Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A fundamental principle of circulatory function is that most tissues have the ability to control their own local blood flow in proportion to their specific metabolic needs. Some of the specific needs of the tissues for blood flow include the following:
Delivery of oxygen to the tissues
Delivery of other nutrients such as glucose, amino acids, and fatty acids
Removal of carbon dioxide from the tissues
Removal of hydrogen ions from the tissues
Maintenance of proper concentrations of ions in the tissues
Transport of various hormones and other substances to the different tissue.
Certain organs have special requirements. For example, blood flow to the skin determines heat loss from the body and, in this way, helps control body temperature. Also, delivery of adequate quantities of blood plasma to the kidneys allows the kidneys to filter and excrete the waste products of the body and to regulate body fluid volumes and electrolytes.
We shall see that these factors exert extreme degrees of local blood flow control and that different tissues place different levels of importance on these factors in controlling blood flow.
Note the very large blood flows listed in Table 17-1 for some organs—for example, several hundred milliliters per minute per 100 grams of thyroid or adrenal gland tissue and a total blood flow of 1350 ml/min in the liver, which is 95 ml/min/100 g of liver tissue.
| Percentage of Cardiac Output | ml/min | ml/min/100 g of Tissue Weight | |
|---|---|---|---|
| Brain | 14 | 700 | 50 |
| Heart | 4 | 200 | 70 |
| Bronchi | 2 | 100 | 25 |
| Kidneys | 22 | 1100 | 360 |
| Liver | 27 | 1350 | 95 |
|
(21) | (1050) | |
|
(6) | (300) | |
| Muscle (inactive state) | 15 | 750 | 4 |
| Bone | 5 | 250 | 3 |
| Skin (cool weather) | 6 | 300 | 3 |
| Thyroid gland | 1 | 50 | 160 |
| Adrenal glands | 0.5 | 25 | 300 |
| Other tissues | 3.5 | 175 | 1.3 |
| Total | 100.0 | 5000 |
Also note the extremely large blood flow through the kidneys—1100 ml/min. This extreme amount of flow is required for the kidneys to perform their function of cleansing the blood of waste products and regulating composition of the body fluids precisely.
Conversely, most surprising is the low blood flow to all the inactive muscles of the body—only a total of 750 ml/min—even though the muscles constitute between 30% and 40% of the total body mass. In the resting state, the metabolic activity of the muscles is low, as is the blood flow—only 4 ml/min/100 g. Yet, during heavy exercise, muscle metabolic activity can increase more than 60-fold and the blood flow as much as 20-fold, increasing to as high as 16,000 ml/min in the body’s total muscle vascular bed (or 80 ml/min/100 g of muscle).
The following question might be asked: Why not continuously provide a very large blood flow through every tissue of the body that would always be enough to supply the tissue’s needs, regardless of whether the activity of the tissue is small or large? The answer is equally simple; such a mechanism would require many times more blood flow than the heart can pump.
Experiments have shown that the blood flow to each tissue usually is regulated at the minimal level that will supply the tissue’s requirements—no more, no less. For example, in tissues for which the most important requirement is delivery of oxygen, the blood flow is always controlled at a level only slightly more than that required to maintain full tissue oxygenation but no more than this. By controlling local blood flow in such an exact way, the tissues almost never experience oxygen nutritional deficiency, and the workload on the heart is kept at a minimum.
Local blood flow control can be divided into two phases, acute control and long-term control. Acute control is achieved by rapid changes in local vasodilation or vasoconstriction of the arterioles, metarterioles, and precapillary sphincters that occur within seconds to minutes to provide rapid maintenance of appropriate local tissue blood flow. Long-term control means slow, controlled changes in flow over a period of days, weeks, or even months. In general, these long-term changes provide even better control of the flow in proportion to the needs of the tissues. These changes come about as a result of an increase or decrease in the physical sizes and numbers of blood vessels supplying the tissues.
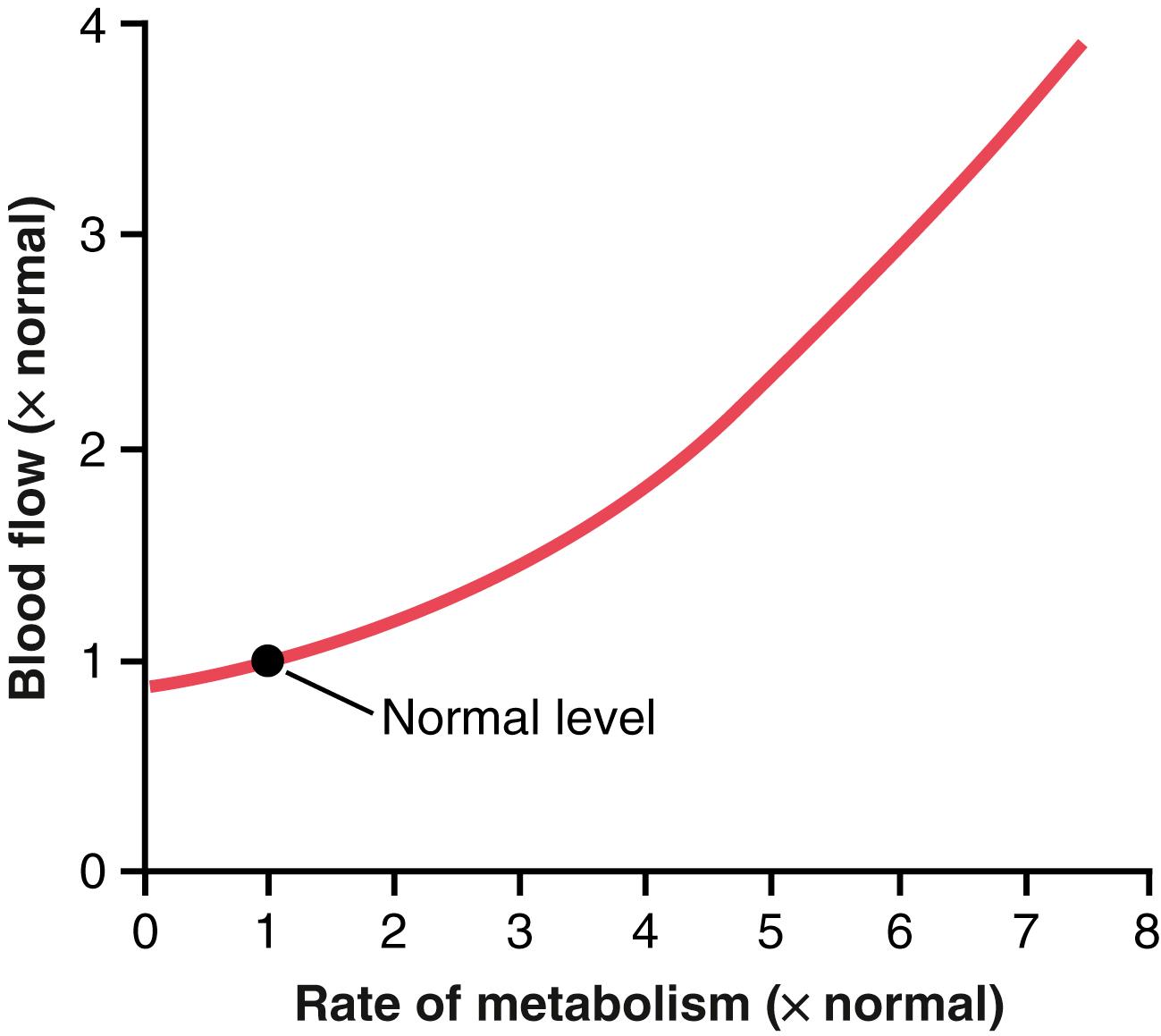
Figure 17-1 shows the approximate acute effect on blood flow of increasing the rate of metabolism in a local tissue, such as in a skeletal muscle. Note that an increase in metabolism up to eight times normal increases the blood flow acutely about fourfold.
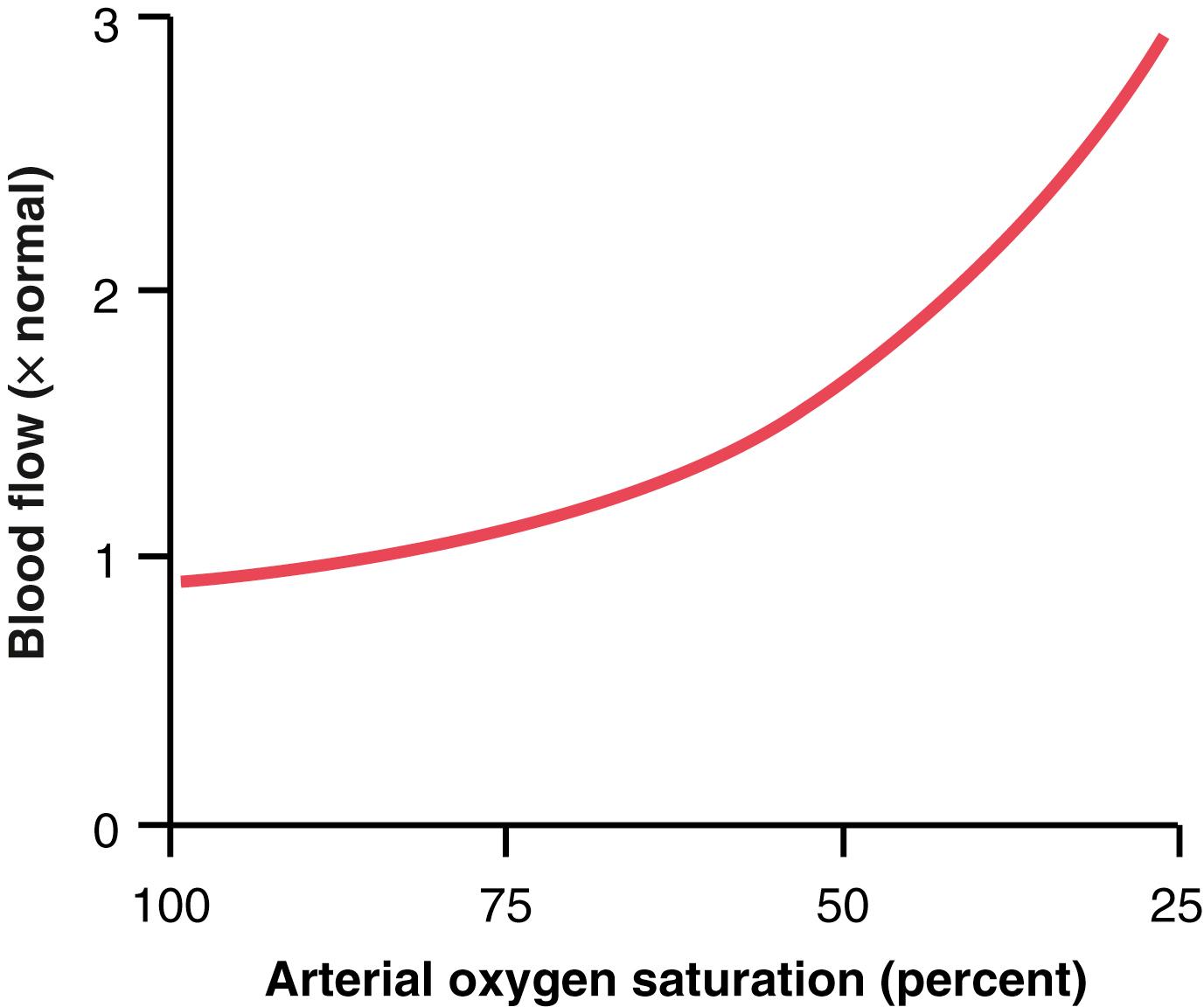
One of the most necessary of the metabolic nutrients is oxygen. Whenever the availability of oxygen to the tissues decreases, such as during the following: (1) at a high altitude at the top of a high mountain; (2) in pneumonia; (3) in carbon monoxide poisoning (which poisons the ability of hemoglobin to transport oxygen); or (4) in cyanide poisoning (which poisons the ability of the tissues to use oxygen), the blood flow through the tissues increases markedly. Figure 17-2 shows that as the arterial oxygen saturation decreases to about 25% of normal, the blood flow through an isolated leg increases about threefold; that is, the blood flow increases almost enough, but not quite enough, to make up for the decreased amount of oxygen in the blood, thus almost maintaining a relatively constant supply of oxygen to the tissues.
Total cyanide poisoning of oxygen usage by a local tissue area can cause local blood flow to increase as much as sevenfold, thus demonstrating the extreme effect of oxygen deficiency to increase blood flow. The mechanisms whereby changes in tissue metabolism or oxygen availability alter tissue blood flow are not fully understood, but two main theories have been proposed, the vasodilator theory and the oxygen demand theory.
According to the vasodilator theory, the greater the rate of metabolism or the less the availability of oxygen or some other nutrients to a tissue, the greater the rate of formation of vasodilator substances in the tissue cells. The vasodilator substances are then believed to diffuse through the tissues to the precapillary sphincters, metarterioles, and arterioles to cause dilation. Some of the different vasodilator substances that have been suggested are adenosine, carbon dioxide, adenosine phosphate compounds, histamine, potassium ions, and hydrogen ions.
Vasodilator substances may be released from the tissue in response to oxygen deficiency. For example, experiments have shown that decreased oxygen availability can cause adenosine and lactic acid (containing hydrogen ions) to be released into the spaces between the tissue cells; these substances then cause intense acute vasodilation and therefore are responsible, or partially responsible, for the local blood flow regulation. Other vasodilator substances, such as carbon dioxide, lactic acid, and potassium ions, also tend to increase in the tissues when blood flow is reduced and cell metabolism continues at the same rate, or when cell metabolism is suddenly increased. An increase in the concentration of vasodilator metabolites causes vasodilation of the arterioles, thus increasing the tissue blood flow and returning the tissue concentration of the metabolites toward normal.
Many physiologists believe that adenosine is an important local vasodilator for controlling local blood flow. For example, minute quantities of adenosine are released from heart muscle cells when coronary blood flow becomes too little, and this release of adenosine causes enough local vasodilation in the heart to return coronary blood flow to normal. Also, whenever the heart becomes more active than normal, the heart’s metabolism increases, causing increased utilization of oxygen, followed by (1) decreased oxygen concentration in the heart muscle cells with (2) consequent degradation of adenosine triphosphate (ATP), which (3) increases the release of adenosine. It is believed that much of this adenosine leaks out of the heart muscle cells to cause coronary vasodilation, providing increased coronary blood flow to supply the increased nutrient demands of the active heart.
Although research evidence is less clear, many physiologists also have suggested that the same adenosine mechanism is an important controller of blood flow in skeletal muscle and many other tissues, as well as in the heart. However, it has been difficult to prove that sufficient quantities of any single vasodilator substance, including adenosine, are formed in the tissues to cause all the measured increase in blood flow. It is likely that a combination of several different vasodilators released by the tissues contributes to blood flow regulation.
Although the vasodilator theory is widely accepted, several critical facts have made other physiologists favor another theory, which can be called the oxygen demand theory or, more accurately, the nutrient demand theory (because other nutrients besides oxygen are involved). Oxygen is one of the metabolic nutrients required to cause vascular muscle contraction, with other nutrients required as well. Therefore, in the absence of adequate oxygen, it is reasonable to believe that the blood vessels would relax and therefore dilate. Also, increased utilization of oxygen in the tissues as a result of increased metabolism theoretically could decrease the availability of oxygen to the smooth muscle fibers in the local blood vessels, causing local vasodilation.
A mechanism whereby oxygen availability could operate is shown in Figure 17-3 . This figure shows a tissue vascular unit, consisting of a metarteriole with a single sidearm capillary and its surrounding tissue. At the origin of the capillary is a precapillary sphincter and around the metarteriole are several other smooth muscle fibers. When observing such a tissue under a microscope, the precapillary sphincters are normally completely open or completely closed. The number of precapillary sphincters that are open at any given time is roughly proportional to the requirements of the tissue for nutrition. The precapillary sphincters and metarterioles open and close cyclically several times per minute, with the duration of the open phases being proportional to the metabolic needs of the tissues for oxygen. The cyclical opening and closing is called vasomotion .
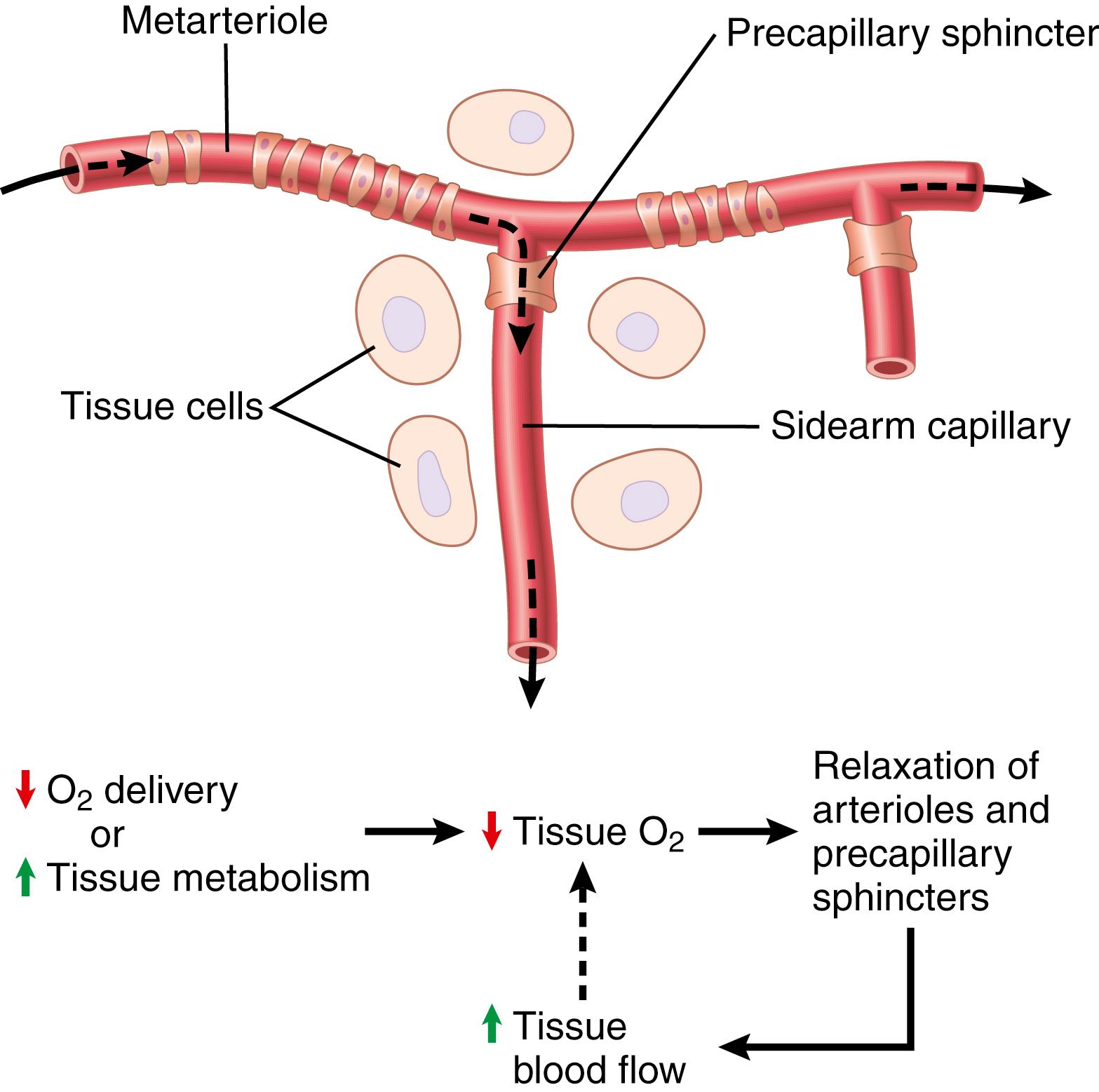
Because smooth muscle requires oxygen to remain contracted, one might assume that the strength of contraction of the sphincters would increase with an increase in oxygen concentration. Consequently, when the oxygen concentration in the tissue rises above a certain level, the precapillary and metarteriole sphincters presumably would close until the tissue cells consume the excess oxygen. However, when the excess oxygen is gone and the oxygen concentration falls low enough, the sphincters open once more to begin the cycle again.
Thus, on the basis of available data, either the vasodilator substance theory or oxygen demand theory could explain acute local blood flow regulation in response to the metabolic needs of the tissues. It is probably a combination of the two mechanisms.
Under special conditions, it has been shown that the lack of glucose in the perfusing blood can cause local tissue vasodilation. It also is possible that this same effect occurs when other nutrients, such as amino acids or fatty acids, are deficient, although this is still uncertain. In addition, vasodilation occurs in the vitamin deficiency disease beriberi , in which the patient has deficiencies of the vitamin B substances thiamine, niacin , and riboflavin. In this disease, the peripheral vascular blood flow almost everywhere in the body often increases twofold to threefold. Because all these vitamins are necessary for oxygen-induced phosphorylation, which is required to produce ATP in the tissue cells, one can understand how deficiency of these vitamins might lead to diminished smooth muscle contractile ability and therefore local vasodilation as well.
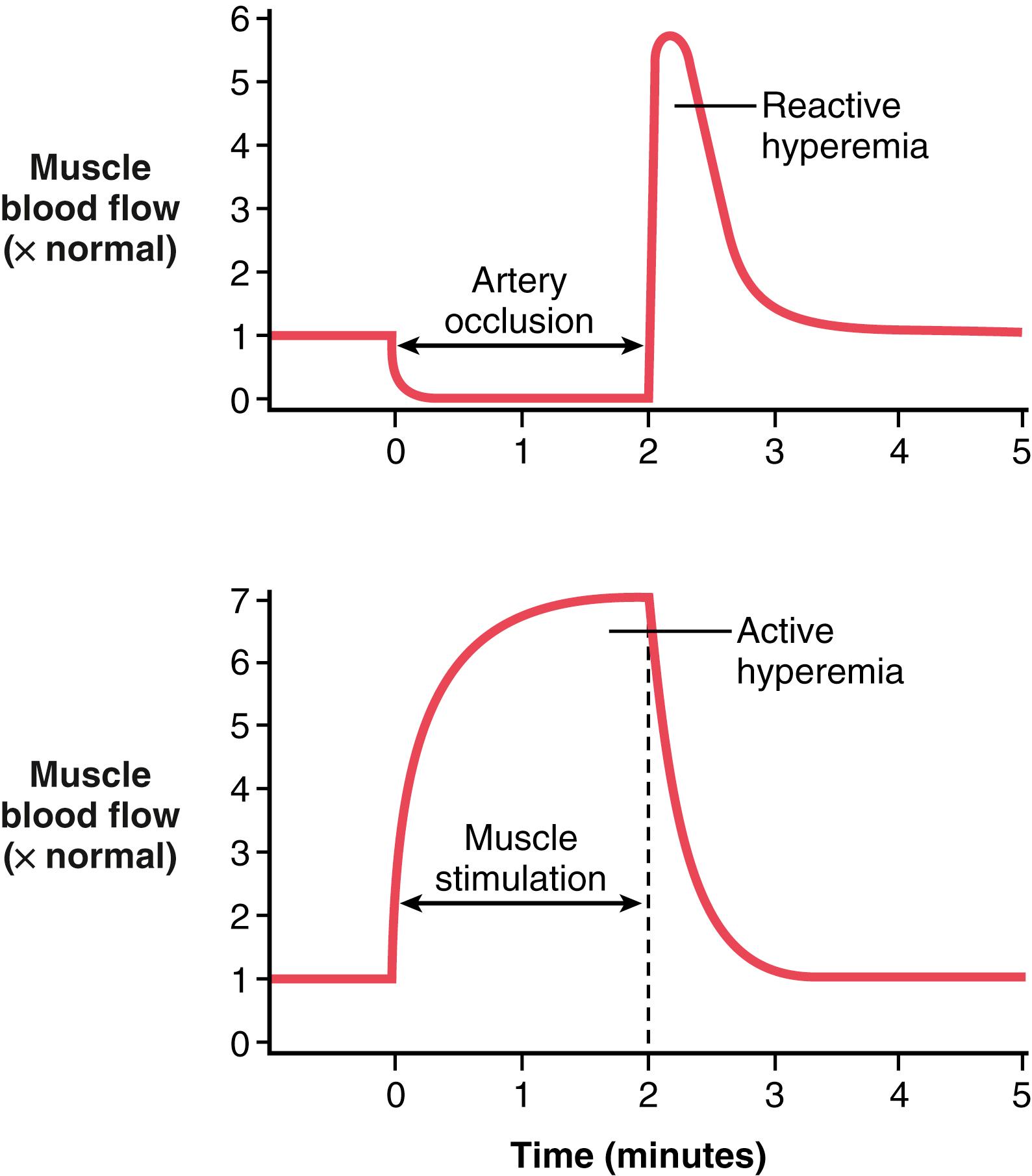
The mechanisms we have described thus far for local blood flow control are called metabolic mechanisms because they all function in response to the metabolic needs of the tissues. Two additional special examples of metabolic control of local blood flow are reactive hyperemia and active hyperemia ( Figure 17-4 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here