Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lung organogenesis is organized into five stages, beginning at embryonic day 25 in humans.
The mechanisms of lung organogenesis, including branching morphogenesis, stretch/mechanotransduction, alveolarization, microvascular maturation, and cellular differentiation, depend on transcriptional regulation of cell–cell and cell–extracellular matrix signaling networks.
Alveolarization and lung growth are primarily postnatal processes that extend to early adulthood to establish a surface area that matches growing metabolic needs.
Disruption in the lung developmental program results in congenital lung malformations.
The transition from intrauterine to extrauterine life requires the maturation of the surfactant system and the development of lung defense systems against infection and environmental injury.
The primary function of the lung is to exchange oxygen for carbon dioxide to meet the demands of aerobic cellular respiration. The oxygen consumption of the adult human ranges from 250 mL/min at rest to 2630 mL/min at peak exercise. To accommodate these metabolic needs, a large surface area and a thin alveolocapillary membrane are required to enable efficient diffusion of oxygen, more so than carbon dioxide. Ultimately, the zone of gas exchange will attain a surface area of 50 to 100 m 2 and a volume of 2.5 to 3.0 L in the adult human. Therefore, the lung must develop in such a way to maximize the alveolar surface area to meet these needs. While a substantially large surface area is critical for oxygen uptake, the diffusion distance from the alveolus to the red blood cell must be relatively short to facilitate release of carbon dioxide. Meanwhile, a protective aqueous barrier protects the delicate alveolar epithelium, working in tandem with surfactant to mitigate surface tension and alveolar collapse. The result of normal lung development is a thin, expansive alveolar epithelial surface area intermingled with a well-approximated capillary network that facilitates the exchange of oxygen and carbon dioxide.
The trachea, airways, and alveoli are in constant contact with the external environment. Every inhalation brings large numbers of microorganisms, as well as potentially toxic particles and gases, into direct contact with epithelial surfaces. Lung organogenesis must also incorporate mechanisms for clearance of microorganisms and allergens that may result in epithelial infection or injury. Similarly, the lung must defend against nonparticulate gases that are potentially harmful. Oxygen, so critical to cellular function, can be the source of harmful reactive oxygen species that require detoxification, as do inhaled pollutants. The appropriate development and maintenance of these lung functions are critical to the health and survival of newborns.
Lung organogenesis begins early in human gestation (by day 25), and growth extends well into early adulthood. Lung development is organized into 5 sequential stages (embryonic, pseudoglandular, canalicular, saccular, and alveolar), although the timing of these stages is somewhat imprecise, and considerable overlap may occur. Fig. 38.1 shows a timeline of fetal and postnatal lung development in the mouse lung versus the human lung.
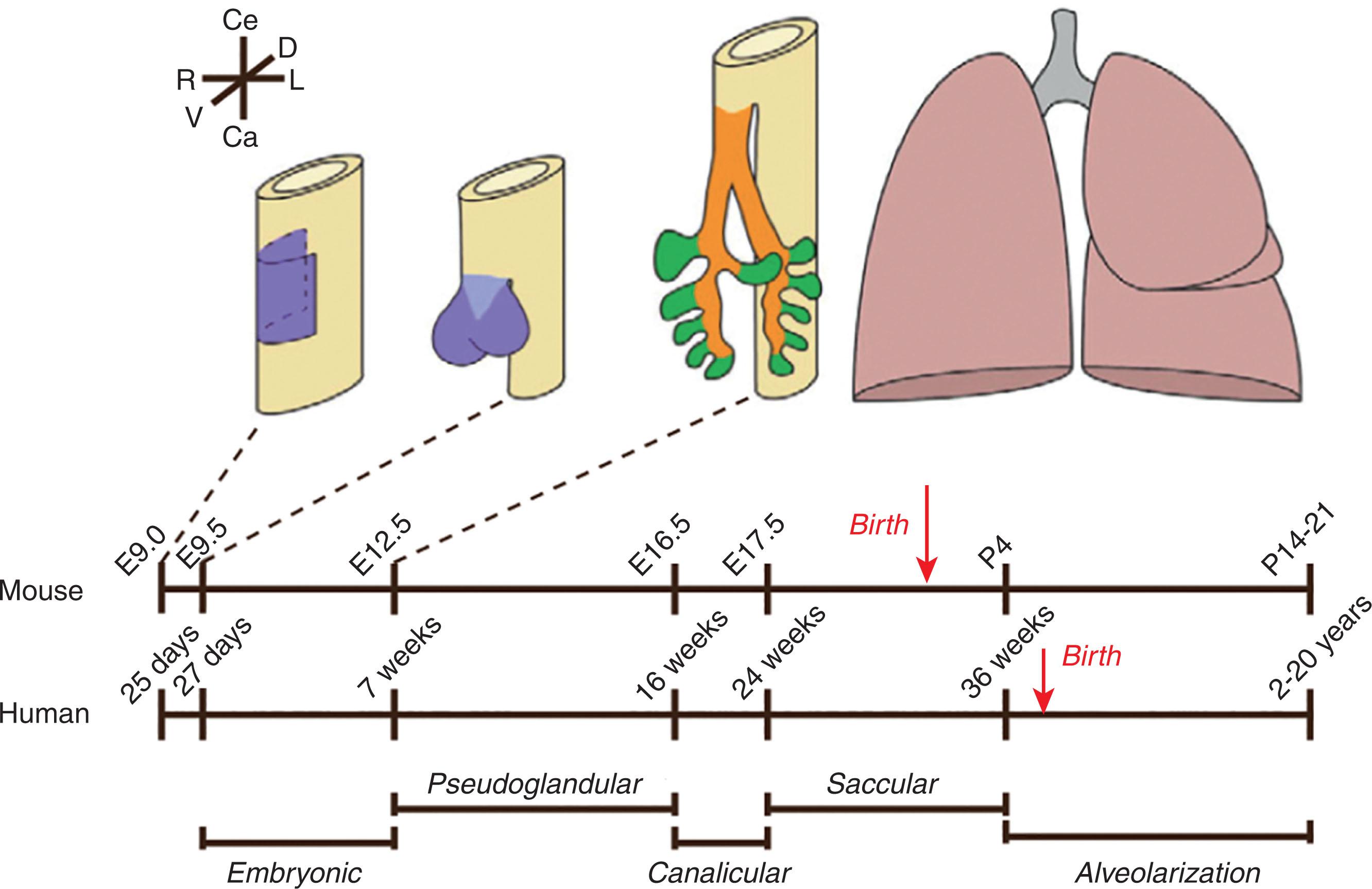
The initial phase of lung development, the embryonic phase , is marked by the formation of the lung bud and the initial branching events. The lung bud is first recognizable as a laryngotracheal groove of the ventral foregut at 25 days of human gestation. The lung bud consists of epithelium and surrounding mesenchyme and begins the first in a series of dichotomous divisions that give rise to the conducting airways and five primordial lung lobes (two on the left and three on the right in humans). Tracheoesophageal fistulae, tracheal atresia, and tracheal stenosis result from errors in separation of the laryngotracheal groove, whereas failure to form the initial branches can result in pulmonary agenesis, most typically of the right lung. Branching continues into the pseudoglandular stage of lung development. By 7 weeks of human gestation, the trachea, segmental bronchi, and subsegmental bronchi are evident. By the end of 16 weeks, all bronchial divisions are completed. It is important to remember that although the conducting airways will certainly enlarge as the fetus and newborn grow (airway diameter and length increase twofold to threefold between birth and adulthood), large airway branching ceases after 16 weeks of human gestation.
Closure of the pleuroperitoneal folds is a critical event of the pseudoglandular phase, reaching completion by 7 weeks and resulting in separation of the thoracic cavity from the peritoneal cavity. Failure to close the pleuroperitoneal folds results in congenital diaphragmatic hernia. Continuity is retained between these cavities, resulting in herniation of abdominal contents into the thorax when the midgut returns to the peritoneal cavity from the umbilical cord at 10 weeks of human gestation. The structural consequence of congenital diaphragmatic hernia is pulmonary hypoplasia of the lung ipsilateral to the diaphragmatic defect as the bowel and solid viscera migrate into the thorax. Pulmonary hypoplasia may also extend to the contralateral lung as the mediastinum shifts because of accumulating abdominal viscera in the thorax.
The canalicular phase is marked by completion of the conducting airways through the level of the terminal bronchioles. Terminal bronchioles give rise to the pulmonary acinus, the rudimentary gas exchange unit, which is in turn comprised of a respiratory bronchiole and all of its associated alveolar ducts and alveoli. A terminal bronchiole and all the distal acinar structures constitute a lobule. Branching of these distal air spaces continues on a more limited basis during the canalicular phase, finally achieving a total of 23 airway subdivisions.
The saccular phase of lung development (24 to 38 weeks of human gestation) refines the relationships between the air spaces, capillaries, and mesenchyme, enabling an alveolocapillary membrane sufficient to participate in gas exchange (0.6 μm) by approximately 24 weeks of human gestation. Beyond this point, the efficiency of gas exchange is determined by the available surface area. Lengthening and widening of the terminal sacs expand the gas-exchange surface area. Each saccule consists of smooth-walled air spaces with thickened interstitial spaces containing a double capillary network. These will give rise to two or three alveolar ducts, further expanding the available surface area. Expansion of these acinar structures continues well into the third trimester of human gestation.
Postnatal lung development can be subdivided into additional stages. True alveoli become evident as early as 36 weeks of gestation in the human fetus, initiating the alveolar phase of lung development. The development of primary alveoli is followed by a further expansion of the gas-exchange surface area through the formation of septa, or secondary crests (described further later). Postnatal alveolarization extends from term through 2 years of age. An initial phase of “ bulk alveolarization ” occurs within the first 6 months postnatally, with a more modest addition of secondary alveoli through the remainder of this period. The alveoli of the infant lung are different from adult alveoli. These immature secondary alveoli contain a double capillary bed, whereas adult alveoli are invested by a single capillary bed. Microvascular maturation , the next phase of postnatal lung development, occurs between the first few postnatal months of life through 3 years of age (discussed later).
There is considerable controversy regarding when the lung ceases to add alveoli. Estimates have ranged from as early as 2 years to as late as 20 years of age in humans. This is further complicated by the observation that alveolar expansion can occur in response to pneumonectomy in adult animals and humans. The acquisition of alveoli after the maturation of the microvasculature has been termed late alveolarization . This activity has been most often demonstrated in subpleural regions of the lung and likely invokes mechanisms similar to secondary crest formation.
The addition of alveoli is not the only means for expanding the surface area of the lung. While alveolarization wanes over the first 3 years of life in the human, growth of the lung continues to expand the gas-exchange surface area. Between 2 years of age and adulthood, lung tissue expands with lung volume proportionately to the increase in body weight of the child. From four years of age to adolescence, lung volume increases at a pace disproportionately greater than body weight despite a slowed rate of alveolarization, suggesting that increased size of alveoli makes a significant contribution to expanding lung volume in later childhood. Thus owing to the combined processes of prenatal lung development, postnatal lung development, and lung growth, there is tremendous potential for expansion of the gas-exchange surface area that is developmentally programmed into the fetal lung to account for the growing needs of the infant, child, and adult for aerobic cellular respiration. The extent to which these developmental mechanisms can be harnessed after premature birth, with or without superimposed lung injury, is a topic of active investigation that relies on extrapolation of experimental data from mice.
As branching morphogenesis proceeds, the airway and alveolar epithelium gives rise to specialized cells that participate in gas exchange, surfactant production, mucociliary clearance, detoxification, and host defense through a process called differentiation. Differentiation occurs in proximal air spaces first, then proceeding to distal air spaces, lagging behind branching. Temporal as well as contextual signals foster the regionalization of epithelial cell types.
The proximal airway epithelium is tall and columnar, decreasing in height to a more cuboidal appearance more distally. Four different epithelial cell types line the trachea and bronchi: undifferentiated columnar, ciliated, secretory/goblet, and basal cells. Undifferentiated columnar epithelial cells are joined by multi-ciliated cells between 11 and 16 weeks of human gestation. These cells are more prevalent in proximal airways and possess multiple motile cilia at the apical surface that beat in a coordinated fashion to clear mucous. Secretory cells can be seen as early as 13 weeks of human gestation and contain either mucous or serous granules, or both. The number of secretory cells with mucous granules peaks at midgestation during fetal lung development and declines in the third trimester relative to adulthood. Finally, immature basal cells expressing epidermal keratin have been noted as early as 12 weeks of human gestation. Basal cells play a critical role in regenerating injured large airway epithelium.
Cartilaginous support of the tracheobronchial tree begins and also proceeds in a centrifugal fashion beginning in the primitive trachea at 4 weeks, reaching the main bronchi by 10 weeks, and proceeding to the most distal terminal bronchioles by approximately 25 weeks of human gestation. Cartilaginous investment of airways is complete by the second month postnatally. Submucosal glands found between the cartilaginous tissue and surface epithelium play a major role in airway host defense. Submucosal gland development can be characterized by five stages: epithelial budding and invasion of the lamina propria, development of a lumen, initiation of tube branching, and repetitive dichotomous branching. The airways of infants and children contain relatively more submucous glands than those of adults. The glands are lined by mucous cells proximally and serous cells more distally, the latter constituting 60% of the total epithelial cell content of the glands. Serous cells secrete water, electrolytes, and proteins with antimicrobial, anti-inflammatory, and antioxidant properties, while the mucous cells produce primarily mucins. In addition to this host defense role, submucosal glands also contain a population of basal cells that respond to injury of the airway by replenishing the airway epithelium.
Muscular investment of the airways begins as early as 6 to 8 weeks of gestation as smooth muscle cells are identifiable around the trachea and large airways. Fetal airway smooth muscle is innervated and able to contract during the first trimester. Muscularization increases throughout fetal life and childhood such that there is an increased amount of smooth muscle relative to airway size when compared with adult airways. Moreover, the rapid increase in the amount of bronchial smooth muscle immediately after birth occurs regardless of the timing of delivery, term or preterm.
An additional airway cell deserves mention because of its association with a wide variety of pediatric diseases. Pulmonary neuroendocrine cells (PNECs) are found throughout the airways, often in innervated clusters known as pulmonary neuroepithelial bodies (NEBs) located at branch points in the bronchial tree. Although they arise from foregut endoderm, the cell of origin is distinct from other epithelial components of the lung. PNECs have large numbers of dense core vesicles containing neuropeptides, including serotonin and calcitonin gene–related peptide/bombesin, and regulate bronchial tone by releasing their contents in response to stretch- and hypoxia-mediated stimuli. Recent evidence suggests that PNECs also function as airway sensors that trigger immune responses. Pathologic conditions associated with PNEC/NEB hyperplasia include bronchopulmonary dysplasia (BPD), disorders of respiratory control (congenital central hypoventilation syndrome and sudden infant death syndrome), cystic fibrosis, chronic obstructive pulmonary disease, congenital diaphragmatic hernia, and pulmonary hypertension. Neuroendocrine hyperplasia of infancy is a rare form of interstitial lung disease of infancy associated with expansion of the number of PNECs and NEBs. Although the associations are strong, it remains unclear whether PNECs/NEBs play a primary role in these diseases or a responsive secondary role.
The bronchiolar epithelium differs from the more proximal airway epithelium. In addition to being more cuboidal in appearance, the epithelium contains progressively fewer ciliated cells and goblet cells, which are ultimately absent from the terminal bronchioles. Instead, the nonciliated, secretory club cell is found in increasing numbers and density down the conducting airways, such that the club cell is the most abundant cell of the terminal bronchiole. Club cells are first evident by 16 to 17 weeks of human gestation, initially exhibiting large glycogen stores that are replaced by secretory granules. Between 23 and 34 weeks of gestation there is a dramatic increase in club cell numbers in distal bronchioles. Club cells are critical to host defense and detoxification functions of the lung by producing high levels of cytochrome P450 and flavin monooxygenases in the lung. The club cell also plays an important role in immunoregulation in the distal airways. Important host defense products of the club cell include club cell secretory protein, surfactant protein A (SP-A), surfactant protein D (SP-D), leukocyte protease inhibitor, and a trypsin-like protease. Club cells produce a precursor form of surfactant protein B (SP-B) that may contribute to host defense. The secretion of antiproteases from club cells suggests that they modulate the protease–antiprotease balance in the distal part of the lung.
During the fourth through sixth month of human gestation the epithelial cells lining the acini begin to differentiate further. The cuboidal epithelial cells accumulate large glycogen stores and develop small vesicles containing loose lamellae. The large glycogen pools provide a ready source of substrate required for the production of increasing amounts of surfactant phospholipids, and they decrease in size as surfactant production advances in the fetal lung. In cells destined to become type 2 cells, lamellar bodies become larger, more numerous, and more densely packed with surfactant phospholipids and proteins, whereas those cells destined to become type 1 cells, on losing their prelamellar vesicles and becoming progressively thinner, adopt a phenotype more suitable for gas exchange. Type 1 and type 2 alveolar epithelial cells are readily identified early in the saccular stage of fetal lung development. There remains considerable controversy regarding the origin of type 1 epithelial cells. These cells in culture demonstrate very slow turnover, with a doubling time estimated to be between 40 and 120 days, suggesting that functionally they are terminally differentiated in vivo. Furthermore, in response to epithelial denudation occurring with lung injury, type 2 cells rapidly proliferate to reestablish epithelial continuity and then lose phenotypic features such as lamellar bodies and acquire markers of type 1 cells, suggesting that rapid repopulation of type 1 cells requires a type 2 cell intermediary. More recent studies in animals have suggested that alveolar type 1 cells can be induced to exit their terminally differentiated state and proliferate.
There is increasing appreciation for type 1 alveolar epithelial cells as more than just a passive membrane for gas exchange. While a large surface area and small cytoplasm-to-nucleus ratio provides a thin alveolocapillary membrane to facilitate gas exchange, it also provides a large absorptive surface in the lung. The presence of water and ion channels, some distinct from those in type 2 cells, facilitates the maintenance of a relatively dry alveolus. Type 1 cells may also regulate cell proliferation locally, signal macrophage accumulation, and modulate the functions of local peptides, proteases, and growth factors.
While most notable for its role in surfactant production (discussed later), the type 2 alveolar epithelial cell provides other important functions in the alveolus. Type 2 cells are local progenitor cells and, like type 1 cells, contain ion and water channels as well as ion pumps that contribute to the movement of water and ions across the epithelium. Type 2 cells also contain and secrete important antioxidants (superoxide dismutases 1, 2, 3 and glutathione) and molecules of innate host defense (SP-A, SP-D, and lysozyme) to participate in detoxification and sterilization of the alveolar microenvironment.
More recently, it is becoming clear that alveolar type 2 cells may play a part in exacerbating alveolar disease. The type 2 cell participates in the coagulation–fibrinolysis cascade through the production of fibrinogen, urokinase-type plasminogen activator, and tissue factor, especially under pathologic circumstances. Type 2 cells are increasingly recognized as a source of cytokine and chemokine production in the lung, as well as growth factors that can promote fibrosis. Finally, cross talk between epithelial cells, cell matrix, interstitial cells, and local inflammatory cells can foster the resolution of injury and inflammation or prolong lung remodeling after injury, with detrimental effects such as lung destruction and fibrosis. Therefore, while previously heralded as the defender of the alveolus, type 2 alveolar epithelial cells play a much more complex role in alveolar health and disease.
Pulmonary surfactant is essential for maintenance of alveolar health. The alveolar epithelial cells secrete a thin layer of liquid to protect the gas-exchange surface. The surface tension generated by this aqueous layer opposes alveolar inflation and promotes alveolar collapse at the end of expiration owing to the law of Laplace, whereby the collapsing pressure on the alveolus is directly proportional to the surface tension and inversely proportional to the radius of the alveolus. The film of pulmonary surfactant at the air–liquid interface lowers surface tension as the alveolar surface area decreases with exhalation, thereby preventing end-expiratory atelectasis, maintaining functional residual capacity, and lowering the force required for subsequent alveolar inflations.
Pulmonary surfactant is a complex mixture of phospholipids, neutral lipids, and proteins that is synthesized, packaged, and secreted by type 2 alveolar epithelial cells. The life cycle of surfactant is depicted in Fig. 38.2 . Storage of surfactant occurs in the lamellar body, a lysosome-derived membrane-bound organelle that undergoes regulated secretion in response to a variety of stimuli, including stretch. In the alveolus, surfactant phospholipids transition through an extracellular storage form, tubular myelin. Phospholipid and protein components are recycled out of the surfactant monolayer at the air–liquid interface and taken back into the alveolar type 2 cell, where they can be repackaged into lamellar bodies. Alternatively, alveolar macrophages are able to engulf and degrade surfactant components.

The predominant surfactant phospholipid is saturated dipalmitoyl phosphatidylcholine (DPPC), with the remaining phospholipids consisting of monounsaturated phosphatidylcholine, phosphatidylglycerol, and other phospholipids ( Table 38.1 ). DPPC is the only surface-active component of lung surfactant capable of lowering surface pressure to nearly zero. The presence of unsaturated phospholipids and other lipid components such as cholesterol enables the monolayer to remain fluid at body temperature during the respiratory cycle. Phospholipid content in the fetal lung increases with advancing gestation because of increased activity of enzymes responsible for phospholipid synthesis within type 2 cells. The expression and activity of enzymes of the choline incorporation pathway, the predominant pathway for surfactant phospholipid synthesis, are not only developmentally regulated but are also induced by hormones. The inductive hormones that have direct clinical relevance are glucocorticoids and agents that increase intracellular cyclic adenosine monophosphate (cAMP) levels such as the β-adrenergic agonist (and tocolytic) terbutaline.
| Component | Percentage (by Weight) |
|---|---|
|
92 |
|
41 |
|
25 |
|
9 |
|
4 |
|
8 |
|
5 |
|
10 |
|
8 |
Surfactant contains a group of specific proteins with importance for surfactant function and host defense. The four surfactant proteins, SP-A, SP-B, surfactant protein C (SP-C), and SP-D, are subdivided on the basis of their physical characteristics into either hydrophobic (SP-B and SP-C) or hydrophilic (SP-A and SP-D) proteins. The hydrophobic surfactant proteins play a major role in the surface-active properties of surfactant, whereas the primary roles of the hydrophilic surfactant proteins are in host defense, immunomodulation, and surfactant clearance and metabolism.
Together the hydrophobic proteins, SP-B and SP-C, facilitate the mobilization of surfactant phospholipid from tubular myelin to the surface monolayer, promote spreading of phospholipids in the surfactant film, and assist in film stability at the end of expiration. SP-B plays a central role in alveolar health because of its critical function in surfactant homeostasis. SP-B is a secretory protein that exhibits strong association with membranes, unlike SP-C, which contains a membrane-spanning domain and covalently attached fatty acids (palmitate) that render it integral to phospholipid membranes. Both SP-B and SP-C are synthesized as large precursor proproteins that undergo extensive posttranslational processing as they pass through the secretory pathway, ultimately reaching the lamellar body. SP-B is essential for the process of lamellar body formation, and the type 2 alveolar epithelial cells of infants with an inherited deficiency of SP-B are devoid of lamellar bodies. Because the lamellar body is where SP-C proteolytic processing is completed, infants with inherited deficiency of SP-B are also deficient in mature SP-C, instead accumulating a larger, nonfunctional precursor of SP-C. Thus, patients with inherited deficiency of SP-B, despite having relatively normal surfactant phospholipid profiles, make a pulmonary surfactant with very poor surface tension properties because of the combined defects in SP-B and SP-C. Conversely, because SP-C does not play either a direct or an indirect role in SP-B processing, animals with SP-C deficiency have normal SP-B, have normal lamellar bodies, and do not exhibit perinatal lethality due to surfactant dysfunction.
Like the enzymes of surfactant phospholipid production, SP-B and SP-C exhibit developmental and hormonal regulation of expression. In human fetuses, SP-C messenger ribonucleic acid (mRNA) is detected as early as 12 weeks of gestation and SP-B mRNA by 14 weeks, yet the mature proteins are not detectable in fetal lung tissue until after 24 weeks. SP-B protein is not detectable in amniotic fluid until after 30 weeks of gestation, the amount increasing toward term. This is due to developmental regulation of posttranslational events in the proteolytic processing of proSP-B and proSP-C. Consequently, infants delivered prematurely have reduced levels of both surface-active components of surfactant—phospholipid and hydrophobic surfactant proteins. The rate of type 2 cell differentiation, and secondarily surfactant production, is modulated by the levels of endogenous corticosteroids and can be accelerated by prenatal administration of glucocorticoid to women in preterm labor. The response of the surfactant system to prenatally administered glucocorticoids involves all key lipid and protein components, and occurs primarily through increased gene expression, thus representing precocious maturation mimicking the normal developmental pattern. Endogenous thyroid hormones, prostaglandins, and catecholamines also have stimulatory effects on type 2 cell maturation as well as on clearance of lung fluid at birth. Certain proinflammatory cytokines (e.g., tumor necrosis factor [TNF]-α and transforming growth factor [TGF]-β) inhibit surfactant production in experimental systems and may downregulate surfactant in conditions such as sepsis and inflammation.
The primary role of the pulmonary vasculature is to supply blood flow to the acini for gas exchange. During early fetal life the airways act as a template for pulmonary blood vessel development. The earliest pulmonary vessels form de novo in the tissue surrounding the lung bud in a process known as vasculogenesis . Mesodermal cells within the mesenchyme investing the developing lung tube differentiate into endothelial cells, proliferate, organize into chords, and develop a central lumen. As each new airway buds into the mesenchyme, a new plexus forms that adds to the pulmonary circulation, thereby extending the network of arteries and veins. By the fifth week of human gestation, a capillary network surrounds each bronchus, and circulation of blood between the right ventricle and the left atrium via this network is evident.
During the canalicular stage of lung development, new blood vessels form from preexisting vessels, a process known as angiogenesis . In contrast to vasculogenesis, angiogenesis is initiated by endothelial cell proliferation and sprouting from established vessels, resulting in extension of a vascular network into undervascularized regions. Vasculogenesis is the primary mode of pulmonary vascular development until the 17th week of gestation, when all preacinar airways and their accompanying vessels are present, whereas angiogenesis becomes the predominant mode of vascular development in the later stages of lung development. Although originally thought to be sequential processes, it is generally accepted that both occur concurrently during lung development, with angiogenesis dominating in the central part of the lung and vasculogenesis dominating in the periphery. Interconnections between vascular networks arising from both angiogenesis and vasculogenesis increase in the saccular phase of lung development.
In the human lung a second circulatory system, the bronchial circulation, arises from the dorsal aorta and nourishes the cellular constituents of the lung itself. The bronchial vasculature develops after the pulmonary circulation, with bronchial vessels first apparent by 8 weeks of gestation. The network of bronchial vessels is extensive, with bronchial arteries demonstrated as distal as the alveolar ducts in the adult respiratory tree.
Vasculogenesis and angiogenesis are the primary mechanisms of vascular development throughout intrauterine life. The human lung at term contains only a small portion of the adult number of alveoli, and the air space walls are represented by a thick “primary septum” consisting of a central layer of connective tissue surrounded by two capillary beds, each of them facing one alveolar surface. As alveolar architecture changes with the appearance of secondary septa, or secondary crests, folding of one of the two capillary layers occurs within the secondary septa. Microvascular maturation involves fusion of the juvenile double capillary network into a single capillary system present in the adult lung. Fusion is facilitated by the expansion of alveolar surface area and luminal volume, which compresses the interstitium, bringing the capillary networks in close proximity. This process is evident in the third postnatal week, during which lung volume increases by 25%, with a concomitant 27% decrease of the interstitial tissue volume. Subsequently, preferential growth of areas with a mature, fused capillary system continues.
Lastly, it is well known that lung volume increases about 23-fold between birth and young adulthood, while capillary volume expands 35-fold. It has been shown recently that this increase in capillary volume occurs by a third mechanism of vascular development: intussusceptive microvascular growth. This new concept in capillary network growth involves the formation of transluminal tissue pillars within capillaries that then expand, resulting in a net increase in capillary surface area.
Muscularization of the pulmonary arteries begins early in development. Initially the muscular investment of the vasculature is derived from the migration of bronchial smooth muscle cells from adjacent airways. Muscularization of the pulmonary arteries begins in the canalicular stage and continues through the remainder of gestation. Smooth muscle cells develop from the surrounding mesenchyme, altering their cellular shape and initiating expression of α-smooth muscle actin, a marker of their transformation into smooth muscle cells. A third phase of vascular muscularization, largely restricted to the distal part of the lung, involves the process of endothelial–mesenchymal transition, marked by endothelial cell division, separation and migration away from the endothelial layer, and expression of smooth muscle cell markers.
Muscularization of pulmonary arteries normally extends to the level of the terminal bronchiole and is minimal to absent in blood vessels surrounding respiratory bronchioles. Abnormal extension of smooth muscle along arterioles supplying acinar structures occurs in infants dying of persistent pulmonary hypertension of the newborn, as well as in infants with congenital diaphragmatic hernia and severe BPD.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here