Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Allotransplantation, defined as the transfer of tissues between genetically nonidentical individuals, has evolved into a highly successful therapy for end-stage organ failure in the modern era. However, unless some modification of the recipient immune system is made, transplanted organs are invariably destroyed through a process broadly known as rejection .
Over the last several decades, the ability to manipulate the immune response has become increasingly selective and less morbid. Through a general understanding of alloimmunity and the unique properties of the liver and pancreas, transplantation results have steadily improved. , This chapter provides a synopsis of the principles governing immune management of liver and pancreatic transplant recipients, highlighting agents and strategies available for clinical use.
Allograft rejection is mediated by the normal elements of the physiologic immune system but arises in response to the nonphysiologic practice of solid organ transplantation . As with most immune responses, rejection requires a specific recognition event combined with a context that signifies that this recognition warrants a response (see Chapter 10 ).
Immune recognition is mediated through one of two types of lymphocyte receptors: the T-cell receptor (on the surface of T cells) or the immunoglobulin (Ig) receptor (on the surface of B cells). Immune specificity is dictated by the ability of these receptors to bind a complementary epitope. In the setting of transplantation, the recipient immune system recognizes specific molecules present on the surface of graft cells, known as major histocompatibility complex (MHC) molecules. In humans, these proteins are also referred to as human leukocyte antigens (HLAs). MHC molecules arise from a cluster of highly polymorphic genes on chromosome 6, with tens of thousands of different MHC alleles identified in humans. Graft MHC molecules serve as the primary immune targets after transplantation, as described further in the chapter.
The context of an immune response is governed through another set of receptors known as costimulation receptors. Broadly speaking, these receptors provide signals that determine whether antigen recognition should evoke an immune response and whether that response should be aggressive or attenuating. These receptors are also involved in the mechanisms of response termination, ensuring that immune responses are contained within physiologic parameters. By separating the signals for specificity and context, the immune response to pathogens, and allografts, can be tightly regulated and finely manipulated.
Typically, the ligands for costimulation receptors are most prominently expressed on antigen-presenting cells (APCs), including dendritic cells and macrophages, which play a central role in initiating and sustaining an immune response. The interactions between APCs and lymphocytes usually take place in lymphoid organs, such as the spleen or lymph nodes. The requirement for APCs and the necessity for secondary lymphoid organs provide additional opportunity for regulation and reduce the risk of autoimmune responses.
The immune system evolved to protect the body from pathogens, not to mediate transplant rejection. Although alloimmunity and physiologic immunity differ, it is critical to understand the components of the alloimmune response in their physiologic roles (see Chapter 10 ).
The immune system is organized into two complementary arms, innate and acquired. The innate immune system is activated by heterogeneous molecular patterns derived from either microbial products (for example, lipopolysaccharide [LPS]) or tissue damage arising from sterile inflammatory states including ischemia, necrosis, and trauma. The acquired immune system subsequently recognizes and eliminates pathogens in a specific manner through antigen presentation and recognition. Both systems interact to maintain overall homeostasis. Typically, innate responses generate localized inflammation at sites of injury and are less overtly regulated. In contrast, acquired immune responses lead to carefully regulated destruction of antigen-expressing tissue. The regulatory checks on acquired immunity prevent autoimmunity and uncontrolled lymphocyte proliferation. It is important to recognize that the acquired immune system is tailored for each individual based on their unique MHC makeup. Evolutionarily, this diversity reduces the chance that a particular pathogen can evade all individuals within a population. However, this also means that an individual’s acquired immune response may be deleterious when exposed to foreign MHC in the setting of allotransplantation.
The cellular components of the innate immune system include dendritic cells, macrophages, neutrophils, and natural killer cells. These cells are activated by binding of either microbial by-products (pathogen-associated molecular patterns [PAMPs]) or products of sterile tissue injury (damage-associated molecular patterns [DAMPs]) to pattern-recognition receptors located in both intracellular and cell surface compartments. Perhaps the most well-described pattern-recognition receptor is the Toll-like receptor (TLR). This highly conserved family of receptors responds to PAMPs associated with microbial infection, including LPS as well as DAMPs generated by cell death, including high mobility group box-1 (HMGB1). Interestingly, the TLRs expressed in the liver differ from those expressed in the periphery and tend to be less responsive to ambient LPS. This is likely an adaptation to portal bacteremia and is thought to make the liver more tolerant of minor perturbations that would evoke an innate response in other organs. It is important to note that innate immune receptors are conserved between individuals and do not demonstrate the polymorphic nature of MHC as described previously.
Binding of PAMPs and DAMPs to innate immune receptors initiate signaling cascades resulting in generalized inflammation and activation of complement. , , Of particular relevance in transplantation, ischemia-reperfusion injury and associated cell death results in the release of multiple DAMPs derived from different subcellular compartments including the nucleus (extracellular DNA, HMGB1) and mitochondria (mitochondrial DNA, reactive oxygen species). Recently, greater attention has been paid to the effects of DAMP-mediated inflammation on both early graft injury and function and the connection with the subsequent adaptive immune response. ,
The complement system acts as the primary mediator of cytolysis, and the by-products of complement help link the innate and acquired immune responses. , Platelets have also been increasingly recognized as serving an innate immune role through the release of chemotactic proteins and other immunostimulatory molecules.
Specific recognition is the hallmark of the acquired immune system. The immense structural diversity of the lymphocyte receptors (T-cell and B-cell receptors) facilitates recognition of a vast array of antigens. Furthermore, initial antigen recognition induces physiologic change in the lymphocyte, lowering the threshold for activation in response to subsequent encounters. This phenomenon underlies the concept of immunologic memory, which results in a more rapid (anamnestic) response to subsequent antigen encounters. T-cell receptors (TCRs) bind peptide antigens that have been processed and presented in combination with MHC. B-cell Igs bind antigens in their native conformation and can be secreted in soluble form as circulating antibodies that act remotely.
Understanding the unique nature of the TCR is fundamental to understanding its function. , T cells, formed in the bone marrow and fetal liver, migrate to the thymus during development, where they undergo rearrangement of the DNA that encodes the TCR. Each gene rearrangement results in the generation of a TCR with specificity generally restricted to one epitope, although some cross-reactivity can occur with similar epitopes. The sum of all random TCR gene rearrangements generates TCRs with approximately 10 9 unique specificities. If such a vast array of T cells was to be released immediately into the periphery, they would mediate widespread and fatal autoimmune processes. Accordingly, the process of thymic selection eliminates those T cells likely to induce autoimmunity. ,
The initial phase of thymic selection occurs through the interaction of cortical thymic epithelial cells with the cohort of developing T cells. These T cells express the accessory cell surface markers CD4 or CD8, which facilitate stronger binding interactions between the TCR and MHC. If T-cell binding does not occur to the self MHC molecules presented by the thymic epithelium, the T cells are eliminated because they are unable to interact with self MHC molecules, which is required for normal immune surveillance and function. This process is known as positive selection.
Positive selection produces a repertoire of T cells capable of recognizing and binding to self MHC, which is critical for normal functioning in the periphery. However, this step does not distinguish T cells with normal affinity from those with inappropriately strong affinity to self MHC, which would predispose to autoimmunity. To eliminate cells with a high risk of autoimmune recognition, the developing T cells then move into the thymic medulla, where either CD4 or CD8 expression is lost. If binding to self MHC in the medulla results in a high-affinity interaction, these T cells are also eliminated, a process known as negative selection. Therefore, after this step the majority of T cells released from the thymus bind to self MHC but fail to become activated in the absence of additional costimulatory molecules. This process is imperfect, and autoreactive cells occasionally escape thymic selection, contributing to disease processes such as primary sclerosing cholangitis (see Chapters 10 and 41 ), autoimmune hepatitis (see Chapters 10 and 68 ), and type 1 diabetes. However, the bar for activation remains high and thus limits the risk of autoimmunity. For example, a single interaction of TCR and antigen-bearing MHC is inadequate to trigger T-cell activation; rather, approximately 8000 TCR-MHC interactions over the course of several hours are needed to initiate activation, which further limits the likelihood of autoimmunity. Costimulatory molecules greatly alter this need for redundancy, as discussed subsequently.
T cells are further distinguished by the accessory cell surface molecules CD8 and CD4 that influence the cell types that they are capable of interacting with and their roles in the immune response. , CD8 + T cells, known as cytotoxic T cells , bind to cells expressing class I MHC molecules. This binding interaction is facilitated by CD8 stabilization of TCR-MHC class I ligation. All parenchymal cells express class I MHC and display internal cellular peptides within the binding groove of this molecule. Cytotoxic T cells recognize diseased or infected parenchymal cells and activate cytolytic mechanisms. T-cell killing can occur through either calcium ion (Ca 2+ )-dependent secretory mechanisms or Ca 2+ -independent direct cell contact mechanisms.
CD4 + T cells, also known as helper T cells, interact with APCs expressing MHC class II including dendritic cells, macrophages, and activated endothelial cells. This interaction is mediated by CD4 stabilization of TCR-MHC class II ligation. MHC class II displays peptide fragments that have been phagocytized from the extracellular space. , Interestingly, resting sinusoidal endothelial cells of the liver are capable of presenting antigen to T cells, making the liver an organ with considerable ability to evoke or suppress an immune response. The interaction between CD4 + T cells and APCs influences subsequent activation of CD8 + T cells. , This process is mediated through upregulation of cell surface molecules on APCs known as costimulation receptors. Thus while APCs act as initiators of an immune response, they require CD4 + T cells to activate the primary effector arm of the acquired immune system mediated by CD8 + T cells.
An additional subset of T cells, regulatory T cells (Treg), further limit promiscuous immune responses. Treg cells have the ability to suppress cytokine secretion, adhesion molecule expression, and costimulatory signaling. The most extensively studied population of Treg cells express CD4 and CD25, the high-affinity α-chain of the interleukin (IL)-2 receptor. Animal models suggest that these cells play a critical role in controlling immune activation. , The prevailing evidence suggests that Treg cells are responsive to established inflammation, rather than serving a prophylactic role in preventing inflammation. However, harnessing the power of Treg cells to quell counteradaptive immune responses such as rejection is an ongoing area of research in autoimmunity and alloimmunity.
B cells recognize antigen in its native, unprocessed form. When antigen binds to two cell surface antibody receptors, the antibodies are brought together in a process known as cross-linking, stimulating B-cell proliferation and differentiation into an antibody-secreting plasma cell. The activation threshold for a resting B cell is relatively high, and similar to TCR recognition, costimulation can lower this threshold substantially. B cells also have the ability to internalize antigen bound to surface immunoglobulins and process them for presentation to T cells along with costimulation molecules.
Antibody structure is determined in the bone marrow through mechanisms similar to those that govern the generation of TCR diversity in the thymus. , Five different heavy-chain loci (μ, γ, α, ε, and δ) on chromosome 14 and two different light-chain loci (κ and λ) on chromosome 2, each with V, D, and/or J, and C regions, are brought together randomly by the RAG1 and RAG2 apparatus to form a functional antigen receptor. The basic antibody structure consists of two identical heavy chains and two identical light chains. The type of heavy chain dictates the Ig class: IgM, IgG, IgA, IgE, or IgD. The overall structure of the antibody results in two identical antigen-binding sites and a common region, the Fc portion. Bound antibody triggers activation of the complement cascade. In addition, most phagocytic cells have receptors for the Fc portion of IgG, allowing them to actively engulf antibody-coated cells.
Unlike the TCR, B-cell immunoglobulin loci undergo several forms of alteration after stimulation to improve the functionality of the secreted antibody. Isotype switching is the process of shifting from the initial heavy-chain IgM to one of four types to improve function and specialization of the secreted antibody. IgG is the most significant soluble mediator of opsonization and is the dominant antibody produced in response to alloantigen. IgA is important in mucosal immunity, IgE is involved in mast cell–mediated immunity, and IgD is primarily cell bound. After a B cell is activated, the specific D and J regions of the heavy- and light-chain genes undergo random alterations of the antigen-binding site. The resultant B-cell clones have altered antigen affinity, and this process is termed affinity maturation. Clones that have higher affinity for the target antigen have a selective survival advantage and form the basis for a more vigorous response on reexposure to the antigen.
A hallmark of immunity is the ability to adapt based on prior immune experience, such that initial immune responses are less robust than anamnestic responses. As humans age, immune experience grows, manifesting in an observed loss of naive T and B cells and a commensurate accumulation of cells expressing a memory phenotype. This transition is accentuated by physiologic thymic atrophy, which slows the production of naive T cells. In fact, the pool of available naive T cells is a function of thymic involution and the ongoing conversion to memory and senescent CD8 T cells. This transition with aging imparts a significant effect on the immune system that is particularly evident in elderly persons but measurable in most people by the fourth decade of life.
By using established markers of resting naive T cells and memory cell activation, four T-cell subpopulations have been described that appear to differ in terms of their degree of antigen experience, prior activation history, and migratory capabilities. These can be defined as naive T cells, effector memory T cells, central memory T cells, and terminally differentiated effector cells. During the last decade, numerous surface markers have been used to assess the myriad differentiation pathways seen after antigen exposure, and it is now clear that the lymphocyte repertoire is dynamic over time. Although it is beyond the scope of this chapter to define the specifics of this migration from naiveté to experience, it should be recognized that each individual’s immune repertoire is a product of both their inherited molecular makeup and their prior immune exposures. This is increasingly important because many of the molecules targeted in transplantation, particularly costimulatory molecules, are altered with immune experience such that optimal immune management is likely to require cognizance of these changes.
Isolated TCR binding with an MHC-peptide complex or antibody ligation with an antigen is not usually sufficient for naive lymphocyte activation. Receptor-ligand pairs on T and B cells and APCs, known as costimulation receptors, determine the character of the T-cell response ( Fig. 104.1 ). The type of costimulatory signal received by the lymphocyte determines whether the cell will become activated, remain quiescent, die, or become resistant to subsequent immune stimulation.
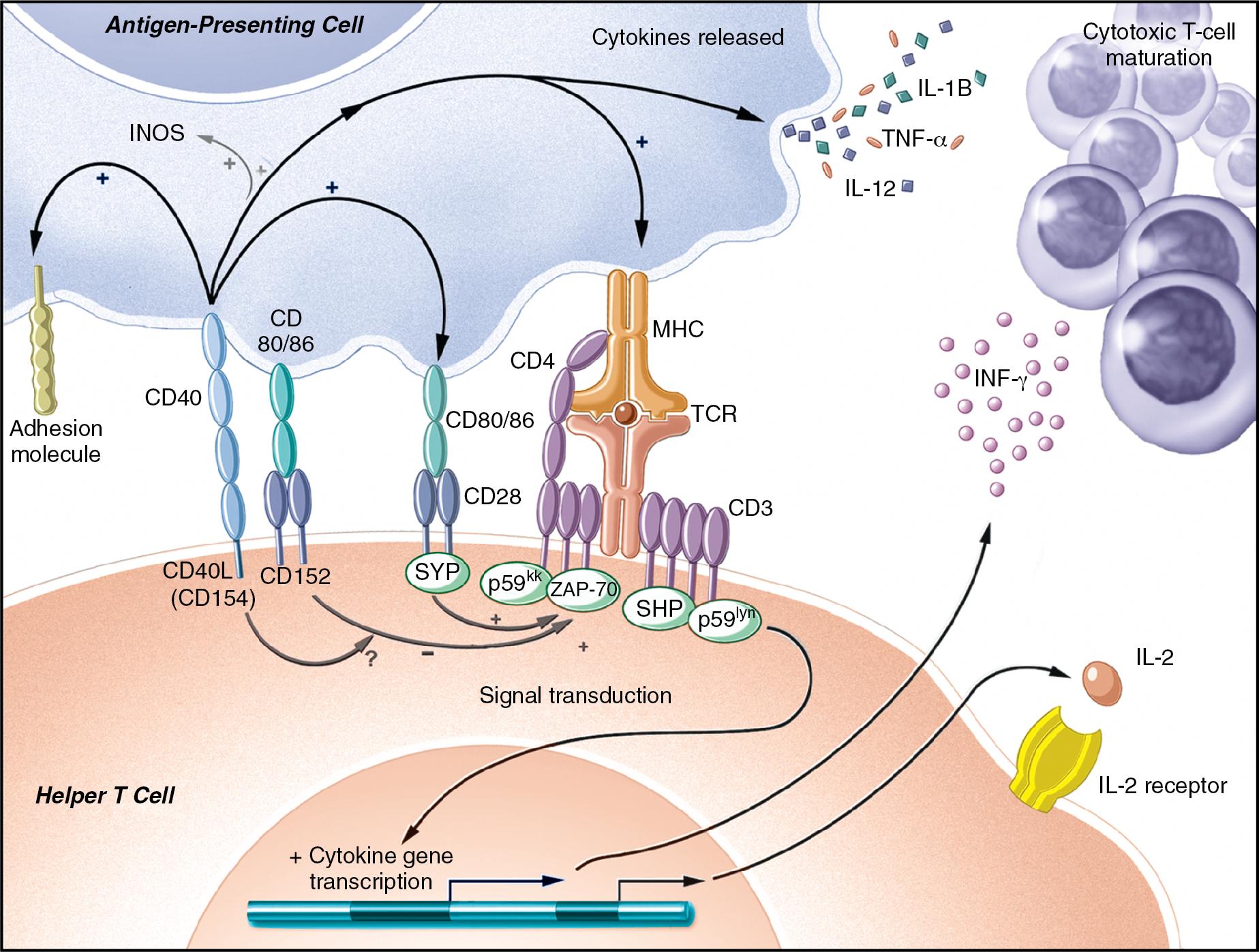
The biology of T-cell costimulation is substantially more developed than that of B-cell costimulation. Examples of T-cell costimulation receptors include CD28 and CTLA-4 (CD152). CD28 promotes T-cell activation and leads to the expression of CTLA-4, which then promotes downregulation of the T-cell response; thus activation typically begets deactivation. The B7 molecules CD80 and CD86, found on APCs, are the ligands for CD28 and CTLA-4. Although B7 molecules can bind to either receptor, their affinity for CTLA-4 is much greater; when B7 is in limited supply, the higher-affinity interaction with CTLA-4 predominates. Because B7 molecules are not expressed by normal tissues, CD8 + T-cell interaction with class I self MHC does not elicit a proliferative response; instead it reinforces quiescence of autoreactive T-cell clones.
Although the mechanisms of costimulation have not been completely elucidated, it is known that binding of CD28 allows more efficient T-cell signal transduction. Through CD28-B7 interactions, the number of binding events required to trigger activation of a T cell decrease from 8000 to 1500. , In contrast, when CTLA-4 binds B7, the T cell becomes incapable of producing IL-2 during the encounter and even in subsequent interactions. The CD19-CD21 complex provides comparable control of antigen receptor binding for B cells.
Additionally, costimulation is mediated through another pair of receptors: CD40, found on dendritic cells, endothelium, B cells and other APCs, and CD154 on T cells and platelets. The ability of APCs to stimulate a cytotoxic T-cell response is greatly augmented by the effects of CD40 binding. After CD40 ligation, activating cytokines are released and B7 molecules are upregulated. , CD154 is upregulated after TCR ligation and provides positive feedback to the APC. In addition, CD154 is found in and released by activated platelets at sites of endothelial injury. Therefore sites of trauma that recruit platelets create an environment of activating costimulatory molecules, thereby bridging the innate and acquired immune systems.
Direct cell-to-cell contact is not the only means by which immune cells communicate. Soluble mediators of communication known as cytokines, or interleukins, are polypeptides that are released from many cells; they can either activate or suppress adjacent cells, and the pattern of cytokine expression is thought to influence the resultant type of T-cell response. , Once activated, T cells have been described by one of several cytokine-secretion phenotypes. T cells that promote cytotoxic responses are characterized by expression of IL-2, IL-12, IL-15, and interferon (IFN)-γ and are called helper T type 1 (Th1) cells; T cells that promote humoral or eosinophilic responses are characterized by secretion of IL-4, IL-5, IL-10, and IL-13 and are called helper T type 2 (Th2) cells. More recently, additional cytokine lineages have emerged; the most relevant to this chapter is the Th17 lineage, involved in gut immunity and driven by IL-6, IL-23, and transforming growth factor (TGF)-β.
In addition to cytokines, other soluble mediators of inflammation are capable of promoting increased blood flow and improved exposure of an area of injury to innate and acquired immune elements. Some suggest that the APCs of the liver are more efficient in generating Th2 responses, which may be a mechanism by which liver allografts avoid late cellular rejection.
T-cell responses to allogeneic organs are largely the result of nonphysiologic TCR-MHC interactions. During development, T cells are initially selected to bind to self MHC and are then eliminated if that binding event leads to activation. However, this sequence of events does not preclude the survival of cells that, through chance, bind to the MHC molecules of another individual, perhaps with high affinity. The discrepancies between self TCR selection and allo-MHC interactions form the basis for most transplant recognition. This nonphysiologic recognition provides the foundation for an antagonistic immune response but does not mandate it in the absence of additional contextual requirements such as expression of costimulatory molecules. Thus alloimmunity is more likely than autoimmunity, but also requires costimulation and innate immune activation to elicit a physiologic immune response.
Most of the significant sequence polymorphism of MHC is located in the areas of the molecule that interact with the TCR, and individual variation in the sequence at the MHC-TCR interface defines alloreactivity. The lack of recipient T-cell thymic education with donor MHC leads to a high frequency of alloreactive peripheral T cells. Many of these cells are cross-reactive with antigen encountered during prior viral exposures, or even with autoantigens in the case of autoimmune disease. This is known as heterologous immunity and results in a situation whereby recipients have allospecific memory without having prior exposure to the alloantigen. , Thus a person’s immune response to a donor is the composite product of the individual’s MHC makeup and past immune exposures. This can lead to vigorous early rejection in apparently nonsensitized recipients.
T cells recognize alloantigen through their TCR in two distinct pathways. First, in the direct pathway, donor APCs expressing donor MHC and costimulatory molecules migrate from the graft and bind to recipient T cells, leading to activation. Conversely, in the indirect pathway, recipient APCs phagocytize and process alloantigens derived from the graft and present them bound to self MHC. In the case of transplanted organs, surgical trauma and ischemia exacerbate the potential for T-cell activation by causing an upregulation of class I and II MHC molecules. Moreover, adhesion and costimulation molecules are upregulated in the perioperative period. ,
Initial T-cell binding to donor cells is nonspecific, mediated by adhesion molecules upregulated during donor cell activation. CD40 on donor APCs and endothelial cells is important in mediating cell activation in this setting via CD154 on T cells and activated platelets. Following nonspecific adhesion, MHC recognition occurs in the relatively high costimulatory environment induced by surgical trauma and ischemia. Once alloreactive T cells are activated, they secrete cytokines, including IL-2 and IFN-γ, and they stimulate APCs to secrete IL-12. , , The resultant cytokine milieu recruits more T cells to the site of injury and potentiates clonal expansion. Secretory perforin/granzyme mechanisms and cell contact–dependent Fas mechanisms are involved in T-cell cytotoxicity within the graft, resulting in graft destruction. Although acute rejection is the result of T-cell activation, antibody responses accompany many episodes. Cellular and soluble components of immunity mediate multiple distinct clinical rejection syndromes through cytokine-mediated toxicity, cellular cytotoxicity, and direct effects of antibody and complement.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here