Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
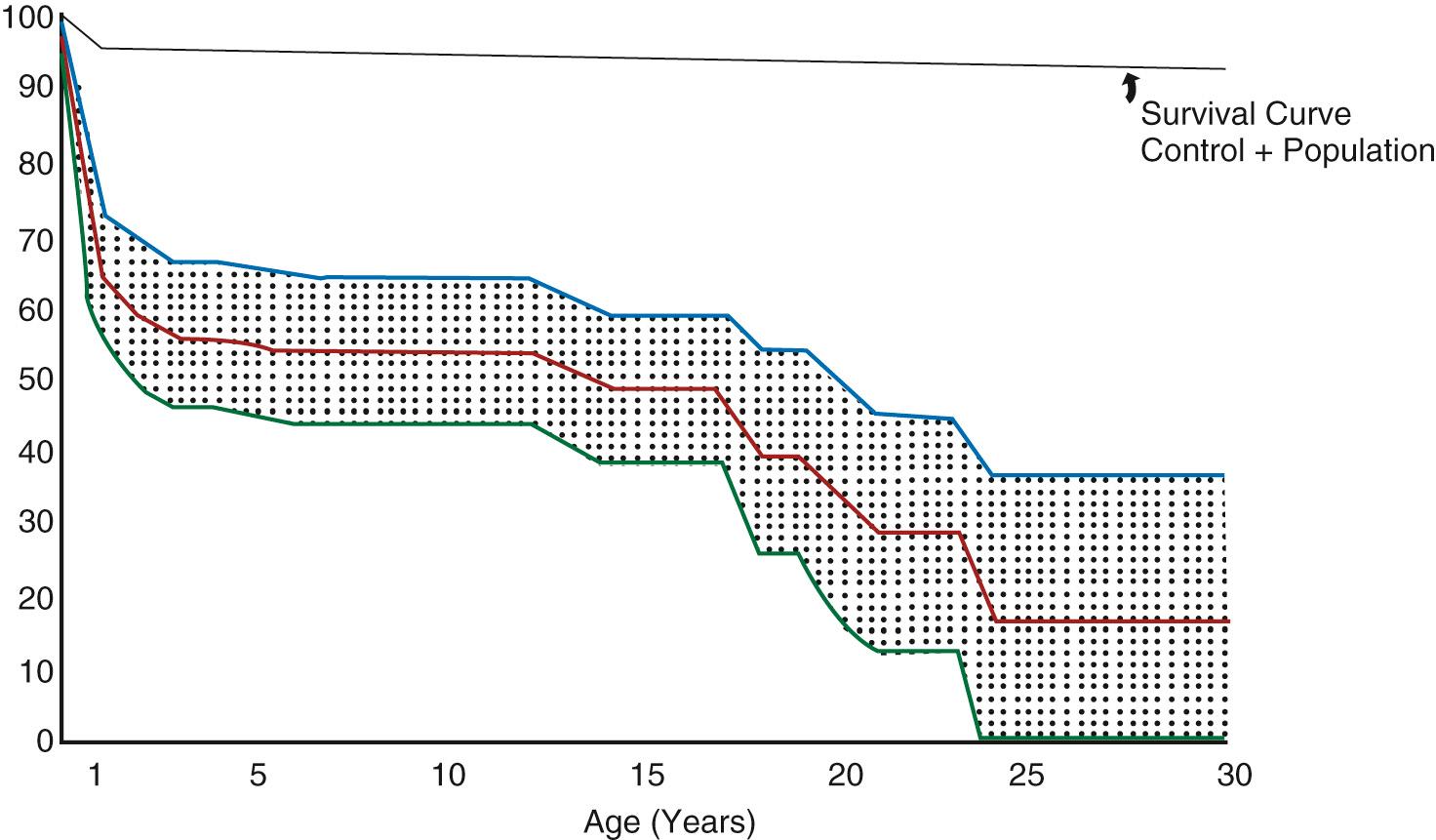
The Fontan operation is the treatment of choice for patients with a single anatomic or functional ventricular chamber. From the late 1940s, survivors with a functionally univentricular heart could be palliated with a systemic-to-pulmonary artery or Glenn shunt, but by the early 1970s, only 50% of those with tricuspid atresia—the most favorable form of functionally univentricular heart—survived 15 years ( Fig. 73.1 ). In that era, complications of cyanotic heart disease including stroke and cerebral abscess, and progressive ventricular failure and atrioventricular valve regurgitation due to chronic volume loading were the commonest causes of death.
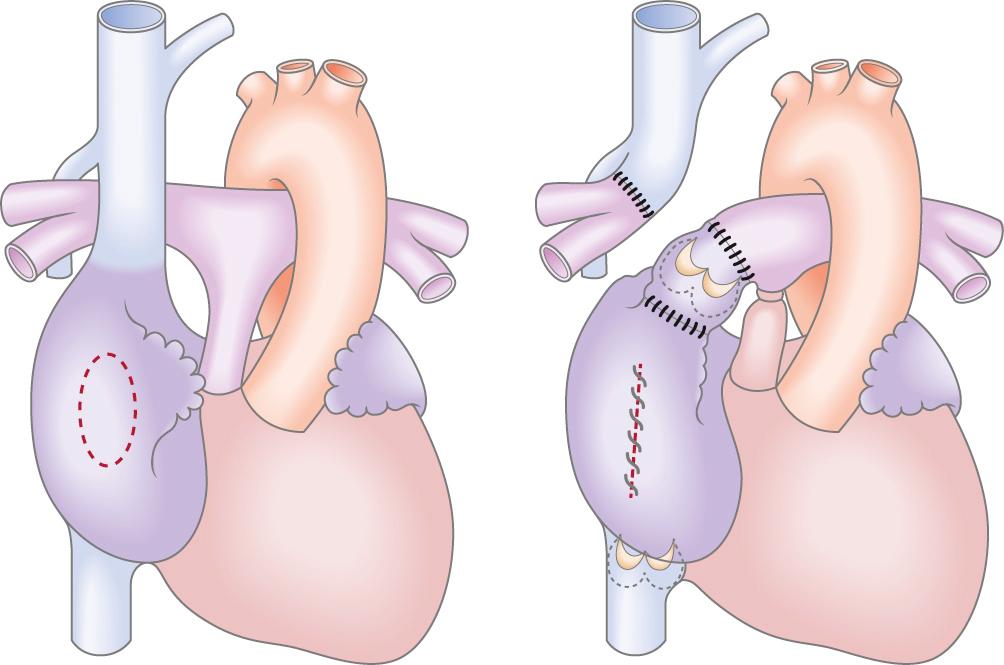
The understanding that blood could flow through the lungs without a subpulmonary ventricle led to development of the Fontan operation, first described by Fontan and Baudet as “corrective surgery” for tricuspid atresia in 1971. The original procedure consisted of division of the right pulmonary artery from the main pulmonary artery and anastomosis of the superior vena cava to the right pulmonary artery. The left pulmonary artery was then anastomosed via a homograft valve to the right atrium, and the main pulmonary artery was disconnected from the hypoplastic ventricle. The valve was used to promote the “ventriculization” of the right atrium so that it might generate sufficient pressure to augment pulmonary flow ( Fig. 73.2 ). The atrial septal defect was closed and a valve placed at the junction of the inferior vena cava and the right atrium to prevent retrograde flow during atrial contraction. Six months later, Dr. Guillermo Kreutzer and team carried out their first atriopulmonary Fontan anastomosis. This procedure included a 6-mm fenestration in the interatrial septum and as such was the first fenestrated Fontan. No valve was positioned in the venous circulation because it was thought that this would result in a degree of obstruction—a hypothesis that was proven correct because it soon became apparent that valves within the Fontan circulation were associated with a high risk of stenosis. This technique evolved to generate an atriopulmonary anastomosis as wide as possible without the use of a valved conduit ( Fig. 73.3 ).
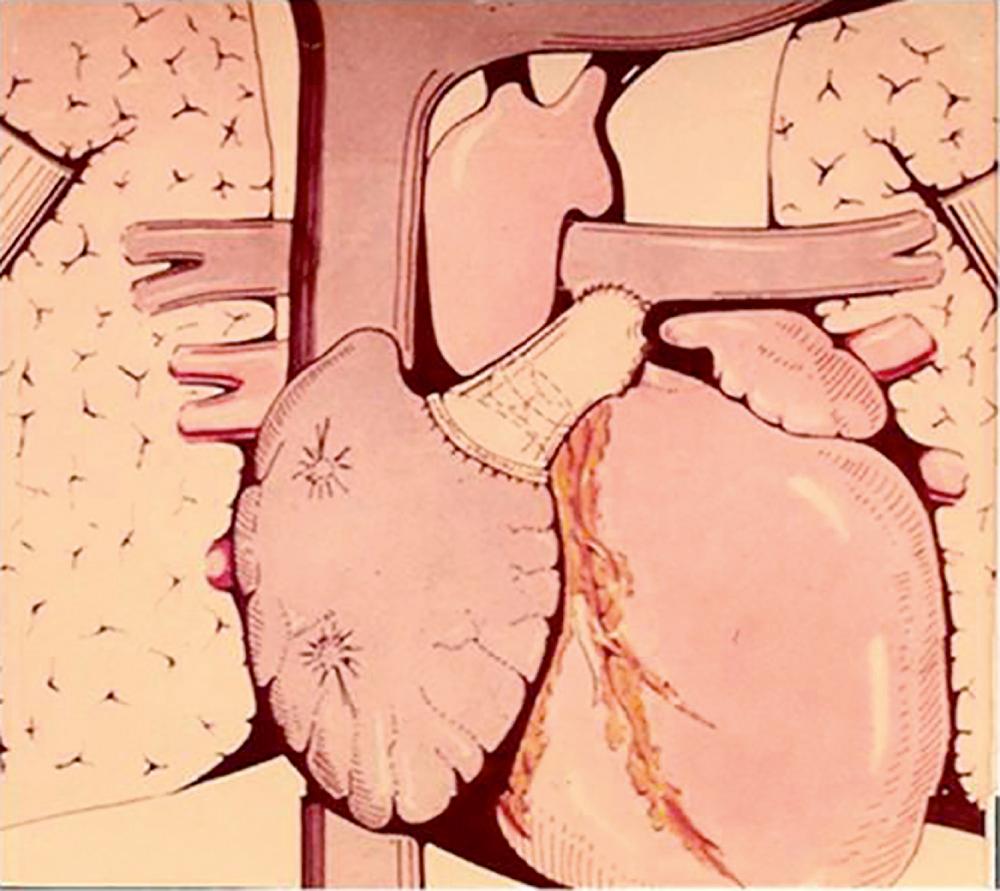
The atriopulmonary connection became the standard Fontan modification through to the late 1980s. However, over the long term, this circulation was associated with progressive dilatation of the systemic venous atrium, atrial thrombus, and intractable atrial arrhythmia. In a series of elegant hydrodynamic experiments, de Leval demonstrated the energy loss associated with the atriopulmonary anastomosis and potential for greater circulatory efficiency if much of the right atrium was excluded from the systemic atrial pathway by using an interatrial patch. This technique—termed the total cavopulmonary connection or lateral tunnel Fontan—reduced the degree of turbulence and energy loss and improved overall hemodynamics. Shortly after, the extracardiac conduit was introduced by Marcelletti et al. by interposing a prosthetic valveless conduit to connect the inferior vena cava to the pulmonary artery. This aimed to avoid progressive atrial dilation, late tachyarrhythmia, and sinus node dysfunction by reducing the number of suture lines and the pressure load within the right atrium ( Fig. 73.4 ).
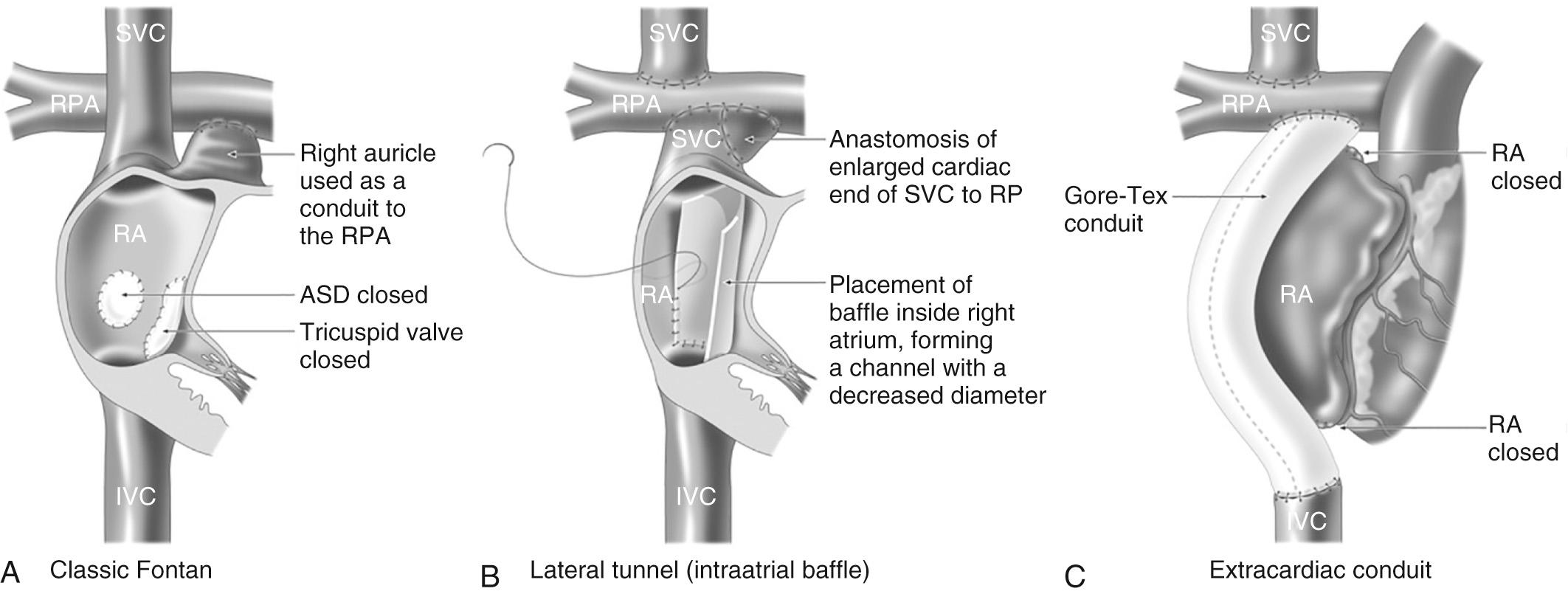
Currently, both the lateral tunnel and extracardiac conduit are widely used, some preferring the former technique in younger patients and those with anomalous drainage of their hepatic veins. Studies demonstrate comparable hemodynamics in both circulations. Nevertheless the extracardiac conduit is the preferred technique in many centers because of the perception that it will be associated with a reduced late arrhythmia burden, although to date this has not been reliably demonstrated.
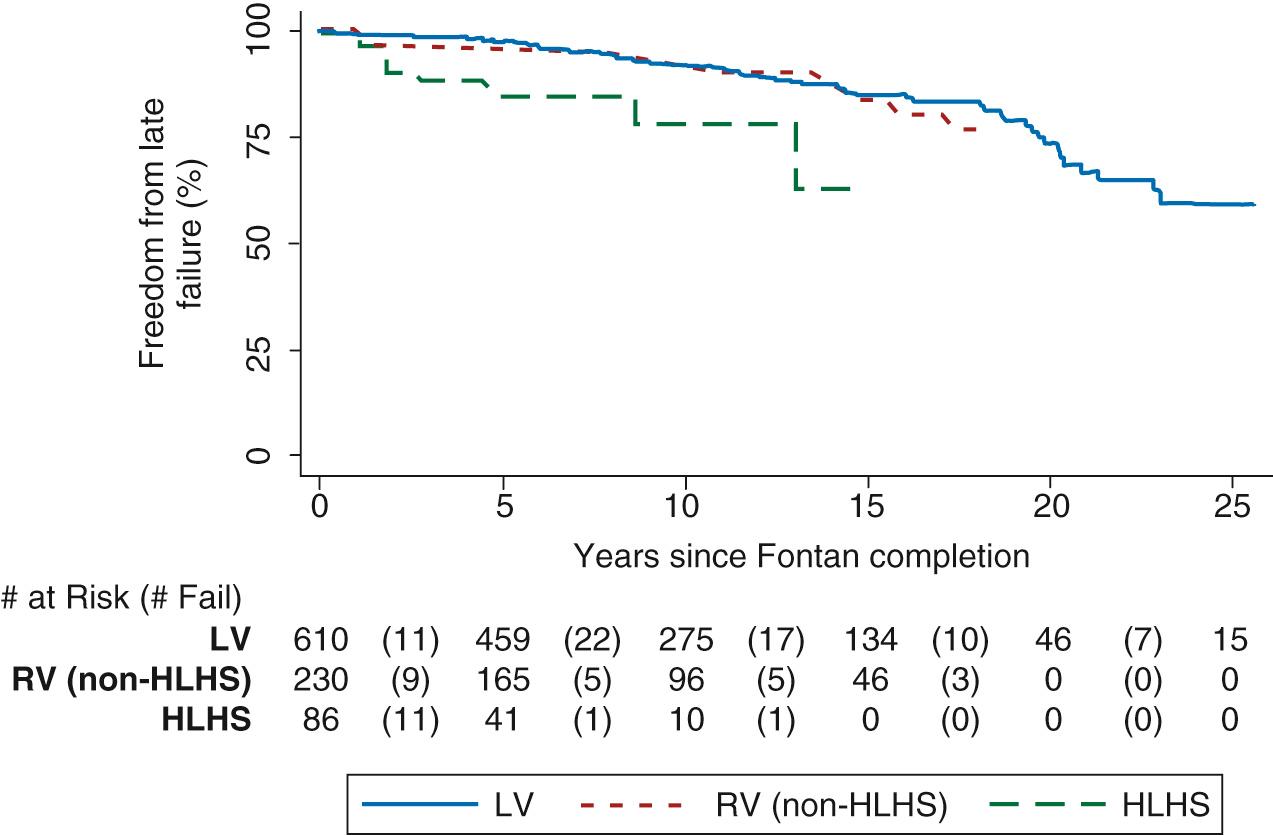
As the fifth decade of Fontan surgery approaches, the burden of late morbidity and mortality has become apparent, with the risk of complications and death increasing the longer the duration of the Fontan circulation. Late outcome studies report a survival rate of 60% to 80% 20 years post-Fontan surgery. Variable case selection and duration of follow-up likely account for this range in outcome. At 25 years after Fontan surgery, almost half the cohort is predicted to face Fontan failure, defined as circulatory dysfunction with limited functional capacity (New York Heart Association [NYHA] class III or IV), Fontan takedown or conversion, the development of debilitating complications including protein-losing enteropathy (PLE) and plastic bronchitis, the need for cardiac transplantation, or death ( Fig. 73.5 ).
Identification of risk factors for long-term outcome and the development of models for risk stratification have the potential to target treatments to the highest risk patients and to guide the development of new treatment strategies. To date, risk stratification has been hampered by a relatively small number of patients and by institutional differences in patient selection and treatment regimens. However, with the increasing numbers of patients and the trend toward multiinstitutional networks and registries, it is likely that risk stratification will become an essential tool to guide the development of effective surveillance regimens and targeted interventions.
Preoperative risk factors for late death include male gender and the diagnosis of hypoplastic left heart syndrome. A higher pre-Fontan mean pulmonary arterial pressure is an important predictor of morbidity and mortality in both the early perioperative and late stages, with a threshold of 15 to 17 mm Hg or less being associated with a better outcome. A higher pulmonary artery pressure is also associated with prolonged pleural effusions in the early postoperative period, as well as the development of PLE in the late stage, both of which independently predict late mortality. Having a common atrioventricular valve (CAVV) is also a predictor of late death, with almost 50% of CAVVs having failed 20 years after Fontan surgery. Moreover, a CAVV is frequently associated with heterotaxy syndrome and anomalies of pulmonary and systemic venous drainage, both of which are also risk factors for late failure ( Table 73.1 ).
| Preexisting (pre-Fontan) factors | Male gender Hypoplastic left heart syndrome Common atrioventricular valve Higher mean pulmonary artery pressure (>16–18 mm Hg) |
| Perioperative factors | Type of Fontan (atriopulmonary worse) Older age at Fontan operation (>7 years) Operative complexity (e.g., aortic cross clamp time, bypass time, concomitant atrioventricular valve replacement) |
| Early postoperative factors | Elevated Fontan circulation pressure (>20 mm Hg) Elevated ventricular filling pressure (>13 mm Hg) Prolonged pleural drainage (>3 weeks) |
| Late postoperative factors | Protein-losing enteropathy Tachyarrhythmia Ventricular pacing Reduced exercise capacity (peak VO 2 ) |
Those with an atriopulmonary Fontan are at greater risk of late death when compared with the more recent variations ( ). However, a survival advantage of the extracardiac conduit over the lateral tunnel has not been demonstrated. When Fontan and colleagues reviewed 160 Fontan surgeries from 1968 to 1988, they found older age at Fontan surgery was predictive of late death. A more recent experience similarly demonstrated a poorer late survival when the Fontan operation was undertaken after 7 years of age. Surrogate markers for surgical complexity including longer aortic cross-clamp time, bypass time, and concurrent atrioventricular valve replacement also impact on late survival.
The main factors in the postoperative course that influence late mortality relate to the presence of elevated pulmonary arterial or Fontan pathway pressure. A postoperative left atrial pressure greater than 13 mm Hg or Fontan pressure greater than 20 mm Hg is associated with a twofold increase in risk of late death. Prolonged pleural effusions, usually described as chest tube drainage for more than 3 weeks after surgery, is one of the strongest predictors of late death. Besides being a marker for elevated pulmonary arterial pressures, it may also be influenced by other factors, including longer cardiopulmonary bypass time, the presence of aortopulmonary collateral vessels, and the absence of a fenestration.
Beyond the perioperative period, the identification of risk factors becomes more challenging due to the insidious nature of disease progression. The development of late complications, including PLE and arrhythmia, and the requirement for ventricular pacing are markers for late failure and are described in detail later in this chapter.
Cardiopulmonary exercise stress testing is an important prognostic tool in the Fontan population. Of all the measured exercise variables, peak VO 2 is the most robust in predicting late morbidity and mortality. Those with a peak VO 2 of less than 16.6 mL/kg/min have a mortality risk seven times of those with a higher peak VO 2 ( Fig. 73.6 ). A lower peak heart rate or reduced heart rate reserve, defined as the difference between peak exercise and resting heart rates, has also been identified as a useful marker of function and prognosis. However, it is important to recognize that confounding factors such as antiarrhythmic therapy and pacemaker-dependence may influence exercise capacity and reduce its prognostic power.

Consequent to improved survival the Fontan population is becoming older, with the average age predicted to increase from 18 years in 2014 to 23 in 2025 and 31 years in 2045. The effective management of these patients will depend on the identification of those at greatest risk of decline, as well as potentially modifying the current approach to staged reconstruction on an individual basis. Well-defined patient surveillance strategies will allow physicians to deliver timely targeted interventions with the aim of increasing longevity and quality of life (QOL).
The Fontan circulation is characterized by elevated central venous pressure (CVP) and a low or low-normal cardiac output with a limited capacity to increase cardiac output with exercise. Arrhythmias are common and may be caused by atrial distension, especially in the case of the atriopulmonary Fontan, or by scarring subsequent to surgical interventions. Elevated CVP and reduced cardiac output adversely affect the function of a number of organs, including the hematologic, renal, liver, and lymphatic systems. Many of the resulting problems have an insidious onset, but, as the time passes, they contribute very significantly to morbidity, mortality, and QOL late after the Fontan procedure.
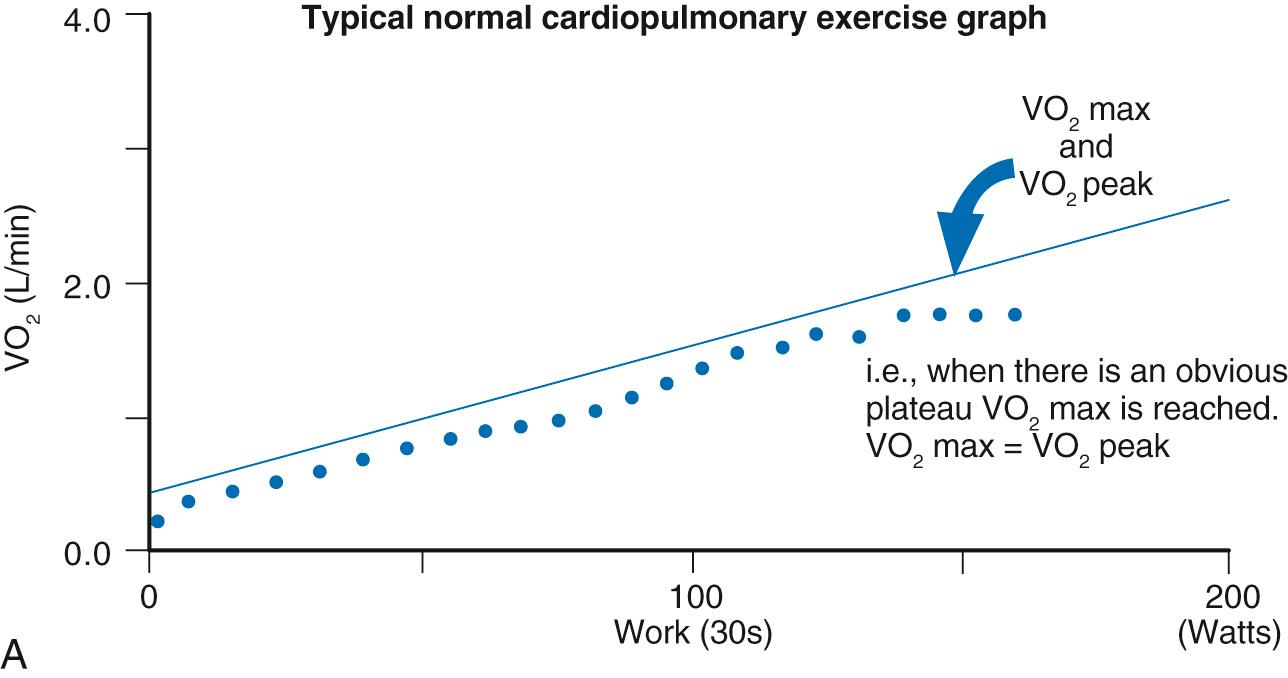
Performance of the Fontan circulation is limited at rest and with exercise, even with optimal anatomic and circulatory conditions. This remains an issue regardless of the type of Fontan procedure and suggests that the problem relates in a large part to the inherent limitations of the circulation itself. In the normal circulation, the subpulmonary ventricle has an important role to play in augmenting cardiac output with exercise. Its absence is central to the limited exercise capacity observed in the Fontan population ( Fig. 73.7 ). The magnitude of the reduction in exercise capacity is best demonstrated by cardiopulmonary exercise testing. Maximal exercise capacity is determined by the highest uptake and utilization of oxygen by the body during maximal exercise (VO 2 max) based on achieving a plateau of VO 2 despite an increase in workload. The highest achieved VO 2 value (VO 2 peak) is used as a substitute when this plateau is not achieved; a common occurrence in the Fontan population ( Fig. 73.8 ). In a structurally normal heart, the major factor limiting VO 2 max is cardiac output, which accounts for 70% to 85% of variance, with the remainder being derived by other factors, including pulmonary and skeletal muscle function and cellular metabolism. Multiple studies have demonstrated reduced VO 2 peak or VO 2 max in Fontan patients. Importantly, a lower VO 2 peak is associated with an increased risk of morbidity and mortality. There is also reduced workload at maximal effort, a variable reduction of VO 2 at ventilatory anaerobic threshold, a reduced peak O 2 pulse, and chronotropic incompetence with a blunted peak heart rate response.
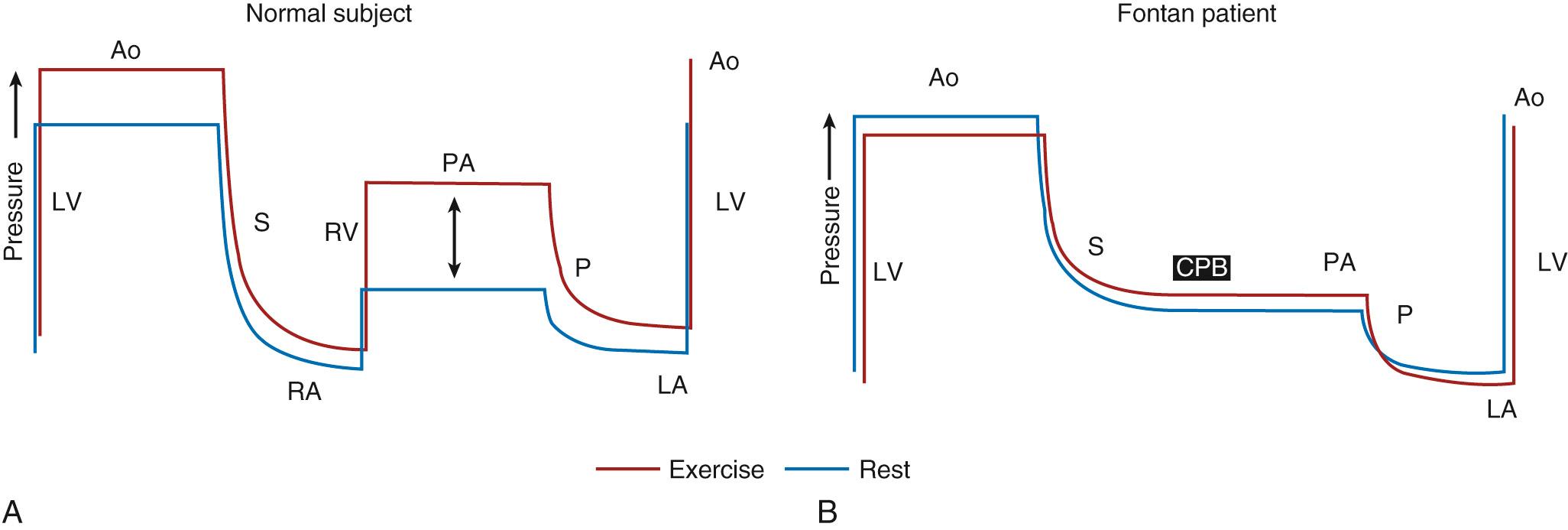
There are several aspects of the Fontan circulation that contribute to impaired exercise capacity ( Fig. 73.9 ). Under normal conditions, cardiac output is augmented by increases in preload, heart rate, and myocardial contractility and a reduction in afterload. Stroke work is increased substantially more in the subpulmonary (right) ventricle than the systemic (left) ventricle. In the absence of a subpulmonary ventricle many of these adaptive responses are absent or compromised.
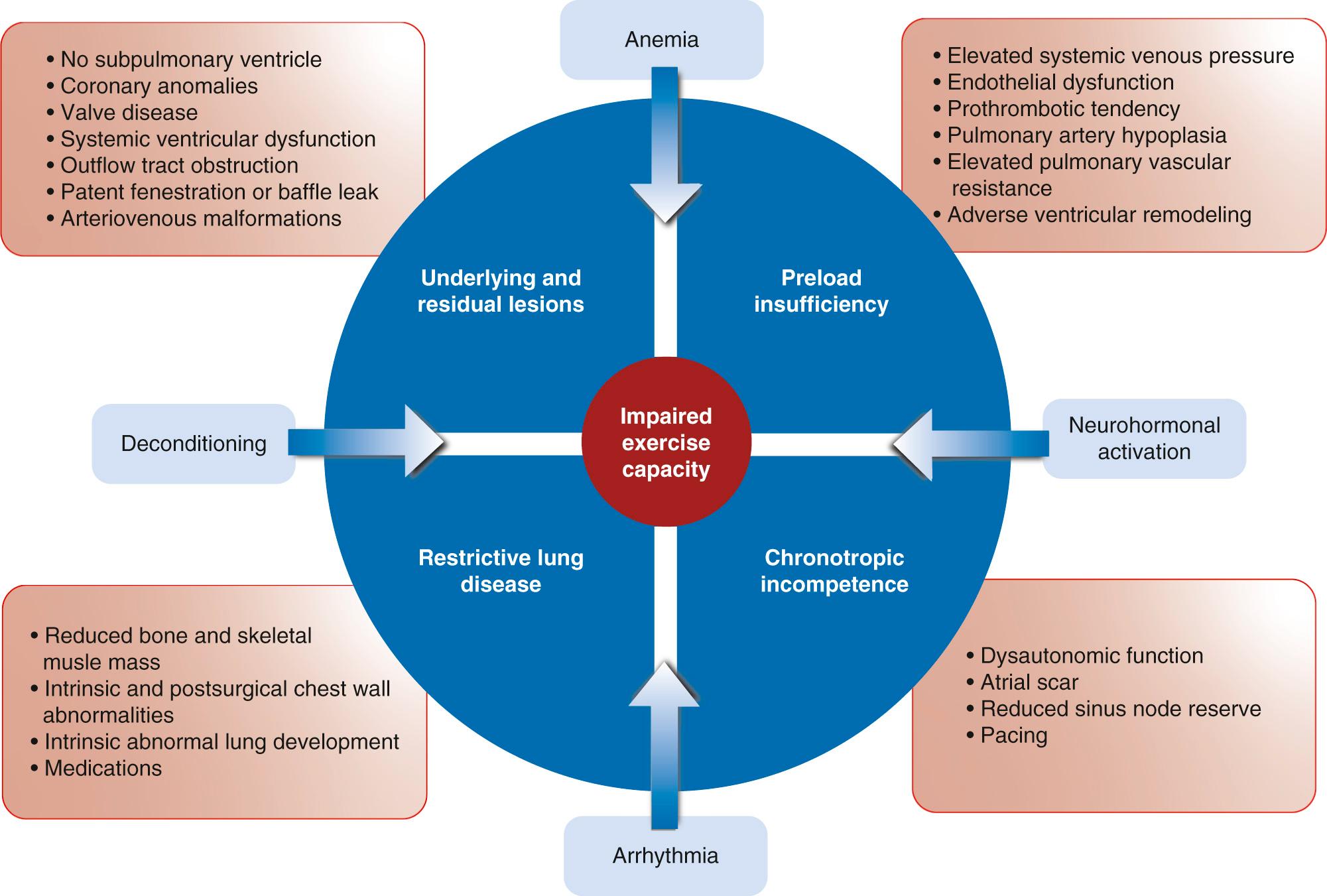
In the Fontan circulation, preload is chronically depleted, and this effect is magnified under exercise conditions. In the absence of a subpulmonary ventricle, systemic ventricular filling is dependent on diastolic function and low pulmonary vascular resistance to pull blood through the pulmonary circulation. These factors are the primary drivers of exercise capacity in the Fontan circulation. Following volume unloading during staged surgical reconstruction, the functionally univentricular heart reduces in size by 25% to 70%. Although remodeling could compensate for this change by reducing myofiber length, diastolic dysfunction predominates from early on in the majority of Fontan patients, suggesting that remodeling is inadequate. Diastolic dysfunction transmits increased filling pressures to the pulmonary veins. This has a progressive negative impact on the pulmonary vascular bed and systemic venous return, leading to further restriction of exercise performance. Increased systemic venous stiffness and reduced capacitance augment systemic return in the Fontan circulation at rest, but these adaptive mechanisms are less effective with exercise.
Pulmonary vascular resistance limits systemic ventricle preload and cardiac output in the Fontan circulation because it sets the level of energy required to deliver blood from the systemic veins to the systemic ventricle. A number of factors have the potential to adversely impact on pulmonary vascular resistance. For example, vascular compliance may be compromised by the lack of pulsatile flow, whereas reduced wall shear stress may lead to maladaptive changes in lung vasculature and increased pulmonary vascular resistance. Abnormal microscopic pulmonary vascular changes have been described in the Fontan circulation, as has impaired pulmonary artery growth. Others have described abnormal pulmonary vascular reactivity with exercise.
Although the mechanism of abnormal pulmonary vascular behavior is unclear, even a small increment of pulmonary vascular resistance has the capacity to reduce the ability to augment cardiac output with exercise. This has generated interest in the use of pulmonary vasodilator therapy in the Fontan population. However, the mixed results of these medications on Fontan exercise performance suggest that the factors responsible for exercise restriction are complex.
The inability of the heart to increase rate commensurate with demand is a recognized predictor of future cardiovascular events and overall mortality in other settings of cardiovascular disease, including heart failure. Chronotropic incompetence is a marker of dysautonomic function and reduced sinus node reserve. In the Fontan population an impaired heart rate response to exercise is a common finding. There is some debate as to its relative contribution to impaired exercise capacity, with some even suggesting that it may form a useful adaptive response when there is diastolic dysfunction. Sinus node dysfunction may be related to damage to the sinus node and its arterial supply during cardiac surgery. More modern cavopulmonary connections have resulted in better preserved chronotropy, but sinus node dysfunction is not eliminated completely because contributors may include prior bidirectional Glenn or hemi-Fontan surgeries. The prognostic implication of chronotropic incompetence within the Fontan population is unclear.
In the absence of a subpulmonary ventricle, the Fontan circulation relies on efficient lung mechanics, with changes in intrathoracic pressure during respiration acting as a suction pump to draw blood through the lungs. It is increasingly recognized that optimized lung parenchyma is an important positive contributor to the Fontan circulation both at rest and under exercise conditions. Restrictive pulmonary function, as demonstrated by reduced forced vital capacity and forced expiratory volume in 1 second is well described in patients with congenital heart disease and is particularly apparent in those with a Fontan circulation. This pattern of abnormal respiratory function is multifactorial with contributions from thoracic surgeries, pleural stiffness, intrinsic lung development abnormalities, and Fontan pulmonary vascular flow dynamics. Side effects from the use of medications, especially amiodarone, may also play a role. Under exercise conditions, restrictive lung function can manifest with reduced O 2 pulse, higher peak minute ventilation (V E ), and reduced ventilation efficiency with higher V E /VCO 2 slope. In addition, several studies have found a significant correlation between impaired resting pulmonary function and reduced peak VO 2 max on exercise in the Fontan population. A small interventional study focused on reduced inspiratory muscle strength in Fontan patients by instituting inspiratory respiratory muscle training. Following training, resting inspiratory muscle strength, cardiac output, and ejection fraction increased, whereas during exercise there was an improvement in ventilatory efficiency.
The relative contribution of the pulmonary circulation and cardiovascular mechanics to exercise restriction in Fontan patients is yet to be fully understood. Further research will improve the understanding of these limitations and lead to new ways of “empowering” the Fontan circulation under exercise conditions.
Somatic growth is impaired during and following staged reconstruction for children with a functionally univentricular heart. Weight and height parameters are most often within the normal range at birth in the absence of prematurity or genetic abnormalities. Following the initial surgical procedure, there is a significant decrease in both height and weight z score. Weight follows a trajectory of relative recovery after the bidirectional Glenn or hemi-Fontan operation, and following Fontan completion in most children. However, height does not demonstrate the same trend. Heart failure, PLE, the presence of venovenous collaterals, and significant atrioventricular valve regurgitation may have a negative impact on weight and height trajectory, although these associations have not been demonstrated in a consistent way. By adulthood, males with a Fontan are shorter than normal population. This relationship is less striking in females. Mechanisms for the reduction in height potential may include lower bone density and reduced muscle mass. Of interest, but as yet unconfirmed, lower exercise participation in Fontan patients may impact on bone growth and subsequent bone/muscle development. Another potential contributor to reduced bone growth is prolonged hypoxemia prior to Fontan completion, although a fenestration after the Fontan operation (a marker for hypoxemia) does not appear to influence height recovery. The impact of the Fontan circulation on insulin-like growth factors (IGFs) and growth hormone and their interaction with somatic growth is yet to be established. In a cross-sectional study, lower IGF was found in Fontan patients with a higher brain natriuretic peptide (a marker for heart failure) and lower cardiac output. However, there was no relationship between IGF and somatic growth. The authors concluded that longitudinal studies were required to determine if these relationships contributed to impaired somatic growth in this population.
There is concern that obesity trends seen in the general population will be similarly seen in the Fontan population. Although obesity is less prevalent in Fontan cohorts compared with the general population, as well as other forms of congenital heart disease, the rate is not insignificant and ranges from 8% to 30%. Moreover, there is a tendency toward increasing weight and body mass index further out from Fontan surgery. Given the reliance of the Fontan circulation on optimal ventricular and vascular function, additional acquired cardiovascular risk factors can contribute only to a worse outcome.
Arrhythmia is a common problem in the Fontan population, has an increasing prevalence in older patients, and is often associated with Fontan failure. The two most frequent arrhythmias are bradycardia due to sinus node dysfunction, and atrial flutter. The latter is more correctly termed intraatrial reentrant tachycardia (IART). Both of these arrhythmias become more prevalent with time but are not necessarily linked to each other ( Fig. 73.10 ). In a population-based report, bradyarrhythmias are present in 7% at 10 years and 15% at 20 years after the Fontan procedure, and tachyarrhythmia in 9% and 31%, respectively. Tachyarrhythmia is commoner in those with functional limitations, isomerism, and an atriopulmonary Fontan connection when compared with the extracardiac Fontan. A contemporary series suggests atrial tachyarrhythmia is present in most if not all patients 25 years after the atriopulmonary Fontan procedure. The extracardiac Fontan may result in less IART than the lateral tunnel, although the evidence for this is less conclusive. IART is also more common when atrioventricular valve repair or pulmonary vein surgery is required at the initial surgery.
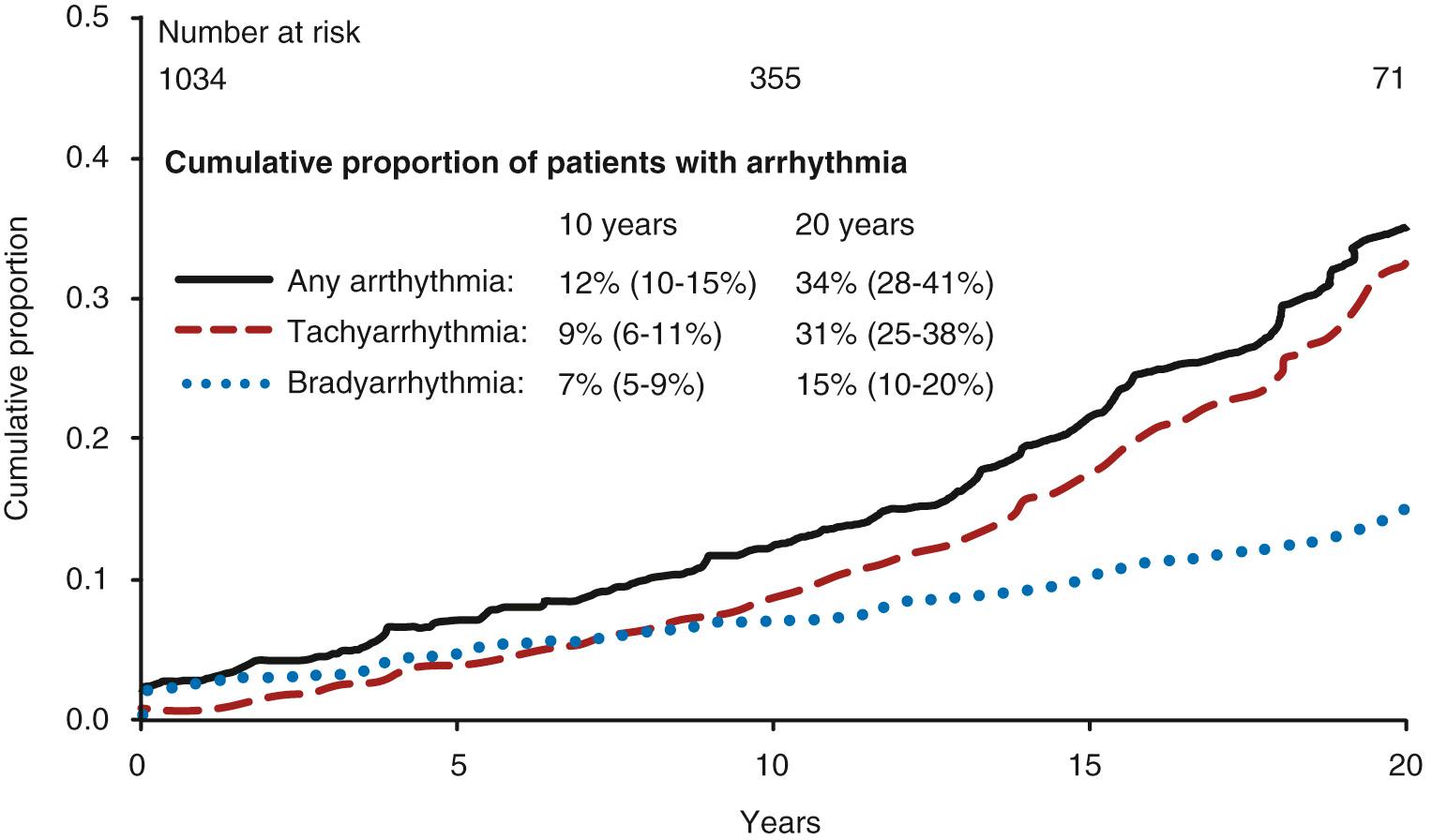
Focal, atrial ectopic tachycardias occur in approximately 13% of patients over long-term follow-up, many in the same patients who have IART. Atrial fibrillation is becoming more frequent in older patients (19% in one series) with risk factors overlapping those of the aging population (such as overweight and hypertension).
The occurrence of bradyarrhythmia or tachyarrhythmia signals a 50% to 60% risk of Fontan failure over the next 10 years.
Ventricular tachycardia (VT) is relatively uncommon and usually asymptomatic, with Holter recordings suggesting a prevalence of approximately 6% 10 years after Fontan. However, symptomatic VT or ventricular fibrillation can occur in up to 3%. The presence of VT correlates with larger ventricular volumes, reduced ejection fraction, and magnetic resonance imaging (MRI) evidence of myocardial fibrosis.
Sudden cardiac death occurs at late follow-up in 5% to 9%. Risk factors include the presence of atrial tachyarrhythmia, atrioventricular valve replacement at the time of the Fontan surgery, and an immediate postoperative systemic venous pressure greater than 20 mm Hg. Preoperative sinus rhythm is protective.
Pacemakers may be used in up to 25% of cases at late follow-up, including those implanted for the management of atrial tachycardia. Pacing for bradyarrhythmia is required in approximately 7% to 15% of patients during long-term follow-up. In approximately two-thirds, the indication is sinus bradycardia and, in one-third, atrioventricular block. The latter is more common among patients with congenitally corrected transposition of the great arteries ( Fig. 73.11 ). Pacemakers are commonly placed when an atriopulmonary Fontan is converted to an extracardiac Fontan. This procedure usually includes antiarrhythmia surgery. Some centers will implant biatrial antitachycardia pacing devices prophylactically during the same procedure.
Bradyarrhythmia is more common after the atriopulmonary Fontan than the lateral tunnel or external cardiac conduit ( Fig. 73.12 ). Heart rate variability, a subtle marker of sinus node dysfunction, is reduced in lateral tunnel and external conduit Fontan in equal measure when compared with healthy controls. There is some suggestion that the external conduit may be associated with more sinus node dysfunction than the lateral tunnel, but this is not a consistent finding. Sinus node dysfunction may relate more to the nature of the prior superior vena cava pulmonary anastomosis (as well as native sinus node function) because that surgery is close to the sinus node and sinus node artery. Atrial pacing, which usually must be epicardial and may require extensive thoracic surgery to be achieved, is generally reserved for those with symptomatic chronotropic incompetence. Depending on the anatomy, it may be feasible to place transvenous atrial leads; however, it is not unusual to have to place leads in nonstandard positions because areas of viable myocardial tissue can be limited ( Fig. 73.13 ).
Ventricular pacing should be avoided or minimized as far as possible because of the risk of causing ventricular dyssynchrony and pacemaker-induced cardiomyopathy, cardiac failure, and atrioventricular valve regurgitation. Fontan patients with ventricular pacing have a fivefold risk of transplant or death compared with matched nonpaced controls. The value of cardiac resynchronization therapy is being explored; results are generally disappointing, but there may be a place in specific cases such as postpacing cardiomyopathy or when there is a systemic left ventricle and left bundle branch block ( Fig. 73.14 ).
Sudden death after the Fontan procedure is not that uncommon, usually occurring in the context of end stage of the Fontan circulation failure. It may be related to events such as pulmonary and cerebral embolism or poorly controlled atrial tachycardias. Implantable cardioverter-defibrillators are a class IB indication for secondary prevention following resuscitated cardiac arrest due to sustained VT or ventricular fibrillation. However, implantation carries a significant risk in those with Fontan failure given that it entails thoracic surgery. If pacing is not required, a subcutaneous implantable cardioverter-defibrillator may be an option in some patients. Careful consideration should be made regarding defibrillation threshold testing at the time of implantation because this process can be lethal in those with severe ventricular failure.
The association of atrial tachycardias with poor outcome is at least partly correlated to the underlying substrate of the arrhythmia, rather than the arrhythmia per se. In the atriopulmonary Fontan, there is an electromechanical correlation between the occurrence of arrhythmia and the degree of atrial dilation and thickening. Risk factors also include previous pulmonary artery banding, isomerism, and a systemic right ventricle.
The observation that atrial dilatation was associated with IART led in part to the adoption of the lateral tunnel procedure. The prevalence of IART has proven to be lower with this type of Fontan connection. The external cardiac conduit approach reduces intracardiac surgery and avoids progressive dilation of the atrial wall, but it is not evident that this operative strategy is associated with a reduced prevalence of IART compared with the lateral tunnel.
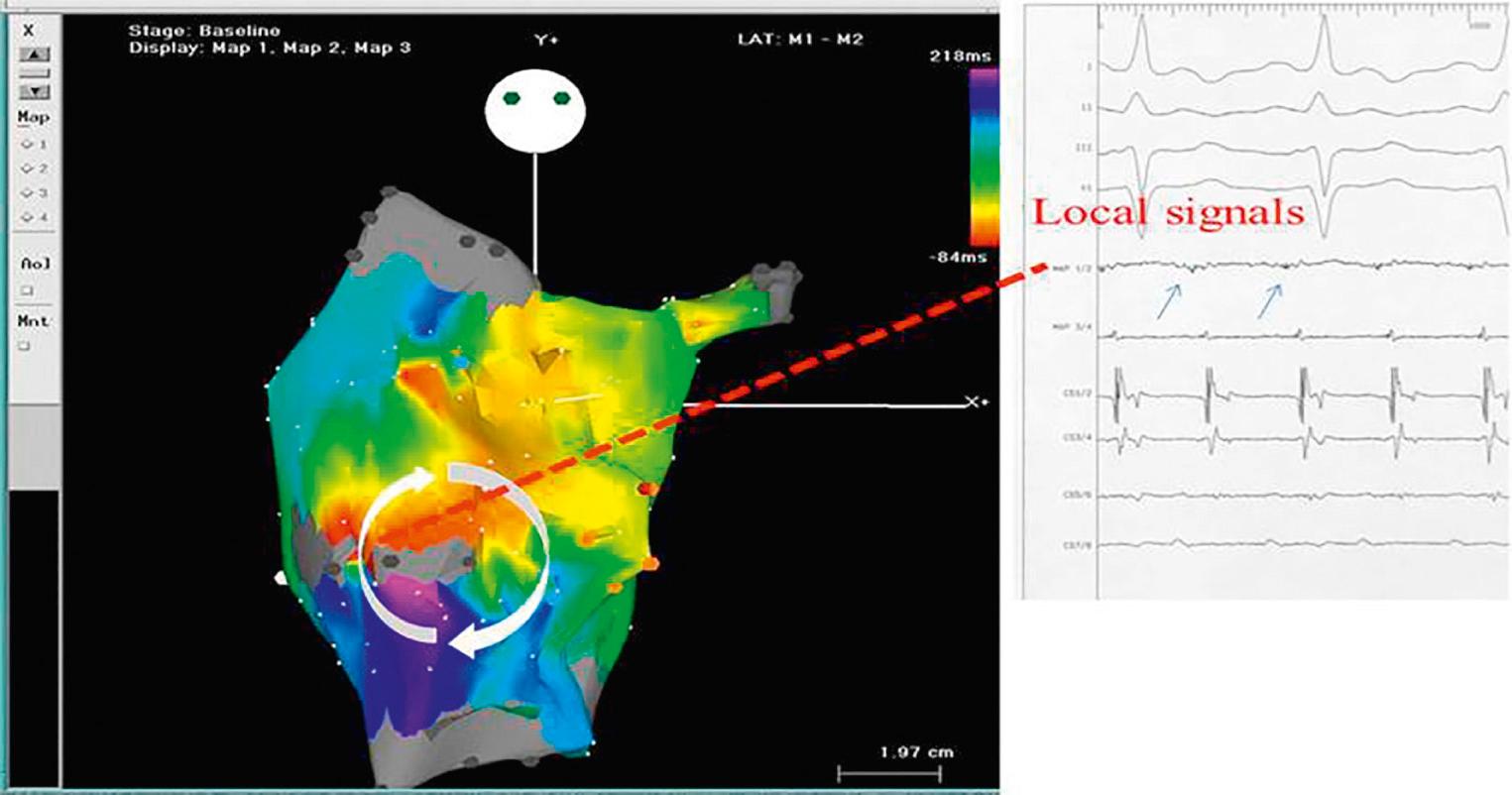
Invasive electrophysiologic studies demonstrate that the mechanism of IART commonly involves surgical scars created during suturing of the lateral tunnel. These studies reveal large areas of low-voltage diseased atrial myocardium, with fractionated signals demonstrating delayed and nonhomogeneous electrical conduction. This substrate is ideal for the development of intraatrial reentry ( Fig. 73.15 ).
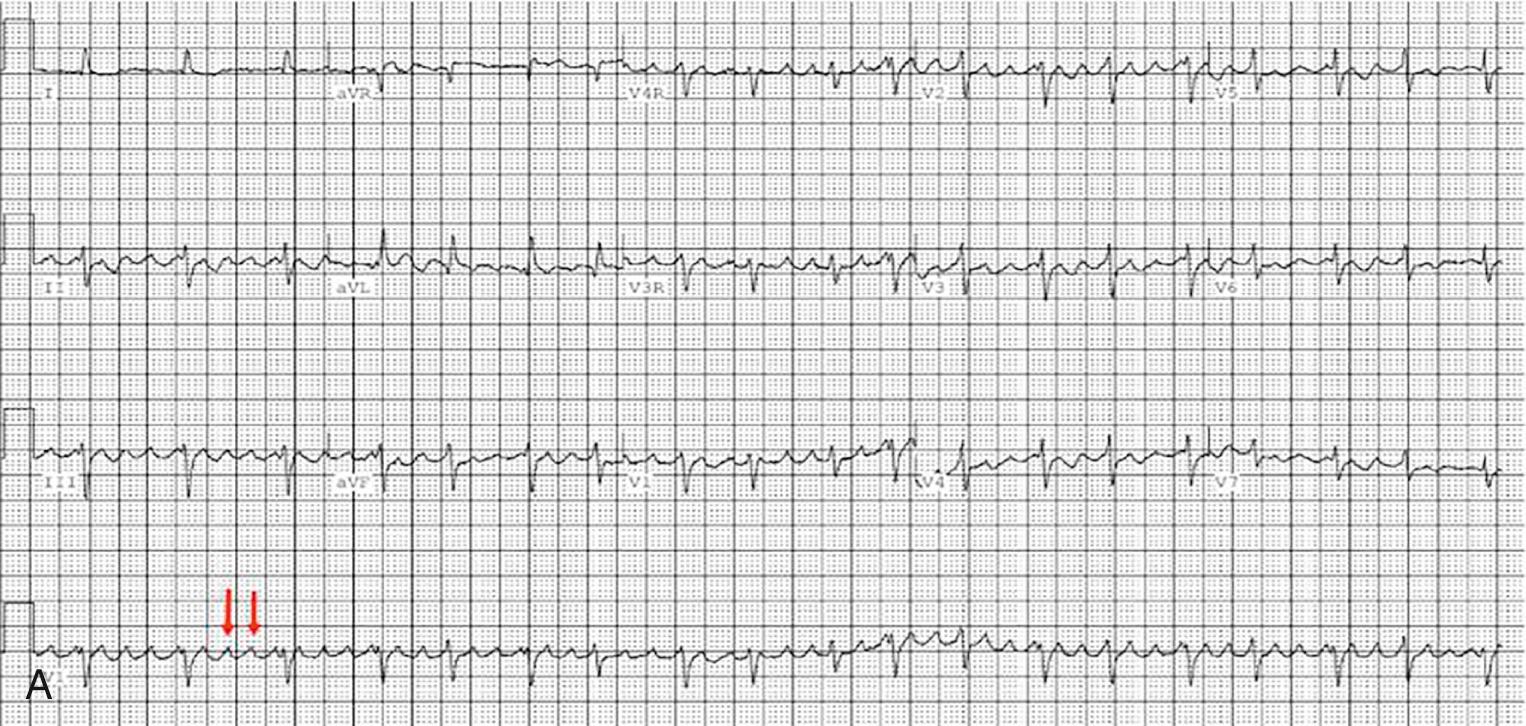
Atrial reentry circuit depends on areas of slow conduction in diseased atrial myocardium with electrically silent tissue on each side. This results in a slow conducting bridge, or isthmus. An electrical signal enters the isthmus, and by the time the electrical signal is released from this isthmus, the healthy myocardium is able to conduct again; the signal propagates around the atrium and back to the entry point of the isthmus. These areas of slow conduction usually develop around areas of scar either surgical or due to progressive atrial fibrosis ( Fig. 73.16 ). After all forms of Fontan, the commonest position for such an isthmus is between the bottom end of a right atriotomy scar and the inferior vena cava (“pericaval origin”). This is different to other postoperative congenital heart groups and the structurally normal heart with atrial flutter, where the isthmus commonly runs across the anatomic cavotricuspid junction. It has been proposed that an additional surgical line should be made at the time of the lateral tunnel surgery, to prevent such an isthmus developing. Unfortunately, given the time lag in the development of IART after the Fontan procedure, it will be decades before we know if this has been successful. As mentioned before, some modifiable surgical techniques may help to prevent IART. Certainly, the move away from atriopulmonary connection has been beneficial, as has reduced age at the time of the Fontan operation.
Other congenital arrhythmia substrates such as accessory pathway–mediated tachycardias and atrioventricular nodal reentrant tachycardias account for up to 30% of the tachycardias in Fontan patients treated in a tertiary electrophysiology laboratory. These tachycardias are more responsive to medical and ablative therapy than IART.
In the acute setting, medical management can be difficult, and the patient may have decompensated cardiac failure, as both a cause and effect of the IART. Direct current cardioversion can fail in a quarter of patients, with increased success rate if type I or III antiarrhythmic medications are started prior. Medication for rate control can be difficult to manage because of the commonly associated sinus node dysfunction, and although amiodarone can be effective, side effects can be harmful when this medication is used long term. Thus, in the adult patient with an atriopulmonary connection, medical management is frequently unsuccessful. Interventional strategies involve a choice of (or combination of) a catheter ablation strategy versus a surgical takedown to a lateral tunnel or extracardiac conduit with concomitant surgical ablation techniques, usually a maze procedure.
Catheter ablation for IART in the atriopulmonary Fontan can be successful in the short term, but there is a high recurrence rate. This is not surprising given the fact that the underlying substrate—the atrial dilation and wall thickening with large areas of scarred and electrically inhomogeneous tissue—is not altered. The grossly dilated atrium is also a nidus for thrombus formation and is hemodynamically inefficient. The early Fontan conversion experience was one of considerable mortality outside of several high-volume centers. The results of this surgery are improving, and this improvement relates at least in part to a better appreciation of the indications for operation. Many centers have published favorable results, with an early mortality rate of approximately 5%, improved NYHA functional class, and reduction in arrhythmia incidence over 10 years (see later, “ Surgical Management of Fontan Failure ”).
Atrial fibrillation commonly occurs earlier in the Fontan population than in other patients with postoperative congenital heart disease and is generally poorly tolerated. Onset often occurs in the third decade, usually as an intermittent arrhythmia that commonly coexists or alternates with other atrial tachycardias. Progression to sustained atrial fibrillation is common within 5 years of the first episode. The inclusion of left atrial (Cox) maze with right atrial maze at the time of Fontan conversion may prove effective in reducing the recurrence rate of this arrhythmia, especially in older patients and those who already have atrial fibrillation. However, it is not known what proportion of atrial fibrillation has a left atrial/pulmonary vein origin in the Fontan circulation, even though this is the commonest mechanism in the structurally normal heart. There is anecdotal evidence, and it makes intuitive sense, that some atrial fibrillation in these patients has a right atrial origin.
Although a surgical approach may be most appropriate for those with atrial tachycardia with an atriopulmonary Fontan, catheter ablation has a role in other cases. An ablation can be a useful palliation where conversion is contraindicated, atrial dilation is not excessive, or the patient has declined surgery. Focal atrial tachycardias can be relatively straightforward to ablate, along with congenital arrhythmia such as accessory pathways, atrioventricular node reentrant tachycardia, and rare cases with twin atrioventricular nodes. With the extracardiac conduit or lateral tunnel Fontan, the critical isthmus is usually on the cardiac side of the baffle, so that access for ablation catheters is difficult. However, there has been increasing confidence in the use of transbaffle puncture technique in these cases because there is commonly a safe puncture point at the lower end of the baffle at the junction with the inferior vena cava/atrial border.
The dominant arrhythmia post Fontan is atrial tachycardia, with complex atrial reentry circuits. Their appearance is commonly coincident with hemodynamic deterioration and the arrhythmia typically contributes further to lower cardiac output, forming a vicious cycle that may be lethal. Atrial fibrillation commonly alternates with other atrial tachycardias. Current management is tending toward early conversion of the atriopulmonary Fontan to an extracardiac conduit, with concurrent atrial arrhythmia prevention surgery.
Following all types of Fontan surgeries, medical management is usually not sustainable for more than 2 to 3 years and, although amiodarone is the most effective medication, side effects are common. Invasive electrophysiology studies and catheter ablation strategies can be very helpful and are recommended early in the absence of gross atrial dilation and to diagnose and treat concurrent congenital arrhythmia substrates including accessory pathways.
It is well recognized that the Fontan circulation presents a hypercoagulable state, with the incidence of thromboembolic events reported to vary between 8% and 20% of the population. This is likely an underestimation, in view of the occurrence of silent thromboembolism in this group. A prospective multicenter randomized controlled trial assessing several anticoagulant regimens reported a total thrombosis rate of 23% over 2 years. Only one-third of these events (8%) were symptomatic, with the remainder being detected during intensive surveillance as part of the study design. Studies have described a peak thrombotic risk in the first year following Fontan completion, which plateaus over the next 3 to 4 years, before a second peak after 10 years. Moreover, the incidence of thromboembolic complications is higher in adults compared with children, suggesting an increase in risk with time that might relate to a gradual deterioration in vascular and liver function, exacerbated by a tendency to a more sedentary lifestyle in older and more debilitated patients.
The etiology of this prothrombotic state is multifactorial and involves all three factors of the Virchow triad, namely abnormal hemodynamics, a hypercoagulable state, and endothelial dysfunction ( Fig. 73.17 ). Potential factors include the low-velocity flow in the systemic veins, cavopulmonary connection and pulmonary arteries, atrial arrhythmias, persistent cyanosis related to right-to-left shunts, and an imbalance of intrinsic procoagulant and anticoagulant factors.
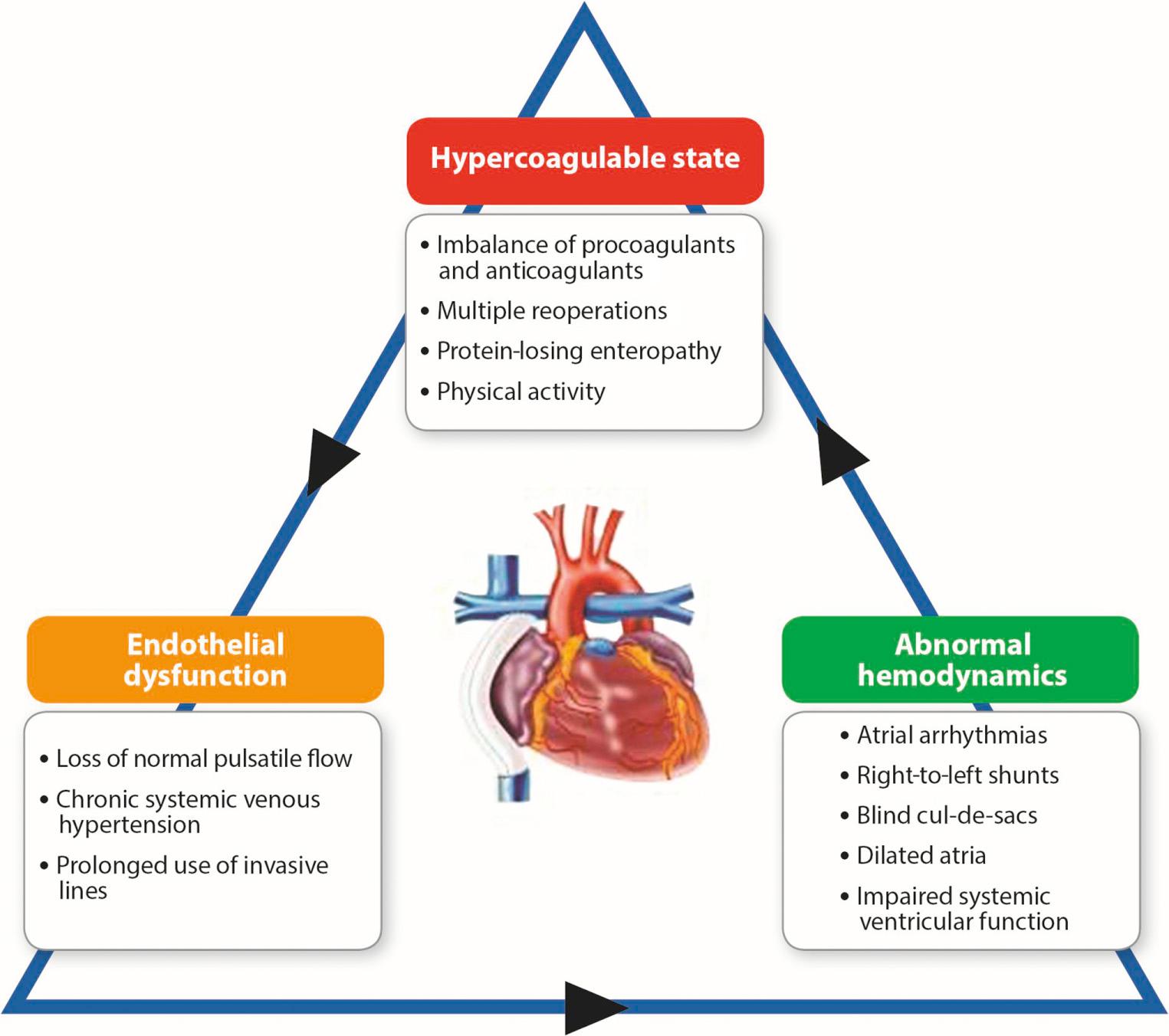
Older age at the time of the Fontan operation is a risk factor for silent thromboembolism. Surprisingly, there appears to be a similar risk of thromboembolism among the different variants of the Fontan ( Fig. 73.18 ; ). Although the presence of a right-to-left shunt is known to increase the risk of cerebral vascular embolization, the presence of a fenestration has not been associated with increased thromboembolic or stroke risk. This suggests that intrinsic hematologic abnormalities may be the most significant factors in the prothrombotic state in the Fontan circulation. Compared with healthy controls, Fontan patients have reduced levels of procoagulant factors, including factors II, V, VII, and X, and coagulant inhibitors, such as protein C, protein S, plasminogen, and antithrombin III. An elevated level of factor VIII is a strong risk factor for venous thromboembolism in the normal adult population, with a predicted incidence of recurrent thrombosis of more than 10% per year in those with increased serum levels. Longitudinal studies monitoring serum factor VIII levels in patients with a functionally univentricular heart have demonstrated a conversion from low serum levels early in the course of staged reconstruction, to significantly raised levels after Fontan completion. Increased factor VIII activity correlates with higher superior vena cava pressure in the Fontan circulation. As such, it is hypothesized that increased pressure transmitted to the liver sinusoidal endothelium leads to the upregulation of factor VIII synthesis. Thrombocytopenia may also contribute, particularly if related to heparin treatment, in which it may be associated with a high risk of thrombosis, or when associated with portal hypertension and failure of the Fontan circulation (see Fontan Failure). Lastly, progressive endothelial dysfunction develops with prolonged exposure to the Fontan circulation. Even well-functioning adult patients may have underlying endothelial dysfunction, indicated by increased plasma concentrations of endothelin-1 and abnormal digital pulse amplitude tonometry. This multitude of factors leads to the high incidence of thromboembolic events that contribute to significant morbidity and mortality early and late after Fontan surgery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here