Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We appreciate the contributions of Dr. Jacqueline Wolf to the previous editions of this chapter.
Pregnancy is an altered physiological state designed to support the developing fetus, and gastrointestinal complaints are common during pregnancy. Although de novo abnormalities of the liver occur infrequently, they require prompt diagnosis and treatment to avoid the potentially high rates of maternal and fetal morbidity and mortality associated with them.
This chapter focuses on the pathophysiology and diagnosis of liver diseases that are unique to pregnancy. In most cases, the panoply of diseases discussed constitutes the differential diagnosis. In practice, some of the most frequent causes of liver disease in pregnancy are common disorders such as viral hepatitis and gallstone disease ( Table 54.1 ), and the differential diagnosis of liver pathology in pregnancy must always be broadened to include these abnormalities.
| Condition ∗ | 1st Trimester | 2nd Trimester | 3rd Trimester |
|---|---|---|---|
| Hyperemesis gravidarum | X | X | |
| Gallstones | X | X | X |
| Hepatitis (various etiologies) | X | X | X |
| Intrahepatic cholestasis of pregnancy (ICP) | X | X | X |
| Preeclampsia/eclampsia | X | X | |
| Hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome | X | X † | |
| Acute fatty liver of pregnancy (AFLP) | X † | ||
| Budd-Chiari syndrome | X † | ||
| Hepatic rupture | X |
∗ Conditions are ordered by the typical time of presentation during pregnancy.
Hepatic histopathology in pregnancy is often nonspecific, and knowledge of the clinical history and physical signs and symptoms is essential for the diagnosis of liver disease. Evaluation of liver disease is further complicated by normal physiological changes in liver function test (LFT) results as a function of gestational age. Despite these well-known physiological alterations, most clinical laboratories use data from a normal adult population as reference ranges, leaving the correct evaluation of liver function abnormalities in the pregnant patient to the subjective judgment of clinicians and pathologists. A few important physiological changes in LFTs as a function of gestational age are depicted in Fig. 54.1 and discussed later to demonstrate the complexity of physiological changes in laboratory values during pregnancy. Given significant variability in technique and patient populations, the original literature and locally developed reference ranges must be consulted for appropriate interpretation of patient-specific laboratory results during pregnancy.
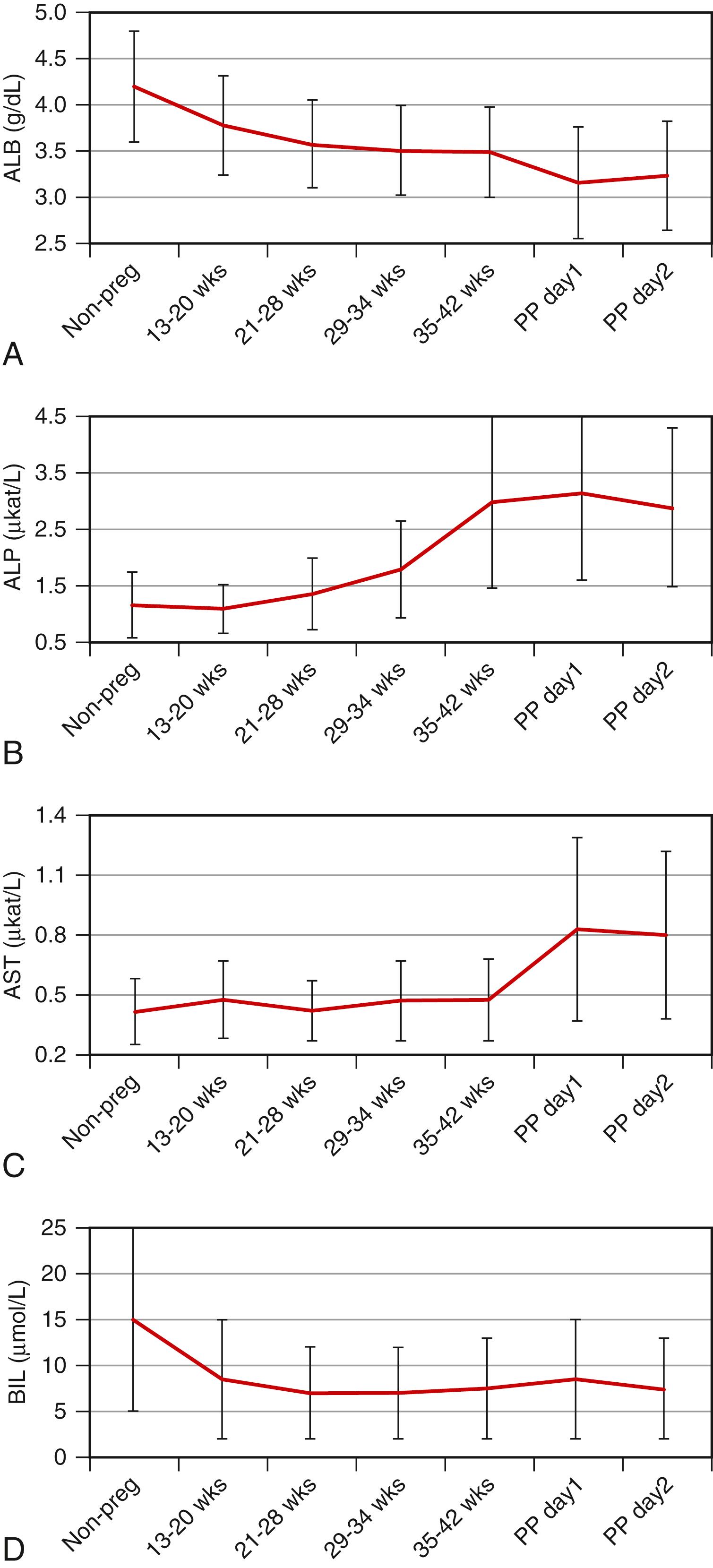
Compared with age-matched, nonpregnant women, the total serum protein and albumin levels are lower during all three trimesters ( Fig. 54.1A ), and the concentration of serum albumin can decrease by as much as 60% by the second trimester. The physiological basis of this change is a subject of debate, although hemodilution during pregnancy is thought to play a role. Among other serum proteins, the levels of coagulation factors VII to X are higher during pregnancy, and fibrinogen levels may increase. Concentrations of α- and β-globulins are also slightly higher than normal, although γ-globulin levels may decrease.
Serum alkaline phosphatase activity increases during pregnancy and is significantly higher during the third trimester ( Fig. 54.1B ), although most of this activity is thought to originate from the placenta. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltranspeptidase (GGT), 5′-nucleotidase, bilirubin, and total bile acids and the prothrombin time remain within typical reference ranges during normal pregnancy, although each of these analytes may be subject to various increasing or decreasing trends during pregnancy ( Fig. 54.1C –D). , Total, free, and conjugated bilirubin levels in all trimesters may be lower than in nonpregnant control patients, with the most significant changes (as much as 50%) occurring in total bilirubin ( Fig 54.1D ) and free bilirubin levels in the second and third trimesters. Serum triglyceride levels are higher in pregnancy, and cholesterol levels may increase by as much as 200% during the third trimester.
The histological appearance of the liver in uncomplicated pregnancy is essentially normal based on limited available evidence. Minor nonspecific changes have been described, including mild nuclear pleomorphism, increased glycogen, mild steatosis, mild portal inflammation, and reactive Kupffer cells, none with a specific diagnostic significance.
The onset of maternal symptoms in relation to the trimester of pregnancy can help in the differential diagnosis of hepatic pathology (see Table 54.1 ). Severe nausea and vomiting during the first trimester are key clinical signs of hyperemesis gravidarum (HG), a disease with a relatively benign course and outcome. Later onset of nausea and vomiting suggests preeclampsia when accompanied by headache and peripheral edema or hepatic rupture when accompanied by abdominal pain with or without systemic hypotension. Pruritus in the third trimester, particularly of the palms and soles, is characteristic of intrahepatic cholestasis of pregnancy (ICP) and typically precedes the clinical manifestation of jaundice. Right upper quadrant and midabdominal pain in the third trimester may indicate acute fatty liver of pregnancy or hepatic rupture, both of which require immediate clinical intervention. Signs and symptoms of acute and chronic viral hepatitis and extrahepatic biliary disease are the same in pregnant women as in nonpregnant women, and their onset is not generally correlated with the gestational age.
With an estimated average incidence of 0.1% to 2% in all pregnancies, ICP is one of the most important pregnancy-associated hepatic diseases. Geographic, ethnic, and familial clustering is well described in ICP and must be considered in the diagnostic evaluation. The average incidence of ICP in Europe and the United States varies from 0.1% to 1.5%, whereas the estimated incidence in South America is up to 4%. However, the declining incidence of ICP in countries such as Chile and Bolivia, as well as the seasonal variations in incidence, suggest an environmental contribution to the disease. , ,
Specific geographic regions also show significant ethnic variations, suggesting a genetic linkage. For instance, the incidence of ICP is significantly higher among the native Araucanian (Chile) and Aimara (Bolivia) people than other populations in the same regions. , Similarly, in northern California, the incidence of ICP is higher among the Latina population than among non-Hispanic whites.
Some studies have suggested that ICP may represent the common clinical manifestation of a pathologically heterogeneous group of disorders, , , but no diagnostic criteria have been proposed for specific subclassification of the group of diseases that clinically manifest as ICP. We will therefore consider ICP a single pathological entity in the present discussions.
ICP is characterized by the triad of pruritus, abnormal LFTs (especially fasting serum bile acid levels greater than 10 μmol/L), and spontaneous resolution of signs and symptoms after delivery or pregnancy termination. ICP occurs most commonly in the late second to third trimesters, although it can manifest at any time during pregnancy.
Pruritus tends to be more severe at night and most often affects the palms of the hands, the soles of the feet, and the trunk. Jaundice occurs in 10% to 25% of cases, making ICP the second leading cause of jaundice in pregnancy after viral hepatitis. When jaundice occurs, it typically follows the onset of pruritus by 2 to 4 weeks. Other symptoms, such as dark urine, light stools or steatorrhea, nausea, vomiting, and abdominal discomfort, may occur but are less specific. Symptoms of ICP typically persist for the duration of pregnancy and resolve within 1 to 3 weeks after delivery or pregnancy termination. ,
Clinical laboratory findings in ICP ( Box 54.1 ) include a mildly elevated serum bilirubin level (mostly direct), mildly elevated levels of serum aminotransferases, and most importantly, markedly elevated levels of fasting serum bile acids. The concentration of cholic acid that is free or conjugated to taurine or glycine progressively increases from 20 to 40 weeks of gestation in women with ICP. The ratio of cholic acid to chenodeoxycholic acid is measurably higher in women with ICP (>1.5:1) than in women with a normal pregnancy. Elevated GGT levels may occur in as many as one-half of cases, providing a clue to the cause of the condition. , Dyslipidemia with elevated levels of total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein B-100 has also been observed. Increased serum autotaxin levels have been proposed to represent a sensitive and specific marker for ICP, distinguishing it from other pruritic disorders that may occur during pregnancy, but the commercial availability of this test is currently limited.
Characteristic time of onset: second to third trimester
Clinical features: pruritus with or without jaundice
Occurs in: <5%
Blood and serum assay results
↑ Serum bile acids (>10 μmol/L)
↑ Aminotransferases (1 to 4 times normal)
↑ Alkaline phosphatase (1 to 2 times normal)
↑ Bilirubin (<6 mg/dL)
↑ Cholesterol and triglycerides
Pathology: intrahepatic cholestasis, predominantly in a pericentral distribution
Pathophysiology: defects in transport across the canalicular membrane
The maternal outcome in ICP is generally favorable, , , although recent studies have suggested an association with chronic liver disease and cirrhosis. , Women with severe ICP may be at increased risk of preterm delivery relative to a healthy pregnancy comparison group (25% vs. 6.5%). The fetal complications of ICP are more serious and consist of prematurity (19% to 60%), fetal distress (22% to 33%), meconium staining (9% to 24%), and perinatal death (1% to 2%). , ,
There is evidence for a direct link between maternal fasting bile acid levels and maternal-fetal complications of ICP. In a prospective cohort study of almost 45,000 pregnancies with 693 cases of ICP, no increase in fetal risk was detected when maternal serum bile acid levels were less than 40 μmol/L, , suggesting the appropriateness of expectant management in mild ICP. Ursodeoxycholic acid (UDCA) is otherwise the treatment of choice for management of pruritus and normalization of liver function. , Delivery as early as the fetal lung maturity allows is the definitive treatment for ICP, and dexamethasone may be used to promote fetal lung maturity.
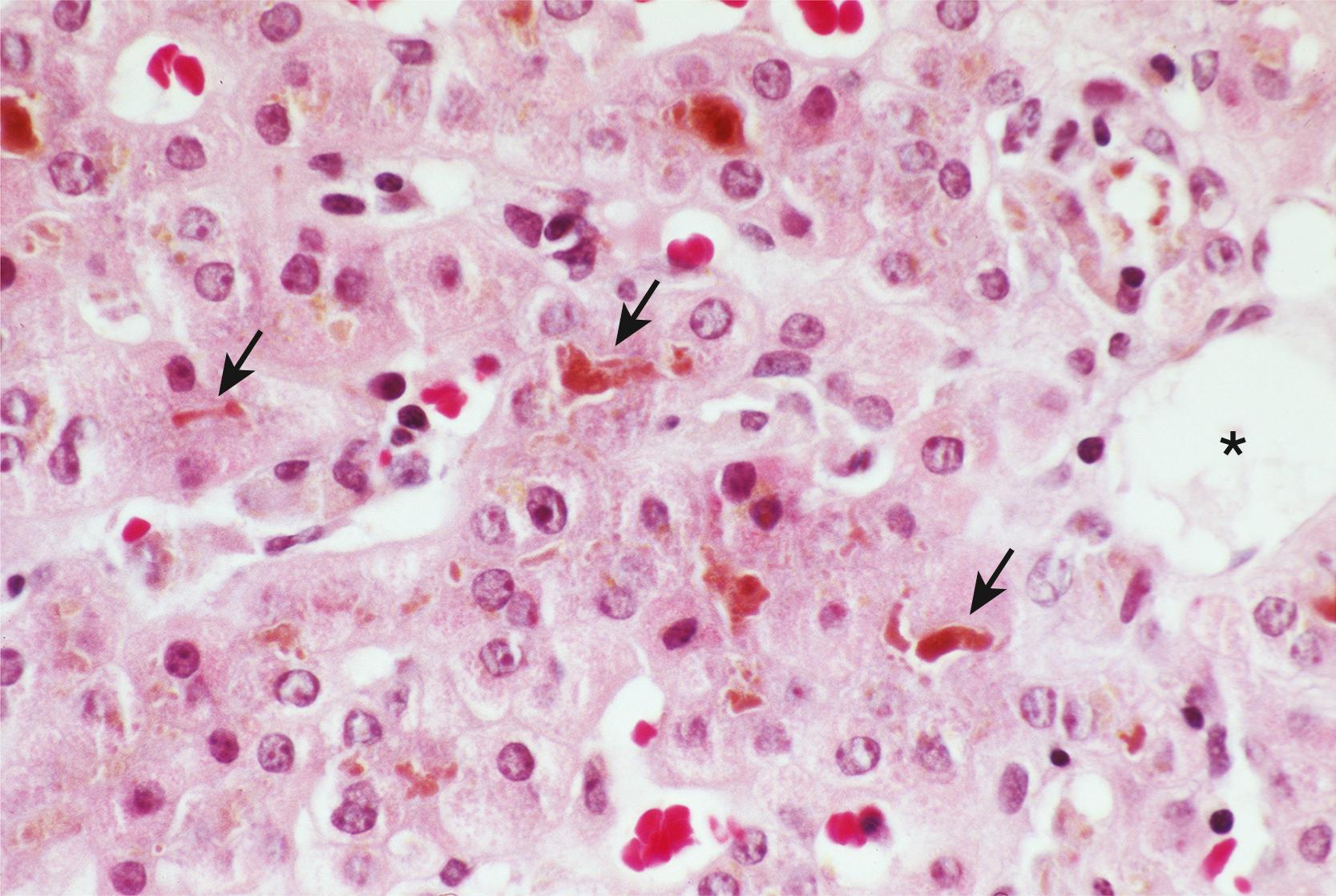
Because a diagnosis of ICP is typically made according to clinical criteria, liver histopathology in ICP has not been extensively reported. Findings are thought to be subtle and consist primarily of hepatocellular bile and canalicular bile plugs, occurring predominantly in a pericentral distribution with minimal or no hepatocellular necrosis and minimal portal inflammation (see Fig. 54.1 ). The histological differential diagnosis of ICP is broad and includes other common entities such as drug-induced hepatocellular injury and early extrahepatic biliary obstruction. Nevertheless, the combination of intrahepatic cholestasis with clinical features (i.e., pruritus and elevated levels of serum bile acids) virtually limits the diagnosis to ICP.
One study showed an association of ICP with abnormalities of the placenta, including syncytial knots, focally thickened amniotic basement membranes, small chorionic villi for gestational age with dense fibrotic stroma, and crowding and congestion of the villi. These abnormalities were proposed to be associated with fetal complications of ICP, but subsequent case-control studies found no significant difference between placental histopathology in ICP versus controls.
The pathogenesis of ICP has been gradually characterized over the past two decades and supports the concept of heterogenous but related abnormalities. Several lines of evidence point to altered metabolism of steroid hormones and bile acids, and various mechanistic roles for disruption of bile acid transport into and out of the hepatocyte by estriol, progesterone, and their intrahepatocellular conjugates have been proposed. , , , , Familial clustering, increased incidence in first-degree relatives of patients with ICP, and linkage to human leukocyte antigens indicate one or more genetic factors. , , Furthermore, it has been suggested that immune dysregulation plays a role in disturbed placental bile acid and serum lipid transportation. , ,
Association with dietary factors such as selenium , and temporal/seasonal variations in incidence , , suggest one or more environmental factors in the pathogenesis of ICP. Demonstration of increased intestinal permeability in patients with ICP during and after pregnancy provides further evidence that the hepatic pathogenesis of ICP may be under the control or influence of extrahepatic factors. Collectively, these somewhat disjointed observations and hypotheses about the pathogenesis of ICP and its associated risk factors suggest that ICP may represent the end result of a heterogeneous group of pregnancy-associated hepatic insults rather than a unique pathophysiological entity.
The genetic basis of ICP began to emerge from studies of patients with ICP and elevated serum GGT activity. Two pedigrees were initially reported, with ICP in the mothers of children who were born with the autosomal recessive form of progressive familial intrahepatic cholestasis (PFIC) and elevated serum GGT activity, a disease commonly referred to as type 3 PFIC. , The affected children had homozygous mutations in the hepatocellular phospholipid transporter ABCB4 gene (also known as multidrug resistance 3 [MDR3] ), whereas ICP developed during pregnancy in mothers who were heterozygous for ABCB4 . ABCB4 is a class III multidrug-resistant P-glycoprotein that mediates translocation (“flipping”) of phosphatidylcholine (lecithin) across the bile canalicular membrane of hepatocytes.
To better characterize the pathogenic role of ABCB4 in the development of ICP, Dixon and associates investigated eight women with ICP and increased serum GGT activity who had no personal or family history of PFIC. DNA sequence analysis revealed a heterozygous missense mutation in ABCB4, resulting in the expression of a nonfunctional protein at the cell surface in one of eight patients. Several subsequent reports on the association between ABCB4 and ICP provide further evidence for a defect in this canalicular transporter protein in the pathogenesis of ICP. , As many as 1 in 5 patients with ICP may have a mutation in the ABCB4 gene, and many different mutations of the gene have now been reported.
In addition to the compelling data regarding the role of ABCB4 in the pathogenesis of a subset of ICP cases, other cases have been associated with benign, recurrent intrahepatic cholestasis (BRIC; Fig. 54.2 ). , BRIC, which is genetically associated with a mutation in the same region of chromosome 18 as type 1 PFIC (Byler disease), is an intrahepatic cholestatic disease in which affected patients have normal serum GGT activity. This sharp biochemical contrast with ABCB4-associated cases of ICP points to alternative mechanistic pathways for another subset of women with ICP. Lastly, the ABCB11 gene, which is associated with PFIC type 2, may also contribute to ICP. Mutations in the bile salt export pump (BSEP), which is the product of the ABCB11 gene, as well as transcription factors driving ABCB11 expression, have been identified in subsets of patients with ICP. , , ,
It is not surprising that different defects in transport across the hepatocyte canalicular membrane can lead to similar clinical phenotypes, which are collectively recognized as ICP. Fundamentally, the phenotype of ICP can result from any disruption in the steady state between the uptake of bile salts into the hepatocytes and transport of bile salts across the canalicular membrane into bile. Estrogens, progesterone, and their conjugates disrupt the steady state by interfering with bile acid transporters at the basolateral membrane and inhibiting efficient bile acid transport across the canalicular membrane. In compromised hosts, such as heterozygous mothers with ABCB4 mutations, the added insult from pregnancy-associated hormones is sufficient to tilt the balance toward a cholestatic disease. Future research will undoubtedly reveal other molecular pathways and canalicular transporters that can result in ICP through similar or related pathways.
Acute fatty liver of pregnancy (AFLP) is a serious and potentially fatal complication for the pregnant mother and fetus. AFLP occurs in an estimated 1 to 3 of 10,000 pregnancies, and the disease is classically associated with first pregnancies, multiple gestations, low body mass index, and a male fetus. AFLP usually occurs late in the third trimester (>30 weeks of gestation) or in the immediate postpartum period. Rare exceptions manifesting as early as 22 weeks of gestation have been reported.
The initial clinical presentation of AFLP is often vague and includes headache, abdominal pain, nausea, vomiting, and a variety of other nonspecific “prodromal” symptoms. , , , , Prodromal symptoms are typically followed by jaundice as the disease progresses. Progressive and severe hepatic failure accompanied by coagulopathy and encephalopathy ensues, typically within 1 to 2 weeks of the onset of jaundice. If untreated, patients may rapidly deteriorate, with gastrointestinal bleeding, seizures, coma, and renal failure with acute tubular necrosis.
AFLP may be associated with preeclampsia in 20% to 40% of patients. , , In these circumstances, the presenting signs and symptoms include those of pregnancy-induced hypertensive disorders (discussed later). A rare association between AFLP and ICP has been reported in which the patient had pruritus at presentation, which is more common for ICP than AFLP, although the significance of this association remains unclear. Other concomitant disorders such as neoplastic lesions and sepsis have been reported, but there is no proof of a definitive association at this time.
Because early diagnosis and prompt treatment of AFLP are essential to maternal and fetal well-being, LFT parameters must be measured promptly in any pregnant woman past 22 weeks of gestation who has any of the aforementioned prodromal symptoms or signs of AFLP. The LFT results for AFLP typically suggest mild to moderate hepatocellular damage with mild cholestasis ( Box 54.2 ). Serum aminotransferase levels usually are elevated in AFLP, although rarely to the extent observed in acute viral hepatitis. The bilirubin level is normal early in the course, but it increases if the pregnancy is not terminated. Alkaline phosphatase levels are also elevated, but distinguishing hepatic from placental isoenzymes may not be practical or fruitful. The peripheral blood analysis may show leukocytosis and thrombocytopenia, and disseminated intravascular coagulation may be present. , Blood urea nitrogen and serum creatinine levels may be elevated, but uric acid levels are disproportionately high, making them diagnostically valuable. Blood glucose levels are typically low, and clinically significant hypoglycemia may occur.
Characteristic time of onset: third trimester
Clinical features
Abdominal pain
Nausea and vomiting
Jaundice
±Coagulopathy
±Encephalopathy
Blood and serum assay results
↑ Aminotransferases (1 to 5 times normal)
↑ Alkaline phosphatase (1 to 2 times normal)
↑ Bilirubin (<10 mg/dL)
↑ Uric acid
± ↑ Prothrombin time and partial thromboplastin time
± ↑ Platelets
± ↑ White blood cells
Pathology: microvesicular steatosis, centrilobular to diffuse
Pathophysiology: mitochondrial fatty acid β-oxidation defects
Historically, liver biopsy has been the gold standard for the diagnosis of AFLP, but coagulopathy often prevents liver biopsy in the acute clinical setting. Fatty infiltration of the liver can also be easily assessed by noninvasive imaging methods such as ultrasonography or magnetic resonance imaging. The Swansea criteria, which use the clinical symptoms and laboratory findings of women with liver disease in pregnancy, constitute a reliable indicator of AFLP, with or without a liver biopsy ( Box 54.3 ). , In one study, application of the Swansea criteria resulted in 100% sensitivity, 57% specificity, an 85% positive predictive value, and a 100% negative predictive value for diffuse or perivenular microvesicular steatosis on liver biopsy.
∗ A minimum of six criteria is required to support the diagnosis. Values in parentheses were suggested by Knight and colleagues.
Vomiting
Abdominal pain
Polydipsia and polyuria
Encephalopathy
Elevated bilirubin (>14 μmol/L)
Hypoglycemia (<4 mmol/L)
Elevated urea (>340 μmol/L)
Leucocytosis (>11 × 10 9 /L)
Ascites or bright liver on ultrasound scan
Elevated transaminases (AST or ALT >42 IU/L)
Elevated ammonia (>47 μmol/L)
Renal impairment (creatinine >150 μmol/L)
Coagulopathy (PT >14 seconds or aPTT >34 seconds)
Microvesicular steatosis on liver biopsy
Regardless of the cause or type of presentation, the mainstay of therapy for AFLP is immediate delivery and supportive care. Liver transplantation, artificial liver support, and plasma exchange have been tried as alternative therapies with various levels of success, and transplantation is the option of last resort in fulminant hepatic failure caused by AFLP. Despite all clinical efforts, AFLP continues to be a serious complication of pregnancy, with maternal or fetal death occurring in 1% to 20% of all cases, depending on the availability and level of care. , ,
Microvesicular steatosis of hepatocytes is the pathological hallmark of AFLP. Classically, steatosis involves the pericentral zone and spares the periportal hepatocytes, although panlobular and periportal involvement may be seen. , In most cases, the fat droplets are large enough and their location well enough preserved to produce readily recognizable vacuolar change on routine sections with hematoxylin and eosin (H&E) staining ( Fig. 54.3A ). Occasionally, individual fat droplets may be too small to be resolved by routine light microscopy or too poorly preserved to result in a classic vacuolar pattern on H&E-stained sections ( Fig. 54.3B ). In these circumstances, hepatocytes may look essentially normal, be somewhat dilated, or exhibit diffuse cytoplasmic ballooning not readily distinguishable from ballooning degeneration or other forms of acute hepatocellular damage. Therefore a portion of any liver biopsy specimen obtained for clinical suspicion of AFLP during pregnancy or in the immediate postpartum period must be processed as a frozen section for oil red O or Sudan black staining, or be processed for electron microscopy ( Fig. 54.3C ).
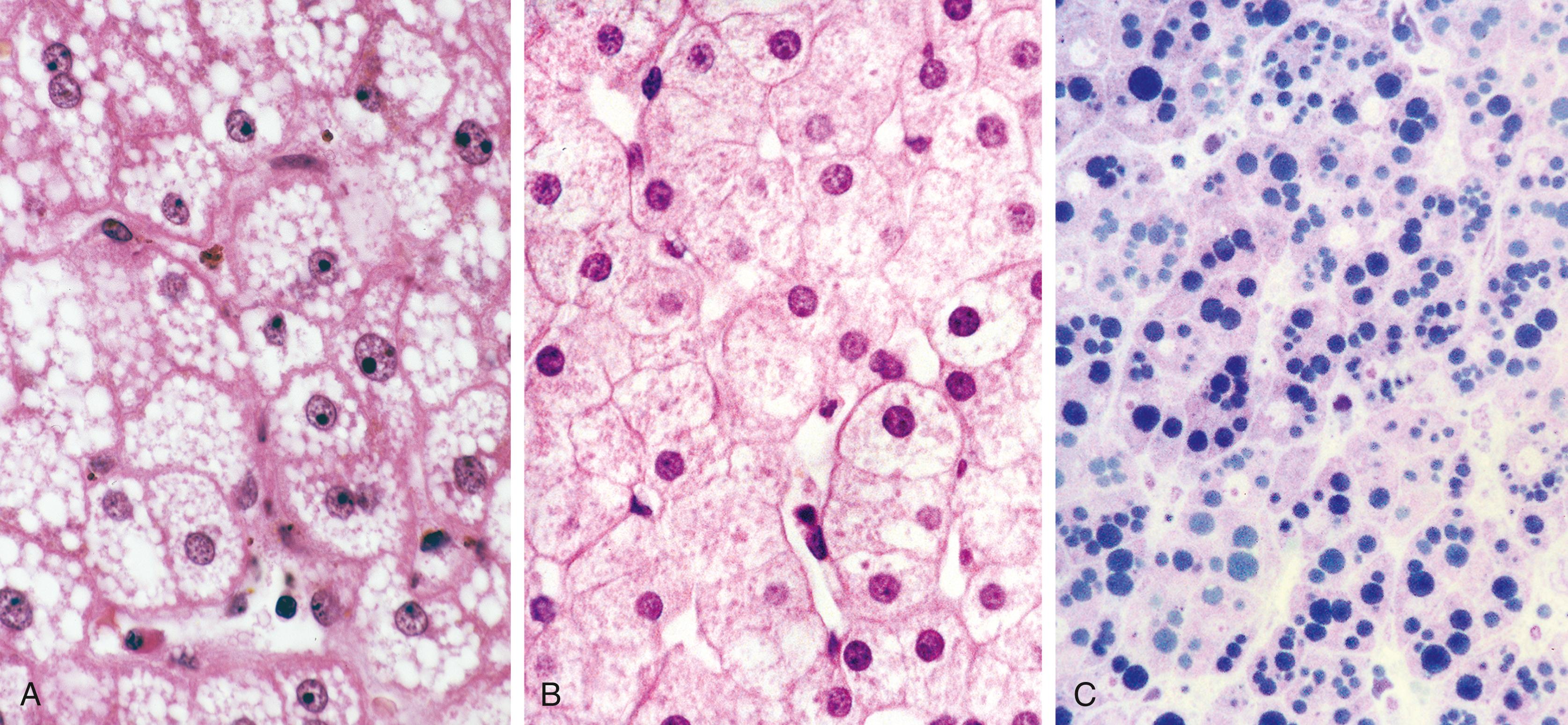
Although fatty change is considered the diagnostic histological hallmark of AFLP, a host of other microscopic abnormalities were reported in a detailed study of 35 cases by Rolfes and Ishak. Hypertrophied Kupffer cells containing lipid or lipofuscin were prominent in areas of fatty change in most cases. Evidence of intrahepatic cholestasis, including bile canalicular plugs and acute cholangiolitis, was seen in two-thirds of cases. Significant mononuclear lobular inflammation (comparable with acute viral hepatitis) and inflammation of the central veins were identified in 25% of cases, and 75% showed evidence of extramedullary hematopoiesis with prominent megakaryocytes and cells of erythroid lineage. Most notable by its absence in this large series of cases was sinusoidal fibrin deposition. Despite frequent clinical signs and symptoms of pregnancy-induced hypertensive disorders in patients with AFLP, the absence of fibrin deposition was considered evidence of a lack of histological overlap between these two entities.
Although fatty infiltration of the liver is an extremely sensitive diagnostic marker for AFLP, it is nonspecific. The histopathological differential diagnosis of AFLP is therefore broad and includes various other forms of fatty liver disease, including essentially all toxic, metabolic, and drug-induced conditions that may lead to microvesicular steatosis of hepatocytes. A definitive diagnosis of AFLP can therefore be made only in conjunction with the appropriate clinical signs and symptoms.
Significant progress has been made in understanding the pathogenesis of AFLP. Most importantly, a strong association between fetal fatty acid oxidation disorders and maternal AFLP has been established, suggesting that AFLP is fundamentally a disorder of mitochondrial fatty acid β-oxidation (FAO).
Mitochondrial β-oxidation of fatty acids is a critical step in intermediary metabolism in hepatocytes. FAO is a source of energy for hepatocytes under physiological stress and results in production of various metabolic intermediates that can be used as a source of energy by other vital organs such as the brain. One of the key enzymes in mitochondrial FAO is long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD), which catalyzes the third step in β-oxidation of long-chain fatty acids on the inner mitochondrial membrane. LCHAD activity occurs on the C-terminal portion of the α subunit of the microsomal triglyceride transfer protein (MTTP, also known as trifunctional protein [TFP]). MTTP also contains the active sites of long-chain 2,3-enoyl-CoA hydratase and long-chain 3-ketoacyl-CoA thiolase, catalyzing the second and fourth steps in fatty acid β-oxidation, respectively.
A recessively inherited defect in LCHAD in the infant is responsible for most of the occurrences of AFLP in the mother. A significant number of reported cases result from a 1528G→C mutation in exon 15 of the α subunit of MTTP, which results in the exchange of glutamic acid for glutamine at amino acid position #474. Although various MTTP deficiencies have emerged as important causes of metabolic disease in children, only limited mutations that typically result in abnormal LCHAD activity have been associated with maternal liver disease in pregnancy. , ,
In addition to MTTP mutations with LCHAD deficiency, other components of mitochondrial FAO have been associated with maternal disease in pregnancy. Fetal short-chain acyl-CoA dehydrogenase (SCAD) deficiency and fetal carnitine palmitoyltransferase deficiency were each reported in association with maternal AFLP several years ago ( Fig. 54.4 ). , Santos and colleagues provided the first description of a normal fetus with a maternal FAO defect in medium-chain acyl-CoA dehydrogenase (MCAD) resulting in AFLP in the late third trimester. In a case-control study comparing fetal oxidation defects with the occurrence of maternal liver disease during pregnancy, two cases of medium chain acyl-CoA dehydrogenase deficiency were associated with AFLP. Remarkably, the maternal liver disease occurring with FAO in the children also included preeclampsia and HELLP syndrome ( h emolysis, e levated l iver enzymes, and l ow p latelets). The risk of liver disease developing in a mother in pregnancy if the child had LCHAD or short- and medium-chain defects was, respectively, 50 times or 12 times more likely than in control patients.

The precise mechanisms through which LCHAD or other FAO deficiencies result in fatty liver or hepatic failure are unknown. It has been postulated that the increasing metabolic demands of the third trimester in a compromised host result in excessive metabolic stress that cannot be handled by the heterozygous mother’s deficient metabolism. The placenta may also be an important factor in the pathophysiology. The genetic makeup of the placenta is identical to that of the fetus. Natarajan and colleagues found that the placentas at birth in mothers with AFLP showed oxidative stress in the mitochondria and peroxisomes and had compromised mitochondrial function compared with controls. The patient’s serum showed elevation of oxidative and nitrosative stress markers with decreased antioxidant levels. The placentas and sera showed increased levels of arachidonic acid, which caused mitochondrial damage in the Chang liver cell line and increased lipid accumulation as identified by Nile red staining. This proposed mechanism is reminiscent of ICP in which normally masked (subclinical) cellular deficiencies present themselves as a disease during the altered physiological state of pregnancy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here