Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lymphedema is the most dreaded complication of breast cancer surgery. It may result in functional, esthetic, and psychological problems, thereby affecting the quality of life of the breast cancer patient. It also predisposes patients to development of infections, decreased functional ability and range of motion, and potential development of malignant lymphangiosarcoma (Stewart-Treves syndrome). It adversely affects quality of life, job performance, and health care costs.
Lymphedema is the result of functional overload of the lymphatic system, in which the volume of lymph is greater than the transport capability of the lymphatic channels. The increase in oncotic pressure in the peripheral tissues due to accumulation of macromolecules leads to accumulation of fluid in the interstitial spaces. This protein-rich environment can lead to infection if the integument is broken by minor abrasion or trauma. Lymphedema can begin insidiously at variable periods after axillary treatment. The swelling can range from mild to seriously disabling enlargement of the arm. Research in this area has been limited because of the variable development over long periods of time and inconsistent subjective versus objective diagnostic definitions of lymphedema. Managing lymphedema has been viewed as less important than eradicating cancer and detecting recurrence. There is little in the literature about the prevalence of breast cancer–related lymphedema (BCRL), but Greene and associates estimated that it was 1 in 1000 patients. Incidence of lymphedema has been reported to be somewhere between 0% and 77% in the literature ( Table 76.1 ). The length of follow-up, type of measurement technique, and definition of lymphedema varies from study to study.
| Reference | Year | Follow-Up (months) | No. of Patients | Measurement | SLNB | ALND |
|---|---|---|---|---|---|---|
| Schrenk et al. | 2000 | 15 | 35, 35 | Not reported | 0 | 57 |
| Sener et al. | 2001 | 24 | 303, 117 | C >20% | 3 | 17 |
| Swenson et al. | 2002 | 12 | 169, 78 | Survey | 9 | 17 |
| Haid et al. | 2002 | >36 | 57, 140 | Subjective, CE | 4 | 27 |
| Blanchard et al. | 2003 | 29 | 894, 164 | Subjective | 6 | 34 |
| Schijven et al. | 2003 | 38%, 50% ≤12 | 180, 213 | Subjective | 1 | 7 |
| Ronka et al. | 2005 | 12 | 43, 40 | Subjective | 13 | 77 |
| ALMANAC | 2006 | 12 | 478, 403 | C | 5 | 13 |
| Francis et al. | 2006 | 12 | 26, 73 | Volume | 17 | 47 |
| ACOSOG Z0011 | 2007 | 12 | 445, 446 | C >2 cm (20%) | 6 | 11 |
| Swiss Multicenter | 2007 | 31, 30 | 449, 210 | C | 4 | 19 |
| McLaughlin et al. | 2008 | 60 | 600, 336 | C | 5 | 16 |
| NSABP B-32 | 2010 | 36 | 2008, 1975 | Volume (≥10%) | 8 | 14 |
| Sagen et al. | 2014 | 30 | 187, 204 | Volume | 3 | 17 |
| Sackey et al. | 2014 | 36 | 140, 280 | Volume | 20 | 45 |
Extent of surgery, patient age, obesity, infection, and adjuvant treatment have been reported to be risk factors associated with the development of lymphedema. The relationship among axillary metastases, use of tamoxifen, presence of preexisting health conditions, tumor size, and time course of the development of lymphedema has not been consistently reported in the literature.
In approximately 1622, anatomic dissections by Gasparo Aselli of Italy revealed mesenteric lacteals. In well-fed dogs, these vessels contained a milky white fluid that drained into a large mesenteric node. Later, the French anatomist Jean Pecquet recognized the thoracic duct and its importance in the understanding of the lymphatic system. In 1953 the Scandinavians Rudbeck and Bartholin were the first to use the term lymphatics, but the function of the lymphatics remained a mystery.
In the 18th century, those in William Hunter’s School of Anatomy in London studied the anatomy and physiology of the lymphatic system. The work, as summarized by Hunter, showed that “the lymphatic vessels are the absorbing vessels all over the body … and constitute one great and general system.” It is probable that Cruickshank (1786), disciple of William Hunter, was the first person to describe in detail the human breast lymphatics in a fresh cadaver of a woman who had died during childbirth.
In 1860 Virchow was one of the first to recognize the importance of the lymphatic system in cancer, believing that lymph nodes acted as a barrier to cancer spread. The radical surgical procedures developed shortly thereafter, including radical mastectomy, radical neck dissection, and abdominal perineal resection, were based on the concept that there was a stepwise progression of cancer spread from the lymph nodes to systemic areas. In fact, Sir Berkeley Moynihan (1865–1936) wrote: “The surgery of malignant disease is not the surgery of organs; it is the anatomy of the lymph node system.”
One of the first articles to describe chronic lymphedema as a complication was written by Matas in 1913. Matas stated the following:
By elephantiasis we mean a progressive histopathologic state or condition which is characterized by a chronic inflammatory fibromatosis or hypertrophy of the hypodermal and dermal connective tissue which is preceded by and associated with lymphatic and venous stasis, and may be caused by any obstruction or mechanical interference with the return flow of the lymphatic and venous currents .
Halsted later expounded on it with his article concerning “elephantiasis chirurgica.”
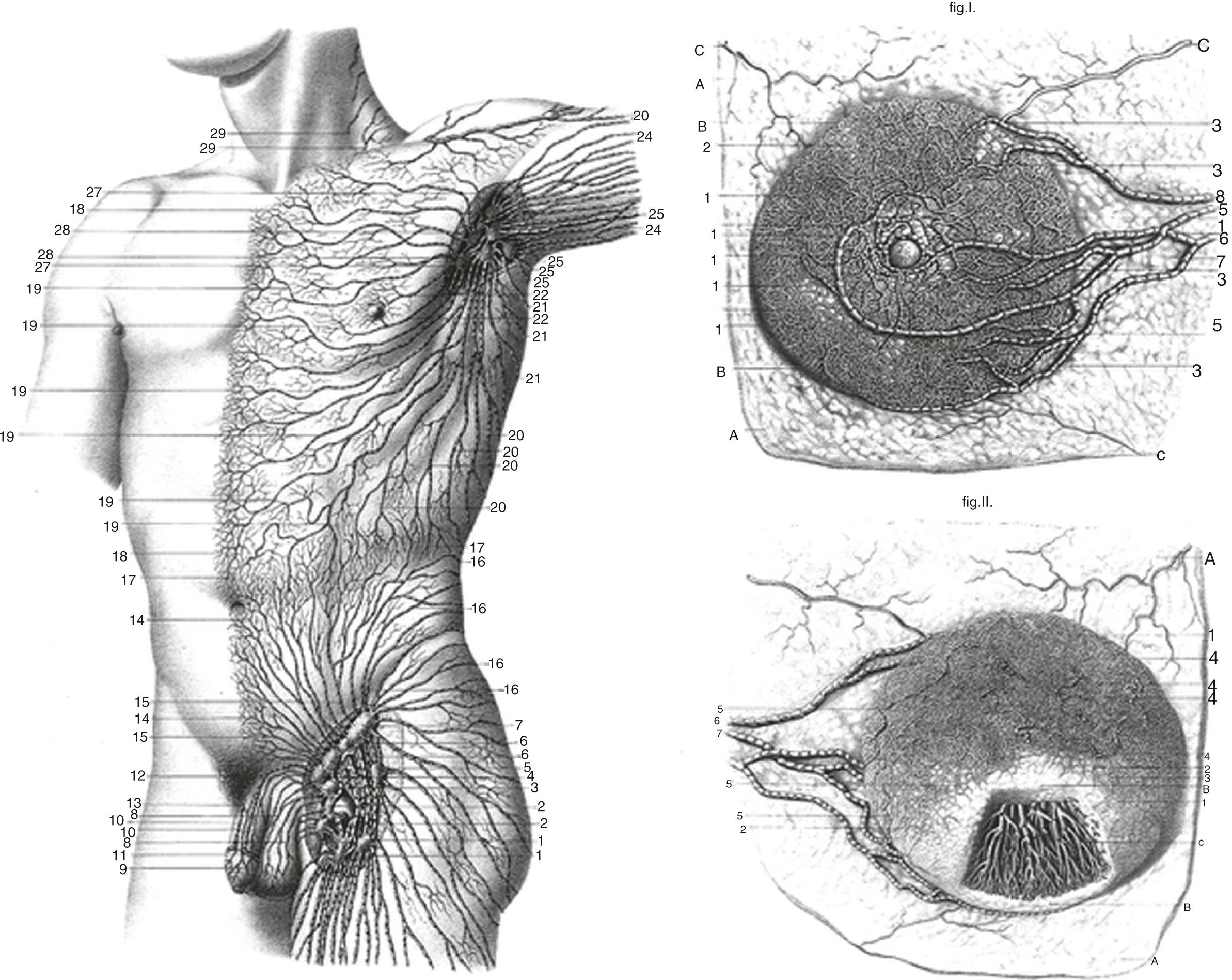
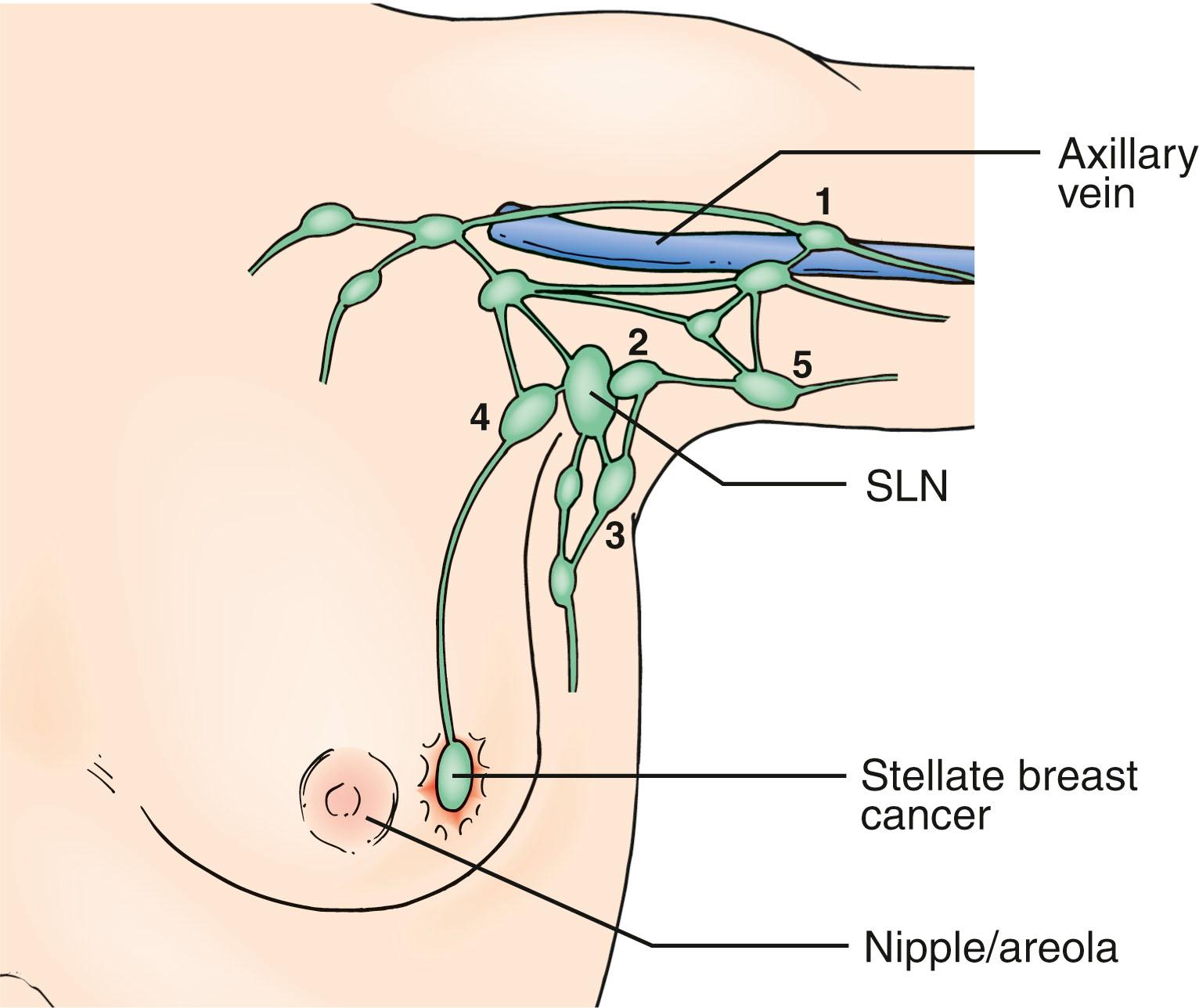
Current understanding of the lymphatic system of the breast is based primarily on the work of Sappey in the 1850s who showed that the upper torso drains from a subareolar plexus to the ipsilateral axilla. In 1903 Poirer and Cuneo summarized the results of Sappey, added their results from fetal studies using Gerota’s method of staining lymphatics with oil painting dye, and published a comprehensive book on the anatomy of the lymphatic system. Figs. 76.1 and 76.2 show the Poirer and Cuneo summary of breast drainage and Sappey’s findings. A recent study published by anatomist Ian Taylor’s group described the anatomy of the breast, and his findings are discordant with the current knowledge of the breast lymphatics. However, Dr. Taylor and colleagues described the lymphatics of the breast by cannulating the lymphatic vessels in cadavers and injecting radiopaque material containing lead oxide, with a repetition of the process until the first lymph node (sentinel lymph node [SLN]) for each collector was reached ( Fig. 76.3 ). The procedure was performed around the entire periphery of the specimen in the subcutaneous plane and around the internal thoracic artery in the vicinity of the perforating branches of the internal thoracic. Blue dye and hydrogen peroxide were used to stain the lymphatic vessels from the nipple and areola. The lymphatic collectors were traced to the first-tier node and color-coded, and all collectors draining to the same first-tier node were given the same color (see Fig. 76.3 ).
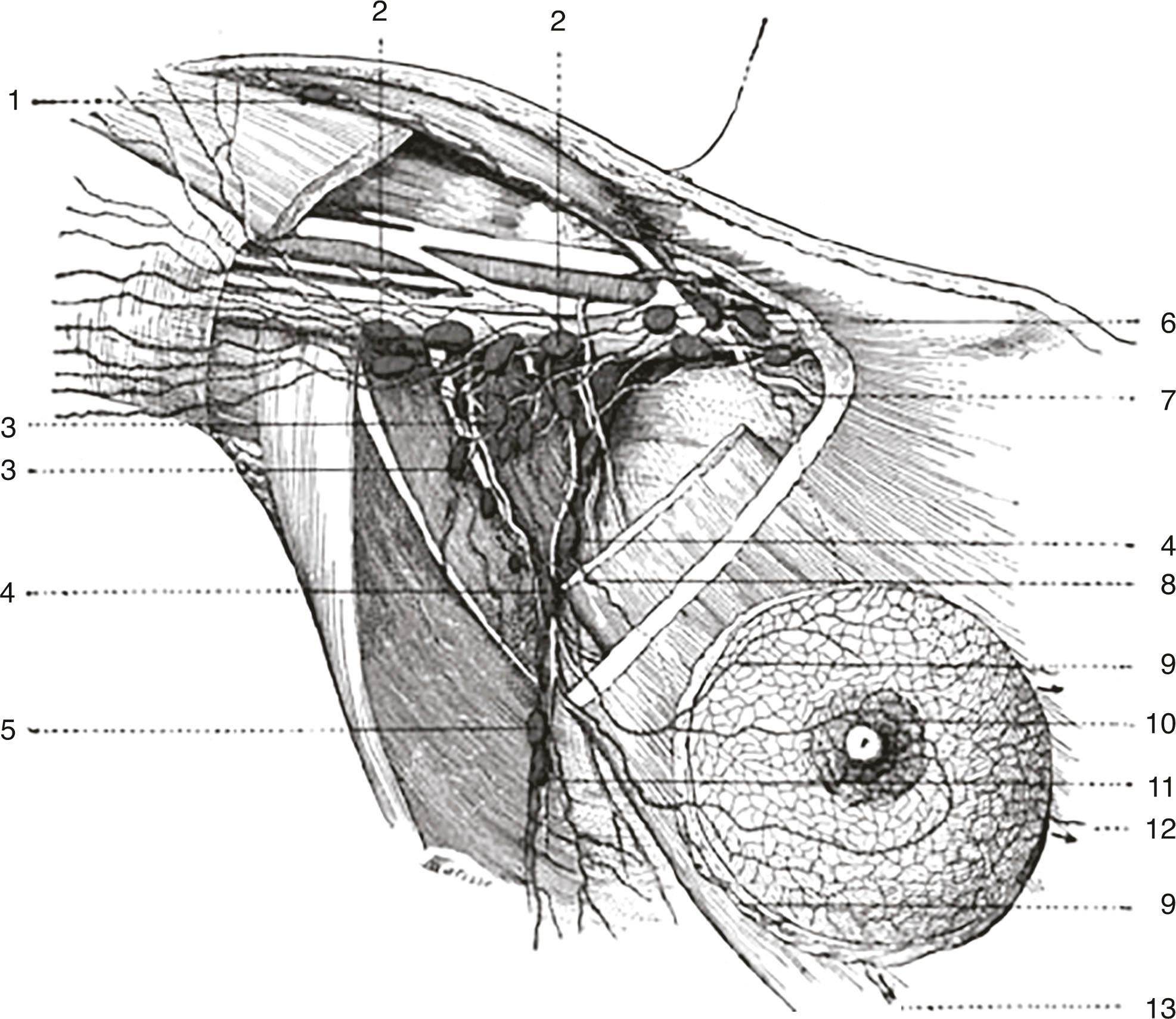
The lymphatics were identified to branch in the peripheral region and then join to form large collectors that remained uniform in diameter until they reached the SLN. All superficial lymphatics entered a lymph node in the axilla close to the lateral edge of the pectoralis minor muscle. Lymphatics that passed over or through the breast ended by draining into the same first-tier lymph nodes. Lymphatics in the areolar region were found to be a dense network of lymph capillaries and precollectors in the dermis ( Fig. 76.4 ). In the breast region, most of the lymphatics ran between the dermis and the breast tissue and traversed the breast tissue to reach axillary lymph nodes.
The internal mammary lymphatics were identified along the internal mammary vessels and lymph nodes identified in the intercostals spaces. No connections between collecting lymphatics along the branches of the internal mammary artery and superficial lymphatics were identified; the superficial lymphatics passed laterally toward the axilla.
The observation that the lymphatics pass through the breast tissue or superficial to it is also supported by the studies done by Turner-Warwick, who demonstrated a direct pathway from the injection site to the axillary lymph node.
The conclusions were as follows:
Lymph vessels passing through the breast contribute to breast drainage.
If the tracer was injected deeply it reached two sets of nodes, as opposed to when it was injected subareolarly or intradermally, in which case it reached one node only. This difference explains the false-negative rate of sentinel node biopsy, because more than one sentinel node can drain the breast.
Another anatomic explanation for false-negative results after sentinel node biopsy is the variation in the distribution of the perforating lymphatics. The perforating system is connected to the deep lymphatic vessels. In all quadrants of the breast, and especially for deep and medial tumors, cancer can spread via internal mammary lymphatics, and intradermal injection does not demonstrate the internal mammary lymphatics.
No evidence of a centripetal anatomic lymphatic pathway draining the breast tissue toward the subareolar plexus and then toward the sentinel node was identified.
Accurate lymphatic mapping requires intraparenchymal injection.
Most but not all lymphatics from the upper limb drain into lymph nodes in the axilla. Some can bypass the axilla and drain directly into the subclavian vein. In addition, axillary nodes receive drainage from an extensive area on the adjacent trunk, which includes regions of the upper back and shoulder, the lower neck, the chest, and the upper anterolateral abdominal wall. Axillary nodes also receive drainage from approximately 75% of the mammary gland. The 20 to 30 axillary nodes are generally divided into five groups on the basis of location ( Fig. 76.5 ):
Humeral nodes are found posteromedial to the axillary vein above the second intercostal nerve and receive most of the lymphatic drainage from the upper limb.
Pectoral nodes occur along the inferior margin of the pectoralis minor muscle along the course of the lateral thoracic vessels and receive drainage from the abdominal wall, the chest, and the mammary gland.
Subscapular nodes on the posterior axillary wall in association with the subscapular vessels drain the posterior axillary wall and receive lymphatic drainage from the back, the shoulder, and the neck.
Central nodes are embedded in axillary fat and receive lymphatic drainage from humeral, subscapular, and pectoral groups of nodes.
Apical nodes are the most superior group of nodes in the axilla and drain all other groups of nodes in the region. In addition, they receive lymphatic vessels that accompany the cephalic vein as well as vessels that drain the superior region of the mammary gland.
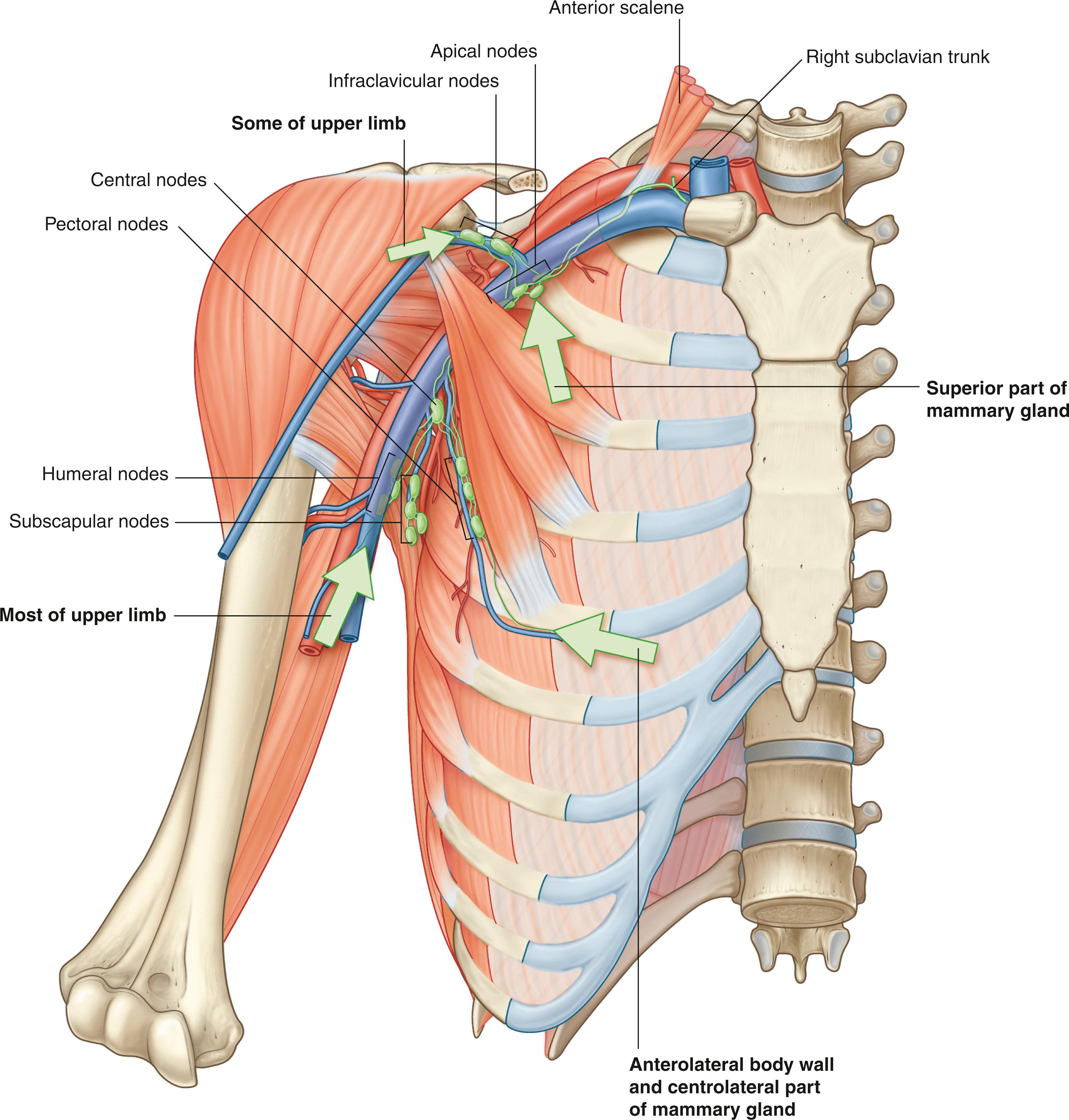
Klimberg and colleagues first described the drainage of the arm within the axilla using a technique called axillary reverse mapping (ARM). Variations in arm drainage were seen, including the traditional teaching of lymphatics from the arm running just beneath the axillary vein either above or below; a sling low in the axilla as much as 3 to 4 cm below the vein; and a lateral or medial apron of nodes that could be separated from the underlying axilla proper and then a cord of lymphatics or even a chain of nodes that came across low in the axilla. Our group demonstrated that, the majority of the time, these lymphatics could be separated from the lymphatics draining the breast within the axilla as well as major variations of that drainage ( Fig. 76.6 ). Efferent vessels from the apical group converge to form the subclavian trunk, which usually joins the venous system at the junction between the right subclavian vein and the right internal jugular vein in the neck. On the left, the subclavian trunk usually joins the thoracic duct in the base of the neck.
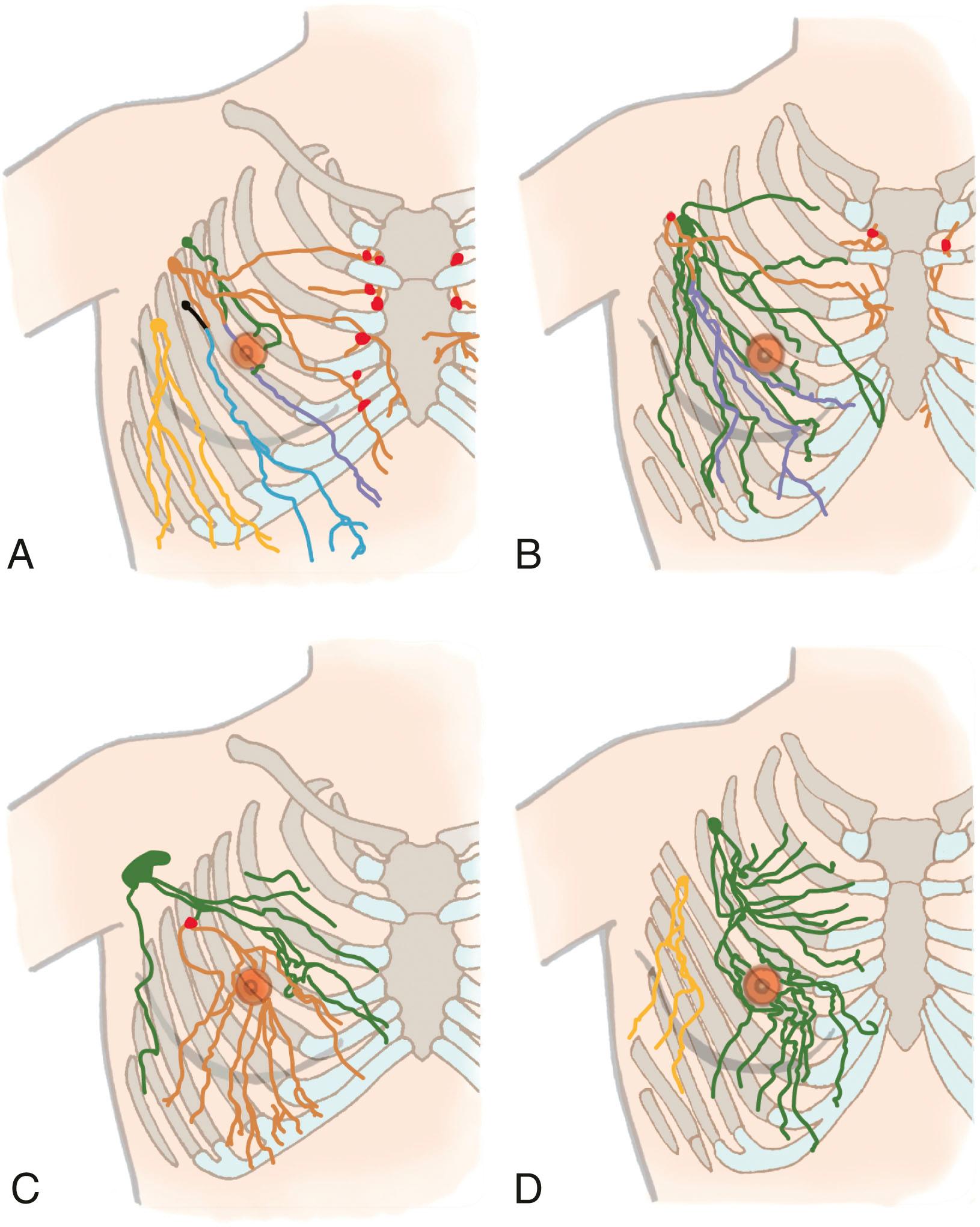
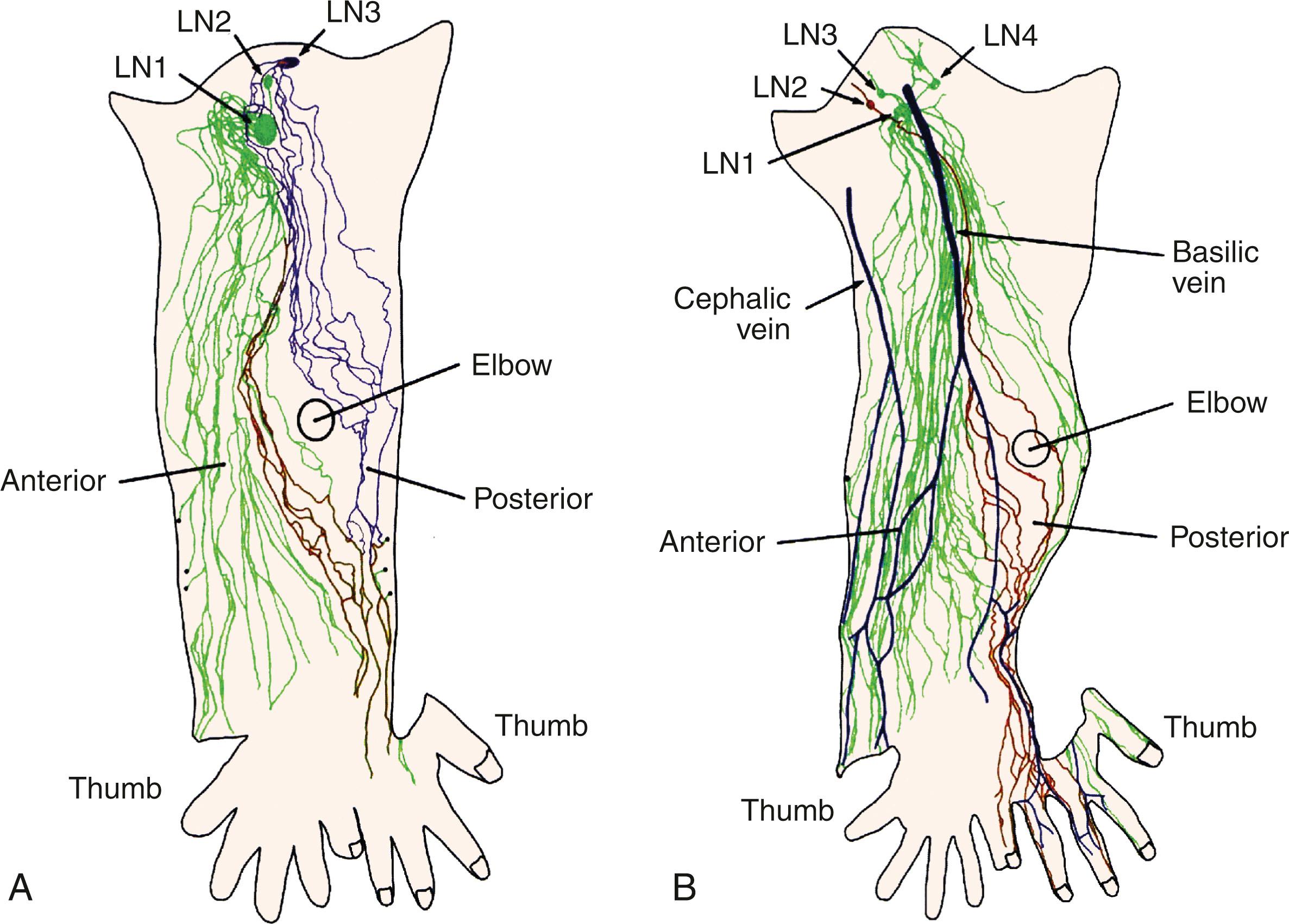
The lymphatics of the upper limb drain directly into a terminal group of lymph nodes in the axilla either directly or passing through a group of lymph nodes. Lymphatics deep to the fascia follow the neurovascular bundles, whereas the superficial lymphatics follow the superficial veins with the exception of the lymphatics of the hand and back of the forearm. Lymphatics from the hand enter the forearm along the cephalic and basilic vein, whereas the deep lymphatics follow the radial, ulnar, interosseous, and brachial neurovascular bundles and end at the lateral or the humeral group of lymph nodes. In some patients, lymphatics accompany the axillary vein. The superficial lymphatics are more numerous, and they appear to communicate with the deep vessel lymphatics along their course in the arm. More recent reports by Suami and Taylor did not identify a communication between the superficial and the deep system. They also reported that most lymphatics from the arm drain into one main lymph node in the axillary region, that some lymphatics run along the posterior arm and bypass the main node and enter other smaller nodes, and that there can be interconnections between these nodes ( Figs. 76.7 and 76.8 ). However, a later report published by Suami and Taylor pointed out that the lymphatics in the limb from the mastectomy side were different compared with the normal arm. They noticed a complete absence of the superficial lymphatic system proximal to the elbow because of blockage of lymphatic channels and identified a circuitous pathway that bypassed these lymphatics to reach the deep system facilitated by backflow through the precollectors and lymph capillaries in the dermis of the forearm. They concluded that previously undetected lymph channels between the superficial and the deep system open due to blockage of the superficial system caused by axillary dissection and reported that these channels may be helpful in prevention of lymphedema.
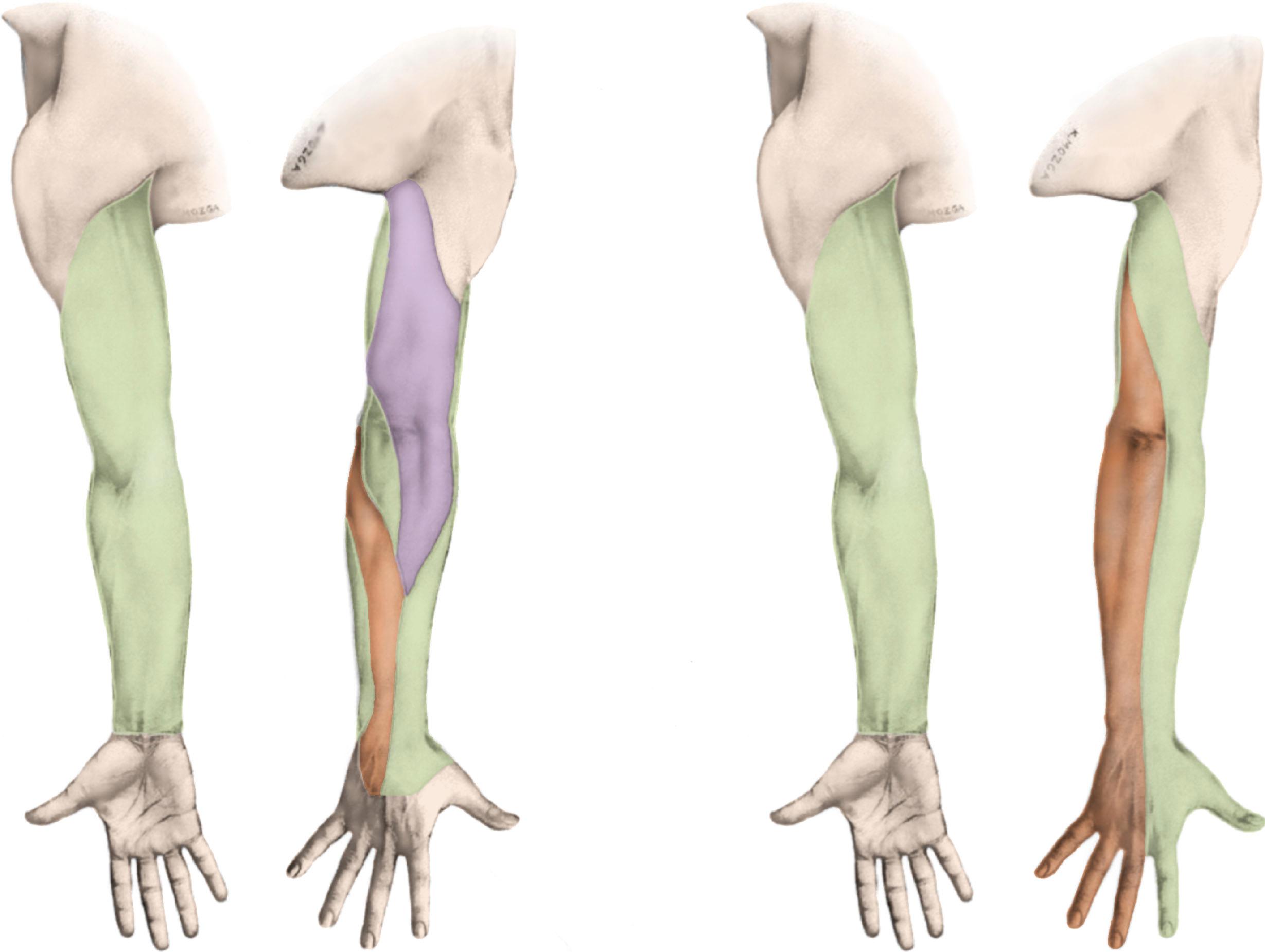
The lymphatic system develops during the embryologic phase as part of the vascular system. The components of the lymphatic fluid include endothelial cells, protein, water, tissue products, and other foreign particles. It is believed that the mammalian lymphatic system evolves from the fusion of clefts in the perivenous mesoderm layers.
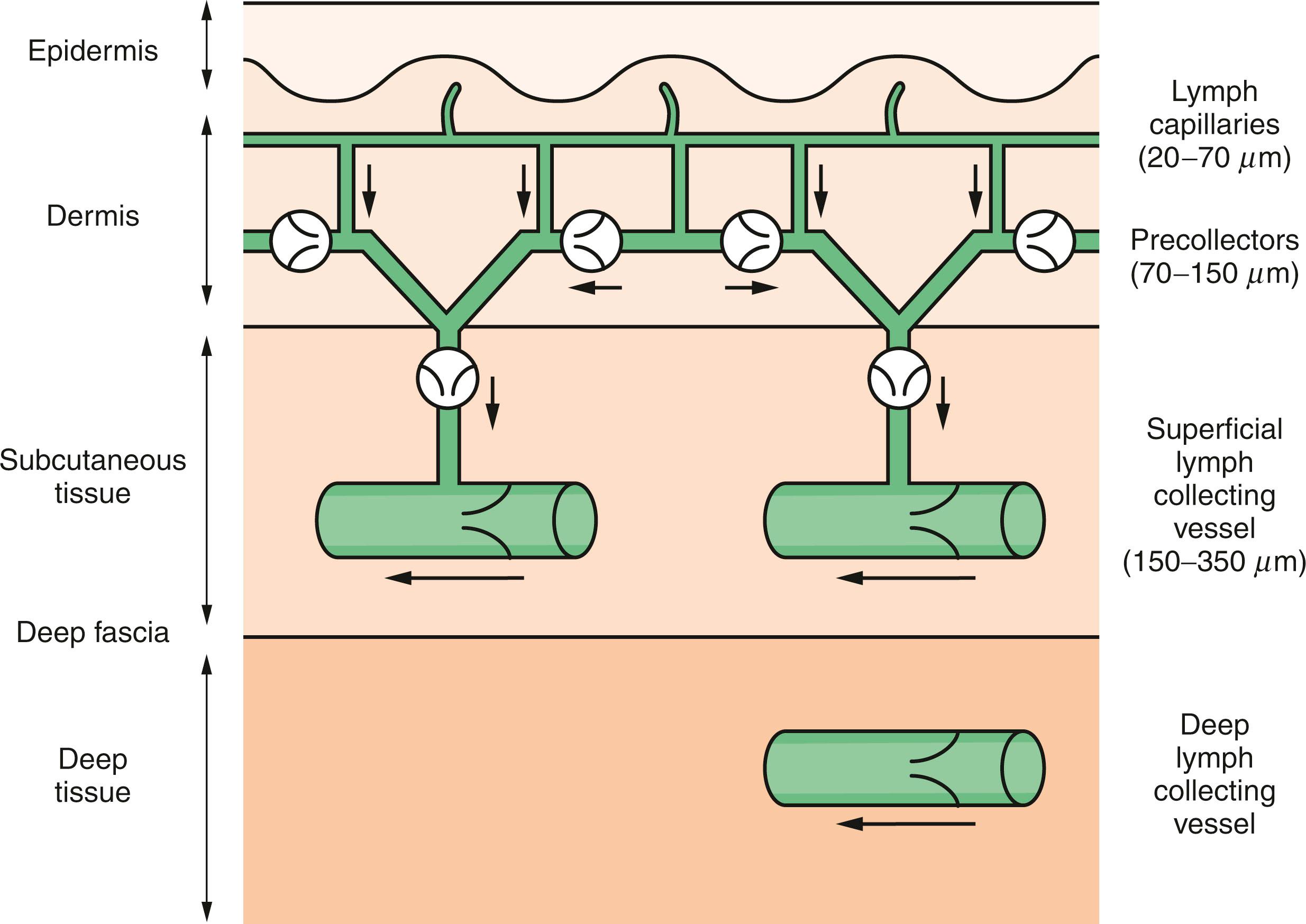
The lymph capillaries form a network more extensive than that of blood capillaries. In some tissues, such as the skin, gastrointestinal tract, and lung, the lymphatics can be quite extensive. Other organs, such as the bone marrow and brain, do not contain any lymphatics. In the breast, the lymphatics may penetrate the pectoralis fascia when coursing to the axilla. The lymph capillaries, which begin as closed saccules, consist of a single layer of endothelium without gaps. Unlike blood vessels, the lymphatic vessels have an absent or poorly developed basement membrane. This facilitates intercellular movement of plasma proteins and lipids that are too large for venous absorption. Lymphatics reabsorb the fluid exuded by the blood capillaries, but they are also capable of taking up material that is quite large to drain into the collecting lymph vessels. Normally, the pressure in the lymphatic vessels is negative or 0 mm H 2 O. After an axillary dissection, intralymphatic pressure becomes positive, and lymphatic flow can be 10 times slower than normal.
The collecting lymph vessels are thin-walled structures with valves every 2 to 3 mm. The smaller collecting vessels have an inner coat of elastic longitudinal fibers and endothelial cells and an outer layer of connective tissue. The larger vessels have an additional middle smooth muscle coat, which aids transport of the fluid. These larger vessels coalesce and travel parallel with the veins to the draining lymph nodes.
In the fetus, the lymph nodes form after the various plexuses develop. Foci of lymphocytes begin to accumulate within a network of lymphatic capillaries and eventually form lymph nodes. The afferent lymphatic enters the outside of the node into the marginal sinus. This structure surrounds the node and has a delicate retinaculum that will trap particulate material, including metastatic cells. Thus the marginal sinus is the first site for metastases to be found. The efferent lymphatic vessel leaves the hilum of the node.
The normal function of the lymphatics is to return proteins, lipids, and water from the interstitium to the intravascular space. High hydrostatic pressures in arterial capillaries force proteinaceous fluid into the interstitium, resulting in increased interstitial oncotic pressure that draws in additional water that then constitutes the lymph fluid. Before returning to the blood, stream lymph passes through lymph nodes, where its composition is altered. Interstitial fluid normally contributes to the nourishment of tissues. Approximately 90% of the fluid returns to the circulation via entry into venous capillaries. The remaining 10% is composed of high–molecular weight proteins and their oncotically associated water, which is too large to readily pass through venous capillary walls. The lymph then flows into the lymphatic capillaries, where pressures are typically low, which can accommodate the large size of the proteins and their accompanying water. The lymph fluid then passes through various lymph nodes to join the venous circulation.
When the lymphatic system is altered either as the result of infection, malignancy, or surgery, the lymphatic transport capacity is reduced. This leads to increased production of interstitial fluid that then exceeds the rate of lymphatic return, therefore leading to stagnation of high–molecular weight proteins in the interstitium. Compared with other forms of edema, this fluid contains a high concentration of proteins, whereas other forms of edema are secondary to hypoproteinemia. The high oncotic pressure in the interstitium favors the accumulation of additional water. The accumulation of protein and fluid is a transitory phase lasting 1 to 3 weeks. This then leads to latent phase, which can last from 4 months to 10 years. There may be no clinical signs and symptoms in the latent phase.
Accumulation of interstitial fluid leads to dilatation of the remaining outflow tracts and valvular incompetence that causes reversal of flow from subcutaneous tissues into the dermal plexus. Persistent swelling and stagnant protein eventually lead to fibrosis and provide an excellent culture medium for bacteria to grow, allowing for repeated bouts of cellulitis.
The lymphatic walls undergo fibrosis, and fibrinoid thrombi accumulate within the lumen, obliterating many of the remaining lymph channels. Spontaneous lymphovenous shunts may form. Lymph nodes harden and shrink, losing their normal architecture.
In the interstitium, protein and fluid accumulation initiates a marked inflammatory reaction that leads to increased macrophage activity and destruction of elastic fibers. Fibroblasts migrate into the interstitium and deposit collagen. As a result of this inflammatory reaction, there is a change from the initial pitting edema to the brawny nonpitting edema characteristic of lymphedema. Local immunologic mechanisms are suppressed, predisposing to chronic infections as well as to malignant degeneration of lymphangiosarcoma (Stewart-Treves syndrome).
The skin becomes thickened and gives the typical peau d’orange appearance of congested dermal lymphatics. The epidermis forms thick, scaly deposits of keratinized debris; cracks and furrows develop, leading to accumulation of bacteria and leakage of lymph from the surface of the skin. The resulting infection and inflammation further aggravate the edema. The clinical treatment of lymphedema is based on prevention of this vicious cycle.
This entire sequence of events develops in only a small percentage of patients after complete axillary lymph node dissection (ALND) or radiation therapy. The precipitating factors for the development of overt lymphedema are unknown. In patients where the lymphatics accompany the cephalic vein instead of the axillary vein, the incidence of lymphedema formation is reduced because in those cases the lymphatics bypass the axilla. Radiation therapy and repeated bouts of infection may be important factors in the development of lymphedema.
Lymphedema becomes clinically apparent when the compensatory mechanisms are inadequate to combat the requirements of lymphatic flow. The process of lymphatic regeneration may be altered in some patients. Another possible mechanism in lymphedema formation could be alterations in blood flow caused by either venous obstruction or increased arterial flow as a result of reflex sympathetic effects of neurologic injury associated with axillary or breast surgery (postmastectomy syndrome).
Six contributing factors have been shown to influence the incidence of brachial edema after treatment for breast cancer: radiation therapy, obesity, age, operative site, incision type, and history of infection. The increased incidence of post-ALND lymphedema formation in older versus younger women may be caused by the formation of lymphovenous anastomoses in younger women that can bridge the lymph and blood circulation systems but that are closed under normal circumstances. When increased pressure in the lymph system causes the lymphovenous anastomoses to open, this alternate route accomplishes lymph drainage. These lymphovenous anastomoses are much less common in older persons. The occurrence of postoperative wound complications, particularly infection, but also hematoma and/or seroma formation or flap necrosis, was identified as an independent risk factor for the development of lymphedema. The presence of axillary metastases and thus the extent of surgery has been found to be associated with increased risk of lymphedema formation by some groups.
Adding radiation therapy has been shown to increase the incidence of lymphedema from 20% to 52%. The incidence of lymphedema is lessened if transverse rather than oblique incisions are used in the axilla. Others have suggested that the extent of axillary dissection is an important contributing factor. Limiting the axillary dissection to level I and II nodes and preserving the level III nodes and lymphatic collateral channels around the shoulder may decrease the incidence of acute and chronic lymphedema. Larson and colleagues reported that the risk of lymphedema was 37% when full level I through III ALND was carried out, compared with 8% when a level I to II ALND was performed. They also found a correlation between the risk of lymphedema formation and the number of removed lymph nodes; when more than 10 lymph nodes were removed, the risk of edema was 28% versus 9% if 1 to 10 lymph nodes were found in the resected specimen. Petrek and associates have described post-treatment weight gain/obesity as a risk factor for lymphedema formation.
Recent adoption of lymphatic mapping and SLN biopsy (SLNB) for women with invasive breast cancer has minimized the risk of lymphedema while providing accurate staging of the axillary nodal basin. It is well documented that the histology of the SLNs reflects the histology of the remaining nodes in the basin. This approach limits the risk of lymphedema mainly to those with positive nodes (see Table 76.1 ).
Postsurgical BCRL is hypothesized to be caused by disruption of the lymphatics draining the arm. Lymphatics from the arm, back, and breast all drain to the same nodal basin under the arm. Such lymphatics are indistinguishable in appearance and for the most part in location. Scarring that occurs with surgery, chemotherapy, and radiation may attribute to this mechanical obstruction. However, primary lymphatic flow does vary with the individual patient and with variants of genes that may influence lymphedema, such as SOX18 , CCBE1 , GJC2 , GATA2 , KIF11 , and VEGFC , making an individual more at risk for lymphedema after surgical disruption of lymphatics.
The risk of lymphedema with ALND averages 28%, with a range that varies in the literature from 11% to 57% in larger trials. It varies over time depending on type of measurement and extent of surgery, but most lymphedema occurs by 3 years and may continue over a lifetime. The variation in lymphedema rates also reflects the great variability in the technique of axillary node dissection between surgeons, how closely lymphedema is monitored, length of follow-up, and, questionably, the number of positive lymph nodes, postoperative irradiation, extent of surgery, body habitus, and a number of other patient characteristics.
Although the lymphedema rate is much lower with SLNB, it is still clinically significant. Because so many more patients undergo SLNB, the actual number of patients who get lymphedema due to SLNB approximates the number that will get lymphedema after ALND. The incidence of lymphedema after SLN mapping averages 6.3%, with a range that has been reported to be between 0% and 23%. In the Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC) trial, lymphedema was observed in about 5% of breast cancer patients after SLNB. In the prospective international multicenter study organized by the American College of Surgeons Oncology Group (ACOSOG), trial Z0010, upper limb lymphedema (as determined by arm circumference measurements with a difference of 1 cm between arms) was observed in about 7% of patients with breast cancer after SLNB performed by a wide range of surgeons. The National Surgical Breast and Bowel Project (NSABP-32) that trained a cohort of surgeons in SLNB and randomized 5400 patients to SLNB followed by ALND versus SLNB alone demonstrated an 8% risk of BCRL with SLNB. Randomized controlled trials reported that SLNB compared with ALND leads to a significant reduction in postoperative complications (see Table 76.1 ).
However, the advent of SLNB does not solve the problem of BCRL, because just as many women get lymphedema from a negative SLNB as from an ALND. Few studies considered incidence, risk factors, and treatment costs of BCRL among working-age women after breast cancer treatment, reporting that the BCRL population had significantly higher rehabilitative medical costs ($14,877–$23,167) with twice as much risk of developing BCRL complications, such as lymphangitis or cellulitis, compared with the “BCRL-free” population (odds ratio = 2.02, P = 0.009).
Francis and colleagues described the applicability of standardized terminology from Common Terminology Criteria for Adverse Events (CTCAE; Table 76.2 ) to the incidence and severity of postoperative acute lymphedema during the first year after SLNB and ALND. According to this report, the incidence of lymphedema was 16.8% after SLNB and 47.1% after ALND. After SLNB, acute lymphedema of severity level I developed in 65% and severity level II in 35%. In the ALND group, the rate of severity level II lymphedema reached 60%.
| Limb | Level I | Level II | Level III | Level IV |
|---|---|---|---|---|
| Edema | 5%–10% interlimb discrepancy in volume or circumference | >10%–30% interlimb discrepancy in volume or circumference | >30% interlimb discrepancy in volume; lymphorrhea; gross deviation from normal anatomic contour; interferes with activities of daily living | Progression to malignancy (i.e., lymphangiosarcoma); amputation indicated; disabling |
a According to the National Cancer Institute, which has standardized reporting of adverse events in clinical trials.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here