, or there is a failure of MRI equipment.
Physiologic movement, including cardiac pulsation, respiration, or gastrointestinal peristalsis, as well as patient motion, can cause a few types of artifact. Physiologically moving objects, such as blood in the heart and vessels, pleural fluid, or bowel fluid, can become blurred and produce duplicated high signal intensity in the phase-encoding direction. This is called ghosting ( Fig. 7.4 ). Ghosting occurs always in the phase-encoding direction, regardless of the direction of the motion. Ghosting can be prevented from obscuring breast tissue by careful selection of phase- and frequency-encoding directions, and by placing a saturation band over the moving structure. Patient motion can result in blurring of the entire image, so it is especially important that the patient hold still and breathe quietly during scanning. Specifically on subtraction images, patient motion can cause alternating bright and dark bands at structure interfaces (such as fat–skin or fat–glandular interfaces) called misregistration artifact, when the patient moves between the images to be subtracted ( Fig. 7.5 ).


One other cause of noise, including unstructured noise or structured ghosting, arises from problems with parallel image reconstruction. Parallel imaging is an image-processing technique that uses spatial information contained in the component coils of an array to partially replace spatial encoding, which would normally be performed using gradients. There have been various parallel imaging techniques developed, such as ASSET, SENSE, mSENSE, GRAPPA, and ARC. The parallel imaging has advantages, including significant reduction in imaging acquisition time and reduction in susceptibility artifacts, but also has disadvantages, including reduction in SNR and increased artifacts caused by the additional imaging processing. These parallel imaging-specific artifacts happen if the acceleration factor and/or coil sensitivity mapping (obtained by the calibration scan) is not correct for the type of breast coil used and are caused by the shape and size of the patient.
Wraparound artifact (also called aliasing or phase wrap ) is caused by aliasing from tissue outside the prescribed imaging FOV that is excited during the image acquisition process. In this artifact, the excited tissue outside the imaging FOV is misregistered and is wrongly superimposed on structures within the FOV in the reconstructed image ( Fig. 7.6 ). Wrapped around artifact is mostly encountered in the phase-encoding direction, because wrapped around artifact in the frequency-encoding direction is usually suppressed by oversampling or the use of a frequency filter. To minimize wrapped around artifact in the phase-encoding direction, increasing the imaging FOV or oversampling in the phase-encoding direction can be used, although these techniques have several disadvantages, such as increasing imaging time and reducing the temporal resolution.

Susceptibility artifact is caused by metallic objects, which induce inhomogeneity in the magnetic field, and is shown as local signal dropout, bright spots, or tissue distortion ( Fig. 7.7 ). Ferromagnetic metals (such as iron, nickel, and cobalt) cause more severe inhomogeneity compared with nonferromagnetic metals (such as titanium). Susceptibility artifacts appear more prominent on gradient-echo images than on FSE images, and at higher field strength (on 3 T than on 1.5 T). These are common around marker clips.

Poor or inhomogeneous fat suppression is usually caused by poor shimming or incorrect choice of the excitation center frequency (usually 220 Hz at 1.5 T), especially in patients with silicone implants or non–MRI-compatible objects, such as metallic BB skin markers, magnetic tissue expanders, metallic scar markers, and metal infusion ports, in or near the breast. Even with optimal shimming, the breast anatomy near the nipple, axilla, chest wall, and interior breast may cause unavoidable variations in B0 field homogeneity and fat suppression failures, resulting in artifacts. Poor fat suppression is easily identified as hyperintense fat on fat-suppressed images ( Fig. 7.8 ).

Chemical shift artifact can be common on nonfat suppressed gradient-echo images. It occurs at the interface of fat:water pixels. Because the fat signal is shifted a few pixels in the read-out direction relative to water, there can be a piling up of combined fat:water signal at some interfaces, and lack of signal at others. This may cause artificial appearance of asymmetric skin thickness, and bright/dark signal around biopsy clips especially on spin-echo images.
Only a good bolus of contrast and timely scanning will translate into breast cancer enhancing rapidly on MRI scans. Failure in DCE studies, including slow bolus, delayed bolus, or late scanning, may result in a nondiagnostic examination and may cause misinterpretation of kinetic curves. Before interpreting dynamic curves, it is important to assess if the technical factors producing the dynamic curves were optimal, resulting in dynamic data good enough to be evaluated.
Cardiovascular kinetic curve obtained with a ROI over large vessels (aorta) or the heart is a useful indicator of failure in DCE studies. The healthy heart/aorta usually shows normal rapid, avid (fast) initial enhancement and rapid late washout ( Figs. 7.9 and 7.10A ). Alternation of the normal cardiovascular dynamic curve pattern tells the radiologist that the DCE study is suboptimal ( Fig. 7.10 B–D ).


Slow or poor bolus of IV contrast may occur when the IV line is injected slowly or may even become detached from the vein, the connector may leak, the catheter may be kinked, there may be a poor saline flush, or heart function is impaired. The contrast either enters slowly, rather than in a bolus, or never enters the patient. Insufficient delivery of contrast media may cause the decrease or absence of the peak of cardiovascular dynamic curve ( Fig. 7.10B ), resulting in poor enhancement of breast lesions and leading to misinterpretation of the washout kinetics as plateau or persistent patterns.
Delayed bolus of IV contrast or poor circulation may cause the delay of the cardiovascular enhancement with or without decrease of the peak enhancement ( Fig. 7.10C ). The first postcontrast scan, which is usually performed to detect rapid peak enhancement, can be obtained earlier than expected, and thus cannot detect a cancer with rapid initial enhancement as different from background.
In late MRI scanning, the early postcontrast scan can miss the peak enhancement ( Fig. 7.10D ). If a cancer has been already washed out at the start of MRI scan, a bright signal from the cancer during the early phase will be missed ( Fig. 7.11 ). Late scanning is caused by an operator error and an incorrect protocol, or when a patient interrupts the scan. Cancers may be obscured by BPE with late scans.
When the cardiovascular dynamic curve pattern is altered, these MRI scans cannot be trusted to show cancer in the breast, and further investigation would be needed to determine the causes of the failed bolus or the late scanning. It is sometimes difficult even for trained technologists to notice the failure in contrast enhancement, thus it is important for radiologists to pay attention to the adequacy of contrast enhancement bolus whenever they assess DCE MRI studies.
Motion artifact is another cause that leads spurious results in DCE analysis. Patient motion can generate faulty kinetic curves if the ROI includes different parts of the lesion or if it includes the adjacent breast parenchyma instead of the target between dynamic images ( ). Particular attention should be paid to identify any patient motion.
Contrast-enhanced breast MRI has high performance in the detection of breast cancer. Initial studies of contrast-enhanced MRI reported a sensitivity of more than 90% for invasive breast cancer. The sensitivity of contrast enhancement has remained high for invasive breast cancer. However, achieving high specificity remains difficult because some benign breast conditions enhance more avidly than normal breast tissue and may resemble breast cancer. Specifically, benign fibroadenoma, papilloma, and proliferative fibrocystic change (FCC) also enhance to a greater degree than normal surrounding breast tissue. Not surprisingly, the highest sensitivity and specificity of contrast-enhanced MRI arise when dynamic enhancement curves are taken into account.
This section first describes the MRI lexicon (except for breast implants) with optimal reporting defined or recommended by BI-RADS 2013. Then, the section discusses essential features of breast lesions on MRI in terms of morphology, DCE kinetics, and signal intensity, which may serve as clues to characterize the lesions. Then presented is a practical systematic image interpretation approach that achieves full assessment of each single MRI study. Finally, diagnostic limitations are discussed.
The provides a valuable standard for the terminology used to analyze breast lesions on MRI ( Table 7.3 ) and is recommended for all breast MRI reporting.
AMOUNT OF FIBROGLANDULAR TISSUE
BACKGROUND PARENCHYMAL ENHANCEMENT
Symmetric or asymmetric
LESION TYPE (SELECT ONE)
FOCUS
Margin
Internal enhancement characteristics
NONMASS ENHANCEMENT
Internal enhancement patterns
|
INTRAMAMMARY LYMPH NODE SKIN LESION NONENHANCING FINDINGS
ASSOCIATED FEATURES
FAT-CONTAINING LESIONS
LOCATION OF LESION
KINETIC CURVE ASSESSMENT
Delayed phase
|
Breast composition should be stated in terms of the amount of FGT, the amount of the BPE, and the position of implants if they are present. The descriptors for important findings include size, location, distribution, morphologic characteristics, and dynamic contrast features.
Abnormal enhancement, which is defined as enhancement significantly different and greater than normal BPE, is categorized as a focus, masses, and nonmass enhancement. Focus is a tiny dot of enhancement (<5 mm) that cannot be characterized otherwise. Masses are 3D space-occupying objects. Mass shapes are categorized as oval, round, and irregular. Mass margins are described as circumscribed or not circumscribed ( irregular or spiculated). Mass internal enhancing characteristics include homogeneous, heterogeneous, rim enhancement, or dark internal septations. If the finding is a nonmass, its distribution should be further classified using the terms focal, linear, segmental, regional, multiple regions, or diffuse. The radiologist further evaluates whether the finding is bilaterally symmetric or if it is asymmetric. Asymmetric findings are more likely to be cancer than are symmetric findings. The internal enhancement pattern of a nonmass should be classified as homogeneous, heterogeneous, clumped, or clustered ring.
In the kinetic curve assessment, the initial signal curve characteristics are classified as slow, medium, or fast. The late signal intensity curve characteristics are classified as either continually enhancing ( persistent ), flat ( plateau ), or washout ( washout ). Details of the BI-RADS definition in the kinetic curve assessment are described in the following section: Dynamic Contrast-Enhancement Kinetic Analysis.
BI-RADS also provides recommendations on the framework of the MRI report and the definition of final category. Reporting should include clinical history, previous imaging examinations, and a brief summary of the scan technique (including the scanner, field strength, pulse sequences used, and the specifics of contrast injection), imaging findings described using the BI-RADS MRI lexicon, final assessment categories, and management recommendations. The radiologists’ final impression of the study is sorted into categories numbered BI-RADS category 0 through 6 ( Table 7.4 ), mostly like mammography. The first BI-RADS category, category 0, is used when additional studies, such as mammogram or targeted ultrasound, are needed at the end of a case to make a final assessment. Categories 1 and 2 are used for normal findings requiring no action, with essentially 0% likelihood of cancer. Category 3 is used for probably benign findings thought to have 2% or less chance of malignancy and for which a short-term, 6-month follow-up may be implemented. Category 4 encompasses a wide variety of findings for which biopsy is recommended. In breast MRI, category 4 is not currently subcategorized into 4A, 4B, and 4C, which are adopted from mammography. Category 5 is reserved for MRI findings highly suggestive of cancer, with 95% or greater likelihood of cancer. Category 6 is intended for cancers for which a known diagnosis has been established before definite therapy such as surgery or chemotherapy. At the end of the report, radiologists should provide a combined summary statement, including an overall assessment of suspicion for cancer, correlation with any other imaging studies, and recommendations for patient management.
| BI-RADS Category | Definition | Likelihood of Malignancy | Management Recommendation |
|---|---|---|---|
| 0 | Incomplete | N/A | Recommend additional imaging |
| 1 | Negative | 0% | Routine breast MRI screening |
| 2 | Benign | 0% | Routine breast MRI screening |
| 3 | Probably benign | >0% but ≤2% | Short-interval (6-month) follow-up |
| 4 | Suspicious | >2% but <95% | Tissue diagnosis |
| 5 | Highly suggestive of malignancy | ≥95% | Tissue diagnosis |
| 6 | Known biopsy-proven malignancy | N/A | Surgical excision when clinically appropriate |
The morphologic characteristics of enhancing malignant and benign lesions are summarized in Box 7.4 , and are shown in Fig. 7.12 . Consistent with mammography, masses with spiculated or very irregular borders are suspicious. Bright enhancement, particularly rim enhancement and enhancing septations, is usually suspicious for tumor angiogenesis. A ductal, linear, or segmental pattern of clumped (cobblestone-like) enhancement is suspicious for DCIS, but it can also be seen in benign duct ectasia or FCC. Clustered ring is worrisome for DCIS. Nonenhancing lesions are benign.
Minimal enhancement
Circumscribed or gently lobulated margin
Homogeneous enhancement
Nonenhancing internal septations
Oriented along Cooper’s ligaments
No enhancement
Center enhances first
Bright enhancement
Spiculated, very irregular margin
Rim enhancement
Heterogeneous enhancement
Linear, linear-branching/segmental enhancement
Clumped or clustered ring enhancement

As with mammography, entirely smooth, oval, or lobulated masses oriented parallel to Cooper’s ligaments suggest benign lesions, whereas lesions traversing Cooper’s ligaments are abnormal and suggest invasive ductal cancer. Nonenhancing dark internal septations in smooth, oval, or lobulated masses are highly specific for a benign fibroadenoma, although they have been reported in mucinous cancers as well. However, it is important to evaluate the dynamic curves of benign-appearing enhancing masses because round or oval homogeneous cancers mimic benign fibroadenomas. Sometimes the suspicious kinetic curves may be the only clue that the morphologically benign mass is a cancer.
When evaluating lesion morphology, including shapes, borders, or internal enhancement patterns, showed a sensitivity and a specificity of contrast-enhanced MRI of 96% and 80%, respectively, for cancer. reported the presence of either skin thickening, or a combination of a spiculated or microlobulated border, with a rim, ductal, linear, or clumped enhancement pattern was 54% sensitive and 94% specific for malignancy.
Breast malignancies usually enhance rapidly after an IV bolus of gadolinium contrast material on DCE MRI ( Fig. 7.13 ). Inflammation, angiogenesis, and tumor necrosis increases tumor vascularity, causing them to be of high signal intensity on the first postcontrast scan. Then, in the later phase, contrast material spreads into various tissues, including intratumoral fibrotic/necrotic portions and normal FGT, while contrast material is often washed out from dense cellular portions consisting of cancer cells within the tumor. In contrast, typical benign lesions poorly or slowly enhance.

These dynamic kinetic curves have initial and delayed enhancement phases ( Fig. 7.14 ). The Lexicon defines initial enhancement as a description of the kinetic curve in the first 2 minutes during the bolus or before the curve shape begins to change. It further defines the initial-phase enhancement as either slow, an increase of signal intensity less than 50% than baseline; medium, an increase of signal intensity 50% to 100% higher than baseline; or fast, an increase of signal intensity >100% higher than baseline. The slow initial rise curve usually indicates benign findings, whereas a fast initial curve may indicate suspicious findings. The Lexicon defines the delayed phase of enhancement as occurring after the first 2 minutes after contrast injection or after the curve shape starts to change. It further defines the delayed-phase enhancement as either persistent if the signal intensity increases by at least 10% of the initial-phase signal intensity, plateau if signal intensity does not change from the initial phase, or washout if the signal intensity decreases by at least 10% of the initial-phase signal intensity. A delayed-phase persistent curve usually indicates benign findings, whereas a delayed-phase plateau or washout curve indicates suspicious findings ( Box 7.5 ).

Curve types may be classified by a five-category system suggested by or by a three-category system ( ; ACR BI-RADS; Fig. 7.15 ). In the five-category Daniel system—nonenhancing (type I); gradually enhancing (type II); or fast initial enhancement with a sustained gradual enhancement, plateau, or early washout (types III, IV, and V, respectively)—types I and II typically indicate benignancy and types IV and V indicate a high likelihood of malignancy. Type III curves are indeterminate. Kuhl/ACR type I curves are gradually enhancing with a late persistent plateau. Kuhl/ACR type II curves have a fast initial enhancement with a late plateau. Kuhl/ACR type III curves have a fast initial enhancement with a late washout.
![FIG. 7.15, Classification of the time course of dynamic contrast enhancement from the most likely benign (type I) through the most likely malignant (type V). No enhancement (Daniel type I) or gradual enhancement (Daniel type II, Kuhl type Ia, and Breast Imaging Reporting and Data System [BI-RADS] type I) suggests a benign lesion. Fast initial enhancement followed by gradual late enhancement (Daniel type III, Kuhl type Ib, and BI-RADS type I) is indeterminate. Fast initial enhancement followed by a plateau signal intensity (Daniel type IV and Kuhl/BI-RADS type II) or early washout of signal intensity (Daniel type V and Kuhl/BI-RADS type III) is suspicious for invasive malignancy. FIG. 7.15, Classification of the time course of dynamic contrast enhancement from the most likely benign (type I) through the most likely malignant (type V). No enhancement (Daniel type I) or gradual enhancement (Daniel type II, Kuhl type Ia, and Breast Imaging Reporting and Data System [BI-RADS] type I) suggests a benign lesion. Fast initial enhancement followed by gradual late enhancement (Daniel type III, Kuhl type Ib, and BI-RADS type I) is indeterminate. Fast initial enhancement followed by a plateau signal intensity (Daniel type IV and Kuhl/BI-RADS type II) or early washout of signal intensity (Daniel type V and Kuhl/BI-RADS type III) is suspicious for invasive malignancy.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MagneticResonanceImagingofBreastCancerandMagneticResonanceImagingGuidedBreastBiopsy/14_3s20B9780323329040000070.jpg)
Generally, the most suspicious curve is one that has a fast initial rise and abrupt transition to a late-phase plateau or washout (see Fig. 7.13 ); the curve shape is also called a square root sign or a cancer corner . When Kuhl types II and type III are used as criteria to diagnose breast cancer, the three-category curve system had a sensitivity of 91% and a specificity of 83% ( ). A geographic distribution of dynamic enhancement also appears to be predictive, with tumors usually enhancing most rapidly at their periphery and benign lesions enhancing most rapidly at the center (see Box 7.5 ).
However, not all cancers or benign findings follow the rules. Not all cancers enhance rapidly, such as invasive lobular carcinoma (ILC). DCIS may exhibit any curve type, including nonenhancing or gradually enhancing curves. Some DCIS lesions may not enhance at all, and are found only as suspicious calcifications on mammography. Benign papillomas may exhibit any kinetic patterns, often initial fast/medium and delayed plateau/washout enhancement, and may mimic cancers. Normal lymph nodes usually enhance fast and wash out like most cancers. Therefore it is important to analyze contrast-enhanced images in combination with morphologic information and T2 signal characteristics.
reported the combined assessment of the dynamic kinetic curves and the margin status improved the diagnostic performance of DCE with a sensitivity of 97% and a specificity of 96%, compared with dynamic kinetic curve assessment alone with a sensitivity of 85% and a specificity of 87%.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here