Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hepatic parenchymal cells (hepatocytes and cholangiocytes) and nonparenchymal cells (hepatic sinusoidal endothelial cells, stellate cells, Kupffer cells, and pit cells) have distinct functions that are integrated through extensive cross-talk. These cells are highly polarized. The distinctive polarization pattern of hepatocytes is unique among glandular epithelial cells. Maintenance of hepatocyte polarization requires energy-consuming processes and is key to the wide array of liver functions. Hepatocytes are organized into functional zones: zone 1 (periportal), zone 2 (midzonal), and zone 3 (pericentral) (see Chapter 71 ). Gene expression and differential function of hepatocytes in the different zones appear to be regulated by a gradient of cell signaling molecules. Although adult hepatocytes are normally relatively quiescent, they retain a lifelong capacity for massive regeneration following liver injury or loss of volume. The critical role of bile acid signaling as a part of the bile acid-farnesoid X receptor-fibroblast growth factor axis in liver regeneration, and eventual cessation of proliferation when a body weight-appropriate liver mass is achieved, is being revealed. In addition to its synthetic and secretory functions, the liver plays a central role in energy metabolism of the body by orchestrating the synthesis, utilization, and catabolism of carbohydrates, proteins, and lipids. The liver’s molecular “clock” synchronizes the body’s energy needs to the availability of nutrients.
Liver cells can be classified into 3 groups: parenchymal cells, including hepatocytes and bile duct epithelia; sinusoidal cells, which are composed of hepatic sinusoidal endothelial cells and Kupffer cells (hepatic macrophages); and perisinusoidal cells, which consist of hepatic stellate cells and pit cells. Hepatocytes comprise 60% of the adult liver cell population, representing approximately 78% of the tissue volume (see Chapter 71 ).
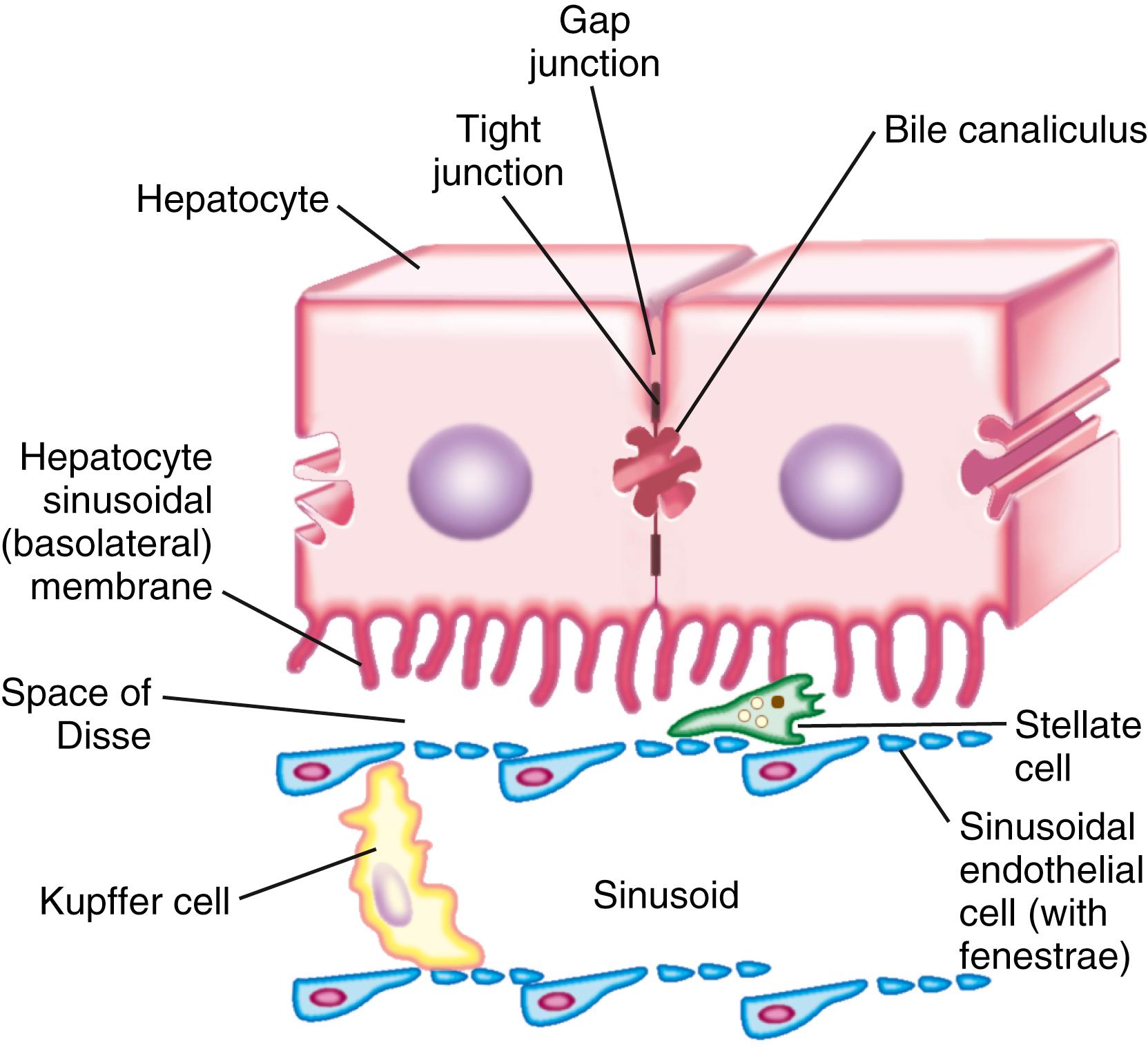
Hepatocytes are large polyhedral cells approximately 20 to 30 μm in diameter. Consistent with their high synthetic and metabolic activity, hepatocytes are enriched in organelles. About 30% of human hepatocytes are binucleate. Hepatocytes are polarized epithelial cells. Their plasma membranes have 3 distinct domains: (1) the sinusoidal surface (∼37% of the cell surface) that comes in direct contact with plasma through the fenestrae of the specialized hepatic sinusoidal endothelial cells; (2) the canalicular surface (∼13% of the cell surface) that encloses the bile canaliculus (BC); and (3) contiguous surfaces. By analogy with glandular epithelia, the sinusoidal, canalicular, and contiguous plasma membrane domains are also termed basolateral, apical, and lateral surfaces, respectively. The sinusoidal and canalicular surfaces contain microvilli, which greatly extend the surface area of these domains.
The unique type of polarization of hepatocytes differs from that of other epithelial cells, such as epithelia of the intestines, bile duct, or renal tubules, that are polarized in the plane of the tissue. By contrast, in hepatocytes, “apical” plasma membranes of 2 adjacent cells join to enclose the BC, which is the smallest tributary of the bile duct system.
Maintenance of both structural and functional polarity of hepatocytes requires mitochondrial energy production, which is regulated by 5’ adenosine monophosphate-activated protein kinase. Structural polarity is supported by (1) the extracellular matrix, which, in addition to serving as an attachment scaffold, functions as a signaling platform needed for maintenance of the differentiated phenotype of hepatocytes; and (2) desmosome (tight junction) proteins, such as claudin 1 and tight junction protein 2, that delimit the bile canalicular space. Functional polarity of hepatocytes is conferred by distinctive localization of various solute carriers, ion channels, water channels, and ATP-driven pumps in specific plasma membrane domains. Basolateral membrane proteins traffic directly to this domain after their synthesis in the endoplasmic reticulum and modification in the Golgi apparatus and the trans-Golgi network (TGN). Some of these proteins are monotopic in that they anchor only to the inner leaflet of the plasma membrane bilayer via glycosylphosphatidylinositol, whereas others are polytopic in that they traverse the membrane bilayer. Canalicular monotopic glucosyl phosphatidylinositol-terminated proteins such as 5’-nucleotidase initially traffic from the TGN to the basolateral domain and are transported from there to the canalicular domain via apical recycling endosomes. By contrast, canalicular polytopic transporters such as the ATP-binding cassette (ABC) proteins traffic from the TGN to the canalicular membrane either directly or via apical recycling endosomes. Protein cargo destined for apical and basolateral sites are thought to be sorted at the TGN and possibly at additional sites.
Plasma membranes consist of lipid bilayers composed of glycerophospholipids, cholesterol, and sphingolipids that act as a barrier to water and most polar substances. The inner and outer leaflets of the plasma membrane differ in lipid, protein, and carbohydrate composition, reflecting their functional differences. Proteins within the leaflets mediate transport of specific molecules and serve as a link with cytoskeletal structures and the extracellular matrix. Hepatocyte plasma membranes consist of 36% lipid, 54% protein, and 10% carbohydrate by dry weight. The outer leaflets of hepatocyte plasma membranes are enriched in carbohydrates.
Lipid rafts are microdomains (∼50 nm diameter) of the outer leaflets of the plasma membrane that are highly enriched in cholesterol and sphingolipids. These are coupled to cholesterol-rich microdomains in the inner leaflet by an unknown mechanism. Raft lipids and associated proteins diffuse together laterally on the membrane surface. Some surface receptors become associated with the rafts on binding to a ligand, or lead to “clustering” of smaller rafts into larger ones. Lipid rafts are important in signal transduction, apoptosis, cell adhesion and migration, cytoskeletal organization, and protein sorting during both exocytosis and endocytosis (see later). Some viruses enter cells via the lipid rafts.
Membrane proteins perform receptor, enzyme, and transport functions. Integral membrane proteins traverse the lipid bilayer once or more or are buried in the lipid. Additional “extrinsic” protein molecules are associated with the plasma membrane. Membrane proteins can rotate or diffuse laterally but usually do not flip-flop from one leaflet to another. Concentration of specific membrane proteins is maintained by a balance between their synthesis and degradation as a result of shedding of membrane vesicles, proteolytic digestion within the membrane, or internalization into the cell. Receptor proteins internalized into the cell may be degraded or recycled to the cell surface.
The space between the endothelia and the sinusoidal villi is termed the space of Disse (see Chapter 71 ). In this space, there is bidirectional exchange of liquids and solutes at the sinusoidal surface between the plasma and hepatocytes. In many cases, the molecular transfer is augmented by proteins that facilitate diffusion along a downhill concentration gradient or use ATP-derived energy to actively pump molecules into the space of Disse. The fluid in the space of Disse drains into hepatic lymphatics, which lead to liver hilum lymphatics, cisterna chyli, the thoracic duct, and, eventually, the central venous circulation. Excess fluid in the space of Disse gains access to Glisson capsule on the liver surface and “sweat out” to form ascites.
Hepatocytes are organized into sheets (seen as cords in 2-dimensional sections) separated by occluding (“tight”), communicating (“gap”), and anchoring junctions ( Fig. 72.1 ). Tight junctions or desmosomes form gasket-like seals around the bile canaliculi, thereby permitting a concentration difference of solutes between the cytoplasm and BC. Desmosomes are specialized membrane structures that anchor intermediate filaments to the plasma membrane and link cells together. Gap junctions are subdomains of contiguous membranes of hepatocytes that comprise approximately 3% of the total surface membrane. They consist of hexagonal particles with hollow cores, termed connexons, made up of 6 connexin molecules. Connexons of one cell are joined to those of an adjacent cell to form a radially symmetrical cylinder that can open or close the central channel. Gap junctions are involved in nutrient exchange, synchronization of cellular activities, and conduction of electrical impulses.
The hepatocyte cytoskeleton supports the organization of subcellular organelles, cell polarity, intracellular movement of vesicles, and molecular transport. It is comprised of microfilaments, microtubules, and intermediate filaments, as well as cytoskeleton-associated proteins. Intermediate filaments are polymers of fibrous polypeptides (cytokeratins and lamins) that provide structural support to cells. In addition, neurofilaments appear in injured hepatocytes and form Mallory bodies (also termed Mallory-Denk bodies or Mallory hyaline). Hepatocytes express 2 cytokeratins, CK8 and CK18. Bile duct epithelial cells express these proteins and CK19. Plectin is a giant protein that cross-links intermediate filaments to each other and to the plasma membrane, microtubules, and actin filaments.
Microtubules are hollow tubular structures (with an outer diameter of 24 nm) composed of polymerized dimers of α- and β-tubulin that are involved in intracellular transport and cellular organization. Microtubules serve as tracks for the movement of cytoplasmic vesicles, mediated by the adenosine triphosphatase (ATPase)-powered motor proteins kinesin, dynein, and dynamin. Depolymerization of the microtubules, by, for example, colchicine treatment inhibits plasma protein secretion without affecting protein synthesis. Microtubules participate in cellular organization by interacting with the Golgi apparatus, intermediate filaments, and F-actin. They also maintain the integrity of the surface membrane during canalicular contraction.
Microfilaments are composed of double helical F-actin strands, which are polymers of G-actin. A large number of actin-associated proteins control the polymerization, depolymerization, and splicing of F-actin. Together with myosins, actins maintain the integrity of the cell matrix, facilitate bile canalicular contraction, and control tight junction permeability. Microfilaments are also important for receptor-mediated endocytosis (RME) and several transport processes. Collapse of the cellular structure of hepatocytes during apoptosis and formation of apoptotic bodies may be related to remodeling of the actin cytoskeleton of hepatocytes.
The nuclei of hepatocytes are relatively large and have prominent nucleoli. The 2 concentric nuclear membranes are stabilized by networks of intermediate filaments, 1 inside the inner membrane and 1 outside the outer membrane. The outer nuclear membrane is in direct continuity with the ER membranes. The perinuclear space between the 2 nuclear membranes surrounds the nucleus and is continuous with the ER lumen. The nuclear membrane contains pores through which molecules are selectively transported to and from the cytoplasm. The ribonucleoprotein network and the perinucleolar chromatin radiate from the nucleolus.
The nuclear chromatin contains the chromosomes and associated proteins. The chromosomes are comprised of a series of genes, interspersed with intragenic DNA. The DNA is transcribed into RNA, which undergoes multiple processing steps, giving rise to messenger RNA (mRNA) molecules that are translocated across the nuclear pores into the cytoplasm, where they become associated with ribosomes. Nuclear DNA also encodes additional RNA types that have accessory roles in protein synthesis and other functions. Ribosomal RNAs (rRNAs) are encoded by DNA within the nucleolus. Transfer RNA (tRNA) binds to amino acids and provides a necessary link between the nucleic acid code and sequential amino acid incorporation in the growing protein chain during translation. Other RNAs are involved in the processing of mRNA, rRNA, and tRNA molecules. Just before cell division, both the DNA and protein components of chromatin are duplicated. The 2 copies of each duplicated chromosome are separated and distributed precisely so that the 2 daughter cells each receive a complete set of genes.
Pores of the nuclear envelope are associated with a large number of proteins, which are organized in an octagonal symmetry. The nuclear pore complex (NPC) is a large macromolecular assembly that protrudes into both the cytoplasm and the nucleoplasm. Bidirectional nucleocytoplasmic transport occurs through the central aqueous channel in NPCs. Histones, DNA and RNA polymerases, transcription factors, and RNA processing proteins are selectively transported into the nucleus from the cytoplasm, where they are synthesized, whereas tRNAs and mRNAs are synthesized in the nucleus and exported to the cytoplasm through the NPCs.
Often, the export and import processes are interrelated. For example, ribosomal proteins are imported into the nucleus from the cytoplasm and, after assembly with ribosomal RNA, are exported to the cytoplasm as a ribosomal subunit. Proteins containing nuclear localization motifs that consist of specific cationic amino acid sequences are recognized by pore complex receptors, termed importins or karyopherins, and are rapidly transported into the nucleus via an energy-consuming process powered by specific ATPase/guanosine triphosphatase (GTPase) enzymes. In other cases, large molecules diffuse slowly through the nuclear pores and are retained in the nucleus by binding to specific intranuclear sites. Molecules that are smaller than 5 kd diffuse freely across the nuclear pores.
The ER is the largest intracellular membrane compartment, consisting of membranous tubules or flattened sacs (cisternae) that enclose a continuous lumen or space and extend throughout the cytoplasm. The domain of the ER in which active protein synthesis occurs has attached ribosomes and is termed the rough ER. The other domain, termed smooth ER, is devoid of ribosomes and is the site of lipid biosynthesis, detoxification, and calcium regulation. The nuclear envelope is a specialized domain of the ER.
The Golgi complex consists of a stack of flat sac-like membranes (cisternae) that are dilated at the margins. Many proteins synthesized in the rough ER are transported to the Golgi apparatus in protein-filled transition vesicles. The aspect of the Golgi complex facing the ER is the cis face; the opposite side is termed the trans face. Glycoproteins are thought to be transported between the Golgi sacs via shuttle vesicles. The highly mannosylated glycosyl moiety of proteins that are N -glycosylated in the ER are processed in the Golgi sacs into mature forms. Some other proteins are O -glycosylated in the Golgi complex. These proteins are then sorted for transport to appropriate cellular organelles (see later discussion of exocytosis and endocytosis).
Lysosomes consist of a system of membrane-bound sacs and tubules that contain hydrolytic enzymes that are active at pH 4.5 to 5. The ATPase-powered proton pump maintains the acid pH by importing hydrogen ions into the lysosomal lumen. Lysosomal enzymes are glycoproteins with N -linked oligosaccharides. Following synthesis in the ER, the carbohydrate moieties are modified in the Golgi apparatus, where their mannose residues are phosphorylated. Recognition of these mannose 6-phosphate (M6P) groups by the M6P receptor in trans-Golgi stacks results in their segregation and translocation into late endosomes, which transform into lysosomes.
Mitochondria constitute about 20% of the cytoplasmic volume of hepatocytes and are responsible for cellular respiration. They contain the enzymes of the tricarboxylic acid (TCA) cycle, fatty acid oxidation, and oxidative phosphorylation. Mitochondria conserve the energy generated by oxidation of substrates as high-energy phosphate bonds of ATP. In addition, parts of the urea cycle, gluconeogenesis, fatty acid synthesis, regulation of intracellular calcium concentration, and heme synthesis take place in the mitochondria, which also play a key role in programmed cell death, or apoptosis (see later).
The outer smooth surface membrane of the mitochondrion is functionally different from the inner membrane, which is highly folded to form cristae. Mitochondria are positioned at major sites of ATP utilization by translocation along microtubules. In addition to soluble enzymes, the mitochondrial matrix includes large intramitochondrial granules that store calcium and other ions and smaller granules that contain mitochondrial ribosomes. Mitochondrial DNA, embedded within the matrix, encodes a number of mitochondrial proteins. The remaining mitochondrial proteins are encoded by nuclear genes.
Glycolysis and fatty acid oxidation in the mitochondria generate chemical intermediates that feed into the TCA cycle of energy-yielding reactions. The TCA cycle breaks down acetyl coenzyme A (acetyl CoA) into 3 molecules of nicotinamide adenine dinucleotide (NADH), 1 molecule of flavin adenine dinucleotide (FADH 2 ), and 2 molecules of carbon dioxide. Electrons derived from NADH and FADH 2 drive an electron transport pathway in the inner mitochondrial membrane, leading to ATP production. Passage of electrons across the inner mitochondrial membrane to the space between the inner and outer membrane generates a proton gradient that drives ATP synthesis.
Peroxisomes are spherical-appearing structures that enclose a matrix that contains a lattice or crystalline core. Peroxisomes are abundant in hepatocytes and are thought to be essential for life. Several oxidative catabolic reactions, as well as anabolic reactions, take place in peroxisomes, which provide important links between the metabolism of carbohydrates, lipids, proteins, fats, and nucleic acids.
Exocytosis and endocytosis are pathways involved in exporting, importing, and intracellular trafficking of molecules. The addition of new proteins and lipids to the plasma membrane by exocytosis and removal of membrane components into cytoplasmic compartments by endocytosis keep the cell surface in a state of dynamic polarization. During exocytosis, secreted proteins, synthesized in the ER, pass sequentially through the cis-, medial -, and trans -Golgi stacks and the TGN and finally appear at the cell surface. This vectorial transport through the Golgi stacks occurs via vesicles that are coated by proteins termed coatamers, or COP (COPI and COPII), which are distinct from clathrin (see later). Guanosine triphosphate-guanosine diphosphate (GTP-GDP) exchange factors and GTP-activating proteins that are specific for each type of vesicle stimulate membrane binding and catalytic activation of small GTPases.
Once bound to the membrane, GTPases induce recruitment of COP proteins. In the ER, the first coat protein to be recruited is COPII, and vesicular/tubular clusters are formed. These clusters are thought to coalesce to form a complex tubular network, termed the ER/Golgi intermediate compartment. Acquisition of COPI proteins by the membranes of this tubular network results in the formation of vesicles that carry out bidirectional protein transport to and from the Golgi stacks. Some vesicles that emerge from the exit side of the Golgi apparatus, termed the TGN, can transport multiple protein molecules simultaneously and release them together into the extracellular medium. Other types of vesicles that carry membrane proteins and enzymes destined for specific intracellular organelles also pass through this secretory pathway. These vesicles are sorted at the TGN, and vesicles carrying specific cargo are delivered to appropriate target organelles.
Endocytosis is the import of extracellular macromolecules by processes that include pinocytosis, phagocytosis, RME, and caveolar internalization. Pinocytosis refers to nonselective bulk-phase uptake of extracellular fluid via engulfment by plasma membrane invaginations. Phagocytosis is the ingestion of particles as well as regions of the cell surface. In contrast to these nonspecific modes of uptake, RME is a mechanism of uptake of specific molecules (ligands). After the ligands bind to their specific cell surface receptors, the ligand-receptor complexes concentrate in “pits” that are coated on the cytoplasmic surface by 3-pronged structures (triskelions) composed of 3 heavy chains and 3 light chains of clathrin. The assembled coats consist of a geometric array of 12 pentagons and a variable number of hexagons, depending on the size of the coat. The coated pits pinch off into the underlying cytoplasm as coated vesicles. In the next step, the vesicles lose their clathrin coat and are termed endosomes. Endosomal vesicles travel along microtubules and can take 3 distinct pathways. Some endosomes return to the cell surface, and the contained ligand-receptor complexes are secreted out of the cells by a process termed diacytosis . Transferrin is a prototype ligand for diacytosis. Some other ligands, such as immunoglobulin A (IgA) oligomers, may traverse the cells to be secreted into bile along with the receptor. This process is termed transcytosis .
The best studied type of RME is the classical endocytotic pathway, in which the interior of the endosome is acidified by the action of a proton pump, thereby leading to ligand-receptor uncoupling. By mechanisms that have not been elucidated fully, the dissociated ligands and receptors are sorted into different vesicles. The ligand-containing vesicles proceed to lysosomes, where the ligand is degraded by lysosomal hydrolases. A majority of the ligand-free receptors translocate to the cell surface and replenish the receptor pool. Some receptors, such as the insulin receptor, do not undergo recycling and are rapidly degraded in lysosomes. In addition to the recruitment of clathrin, initiation of the formation of endocytotic vesicles requires adaptor proteins, particularly AP-2, which localizes between the lipid bilayer and clathrin. Non-scaffold proteins, such as the GTPases and dynamin, are also important in the conversion of a coated pit to a coated vesicle. This function of dynamin requires association with a protein termed amphiphysin. Genetic, cell biological, and biochemical studies are identifying additional proteins that are required for clathrin coat and vesicle formation (reviewed by Stockert ). In addition to physiologic ligands, many viruses use RME to enter cells.
Internalization via caveolae is another pathway by which macromolecules can enter cells. Binding of caveolin to the cytoplasmic aspect of cholesterol-rich lipid rafts on the plasma membrane generates 50- to 60-nm flask-shaped invaginations of the plasma membrane. These invaginations bud off into the cytoplasm to form vesicles, termed caveolae or plasmalemmal vesicles . Caveolae perform several functions, including signal transduction, calcium regulation, non‒clathrin-dependent internalization, and transcytosis. Glucosyl phosphatidylinositol-anchored proteins, the β-adrenergic receptor, and tyrosine kinase are concentrated in caveolae.
Hepatocytes are organized in metabolic zones within the liver cell plates in a manner that optimizes their metabolic function to internalize molecules arriving through the portal vein and hepatic artery and to biotransform, synthesize, and secrete products into the systemic circulation via the hepatic vein and to the intestine through the bile ducts (see Chapter 71 ). The functional unit of the liver consists of a row of 15 to 25 hepatocytes extending from the periportal region (zone 1) toward the central vein (zone 3 or pericentral). For example, hepatocytes in zone 1 that are exposed to highly oxygenated blood are enriched in enzymes involved in energy-demanding functions, such as gluconeogenesis and urea production, whereas zone 3 hepatocytes specialize in glycolysis and xenobiotic metabolism. Correspondingly, zone 1 hepatocytes express Ass110 , As110 , Alb8 , and cyp2f29 , whereas zone 3 hepatocytes express Glul and Cyp2e19 in a nearly mutually exclusive manner. In addition, zone 2 (midzonal) hepatocytes are enriched in the expression of certain genes, such as Hamp and Hamp2 (that encode hepcidin, a liver hormone that regulates systemic iron levels [see Chapter 75 ]), Igfbp2 , Mup3 , and Cyp8b1 . Overall, nearly half of all genes expressed in hepatocytes are spatially zonated.
The developmental mechanism of hepatic zonation appears to be based on the spatial separation and functional antagonism between the adenomatous polyposis coli gene product expressed in zone 1 cells and β-catenin in zone 3, which is activated by Wnt signaling from endothelial cells in zone 3. In the absence of Wnt signals, a degradation complex that contains the products of the tumor suppressor genes adenomatous polyposis coli and axins and the kinases GSK-3β and CK1 promotes phosphorylation and subsequent degradation of β-catenin. The lack of β-catenin signaling in zone 1 and an ascending gradient of the signaling toward zone 3 is thought to generate and maintain the zonation of gene expression and function of the liver.
Bile duct epithelial cells, or cholangiocytes, consist of large and small subpopulations of cells, the cell volumes of which correlate roughly with the diameter of the intrahepatic bile ducts (see Chapter 62 ). Large cholangiocytes have a relatively more developed ER and a lower nucleus-to-cytoplasmic ratio than do small cholangiocytes. Low expression of cytochrome P450 (CYP)-dependent monooxygenase activity imparts a survival advantage to the small cholangiocytes against injury by chemicals. For example, CYP2E1-mediated formation of toxic intermediates of carbon tetrachloride leads to the loss of large cholangiocyte function after administration of the pro-toxin, whereas small cholangiocytes are resistant to the toxin.
Bile ducts are not mere passive conduits for biliary drainage but play an active role in the secretion and absorption of biliary components, as well as regulation of the extracellular matrix composition. Cholangiocytes are highly polarized. A sodium-dependent bile salt transporter (ABAT), located at the apical (luminal) surface of cholangiocytes, mediates the uptake of conjugated bile acids, whereas an alternatively spliced truncated form of the protein (ASBT), located at the basolateral surface, mediates sodium-independent efflux of bile acids (see Chapter 64 ). The sodium-dependent glucose transporter (SGLT1), located at the apical domain, and GLUT1, a facilitative glucose transporter on the basolateral domain, are responsible for glucose reabsorption from bile. Aquaporin-1 at the apical and basolateral surfaces constitutes a water channel that may mediate hormone-regulated transport of water into bile. The purinergic receptor (P 2u ) stimulates chloride ion efflux. Activation of apical P 2u by ATP, which is secreted into the bile by hepatocytes, mobilizes Ca 2+ stores, thereby stimulating Cl − efflux from cholangiocytes. The large, but not the small, cholangiocytes express secretin and somatostatin receptors, the chloride/bicarbonate exchanger, and the CFTR, which may enable this population of cholangiocytes to modulate water and electrolyte secretion in response to secretin and somatostatin.
Cholangiocytes are the only liver cells with primary cilia. “Primary” bile secreted by hepatocytes is subsequently modified by cholangiocytes, which modulate the fluidity and alkalinity of bile by secreting Cl − and HCO 3 − and by absorbing bile salts, glucose, and amino acids, followed by passive movement of water into or out of the bile duct lumen along osmotic gradients. These functions require sensing the flow rate, osmolality, and composition of bile, which is provided by primary cilia of cholangiocytes. Each cholangiocyte has 1 primary cilium that consists of a shaft, termed the axoneme , which is composed of 9 peripheral microtubule doublets arranged around a hollow central core. The axoneme is attached to a centriole-derived microtubule organizing center, termed the basal body . Axonemes of large cholangiocytes are 7.35 ± 1.32 μm long, whereas those of small cholangiocytes are approximately half as long. The primary cilium extends from the apical (luminal) plasma membrane into the bile duct lumen and is, therefore, positioned strategically to serve as a mechanoreceptor, osmoreceptor, and chemoreceptor that modulates the secretory and absorptive functions of cholangiocytes in response to the pulsatile flow of primary bile.
Hepatic sinusoidal endothelial cells (HSECs) account for 20% of total liver cells. HSECs are derived from hemangioblasts and endocardium of the sinus venosus. These cells are distinguished from capillary endothelial cells by the presence of fenestrae (pores) in their flat, thin extensions, which form sieve plates. Unlike capillary endothelial cells, HSECs do not form intracellular junctions and simply overlap each other (see Fig. 72.1 ). The presence of fenestrae and the absence of a basement membrane make these cells the most permeable of all endothelial cells of the mammalian body and permit plasma to enter the space of Disse and come in direct contact with the sinusoidal surface of hepatocytes. Sieve plates are surrounded by microtubules, and the diameter and number of the fenestrae are actively controlled by the actin-containing components of the cytoskeleton in response to changes in the chemical milieu. Thus, the specialized endothelial lining of hepatic sinusoids serves as a selective barrier between the blood and the hepatocytes. HSECs can secrete prostaglandins and a wide variety of proteins, including interleukin (IL)-1 and IL-6, interferon, TNF-α, and endothelin. HSECs regulate hepatic vascular tone, which helps maintain a low portal venous pressure despite major increases in hepatic blood flow during digestion. HSECs keep hepatic stellate cells quiescent, thereby inhibiting intrahepatic vasoconstriction and development of fibrosis. By virtue of an abundance of scavenger receptors and mannose receptors, HSECs have a high endocytotic capacity and clear several metabolites and microbial products.
Following loss of liver mass due to acute liver injury or partial hepatectomy, hepatic vascular endothelial growth factor (VEGF) expression increases, which stimulates bone marrow sinusoidal progenitor cell proliferation and their mobilization to the circulation, followed by engraftment in liver sinusoids and differentiation to mature HSECs. VEGF stimulates liver regeneration through production of hepatocyte growth factor (HGF) by HSECs, which leads to the proliferation of both hepatocytes and HSECs. Additionally, increased shear stress resulting from portal blood flow into a smaller liver volume stimulates HSECs to produce nitric oxide (NO), which in turn augments the effect of HGF on hepatocytes. Platelets recruited to the liver after partial hepatectomy adhere to HSECs and stimulate secretion of molecules that are important in hepatocyte and HSEC proliferation and survival.
Kupffer cells are specialized tissue macrophages that account for 80% to 90% of the total population of fixed macrophages in the body. These cells are derived from bone marrow stem cells or monocytes and are highly active in removing particulate matter and toxic or foreign substances that appear in the portal blood from the intestine. Kupffer cells are located in the sinusoidal lumen and are in direct contact with endothelial cells (see Fig. 72.1 ). They possess bristle-coated micropinocytic vesicles, fuzzy-coated vacuoles and worm-like structures that are special features of cells that are active in pinocytosis and phagocytosis. An abundance of lysosomes reflects their prominent role in degrading substances taken up from the bloodstream. Kupffer cells secrete a variety of vasoactive toxic mediators, which may be involved in host defense mechanisms and in pathophysiologic processes in some liver diseases. Kupffer cells increase in number and activity in chemical, infectious, or immunologic injury to the liver.
Hepatic stellate cells (HSCs) are also known as Ito cells, vitamin A–storing cells, fat-storing cells, and lipocytes. These cells are a part of the stellate cell system, which includes similar cells in the pancreas, lung, kidney, and intestine. HSCs are located between the endothelial lining and hepatocytes (see Fig. 72.1 ). These mesenchymal cells represent 5% to 8% of all liver cells and are important sources of paracrine, autocrine, juxtacrine, and chemoattractant factors that maintain homeostasis in the microenvironment of the hepatic sinusoid. Microfilament and microtubule-enriched flat cytoplasmic extensions of quiescent stellate cells store vitamin A–enriched lipid droplets and spread out parallel to the endothelial lining, thereby contacting several cells. HSCs express receptors for retinol-binding protein, which mediates the endocytosis of retinol-binding protein-retinol complexes.
After chronic liver injury, the slender star-shaped HSCs become activated to elongated myofibroblasts. They lose retinoids and up-regulate the synthesis of extracellular matrix components, such as collagen, proteoglycan, and adhesive glycoproteins. Stellate cell activation is the central event in hepatic fibrosis. Activation of HSCs is initiated by paracrine stimulation by neighboring HSECs, Kupffer cells, other endothelial cells, and hepatocytes, as well as platelets and leukocytes. Endothelial cells participate in activation by producing cellular fibronectin and by converting the latent form of TGF-β to its active, profibrogenic form. Binding of TGF-β to its receptor on HSCs plays a critical role in stellate cell activation. Binding of bacterial lipopolysaccharides (LPS) arriving to the liver from the intestine to Toll-like receptor 4 (TLR4) enhances the effect of TGF-β on HSCs by 2 different mechanisms. First, increased chemokine expression by HSCs results in chemotaxis of Kupffer cells, which secrete TGF-β. Second, binding of LPS to TLR4 activates nuclear factor kappa B (NF-ĸB) via the adapter protein MyD88 (myeloid differentiation response protein), thereby down-regulating the TGF-β pseudoreceptor BAMBI (bone morphogenetic protein and the activin membrane-bound inhibitor) and thereby sensitizing the HSCs to TGF-β signaling. The 3-dimensional structure of the extracellular matrix modulates the shape, proliferation, and function of HSCs, probably by signal transduction via binding to cell surface integrins, followed by changes in cytoskeleton assembly.
Activation of HSCs is perpetuated by the continued effect of these stimuli, leading to several discrete changes in cell behavior, such as proliferation, contractility, overexpression of extracellular matrix proteins (e.g., collagens I, III, IV, V, and VI; laminin; tenascin; undulin; hyaluronic acid; and proteoglycans), matrix degradation by release of metalloproteinases, and release of leukocyte chemoattractants and cytokines. The overall number of HSCs increases during fibrosis because of a change in the balance between proliferation and apoptosis, which is influenced by soluble growth factors and the matrix.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here