Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although the large majority of anesthesia critical incidents and catastrophes involve errors in human judgment, some events involve overt equipment failure or failure of the anesthesia provider to discover an equipment problem. Many equipment problems in anesthesia practice are preventable, and this chapter is intended to help practitioners achieve that goal.
The analogy between administering general anesthesia and piloting a commercial jet may be somewhat overused, but it is singularly relevant in this context. The aviation industry has developed extraordinarily thorough plans involving “acute” and “chronic” interactions with its principal equipment, the commercial passenger airplane. The acute component is the immediate preflight check to verify that a particular aircraft should fly safely that day on a given trip; the chronic component is the elaborate scheme of scheduled preventive maintenance, repair, exchange of old parts for new, and safety inspections of structural components. These are all oriented toward ensuring that the aircraft will fly safely for the designated interval covered by that particular action. In anesthesia practice, the analogy is appropriate to an anesthesia equipment quality assurance (QA) program. The acute effort is the preanesthetic equipment checkout, and the chronic component is the vital and ongoing QA mechanism that involves preventive maintenance, testing for safe function, and the detection of expected wear prior to the failure of a piece of equipment.
Preflight checkout procedures in aviation have changed as technology has advanced. Aircraft systems have become more automated, and modern instrument displays have reduced the workload involved in operating multiple complex systems. This has allowed two crew members to do work that previously required three or more, with increased reliance on automated systems that function with minimal pilot input. Anesthesia equipment has also evolved rapidly, with increasing reliance on systems to function properly with minimal input by the anesthesia professional. This makes it increasingly challenging to maintain a strong understanding of principles of operation and troubleshooting of the workstation. Nevertheless, human vigilance will remain important as the ultimate watchdog and guarantor of machine safety.
Because the practice of anesthesia is heavily dependent on the correct functioning of a large number of diverse pieces of equipment, reports of problems with anesthesia equipment have been prominent in the anesthesia literature virtually since its inception. A great many of the classic traditional problems that had been common since recognizable anesthesia machines came into use—such as fresh gas rotameter leaks, ventilator leaks, and disconnections of poorly designed hose or tubing connectors—have been largely eliminated by the adoption and implementation of rigorous design and fabrication standards by anesthesia machine manufacturers. However, many of the details and the specific problems have changed, and advances have been made in the study of human interaction with the anesthesia system. Improvement of the user friendliness of anesthesia technology and study of human factors in both routine and crisis anesthesia situations have been made possible in part by major growth and development of high-fidelity patient simulators that enable trials and modification of equipment and protocols without patient risk. Current simulators also allow specific training of anesthesia professionals to deal with extremely rare catastrophic situations. Although required to be familiar with and prepared to react to these challenges, one hopes to never encounter them in real life, such as crossed oxygen and nitrous oxide pipelines.
Classic treatises on anesthesia equipment provide useful references for reviewing the spectrum of defects and problems that have historically been reported with anesthesia equipment. Rendell-Baker edited a classic monograph that described 48 specific safety-related problems with anesthesia machines. Among these, in order of frequency, were problems with (1) the vaporizer, (2) the breathing system, (3) the gas flowmeters, (4) the mechanical functions of the machine, and (5) human engineering. In another classic, Spooner and Kirby outlined some of the data collected by the Emergency Care Research Institute (ECRI) regarding the role of equipment in anesthesia accidents. In the American Society of Anesthesiologists (ASA) Closed Claims Study from 1998, one report noted that only 2% of all claims were apparently caused by the anesthesia gas delivery equipment; however, 76% of those involved catastrophic adverse outcomes.
Further studies have attempted to delineate the contribution of human error or oversight to machine failure. Pioneering work by Cooper and associates suggested that among anesthesia critical incidents, 82% involved human error, and only 14% resulted from overt equipment failure. Among the equipment failures, 20% involved the breathing circuit, 18% airway components, 12% laryngoscopes, and 12% the anesthesia machine. Failure to perform a normal checkout was cited 22 times on a list of 481 factors associated with 359 incidents. Follow-up studies of 1089 preventable critical incidents found that only 4% of incidents with substantive negative outcomes involved equipment failure. Of all incidents reported in the various parts of the study, 11% to 19% involved equipment failure. However, 129 (22%) of 583 instances of human error involved anesthesia machine use. These data suggest that a combination of the occasional overt device failure along with a component of various types of human oversight lead to anesthetic mishaps (see Chapter 17 ). More recent data from the ASA Closed Claims Study showed a decrease in anesthesia gas delivery equipment-related claims to only 1%, yet the majority (85%) involved provider error. Of these, 35% were deemed by the authors to be preventable by a preanesthesia check (see Chapter 23 ).
It is well accepted that a thorough and stepwise anesthesia apparatus checkout should be performed prior to the delivery of anesthesia. Even in surveys that identify poor provider compliance with preanesthesia checkout (PAC) procedures, most participants feel that such checks improve patient safety. This perception is correct, as it has been demonstrated that a PAC performed with a checklist and protocol is associated with a decreased risk of perioperative morbidity and mortality. The obvious industry parallel is aviation, in which a strict adherence to pre-event checklists (e.g., before start, at takeoff, on approach) is known to enhance compliance with important steps and procedures and to save lives. Great progress has been made in enhancing the knowledge of the anesthesia workstation and in introducing anesthesia equipment pre-use checkout guidelines. Despite these efforts, our overall performance in “preflighting” the anesthesia workstation seems to be less than optimal. This is both troubling and perplexing given the pivotal role that the anesthesia workstation plays in anesthesia practice and patient safety.
Data predating the first publication of the U.S. Food and Drug Administration (FDA) Anesthesia Apparatus Checkout Recommendations in 1986 demonstrated a low level of proficiency by anesthesiologists in detecting life-threatening machine problems. Using a machine with five intentionally created faults, researchers found that anesthesiologists detected on average only 2.2 serious problems (44%); and 7.3% found no faults at all despite knowing ahead of time that the machine was intentionally altered. At that time, available pre-use checkout procedures for anesthesia machines were provided and promoted by individual machine manufacturers. Given the design and engineering perspectives of the manufacturers and their liability concerns, the machine-specific checkout recommendations were not entirely user friendly nor well suited for clinical application.
Prompted by a series of anesthesia machine–related accidents, in 1984 the FDA met with representatives from the ASA, anesthesia equipment experts, and anesthesia machine manufacturers to discuss methods of reducing patient risk during anesthesia. , During that meeting the FDA was asked to take the lead in the development of the first generic anesthesia apparatus checkout recommendations. This general guideline was intended to instruct users on how to perform a pre-use checkout, to promote the concept of a pre-use checkout, and to create a framework that providers could modify to meet their local needs. This first pre-use checkout was released in August of 1986 and contained 25 primary items, with some having up to six subitems. The guideline intended that a comprehensive checkout be accomplished at the beginning of the day (“day check”) followed by an abbreviated check prior to subsequent cases (“case check”). The 1986 PAC guideline was fairly detailed, proved to be very time consuming, and was not extensively used. ,
The issuance of the 1986 recommendations also did not appear to improve the ability of anesthesiologists to detect anesthesia machine faults. March and Crowley showed that 188 anesthesiologists, using their own methods, could detect only one of four preset faults. When the same subjects used the FDA checklist to assess a different set of failures, detection performance improved only modestly.
Recognizing that there was poor compliance with the 1986 guideline, the FDA revised the PAC in the early 1990s, working once again with anesthesiology professional organizations and industry. Other factors, including the development of monitoring standards by the ASA, the retirement of many older machines, and the introduction of newer-generation anesthesia machines following the new American Society for Testing and Materials (ASTM) specifications also provided impetus for the update of the guidelines. The revised checklist was issued in 1993 and included only 14 major steps, many with several bulleted instructions. Although the checklist was fairly comprehensive and universal, a stated intent of the authors, similar to the 1986 version, was to encourage users to “modify [the guideline] to accommodate differences in equipment design and variations in local clinical practice” and to subject modifications to local peer review. It also encouraged users to “refer to the operator’s manual for the manufacturer’s specific procedures and precautions,” particularly when addressing the machine’s low–pressure system leak test. Much like the 1986 guideline, the FDA did not mandate the use of the 1993 Anesthesia Apparatus Checkout Recommendations—they offered guidance and encouraged modification to accommodate differences in equipment design and variations in local clinical practice.
Although the data are limited, evidence that the 1993 PAC recommendations led to improved user compliance and better detection of machine faults was not forthcoming. When anesthesiology providers of different backgrounds and experience levels were asked to use their own anesthesia pre-use checkout procedures to check a fault-laden machine, and then went on to check another sabotaged machine using the 1993 FDA checkout procedure, researchers detected no difference in the rate of fault detection using either method. In fact, despite having the FDA checklist in hand, 41% of the participants could not identify more than 50% of the faults. Blike and Biddle found that anesthesiology providers missed “easy” anesthesia machine faults 30% of the time and “difficult” anesthesia faults 62% of the time when provided with the FDA checklist. Larson and colleagues observed 87 participants at a “nationally attended anesthesia meeting” when they were asked to perform a checkout on an anesthesia machine with preset faults. The average number of faults detected by all participants was 3.1 of 5 total faults. Interestingly, the authors showed a negative correlation between level of experience and the ability to detect faults. Olympio and colleagues observed anesthesiology residents checking out the machine and noted a low performance rate (69%), which improved by only 12% after focused instructional review even though the residents knew in advance that their performance would be evaluated. In another experimental setting, Armstrong and colleagues observed anesthesiologists in a simulator who knew only that they were involved in a study to evaluate the simulator as a testing tool, and who were aware that simulated patient or technical problems would be presented during the case. The researchers quietly graded the quality of the anesthesia checkout and found that the subjects, on average, checked 50% or fewer of 20 key items. Performance was noted to be poor regardless of the age or experience of the anesthesiologist.
As tempting as it may be to implicate the checklists in these failures, human factors and training issues are more likely to blame. In particular, a lack of cultural discipline in the routine, proper use of a PAC can be implicated. As noted, pivotal work by Cooper and colleagues demonstrated that in 22% of equipment-related mishaps, a failure to check or inspect was identified as an associated factor. Similarly, in a 1981 survey of anesthetic misadventures, human error was found to be more often responsible than equipment failure, and a failure to perform a machine checkout was the factor most likely associated with an equipment-related issue. In 1992 Mayor and Eaton found that almost 41% percent of anesthesia providers admitted to performing inadequate machine checks, and few followed published guidelines. In an Internet-based survey of anesthesiology providers and anesthesia technicians published in abstract form in 2005, 29% of respondents rated their competence in performing the 1993 FDA pre-use checkout as poor. Reasons cited in the same survey for skipping the checkout included unfamiliarity with the procedure, a belief that the machine self-check alone was sufficient, and that checkout took too long to perform. Finally, in a 2007 survey conducted in the United Kingdom, researchers found that most anesthetists admitted to only partially checking the anesthesia machine; only 12% performed a check between cases, and only 27% identified an alternate means for ventilation prior to anesthesia.
It seems that no matter how well-conceived and heavily promoted PAC recommendations have been, the data demonstrate that their adoption and routine use has not been consistent. As anesthesiologists have historically been and remain leaders in patient safety, the cause of this remains speculative and likely multifactorial. Departments may rely on technician-initiated PACs, decreasing user familiarity with the steps of the procedure. Production pressure in a fast-paced operating room (OR) environment disincentivizes a potentially time-consuming check. In concert, improved engineering resulting in a decreased incidence of catastrophic patient harm may instill a sense of comfort in the reliability of machines, whether deservedly or not. What compounds the issue is the growing assortment of anesthesia machine designs and features that depart significantly from the more generic, older-generation gas machines and workstations. Components of newer anesthesia machines are increasingly unavailable for visual inspection ( Fig. 25.1 ). When these factors are combined with a misunderstanding of, and an over-reliance on, automated machine checkout functions, the potential for a suboptimal PAC is significant.

The 2008 guidelines are available at https://www.asahq.org/standards-and-guidelines/2008-asa-recommendations-for-pre-anesthesia-checkout . They were developed with the knowledge that the existing PAC was not well understood, nor was it reliably used by anesthesia providers, and that anesthesia delivery systems have evolved to the point where one checkout procedure is no longer universally applicable. Although anesthesia providers were encouraged to modify the 1993 PAC to meet their own equipment needs, it was essentially applicable to almost any machine when it was published. As a newer generation of machines began to emerge, the PAC became increasingly difficult to apply strictly. These machines differed in their functions and features as well as their checkout procedures, and they have become increasingly diverse even among themselves. For precisely these reasons, the authors of the ASA’s 2008 Recommendations for Pre-Anesthesia Checkout Procedures created a template to develop “checkout procedures that are appropriate for each individual anesthesia machine design and practice setting,” instead of a detailed PAC. Their goal was to provide guidelines so that individual departments could develop their own PAC, which could be performed consistently and expeditiously ( Table 25.1 ). In fact, the footer of the document reads “Guideline for Designing Pre-Anesthesia Checkout Procedures.”
| Item | Task | Responsible Parties |
|---|---|---|
| To Be Completed Daily | ||
| 1 | Verify auxiliary oxygen cylinder and self-inflating manual ventilation device are available and functioning | Provider and technician |
| 2 | Verify patient suction is adequate to clear the airway | Provider and technician |
| 3 | Turn on anesthesia delivery system and confirm that AC power is available | Provider or technician |
| 4 | Verify availability of required monitors, including alarms | Provider or technician |
| 5 | Verify that pressure is adequate on the spare oxygen cylinder mounted on the anesthesia machine | Provider and technician |
| 6 | Verify that the piped gas pressures are ≥50 psig | Provider and technician |
| 7 | Verify that vaporizers are adequately filled and, if applicable, that the filler ports are tightly closed | Provider only |
| 8 | Verify that there are no leaks in the gas supply lines between the flowmeters and the common gas outlet | Provider or technician |
| 9 | Test scavenging system function | Provider or technician |
| 10 | Calibrate, or verify calibration of, the oxygen monitor and check the low-oxygen alarm | Provider or technician |
| 11 | Verify carbon dioxide absorbent is not exhausted | Provider or technician |
| 12 | Perform breathing system pressure and leak testing | Provider and technician |
| 13 | Verify that gas flows properly through the breathing circuit during both inspiration and exhalation | Provider and technician |
| 14 | Document completion of checkout procedures | Provider and technician |
| 15 | Confirm ventilator settings and evaluate readiness to deliver anesthesia care (anesthesia time out) | Provider only |
| To Be Completed Before Each Procedure | ||
| 1 | Verify patient suction is adequate to clear the airway | Provider and technician |
| 2 | Verify availability of required monitors, including alarms | Provider or technician |
| 3 | Verify that vaporizers are adequately filled and, if applicable, that the filler ports are tightly closed | Provider |
| 4 | Verify carbon dioxide absorbent is not exhausted | Provider or technician |
| 5 | Perform breathing system pressure and leak testing | Provider and technician |
| 6 | Verify that gas flows properly through the breathing circuit during both inspiration and exhalation | Provider and technician |
| 7 | Document completion of checkout procedures | Provider and technician |
| 8 | Confirm ventilator settings and evaluate readiness to deliver anesthesia care (anesthesia time out) | Provider |
The 2008 recommendations warn against an overreliance on automated machine checkouts, alerting that anesthesiology providers may be unaware of what is actually assessed by these features and that they may omit important pre-use checkout items if they place all their faith in an automated checkout. When developing a local PAC, it is important to understand what is actually checked by the machine. However, this is not always easy to ascertain by simply reviewing user manuals.
The 1993 version of the PAC placed all of the responsibility of the pre-use checkout on the anesthesia provider. The authors of the 2008 guidelines recognized that using anesthesia technicians and/or biomedical technicians to perform some aspects of the checkout procedures may improve compliance with a department’s PAC and could add redundancy to critical steps. Although the 2008 guidelines suggest which steps may be checked by “a qualified anesthesia technician and/or biomedical technician,” this should indeed be an institutional decision, because skill levels, workflow patterns, and training requirements vary greatly. Additionally, they state that regardless of who participates in the PAC, “the anesthesia care provider is ultimately responsible for proper function of all equipment used to provide anesthesia care.”
The items and rationale statements listed below, excerpted directly from the 2008 Recommendations for Pre-Anesthesia Checkout Procedures, are intended to describe a basic approach to developing sound institution-specific PAC procedures designed “for the equipment and resources available.” They identify items that need to be checked as part of a complete PAC. The method used to check each item will depend on the specific equipment. Also identified in the recommendations are the suggested frequency of the checks, and the suggested responsible parties either individually, alternatively (“or”), or redundantly (“and”). It is important to recognize that the guidelines are not all inclusive; they simply suggest the minimum machine-related items that should be assessed prior to use. A local PAC checklist should represent a workable merger between these guidelines and the manufacturer’s checkout recommendations. As in the prior PAC guidelines, items that require checkout prior to each procedure are distinguished from those that need only to be checked daily.
Item 1: Verify auxiliary oxygen cylinder and self-inflating manual ventilation device are available and functioning
Frequency : Daily
Responsible Parties: Provider and technician
Failure to be able to ventilate is a major cause of morbidity and mortality related to anesthesia care. Because equipment failure with resulting inability to ventilate the patient can occur at any time, a self-inflating manual ventilation device (e.g., Ambu bag) should be present at every anesthetizing location for every case and should be checked for proper function. In addition, a source of oxygen separate from the anesthesia machine and pipeline supply, specifically an oxygen cylinder with regulator and a means to open the cylinder valve, should be immediately available and checked. After checking the cylinder pressure, it is recommended that the main cylinder valve be closed to avoid inadvertent emptying of the cylinder through a leaky or open regulator .
This step was item 1 on the 1993 PAC and remains so in the 2008 recommendations. It is the most important item on the checklist. No matter what happens to the machine, you should always be prepared to keep the patient alive without it. In the event of machine failure, it is critical to ventilate first and troubleshoot later . The auxiliary ventilation device should be self-inflating, which would exclude the disposable Mapleson circuits often found in and out of the operating room (OR); these devices should be located at every anesthetizing location, and the guideline further recommends that they be checked for proper function ( Fig. 25.2 ). The recommendation also states that the auxiliary oxygen source should be separate from the machine and its pipeline supply, “specifically an oxygen cylinder.” Ensuring that properly filled portable cylinders with attached flowmeters are available at specific locations requires an institutional logistic commitment and careful attention to detail by support staff. Incorporating technicians into this step would likely be very useful.

Item 2: Verify patient suction is adequate to clear the airway
Frequency: Prior to each use
Responsible Parties: Provider and technician
Safe anesthetic care requires the immediate availability of suction to clear the airway if needed.
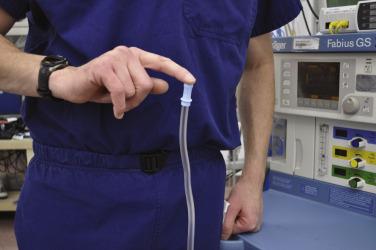
This step moved up from the last position on the 1993 PAC recommendations. Suction is critically important to anesthesia care, because it is the only major piece of equipment used routinely by anesthesiologists whose function cannot be replaced in a life-threatening crisis by the anesthesiologist’s own body. An anesthesiologist can monitor, ventilate, and even intubate if necessary without any equipment at all. An anesthesiologist cannot, however, adequately clear a pharynx full of secretions or vomitus without an adequately functioning suction; thus suction is a genuinely vital piece of anesthesia equipment. One simple way to check the suction is to determine whether there is enough negative pressure for the tubing to attach to the operator’s finger and support its own weight while suspended in the air ( Fig. 25.3 ).
Item 3: Turn on anesthesia delivery system and confirm that AC power is available
Frequency : Daily
Responsible Parties : Provider or technician
Anesthesia delivery systems typically function with back-up battery power if AC power fails. Unless the presence of AC power is confirmed, the first obvious sign of power failure can be a complete system shutdown when the batteries can no longer power the system. Many anesthesia delivery systems have visual indicators of the power source showing the presence of both AC and battery power. These indicators should be checked, and connection of the power cord to a functional AC power source should be confirmed. Desflurane vaporizers require electrical power and recommendations for checking power to these vaporizers should also be followed.
Most anesthesia machines provide some indication that the machine is plugged into AC power, or that it is not and is on battery power ( Fig. 25.4 ). Some newer generation machines perform a battery check automatically and report problems to the operator during start-up checks, whereas some machines require a manual assessment of battery power, such as unplugging the machine from the outlet and pressing a battery test button. If not an automated function, checking the battery power is another example of how an anesthesia technician could unburden anesthesia providers.

Item 4: Verify availability of required monitors and check alarms
Frequency: Prior to each use
Responsible Parties: Provider or technician
Standards for patient monitoring during anesthesia are clearly defined. The ability to conform to these standards should be confirmed for every anesthetic. The first step is to visually verify that the appropriate monitoring supplies (BP cuffs, oximetry probes, etc.) are available. All monitors should be turned on and proper completion of power-up self-tests confirmed. Given the importance of pulse oximetry and capnography to patient safety, verifying proper function of these devices before anesthetizing the patient is essential. Capnometer function can be verified by exhaling through the breathing circuit or gas sensor to generate a capnogram, or verifying that the patient’s breathing efforts generate a capnogram before the patient is anesthetized. Visual and audible alarm signals should be generated when this is discontinued. Pulse oximeter function, including an audible alarm, can be verified by placing the sensor on a finger and observing for a proper recording. The pulse oximeter alarm can be tested by introducing motion artifact or removing the sensor. Audible alarms have also been reconfirmed as essential to patient safety by ASA, American Association of Nurse Anesthetists, Anesthesia Patient Safety Foundation, and The Joint Commission. Proper monitor functioning includes visual and audible alarm signals that function as designed.
Verifying the availability and proper functioning of standard and other required monitors is a relatively straightforward task. However, the process of checking alarm thresholds, and possibly resetting them, can be tedious. It is possible for alarm settings on monitors to vary within individual facilities, because of provider manipulation of alarms for case requirements, a lack of standard default settings, and a failure to routinely reset alarm limits. Departmental alarm default settings can be established and programmed into anesthesia workstation monitors. Alarm limit settings also include anesthesia machine alarms such as volume, pressure, and inspired oxygen concentration limits ( Fig. 25.5 ).

Item 5: Verify that pressure is adequate on the spare oxygen cylinder mounted on the anesthesia machine
Frequency : Daily
Responsible Parties : Provider and technician
Anesthesia delivery systems rely on a supply of oxygen for various machine functions. At a minimum, the oxygen supply is used to provide oxygen to the patient. Pneumatically powered ventilators also rely on a gas supply. Oxygen cylinder(s) should be mounted on the anesthesia delivery system and determined to have an acceptable minimum pressure. The acceptable pressure depends on the intended use, the design of the anesthesia delivery system, and the availability of piped oxygen.
Verification of oxygen cylinder pressure is accomplished by opening the oxygen cylinder(s) on the back of the machine and evaluating the tank gauge pressure located on the front of the machine, although some newer machines may also have a tank gauge located on the back of the machine. The 1993 PAC guideline recommends that the oxygen cylinder be “at least half full (about 1000 pounds per square inch gauge pressure (psig))” during checkout. The current recommendations do not provide a specific value, but some manufacturers’ manuals still suggest the 1000 psig minimum.
It is important to understand that it is theoretically possible for the tank gauge pressure to read higher than the actual pressure in the tank; this is because on many machines, the tank gauge pressure reflects the pressure in the pipeline segment between the yoke check valve and low-pressure side of the pressure-reducing regulator ( Fig. 25.6 ). When the tank pressure falls below the pressure it contained when it was previously opened, the gauge will continue to reflect the higher pressure in the segment unless the wall supply pressure within the machine dips low enough for the pressure-reducing regulator to open to the tank supply route. A situation like this could occur, theoretically, if the provider were to leave the tank valve open during the PAC, and the tank were to drain down low or even empty through a leak at the yoke assembly. If a provider or technician then opened the tank valve, the gauge pressure would read the pressure remaining in the piping segment, and not within the tank. This is due to the one-way nature of the yoke check valve—unless tank pressure can overcome the upstream segment pressure, actual tank pressure will not be reflected on the gauge. In fact, even if the tank is completely removed , segment pressure will still be reflected on the gauge ( Fig. 25.7 ). Some newer generation machines measure tank pressure upstream of the yoke check valve, which eliminates this concern completely. Additional bulleted comments in this item of the 2008 guidelines include:
Typically, an oxygen cylinder will be used if the central oxygen supply fails.


Auxiliary oxygen cylinders will be used if the pipeline supply of oxygen fails or becomes contaminated . During a simulated gas pipeline crossover accident, researchers found that several participants used the machine’s auxiliary oxygen flowmeter as a presumed external source of oxygen, yet none properly disconnected the wall oxygen line while the inspired oxygen concentration decreased in the face of sustained pipeline pressure.
If the cylinder is intended to be the primary source of oxygen (e.g., remote-site anesthesia), then a cylinder supply sufficient to last for the entire anesthetic is required.
The amount of oxygen required for a case begins with estimating the patient’s anticipated needs and then determining the requirements of the mechanical ventilator if driven by gas (see below). It is always wise to estimate finite-source oxygen needs (e.g., a tank) by applying a wide margin on the side of safety.
If a pneumatically powered ventilator that uses oxygen as its driving gas will be used, a full ‘E’ oxygen cylinder may provide only 30 minutes of oxygen. In that case, the maximum duration of oxygen supply can be obtained from an oxygen cylinder if it is used only to provide fresh gas to the patient in conjunction with manual or spontaneous ventilation. Mechanical ventilators will consume the oxygen supply if pneumatically powered ventilators that require oxygen to power the ventilator are used. Electrically powered ventilators do not consume oxygen so that the duration of a cylinder supply will depend only on total fresh gas flow.
Generally speaking, mechanical ventilators using a bellows are typically gas driven, with either oxygen or air, and piston driven and impeller ventilators are electrically driven. This underscores the importance of machine familiarity.
The oxygen cylinder valve should be closed after it has been verified that adequate pressure is present, unless the cylinder is to be the primary source of oxygen (i.e., piped oxygen is not available). If the valve remains open and the pipeline supply should fail, the oxygen cylinder can become depleted while the anesthesia provider is unaware of the oxygen supply problem.
The interface between the oxygen tank and the yoke assembly is very vulnerable to leaking. As alluded to earlier, if the oxygen tank pressure were to steadily decrease during the day, the provider would possibly be unaware, because real-time tank pressure measurement could be blocked by the yoke check valve. If tank pressure on the machine was measured upstream of the yoke check valve, which is currently the exception, actual tank pressure would be continuously displayed, and this problem would be immediately recognized.
Other gas supply cylinders (e.g., Heliox, CO 2 , Air, N 2 O) need to be checked only if that gas is required to provide anesthesia care .
Item 6: Verify that piped gas pressures are 50 psig or greater
Frequency : Daily
Responsible Parties : Provider and technician
A minimum gas supply pressure is required for proper function of the anesthesia delivery system. Gas supplied from a central source can fail for a variety of reasons. Therefore, the pressure in the piped gas supply should be checked at least once daily.
Normal pipeline pressures in the United States for common gases (O 2 , air, N 2 O) are 50 to 55 psig (345 to 380 kPa). Pipeline pressure gauges on anesthesia machines include standard numeric analog gauges, analog gauges that highlight acceptable ranges, and a digital display of pressures measured by a pressure transducer. ( Fig. 25.8 ). Although the guideline suggests verifying gauge pressures, an inspection of the supply hoses and connections is also recommended by some manufacturers. Checking that “hoses are connected” was a checklist item on the 1993 PAC, and a “tug test” can be performed to check for proper insertion of the pipelines. Despite gas-specific connectors, misconnections of gas hoses have been reported. Likewise, medical gas supply lines behind the walls of the OR are not immune from misconnection or contamination.

An important safety item on all machines is an audible and visual alarm that warns the operator of diminishing oxygen supply pressure. However, the only way to evaluate this item is to disconnect the wall oxygen supply and shut off any oxygen supply tanks. Likewise, an evaluation of the machine’s oxygen failure protection device or fail-safe feature would also require disconnection of the O 2 supply hose. These two checks were not included in the 1993 guideline or in the current version, presumably because they are too time-consuming relative to their risk-preventative value. Also, daily removal of the oxygen supply hose may introduce a risk greater than that posed by the potential for failure of these features. An evaluation of these features is usually part of routine preventative maintenance.
Item 7: Verify that vaporizers are adequately filled and, if applicable, that the filler ports are tightly closed
Frequency : Daily
Responsible Parties : Provider and also the technician if redundancy is desired
If anesthetic vapor delivery is planned, an adequate supply is essential to reduce the risk of light anesthesia or recall. This is especially true if an anesthetic agent monitor with a low agent alarm is not being used. Partially open filler ports are a common cause of leaks that may not be detected if the vaporizer control dial is not open when a leak test is performed. This leak source can be minimized by tightly closing filler ports. Newer vaporizer designs have filling systems that automatically close the filler port when filling is completed. High and low anesthetic agent alarms are useful to help prevent over- or under-dosage of anesthetic vapor. Use of these alarms is encouraged, and they should be set to the appropriate limits and enabled .
Although not part of the 2008 guideline, some manufacturers recommend a check of their machine’s vaporizer interlock system, which if present prevents more than one vaporizer from being activated simultaneously. If this step is added to a local checklist, make sure that when one vaporizer handwheel is turned to a setting greater than zero that any other vaporizers remain locked in the zero position.
Item 8: Verify that there are no leaks in the gas supply lines between the flowmeters and the common gas outlet
Frequency: Daily and whenever a vaporizer is changed
Responsible Parties: Provider or technician
The gas supply in this part of the anesthesia delivery system passes through the anesthetic vaporizer(s) on most anesthesia delivery systems. In order to perform a thorough leak test, each vaporizer must be turned on individually to check for leaks at the vaporizer mount(s) or inside the vaporizer. Furthermore, some machines have a check valve between the flowmeters and the common gas outlet, requiring a negative pressure test to adequately check for leaks. Automated checkout procedures typically include a leak test but may not evaluate leaks at the vaporizer, especially if the vaporizer is not turned on during the leak test. When relying upon automated testing to evaluate the system for leaks, the automated leak test would need to be repeated for each vaporizer in place. This test should also be completed whenever a vaporizer is changed. The risk of a leak at the vaporizer depends upon the vaporizer design. Vaporizer designs where the filler port closes automatically after filling can reduce the risk of leaks. Technicians can provide useful assistance with this aspect of the machine checkout, since it can be time consuming.
This step checks the integrity of the so-called low-pressure system (LPS) of the anesthesia machine, which is traditionally defined as the section downstream from the flow control valves to the common gas outlet. Leaks in this section of the machine are associated with hypoxemia or patient awareness under anesthesia. , Leaks here are commonly related to the anesthetic vaporizer, the vaporizer mounting, or the flowmeter tubes.
Because of significant machine design differences, several tests have been described to check for leaks within the LPS. These tests use either positive pressure, assessing either leak flow or system pressure stability, or negative pressure to facilitate leak detection in this vulnerable part of the anesthesia machine ( Figs. 25.9 and 25.10 ). Historically, selecting the proper test was confusing, because some machines have an outlet check valve between the common gas outlet and the vaporizers (many Ohmeda machines), while others do not. The check valve is meant to minimize the effects of intermittent back pressure on vaporizer output, but is held closed by downstream positive pressure which precludes manual positive-pressure testing of the LPS. For machines without an outlet check valve, positive-pressure tests are usually sufficient and include simple pressurization of the patient breathing circuit or more complex testing using specialized bulbs, manometers, and/or flowmeters. , ,


To eliminate confusion with this, the 1993 PAC’s “Leak Check of the Machine Low-Pressure System” prescribed the so-called universal leak test, a negative-pressure test that checks for leaks in the LPS regardless of whether an outlet check valve is present. When compared to several other LPS leak tests performed on machines with and without outlet check valves, the universal leak test was found to be the most sensitive. This simple-to-perform yet often neglected test requires that the machine be turned off and that the flow control valves be fully closed to prevent any flow of gas into the LPS. A specially configured suction bulb, which can either be constructed or obtained from the manufacturer, is then connected to the common gas outlet via tubing and a 15-mm adapter ( Fig. 25.11 , see also Fig. 25.9 ). The bulb is then squeezed repeatedly until it is fully collapsed. If the bulb does not stay collapsed for a specified period of time, air is being sucked by the bulb into the machine via a leak that will allow gas to escape when the machine is pressurized. The same maneuver is carried out with each vaporizer opened in turn to check for associated leaks.

The specified period of bulb collapse varies by reference from 10 seconds in popular texts to 30 seconds in some workstations’ user manuals. , , Although small leaks may require more than 10 seconds for bulb reinflation, it is likely that the collapsed bulb will be noted to be steadily expanding before that time. The most important aspect about the universal negative-pressure leak test is that it eliminates any potential for error where an operator might mistakenly apply a positive-pressure leak test to a machine with an outlet check valve.
Many of the newer generation anesthesia machines do not have an accessible common gas outlet; therefore, manual LPS testing cannot be performed. These machines presumably test the integrity of the LPS via an automated checkout, but detailed descriptions are not universally available in machine users’ manuals. For example, in the Dräger Apollo user manual the leak test is described to check the integrity of the LPS, which should assess for proper mounting of the vaporizer apparatus. It is specifically stated that internal vaporizer leakage would not be tested and should be performed after filling or changing vaporizers. For those machines that still require a manual LPS leak test, the universal leak test can be applied unless clearly instructed otherwise by the manufacturer ( Table 25.2 ).
| Machine | Test Method |
|---|---|
| Datex-Ohmeda Aestiva™/5 | Manual: negative pressure or positive pressure a |
| Datex-Ohmeda Aisys™ with ACGO | Manual: negative pressure or positive pressure a |
| Datex-Ohmeda Aisys without ACGO | Automatic |
| Datex-Ohmeda S/5 Aespire | Manual: negative pressure or positive pressure a |
| Datex-Ohmeda ADU | Automatic |
| Datex-Ohmeda Avance™ with ACGO | Manual: negative pressure |
| Datex-Ohmeda Avance without ACGO | Automatic |
| Dräger Narkomed 2B | Manual: positive pressure |
| Dräger Narkomed M | Manual: positive pressure |
| Dräger Narkomed MRI | Manual: positive pressure |
| Dräger Fabius MRI | Automatic |
| Dräger Fabius Tiro | Automatic |
| Dräger Narkomed Julian | Automatic |
| Dräger Narkomed 6000 & 6400 | Automatic |
| Dräger Fabius GS | Automatic |
| Dräger Apollo | Automatic |
| Dräger Perseus | Automatic |
| Penlon Prima SP3 | Manual: positive pressure |
| Mindray (Datascope) AS 3000 | Automatic |
a ISO 5358 or BSI standard positive low-pressure leak test. Vaporizers may require manual opening to be checked for internal leaks.
Further complicating the picture, the universal leak test may no longer be applicable to some machines. This underscores the importance of developing a local PAC that indicates when LPS testing is necessary, and if so, the steps required on each specific machine to accomplish this important step. The 2008 guidelines recognize the time-consuming nature of LPS testing and suggest the assistance of technicians to maintain compliance.
Item 9: Test scavenging system function
Frequency : Daily
Responsible Parties : Provider or technician
A properly functioning scavenging system prevents room contamination by anesthetic gases. Proper function depends upon correct connections between the scavenging system and the anesthesia delivery system. These connections should be checked daily by a provider or technician. Depending upon the scavenging system design, proper function may also require that the vacuum level is adequate, which should also be confirmed daily. Some scavenging systems have mechanical positive and negative pressure relief valves. Positive and negative pressure relief is important to protect the patient circuit from pressure fluctuations related to the scavenging system. Proper checkout of the scavenging system should ensure that positive and negative pressure relief is functioning properly. Due to the complexity of checking for effective positive and negative pressure relief, and the variations in scavenging system design, a properly trained technician can facilitate this aspect of the checkout process .
A test of the scavenging system begins by checking the proper assembly and integrity of each component and connection within the system, including the gas-transfer tubes leading from the adjustable pressure-limiting (APL) valve and the ventilator relief valve to the scavenging interface. In the case of many modern machines, a single transfer tube may lead from a compact breathing system to the scavenger interface. The integrity of the vacuum tubing leading from the wall outlet to the scavenger interface should also be checked.
There exist two types of scavenging interface systems, open and closed. Closed scavenging systems are isolated from the environment by pressure relief valves, so the relationship between waste-gas flow, vacuum flow, and the size of the system’s reservoir bag determines the effectiveness of the gas elimination. Some closed systems may contain only a positive-pressure relief valve, which protects the breathing circuit from overpressurization (positive end-expiratory pressure [PEEP]) if obstruction occurs downstream from the scavenger interface. These systems rely on passive outflow of waste gas, not a central vacuum, and they do not require a reservoir bag ( Fig. 25.12 ). They are designed to vent into nonrecirculating heating, ventilation, and air conditioning (HVAC) systems or simply to the building’s exterior (See also Chapter 5 ).

More commonly, closed systems will incorporate positive- and negative-pressure relief valves, a reservoir bag, and active gas elimination via central vacuum ( Fig. 25.13 ). Negative-pressure relief valves within these systems prevent subatmospheric pressure from occurring within the patient breathing circuit as a result of the application of excessive suction. An adjustable needle valve regulates the waste-gas exhaust flow. Generally speaking, the scavenger suction on active systems should be adjusted so the reservoir bag is neither overinflated nor underinflated; rather, it should remain slightly inflated during routine use. Because the volume of gas being passed into the scavenging system varies, it may be necessary to adjust the needle valve. Given the diversity of breathing systems, this check serves as another instance in which users must consider manufacturer specified protocols when developing a local PAC.

The 1993 PAC prescribed a simple procedure for checking the scavenging system that eliminated several steps described in manufacturers’ users’ manuals. The first step is to ensure proper connections between the scavenging system and both the APL and ventilator relief valves. With all flow control valves turned off, the APL valve is opened, and the Y piece is occluded. The scavenger reservoir bag is allowed to collapse completely, and the breathing pressure gauge should be negligible (e.g., no lower than −1.0 cm H 2 O). This tests the integrity of negative-pressure relief, if a negative pressure–relief valve is present. Next, with the O 2 flush valve held open, the scavenger reservoir bag is allowed to fully distend. The breathing pressure gauge should read <10 cm H 2 O, which assesses the integrity of positive pressure relief. Some manufacturers recommend that the suction needle valve be turned off for this step.
Open scavenger systems are simpler to understand and use, and they are easier to check out. Open systems contain no valves and are open to the environment ( Fig. 25.14 ). The patient breathing circuit is much less likely to be subject to overpressure or negative pressure, assuming the conduits are patent, and adequate suction is present. As in the case of the active closed scavenger system, inadequate suction will result in waste anesthetic gases venting into the room. After ensuring that all gas-transfer tubes and suction lines are properly connected, the scavenger suction needle valve is adjusted to place the flowmeter bobbin between the indicator lines. A positive- and negative-pressure test is then conducted as described previously. As stated in the 2008 PAC guidelines, technician support can facilitate proper maintenance of the scavenging system.

Item 10: Calibrate, or verify calibration of, the oxygen monitor and check the low oxygen alarm
Frequency : Daily
Responsible Parties : Provider or technician
Continuous monitoring of the inspired oxygen concentration is the last line of defense against delivering hypoxic gas concentrations to the patient. The oxygen monitor is essential for detecting adulteration of the oxygen supply. Most oxygen monitors require calibration once daily, although some are self-calibrating. For self-calibrating oxygen monitors, they should be verified to read 21% when sampling room air. This is a step that is easily completed by a trained technician. When more than one oxygen monitor is present, the primary sensor that will be relied upon for oxygen monitoring should be checked. The low oxygen concentration alarm should also be checked at this time by setting the alarm above the measured oxygen concentration and confirming that an audible alarm signal is generated .
The importance of the oxygen monitor in preventing administration of a hypoxic gas mixture cannot be overstated. It is the only monitor positioned to detect oxygen delivery problems downstream from the flow control valves. All other oxygen-related safety devices are located upstream from the flow control valves. Traditionally, most machines have used a galvanic cell oxygen sensor located near the patient breathing circuit inspiratory valve. In this position, the sensor is exposed to the gas as it flows toward the patient after the fresh gas flow is introduced. These electrochemical devices have a finite life span that is inversely proportional to the amount of oxygen exposure. They are also vulnerable to drift; therefore, daily verification of calibration is recommended, with recalibration as necessary.
The procedure to verify 21% fraction of inspired oxygen (FiO 2 ) calibration is variable; some machines include a removable sensor housing ( Fig. 25.15A ) which should activate a low-oxygen alarm upon exposure to room air if the limit is set above 21% ( Fig 25.15B ). The steps involved in recalibrating the sensor to room air involve removing the sensor from the breathing circuit. After calibration verification or recalibration, the breathing system is flushed with 100% oxygen. This should result in an oxygen concentration reading of more than 90% ( Fig. 25.15C ).

Some newer generation anesthesia machines do not contain an in-circuit galvanic sensor. These machines rely on the sidestream sampling gas analyzer to measure FiO 2 . They typically employ paramagnetic O 2 sensors, which are less prone to drift and require less frequent calibration (see Chapter 8 ). Removal of the sample line can be done to assess accurate room air calibration, and flushing the system with 100% oxygen should result in a reading of >90%. This serves as another example of growing machine diversity and the importance of machine familiarity as it pertains to daily use and local PAC development.
Item 11: Verify carbon dioxide absorbent is not exhausted
Frequency: Prior to each use
Responsible Parties: Provider or technician
Proper function of a circle anesthesia system relies on the absorbent to remove carbon dioxide from rebreathed gas. Exhausted absorbent as indicated by the characteristic color change should be replaced. It is possible for absorbent material to lose the ability to absorb CO 2 , yet the characteristic color change may be absent or difficult to see. Some newer absorbents do change color when desiccated. Capnography should be used for every anesthetic and, when using a circle anesthesia system, rebreathing carbon dioxide as indicated by an inspired CO 2 concentration >0 can also indicate exhausted absorbent .
It is important for providers to know that absorbent color change is not as reliable as is the presence of inspired CO 2 on capnography in identifying exhausted absorbent. Absorbent “regeneration,” indicator deactivation, inner canister channeling, and coloration of the absorbent canister wall are examples of circumstances that can mislead the practitioner regarding the actual absorptive capacity. , Therefore a normal-appearing absorbent may be significantly degraded in its ability to remove CO 2 . However, it is not advised for providers to manually exercise (i.e., breathe in and out of) the breathing circuit and absorbent to assess the absorbent during pre-use checkout. Visual inspection must suffice.
In addition to the exhaustion of CO 2 absorptive capacity, absorbent desiccation is another potential hazard. Exposure of volatile anesthetics to desiccated carbon dioxide absorbents that contain sodium, potassium, or barium hydroxide may result in severe exothermic reactions and/or the production of toxic byproducts such as carbon monoxide. Currently no consistently reliable steps can be included in a PAC procedure to identify absorbent desiccation. However, certain scenarios increase the risk of absorbent desiccation, and if recognized these should prompt the provider performing the pre-use check to ensure that the absorbent is replaced. Prolonged fresh gas flow during periods of machine nonuse is thought to be the main factor associated with absorbent desiccation. Situations in which gas has been flowing for indeterminate periods—such as over a weekend, with an infrequently used remote-site machine, when gas flows are found during the daily pre-use check—should therefore prompt concern. In 2005 the Anesthesia Patient Safety Foundation (APSF) convened a Carbon Dioxide Absorbent Desiccation Safety Conference and published a consensus statement aimed at reducing the risk of adverse reactions associated with carbon dioxide absorbents ( Box 25.1 ). These recommendations should be referenced in developing a departmental risk-management strategy.
The APSF recommends use of carbon dioxide absorbents whose composition is such that exposure to volatile anesthetics does not result in significant degradation of the volatile anesthetic. The APSF further recommends that there should be institutional, hospital, and/or departmental policies regarding steps to prevent desiccation of the carbon dioxide absorbent, should conventional carbon dioxide absorbents be chosen that may degrade volatile anesthetics when absorbent desiccation occurs. In such circumstances of using absorbents that may degrade volatile anesthetics, conference attendees generally agreed that users could take the following steps, consistent with ECRI Institute recommendations:
Turn off all gas flow when the machine is not in use.
Change the absorbent regularly, on Monday morning for instance.
Change absorbent whenever the color change indicates exhaustion.
Change all absorbents, not just one canister in a two-canister system.
Change absorbent when uncertain of the state of hydration, such as if the fresh gas flow has been left on for an extensive or indeterminate period.
If compact canisters are used, consider changing them more frequently.
Item 12: Breathing system pressure and leak testing
Frequency: Prior to each use
Responsible Parties: Provider and technician
The breathing system pressure and leak test should be performed with the circuit configuration to be used during anesthetic delivery. If any components of the circuit are changed after this test is completed, the test should be performed again. Although the anesthesia provider should perform this test before each use, anesthesia technicians who replace and assemble circuits can also perform this check and add redundancy to this important checkout procedure. Proper testing will demonstrate that pressure can be developed in the breathing system during both manual and mechanical ventilation and that pressure can be relieved during manual ventilation by opening the APL valve. Automated testing is often implemented in the newer anesthesia delivery systems to evaluate the system for leaks and also to determine the compliance of the breathing system. The compliance value determined during this testing will be used to automatically adjust the volume delivered by the ventilator to maintain a constant volume delivery to the patient. It is important that the circuit configuration that is to be used be in place during the test .
It is not rare for either the disposable breathing circuit components or the fixed anesthesia machine components to leak; therefore, a leak check of the breathing system is of paramount importance. Traditionally, this test has been performed manually after an inspection of the breathing circuit, removal of the gas sampling line, and capping of the gas sampling line port. With the machine set in the “bag” or manual mode of ventilation, the gas flows are set to zero (or the minimal settings), the APL valve is closed, the patient Y-piece is occluded, and the breathing system is pressurized with the oxygen flush button to about 30 cm H 2 O ( Fig. 25.16 ). The circuit passes the leak test if it holds this pressure for at least 10 seconds. Some manufacturers may specify a low oxygen flow rate during the test. PAC developers should refer to the user’s manual in this regard. A decrease in pressure during the test should prompt a check of all plug-in, push-fit, and screw connectors and the seal of the absorbent canister along with a careful inspection of the disposable tubing.

A common location of a circuit leak is at the absorbent canister, and it is particularly important for the anesthesia provider to be wary after the absorbent has been changed, because obstructed disposable canisters (plastic wrapper still on) or incorrectly seated or poorly sealed reusable canisters (often an absorbent granule on the rubber gasket) probably constitute the most frequent anesthesia machine problem still occurring. In fact, some machines may pass the automated checkout despite lacking a CO 2 absorber altogether ( Fig. 25.17 ).

On many modern anesthesia machines, breathing circuit leak testing is an automated feature, although manual steps are still required for test preparation. Circuit compliance is often also automatically assessed during this phase to guide ventilator tidal volume delivery. It is therefore important that the test be performed with the circuit that will be used and, if expandable, at the length at which it will be used.
The APL valve can also be assessed at this time by opening it wide after the pressure test and ensuring that the breathing circuit pressure decreases rapidly to zero. A prompt pressure drop should occur regardless of APL valve design. Modern APL valves differ from the traditional variable-resistor valves in that they are designed to maintain a relatively stable circuit pressure through a range of fresh gas flows, living more up to the name “pressure limiting.” Like any mechanical device, however, this feature has been reported to fail.
The ability of a modern APL valve to maintain stable circuit pressure can be easily assessed, if deemed necessary, by setting the APL valve to 30 cm H 2 O, occluding the patient Y-piece in a manual mode of ventilation, increasing gas flow to approximately 5 L/min, and ensuring that the circuit pressure, once stable, remains within a range close to that set on the APL valve. This range may be specified in some users’ manuals and altogether absent in others. Depending on the manufacturer and model, this step may be a component of the automated check or require manual testing, underscoring the importance of a machine-specific PAC. Indeed, failure to deliver positive pressure ventilation due to incomplete closure of the APL valve has been discovered immediately following induction, despite passing an automated machine check.
Item 13: Verify that gas flows properly through the breathing circuit during both inspiration and exhalation
Frequency: Prior to each use
Responsible Parties: Provider and technician
Pressure and leak testing does not identify all obstructions in the breathing circuit or confirm proper function of the inspiratory and expiratory unidirectional valves. A test lung or second reservoir bag can be used to confirm that flow through the circuit is unimpeded. Complete testing includes both manual and mechanical ventilation. The presence of the unidirectional valves can be assessed visually during the PAC. Proper function of these valves cannot be visually assessed, since subtle valve incompetence may not be detected. Checkout procedures to identify valve incompetence that may not be visually obvious can be implemented but are typically too complex for daily testing. A trained technician can perform regular valve competence tests. Capnography should be used during every anesthetic, and the presence of carbon dioxide in the inspired gases can help to detect an incompetent valve.
The original 1986 FDA checklist recommended that the person checking the anesthesia machine inhale and exhale into the patient connector while observing the unidirectional valves for free gas flow in the correct direction and no flow in the opposite direction. While this is no longer recommended, leak testing can be accomplished by placing a “test lung” or an extra reservoir bag at the patient elbow. In the “bag” or manual mode of ventilation, the operator ventilates the artificial “lung” with the breathing circuit reservoir bag then actively “exhales” (squeezes) the test lung back to the breathing circuit reservoir bag in a to-and-fro motion ( Fig. 25.18 ). This is the so-called flow test . During inspiration, the inspiratory valve should open, and the expiratory valve should close, and vice versa for exhalation. It is important to note that leak testing does not reliably identify circuit obstruction or unidirectional valve malfunction. A major malfunction of a unidirectional valve can be visually assessed on older machines, although subtle valve leaks (reverse flow) may only be apparent via capnography during anesthesia or through formal machine evaluation. Obstruction to flow during the flow test manifests as a “tight” reservoir bag on inspiration, whereas expiratory limb obstructions cause impeded exhalation. Undetected circuit obstructions are particularly ominous and can manifest dramatically and sometimes immediately following induction.

It cannot be stated definitively that automated machine checks assess for unimpeded circuit flow. Although most users’ manuals for machines that perform automated aspects of the pre-use checkout describe a leak test function, none were identified that specifically describe a flow test. Some manuals recommend visual inspection of the valves, , but on many newer machines the valves may be completely hidden from view. All three anesthesia machines subjected to a simulated obstruction of the expiratory limb of a circuit using plastic wrapping detected an issue on automated testing, yet one allowed the user to proceed to patient care despite the error.
Recognizing the complexity of testing valve competency, the 2008 guidelines suggest that regular technician-performed tests in lieu of daily checks could be more practical to detect subtle issues and assure compliance. Capnography is essential for every anesthetic and may demonstrate the first signs of valve incompetency. ,
Item 14: Document completion of checkout procedures
Responsible Parties: Provider and technician
Each individual responsible for checkout procedures should document completion of these procedures. Documentation gives credit for completing the job and can be helpful if an adverse event should occur. Some automated checkout systems maintain an audit trail of completed checkout procedures that are dated and timed .
Documentation of completion of the anesthetic checkout procedure by providers should be contained within the anesthetic record. Currently, there is no guidance regarding where anesthesia or biomedical technician documentation of checkout procedures should occur.
Item 15: Confirm ventilator settings and evaluate readiness to deliver anesthesia care (anesthesia time out )
Frequency: Immediately prior to initiating the anesthetic
Responsible Parties: Provider
This step is intended to avoid errors due to production pressure or other sources of haste. The goal is to confirm that appropriate checks have been completed and that essential equipment is indeed available. The concept is analogous to the “time out” used to confirm patient identity and surgical site prior to incision. Improper ventilator settings can be harmful, especially if a small patient is following a much larger patient or vice versa. Pressure limit settings (when available) should be used to prevent excessive volume delivery from improper ventilator settings. Items to check: Monitors functional? Capnogram present? Oxygen saturation by pulse oximetry measured? Flowmeter and ventilator settings proper? Manual/ventilator switch set to manual? Vaporizer(s) adequately filled?
This last step serves as a recommended final preinduction checklist of the machine and other important items, including the application of essential monitors. It is a “pre-takeoff” checklist for anesthesia providers. Some providers rely on final check mnemonic devices such as the “MS MAIDS” checklist ( Box 25.2 ). Regardless of the configuration, a final checklist that verifies key safety items is just as important in anesthesia as it is in aviation.
Machine: The machine checkout is complete; the vaporizers are filled, closed, and set to “0”; all gas flow knobs are set to zero flow; the ventilator is set up for an upcoming patient.
Suction: Patient suction is adequate to clear the airway.
Monitors: All required monitors are present and ready to go.
Airway: Primary airway equipment and appropriate back-up equipment are ready to go.
IV: Lines, fluids, and associated equipment are ready to go.
Drugs: All necessary medications are available and are properly labeled.
Special Items: Any special or unique items required for the case are available and ready.
IV, Intravenous.
Although the 2008 Guidelines for Designing Pre-Anesthesia Checkout Procedures are comprehensive, there are several steps that were part of the 1986 or 1993 recommendations that do not appear in the current guideline yet are sometimes found within machine users’ manuals. The use of these steps should be based on local needs and/or requirements, because the 2008 recommendations are not restrictive or intended to be limiting. Some of these items have been mentioned previously:
Disconnecting the central oxygen supply line to assess the low oxygen supply pressure alarm and to purge the tank pressure gauges to zero
Inspecting the gas supply hoses for cracks or wear
Testing the flowmeters for smooth operation
Testing the proportioning system by attempting to create a hypoxic O 2 /N 2 O mixture
Other Anesthesiology societies have compiled their own recommendations for the PAC, either independently or derived from the ASA. These include the Association of Anaesthetists of Great Britain and Ireland (AAGBI), the Australian and New Zealand College of Anaesthetists (ANZCA), and the Kenya Society of Anaesthesiologists, amongst others. Recognizing that one checklist would be difficult to apply universally, these checklists are similar in scope and philosophy to that provided by the ASA. They are meant to apply to a wide variety of machines and include procedures to be performed at the beginning of the day and between subsequent cases. Key differences can be noted in Table 25.3 .
| Component | ASA a | AAGBI b | ANZCA c | KSA d |
|---|---|---|---|---|
| 1a) Auxiliary Oxygen Cylinder | Daily | Yes | Yes | Yes |
| 1b) Self Inflating Manual Ventilation Device | Each use | Each use | Yes | Yes |
| 2) Suction | Each use | Each use | Each use | Each use |
| 3) Power | Daily | Yes | Yes | Yes |
| 4a) Monitors | Each use | Yes | Each use | Yes |
| 4b) Alarms | Each use | Yes | Each use | Yes |
| 5) Spare Oxygen Cylinder Pressure | Daily | Yes | Yes | Yes |
| 6) Piped Gas Pressures | Daily | Yes, 400–500 kilopascals | Yes | Yes, 400–500 kilopascals |
| Piped Gas Connections | No | Yes, “Tug Test” | No | Yes, “Tug Test” |
| 7) Vaporizers | Each use | Each use | Each use | Each use |
| 8) Low Pressure Leak Test | Daily | Yes | Yes | Yes |
| 9) Scavenging System | Daily | Yes | Yes | Yes |
| 10a) Oxygen Monitor Calibration | Daily | No | No | No |
| 10b) Low FiO 2 Alarm | Daily | Weekly | Yes | Weekly |
| Flowmeters | No | Yes | Yes | Yes |
| 11) CO 2 Absorbent | Each use | Yes | Yes | Yes |
| 12) Breathing Pressure & Leak Test | Each use | Yes | Each use | Yes |
| APL Valve | No | Yes | No | Yes |
| 13) Circuit Flow | Each use | Each use | Each use | Each use |
| 14) Document Checkout Procedure | Each use | Each use | Each use | Each use |
| 15) Anesthesia Time Out | Each use | Each use | Each use | Each use |
| Proportioning N 2 O:O 2 | No | Yes | No | Yes |
a ASA: American Society of Anesthesiology, 2008
b AAGBI: The Association of Anaesthetists of Great Britain and Ireland, 2012
c ANZCA: Australian and New Zealand College of Anaesthetists, 2014
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here