Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Today’s successful electrophysiologist must not only be a competent proceduralist but also a physician who understands the pathophysiology of arrhythmias, new drug targets and developments, complexities of new technologies, and various treatment options. Additionally, an in-depth understanding of cardiac anatomy, common anatomic variations, typical scar patterns with each pathology, and how anatomic arrhythmia substrates relate to diagnostic and procedural nuances is increasingly important. Advanced imaging modalities, including cardiac computed tomography (CT) and magnetic resonance imaging (MRI), are now important adjuncts for the care of patients with cardiac arrhythmia. This chapter describes the basics of cardiac MRI (CMR), diagnostic and prognostic contributions of MRI for common arrhythmia conditions, MRI safety and advances in the setting of implanted devices, the utility of MRI for interventional electrophysiology (EP), and the advantages and deficiencies of MRI versus CT in the EP laboratory.
The basic MRI means to differentiate between tissues is grounded on differences in signal intensity within acquired images, referred to as intertissue contrasts. The main intertissue contrasts are as follows:
T1-weighted : Tissues with shorter T1 relaxation times appear hyperintense, either caused by intrinsic properties (smaller cell size, presence of lipids, presence of collagen) or caused by the presence of gadolinium-chelate agents that that shorten the intrinsic T1.
T2-weighted : Tissues with longer T2 relaxation values (more plasma, larger cells, presence of edema) appear brighter.
Diffusion-weighted imaging (DWI) : Tissues are differentiated based on the time required to diffuse to boundaries in the three spatial directions: restricted motion to, or through, the cell’s membrane; anisotropy of tissue structure; and intercellular connectivity.
T2∗-weighted imaging , : Tissues with longer T2∗ relaxation time (less fibrotic content, less paramagnetic material content, such as iron-oxide compounds) appear hyperintense. More recently, sequences that provide quantitative maps of the various relaxation times in each spatial location (i.e., T1 maps, T2, T2∗, and apparent diffusion constant maps), which typically require multiple acquisitions, are also provided by the system manufacturers.
MR angiography (MRA): Tissues that are part of the vascular system appear hyperintense. MRA is performed using several methods:
Time of flight : This does not require contrast injection and is generally limited to small anatomic regions.
Phase-contrast (similar in principle to Doppler ultrasound): This only displays moving tissues and can be performed before or after contrast injection. It has lately been extended to four-dimensional (4D) phase-contrast MRI, , which can visualize the entire cardiovascular circulation at multiple times in the cardiac cycle, and provide quantitative vectorial (i.e., directional) flow measurements at each time point.
Contrast-enhanced MRA : This relies on vascular signal enhancement because of contrast perfusion into the vascular space.
CMR represents a porting of the MRI principles to the complex anatomy and physiologic conditions encountered in the heart, so dedicated MRI sequences were developed for use in the heart. It is possible to image tissue properties at either a single time in the cardiac cycle, referred to as single-phase imaging, which is used to provide anatomic structure. It is also possible to acquire images at multiple times within the cycle, referred to as multiphase imaging (now commonly referred to as cine imaging ), which is used to observe cardiac function. For cine imaging, a sequence called balanced steady-state free precession (BSSFP), whose image contrast depends on the ratio of T2 over T1, is prevalently used in the heart, because it best differentiates, without contrast administration, between the cardiac chambers’ lumens and walls, delivering strongly hyperintense lumens and dark walls. Most MRI sequences provide images from normal (sinus rhythm) cardiac cycles, although there are now sequences that can display arrhythmogenic heart motion. ,
To address respiratory motion, special sequences have been developed that allow acquiring images only when both the heart and the lungs return to the same locations in their respective cycles (e.g., mid-diastole and end expiration): (1) single breath-hold imaging, wherein imaging is performed during a patient breath-hold (<15 seconds), generally restricted to lower spatial resolution images, and (2) free-breathing methods, wherein imaging is performed using synchronization of the acquisition (e.g., turning the acquisition on selectively) at a predetermined location (e.g., end expiration or end inspiration) in the breathing cycle. Free-breathing methods can be executed for multiple minutes, allowing for the acquisition of the highest spatial resolution images. Older versions of respiratory synchronization use special pulses that measure lung diaphragmatic motion (referred to as respiratory navigator echoes ), , whereas newer ones are synchronized to the actual location of the desired cardiac chamber’s wall during the respiratory cycle (referred to as self-[respiratory]-gated ), , which can be detected by the MRI sequence.
MRA in the heart can be performed with electrocardiography (ECG) synchronization to obtain the shape of cardiac chamber lumens during various portions of the ECG cycle. Contrast MRA is also performed at multiple time delays after contrast injection, which allows selective observation of the venous anatomy (right heart) or arterial anatomy (left heart).
Dedicated T2-weighted sequences are required in the heart because of problems with rapid blood flow and physiologic motion. These include fast spin echo (FSE)-based techniques with added double or triple (referred to as double inversion recovery [IR] FSE or triple IR FSE ) pulses to null the signal from moving blood and fat. They are frequently performed during single breath-holds and typically provide low-resolution images. Also included are T2-prepared BSSFP sequences (BSSFP with a preceding T2-preparation pulse), , which are used to obtain higher resolution images during free-breathing intervals.
T1-weighted imaging, which uses an IR pulse to suppress viable myocardium signals, performed 10 to 15 minutes after contrast administration and referred to as late gadolinium enhancement (LGE), is used for myocardial scar identification and is routinely performed for the ventricles. LGE takes advantage of delayed washout of gadolinium contrast from scar tissue, which results in shortened T1 relaxation and hyperenhancement of scar compared with the surrounding normal myocardium. , Importantly, a recent U.S. population–based study revealed that up to 6% of individuals have ventricular myocardial scar that remains unrecognized by ECG or clinical evaluation. Atrial LGE imaging requires respiratory gating and higher spatial resolution to visualize the thinner muscular tissue. Such images allow the integration of preexisting atrial scar into the procedural space.
When evaluating the ventricles or atria for fibrosis, focal fibrosis is not always the rule. When the myocardial fibrosis is diffuse rather than focal, such as in persistent atrial fibrillation (AF), after gadolinium contrast injection, and after washout, the contrast may be evenly retained throughout the diffusely fibrotic myocardium. Additionally, compared with the normal tissue, the signal intensity variation in diffusely fibrotic areas may be minimal, so LGE may overlook diffuse myocardial fibrosis despite substantial retention of gadolinium.
The T1-mapping technique, , in which measurements are made of the T1 relaxation time of each volume element (voxel) of tissue prior to gadolinium-chelate injection, and after contrast injection, can detect diffuse myocardial fibrosis by providing a quantitative measure of the myocardial T1 relaxation time. Diffuse myocardial fibrosis shortens the T1 relaxation time because of retention of gadolinium contrast in increased interstitial spaces. The T1-mapping technique has been successfully used to quantify diffuse fibrosis in patients with heart failure, aortic regurgitation, adult congenital heart disease, nonischemic cardiomyopathy (NICM), and myotonic muscular dystrophy. It is important to note that histopathologic processes other than diffuse myocardial fibrosis, such as fatty infiltration, edema, amyloid protein deposition, and iron deposition, can also influence the myocardial T1 relaxation time. Additionally, the postcontrast myocardial T1 time may vary as a function of parameters such as contrast dose, delay time of MRI scan after contrast injection, and patient hematocrit and glomerular filtration rate (GFR). Recently the “native” (without contrast) T1 time has been used in multiple clinical scenarios, such as differentiation of hypertrophic cardiomyopathy (HCM) from hypertensive heart disease and characterization of the chronicity of myocarditis.
If an arrhythmia is suspected to be related to myocarditis or active inflammation in the setting of sarcoidosis, visualization of active inflammation may change the management and can be performed with T2-weighted imaging.
The primary advantage of CMR over CT is the lack of ionizing radiation. Despite new techniques to minimize or even eliminate radiation during complex catheter ablation procedures, it is important to minimize the cumulative radiation exposure, particularly for women and young patients. Additionally, for imaging myocardial scar as a substrate for arrhythmia, the signal-to-noise ratio of MRI LGE far surpass those with delayed contrast enhancement cardiac CT, , which is the analogous CT examination. The delayed contrast enhancement CT protocol also requires higher contrast dose than typical CT perfusion or cardiac chamber visualization protocols, so it cannot be performed more than once per patient. Also, newer MRI gadolinium-chelate-based contrast agents avoid nephrogenic systemic sclerosis and have an improved safety profile compared with the iodinated contrast agents used for cardiac CT. In general, patients with mild renal insufficiency, history of multiple myeloma, risk of contrast-induced nephropathy, recent radioactive iodine therapy, and hyperthyroidism would be better served with CMR and gadolinium-chelate contrast than with iodinated CT contrast. Finally, MRI provides substantially higher temporal resolution than CT, which can play an important role in functional cardiac imaging.
On the other hand, cardiac CT has a substantially shorter acquisition time than CMR and may be the preferred alternative for a claustrophobic patient, patients with significant ectopy, and those with breath-holding difficulties. Typical acquisition times for detailed CT images of the entire heart and vasculature are on the order of 15 seconds. In contrast, CMR requires acquisitions that are several minutes long, and that vary in length depending on sequence settings and patient heart rate. It is important to note that newer free-breathing MRI sequences, which employ self-respiratory gating and the retrospective binning of cardiac phases (now referred to as X-GRASP sequences), can be used in patients with significant ectopy (such as premature ventricular contractions) to provide information on cardiac anatomy or function during either sinus rhythm or ectopy. , Additionally, when evaluating chamber dimensions, the pulmonary vein, the coronary artery, and other vascular boundaries, cardiac CT provides superior spatial resolution.
Although cardiac CT is easier to perform in the setting of permanent pacemakers and implantable cardioverter-defibrillators (ICDs), techniques for safe imaging in this setting are available. Importantly, although an ICD generator’s metal artifact is larger on MRI, and may require dedicated sequences to visualize cardiac anatomy close to it, the artifact caused by the ICD leads tends to be significantly smaller on MRI than on CT.
Finally, intraprocedural imaging is only possible with MRI, unless only a single, low-dose and very short imaging session is required. This is a consequence of the lack of ionizing radiation with MRI, and the greatly superior ability of MRI to differentiate between soft tissues, especially during the acute phase of tissue recovery after ablation.
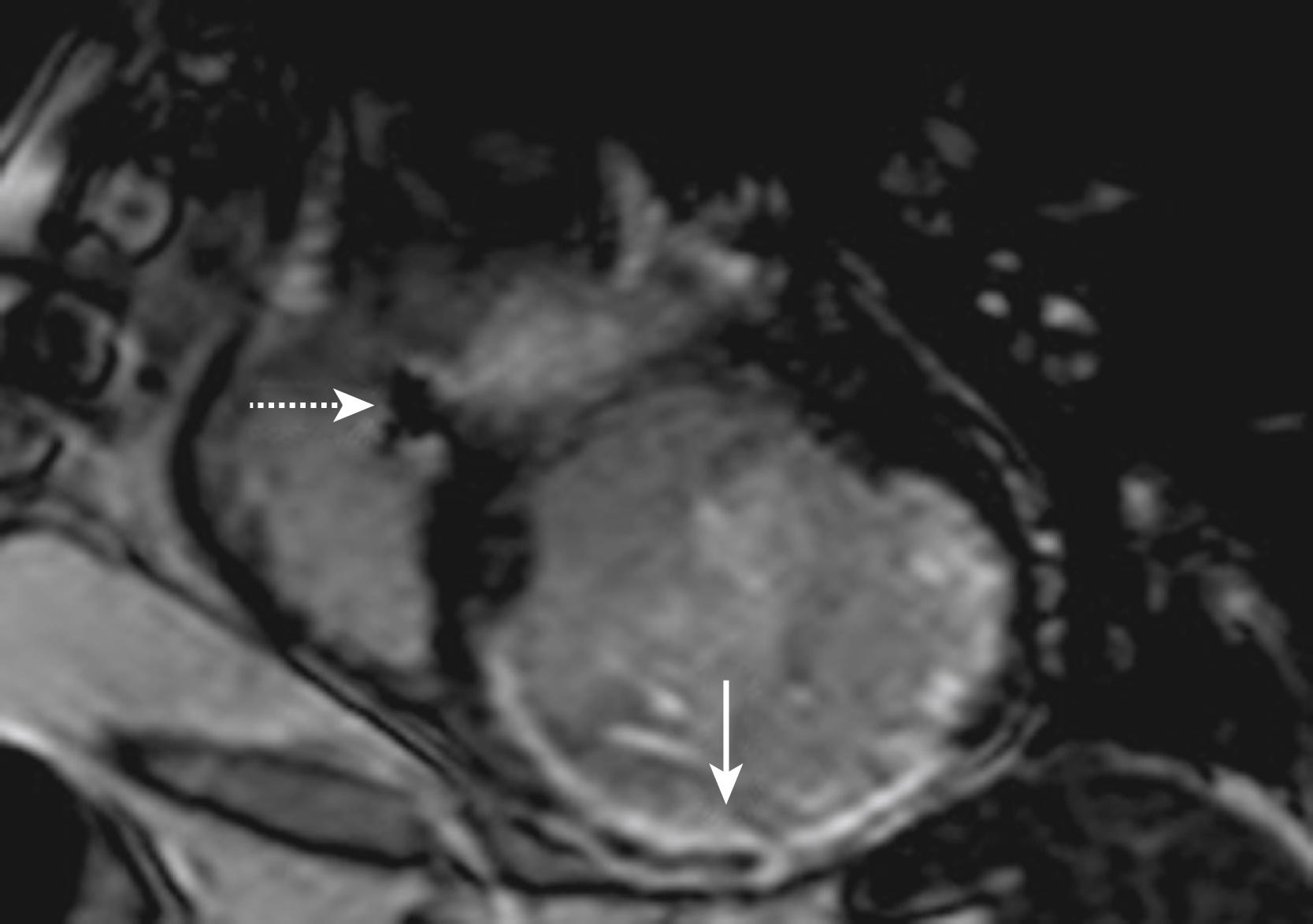
Patients with ischemic cardiomyopathy (ICM) are at risk of ventricular arrhythmia typically caused by reentry involving diseased slow-conducting tissue within the infarct scar. The extent and distribution pattern of scar fibrosis is easily recognizable on CMR ( Fig. 65.1 ). In pioneering work to image the substrate of ventricular tachycardia (VT), Bello and associates showed that measurements of the infarct surface area and mass by CMR could identify the substrate for inducible monomorphic VT. Later, Yan and colleagues provided evidence for the relationship of the scar substrate to clinical events in a study that revealed a strong association of the extent of the periinfarct zone characterized by CMR to all-cause and cardiovascular mortality. Schmidt and coworkers later showed that increased “tissue heterogeneity” in the periinfarct zone correlates with inducible VT in an EP study. Similarly, work with higher-resolution MRI in animal models suggested that the reentry isthmus is characterized by a relatively small volume of viable myocardium bound by the scar tissue at the infarct border zone or over the infarct. Recent work also has suggested that CMR-based computer models of ischemic scar substrates may enable the prediction of specific VT circuits resulting from unique infarct architectures. Studies focusing on the association of VT circuitry with high-resolution infarct scar imaging suggest that critical sites for maintenance of postinfarct VT are often confined to areas with greater than 25% scar transmurality. New studies by our group have also delineated the importance of fat deposition in chronic infarcted myocardium and its effect on electrogram characteristics and VT circuit sites.
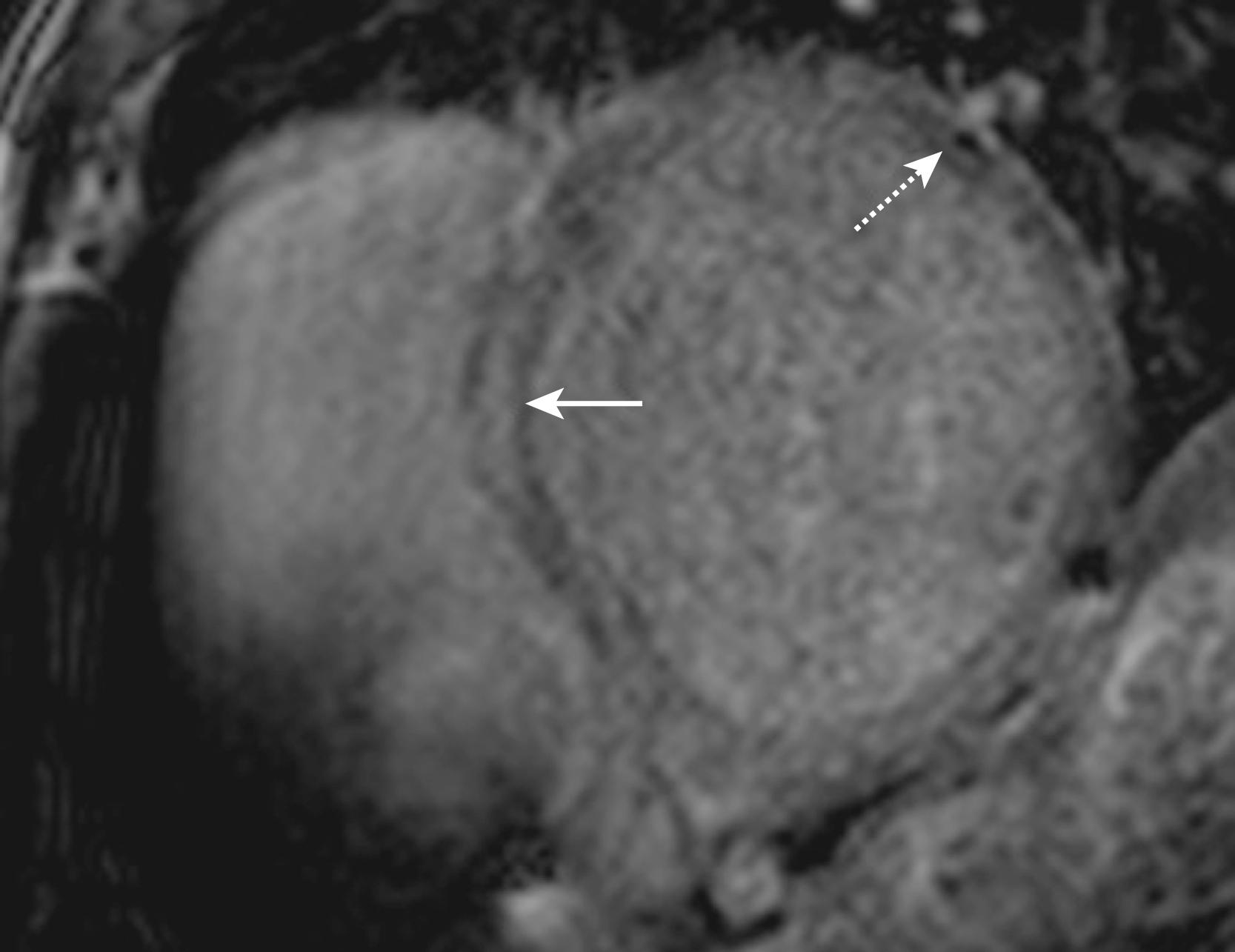
The pathogenesis of NICM with ventricular dilation and reduced cardiac function in the absence of flow-limiting coronary artery disease (CAD) can be genetic, inflammatory, toxic, or viral. However, in the vast majority of cases, the origin is unclear. Although syncope and sudden death are rarely the initial manifestations of the disease, NICM is often associated with VT. CMR readily assesses the anatomic and functional abnormalities related to NICM. LGE using gadolinium contrast can be used to identify scar in the evaluation of patients with NICM. Although absence of LGE is the most common finding in NICM, midwall striae or patches of enhancement can be identified in up to 42% of cases. Compared with ICM, the pattern and location of delayed enhancement in NICM is often atypical, making it difficult to distinguish artifact from true scar ( Fig. 65.2 ). The presence of scar should therefore be verified using multiple planes. Using CMR to delineate scar distribution, we showed that the VT substrate in NICM is often a midwall scar involving greater than 25% of the wall thickness. In a prospective study of patients with NICM, Assomull and colleagues demonstrated that this high-risk midwall fibrosis pattern predicts sudden cardiac death (SCD) and spontaneous VT. In patients with newly diagnosed NICM, the extent of myocardial scar, as quantified by LGE, is independently associated with lack of response to medical therapy, and with the combined end point of mortality and hospitalizations. The transmural extent of LGE predicts inducibility of VT at the time of EP study and the composite end point of hospitalization for heart failure, appropriate ICD firing, and cardiac death. We have shown that electrogram features are associated with scar morphology and distribution in patients with NICM. Additionally, most VT circuit sites were located in NICM scar with more than 25% scar transmurality.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here