Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cholesterol is a 4-ring hydrocarbon structure with an 8-carbon side chain. Cholesterol serves as a key component of cell membranes, and it is the substrate for synthesis of steroid hormones and bile acids. Cholesterol is either synthesized endogenously or obtained exogenously by ingestion of animal fats (e.g., meat, eggs, and dairy products). The biosynthesis of cholesterol starts with three molecules of acetate that are condensed to form 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA). HMG-CoA is then converted to mevalonic acid by HMG-CoA reductase—the rate-limiting step in cholesterol biosynthesis—and mevalonic acid is converted to cholesterol. Competitive inhibitors of HMG-CoA reductase (statins) are used clinically to decrease cholesterol biosynthesis and to lower serum cholesterol concentrations.
Cholesterol is metabolized by the biliary excretion of free cholesterol or by conversion to bile acids that are secreted into the intestine. Approximately 50% of biliary cholesterol and 97% of bile acids are reabsorbed in the small intestine and recirculate to the liver (enterohepatic circulation); the remaining biliary cholesterol and bile acids are excreted in the feces.
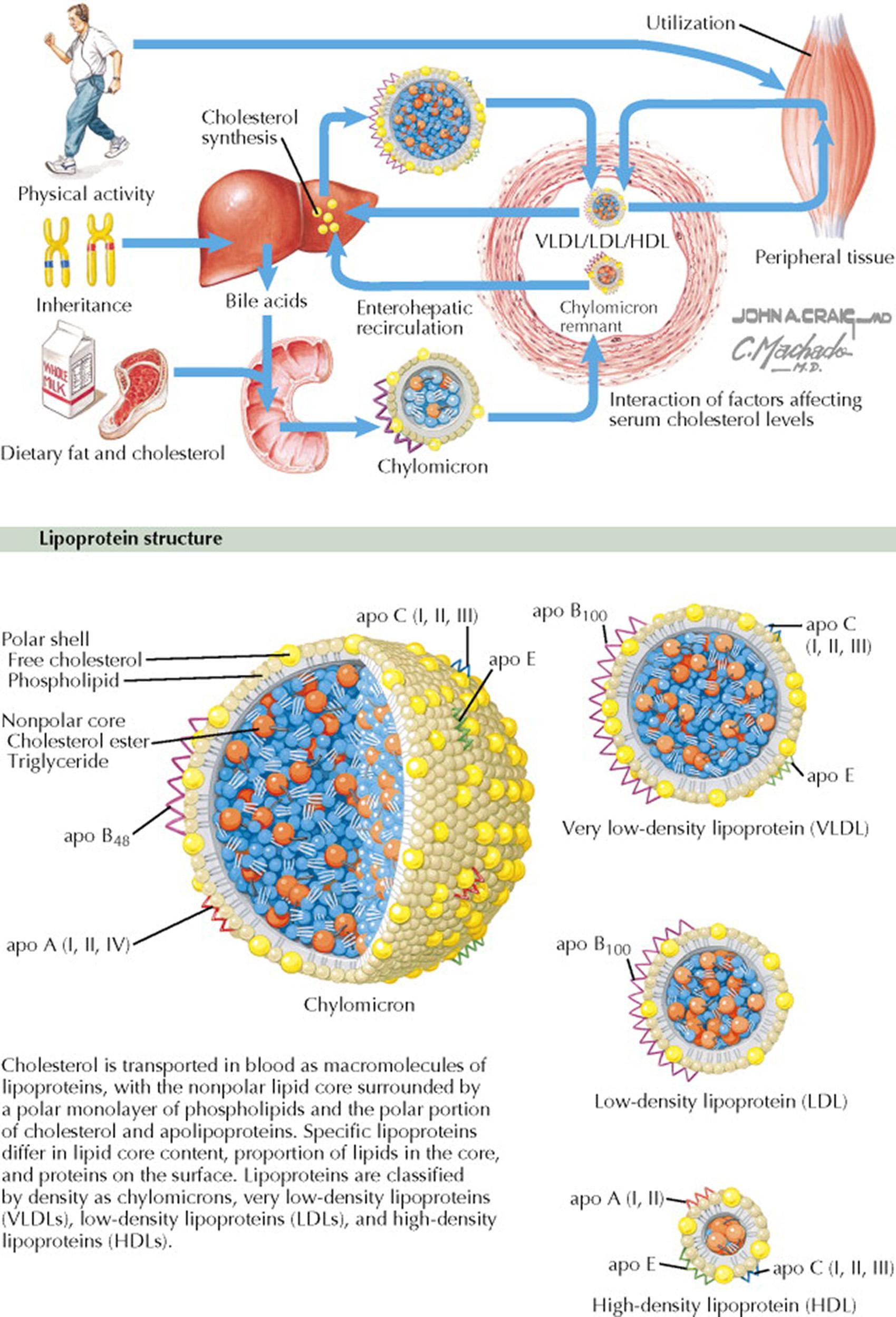
Lipoproteins, which are composed of protein, triglycerides, cholesterol esters, and free cholesterol, are macromolecules that transport cholesterol and triglycerides in the blood to target tissues (for bile acid formation, adrenal and gonadal steroidogenesis, energy production). The 12 proteins in the lipoproteins are termed apolipoproteins (apo) and are given letter designations. The apolipoproteins act as ligands for receptors and as cofactors for enzymes. The lipoproteins have a nonpolar lipid core surrounded by a polar monolayer of phospholipids and the polar portions of cholesterol and apolipoproteins. Specific lipoproteins differ in the lipid core content, the proportion of lipids in the core, and the protein on the surface. Lipoproteins are classified on the basis of density as chylomicrons, very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL).
Chylomicrons are large, low-density particles that transport dietary lipid (see Plate 7-2 ). The associated apolipoproteins include apo A (I, II, IV); apo B 48 ; apo C (I, II, III), and apo E.
VLDL transports primarily triglycerides. The associated apolipoproteins include apo B 100 , apo C (I, II, III), and apo E.
LDL transports primarily cholesterol esters and is associated with apo B 100 .
HDL also transports cholesterol esters. HDL is associated with apo A (I, II), apo C (I, II, III), and apo E.
How lipids are transported and metabolized is determined in large part by the apolipoproteins. For example, apo AI is not only a structural protein in HDL, but it also activates lecithin–cholesterol acyltransferase (LCAT). Apo AII is a structural protein of HDL and activates hepatic lipase. Apo AIV is an activator for lipoprotein lipase (LPL) and LCAT. Apo B 100 is a structural protein for VLDL and LDL and serves as a ligand for the LDL receptor. Apo B 48 is critical for the formation and secretion of chylomicrons. Apo CI serves to activate LCAT. Apo CII is a key cofactor for LPL. Apo CIII inhibits the hydrolysis of triglycerides by LPL. The three genetically determined isoforms of apo E clear lipoproteins (VLDL and chylomicrons) from the circulation by serving as ligands for the VLDL remnant receptor. The presence of two copies of the apo E2 isoform (homozygous) results in less efficient clearance of chylomicrons and VLDL and is clinically referred to as familial dysbetalipoproteinemia (see Plate 7-9 ).
The cholesterol concentration in the blood is controlled by the LDL receptor. The LDL receptor mediates the endocytosis of apo B– and apo E–containing lipoproteins (LDL, chylomicron remnants, VLDL, and VLDL remnants) into cells. The number of LDL receptors on the cell surface is regulated to maintain normal intracellular cholesterol content.
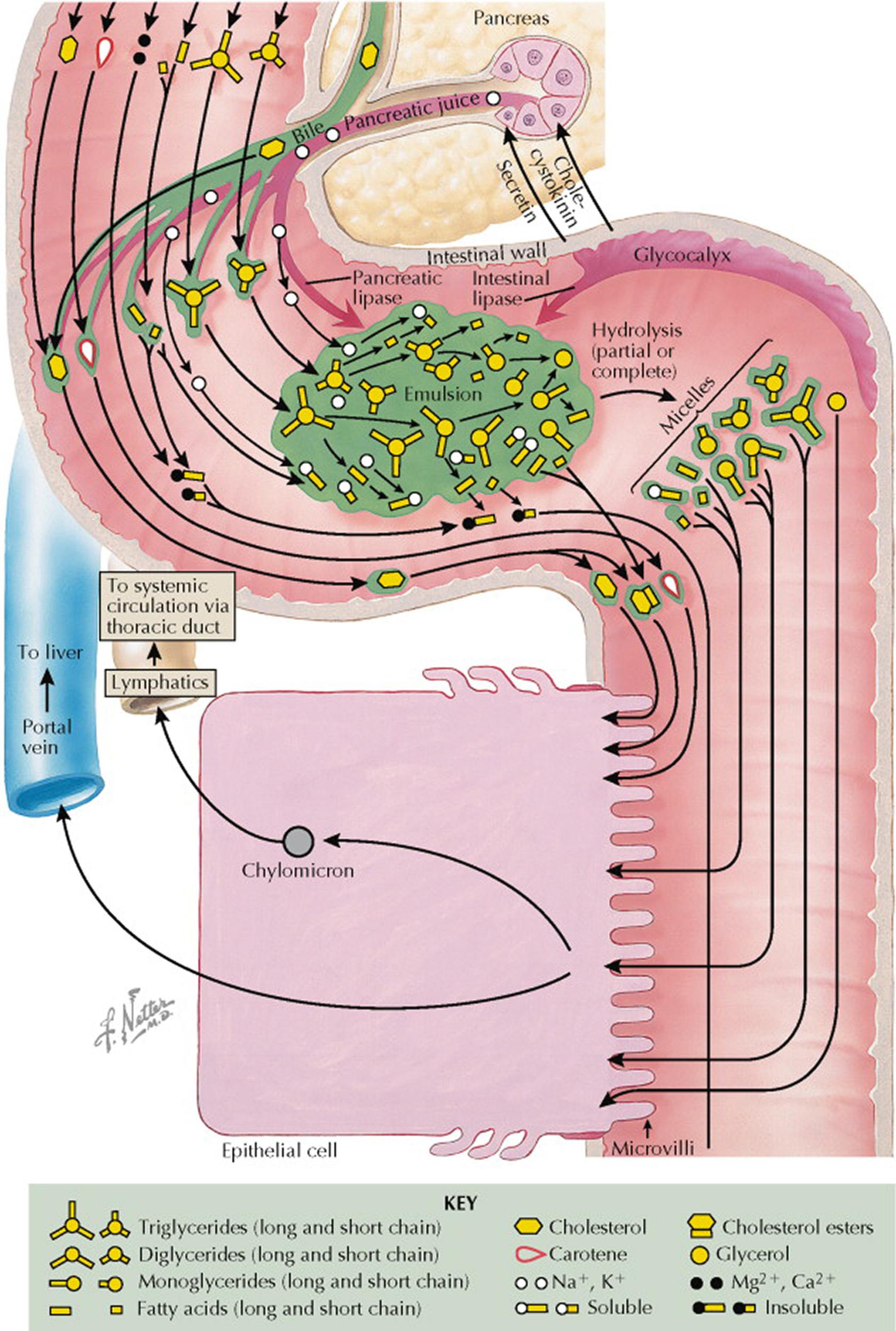
Dietary fat digestion starts in the stomach and is completed in the small intestine. Most dietary lipid is in the form of long-chain triglycerides (three fatty acids [with at least 12 carbon atoms each] that are esterified to glycerol). Other dietary lipids include phospholipids, plant sterols, cholesterol, and fat-soluble vitamins (vitamins A, D, E, and K). Gastric peristalsis and mixing serve to disperse dietary triglycerides and phospholipids into an emulsion. Intestinal (gastric) lipase acts on the oil droplets in the emulsion to generate free fatty acids and diglycerides. The presence of fatty acids in the small intestine leads to the secretion of cholecystokinin. Cholecystokinin promotes the secretion and release of pancreatic enzymes into the intestinal lumen (see Plate 5-2 ); it also promotes contraction of the gallbladder, leading to release of concentrated bile. The pancreatic lipase metabolizes triglycerides to fatty acids and monoglycerides. Another pancreatic enzyme is phospholipase A 2 , which breaks down dietary phospholipids.
By partially solubilizing water-insoluble lipids, bile salt micelles facilitate intestinal transport of lipids to the intestinal epithelial cells (enterocytes) for absorption. Specific carrier proteins facilitate diffusion of lipids across the brush border membrane. In addition, enterocytes in the duodenum and proximal jejunum directly take up long-chain fatty acids by passive transfer. Medium-chain fatty acids (six to 12 carbon atoms) are not esterified, and the enterocytes release these directly into the portal venous system along with other absorbed nutrients. Long-chain fatty acids and monoglycerides are re-esterified into triglyceride in the smooth endoplasmic reticulum of the enterocyte. In addition, cholesterol is esterified by cholesterol acyltransferase. The reassembled lipids are coated with apolipoproteins (apo) (see Plate 7-1 ) to produce chylomicrons in the Golgi apparatus. The primary intestinal apolipoproteins are apo B 48 , apo AI, and apo AIV. Chylomicrons acquire apo C and apo E during transit in the lymph and blood. Approximately 85% of the chylomicron is composed of triglyceride. The chylomicrons are too large to cross intercellular junctions linking to capillary epithelial cells. Thus, chylomicrons are transported across the basolateral membrane by exocytosis into the mesenteric lymphatic system that flows to the thoracic duct, where they enter the systemic circulation. Chylomicrons are therefore present in postprandial plasma but are absent with fasting. Apo CII is a cofactor for lipoprotein lipase, the enzyme that hydrolyzes the core triglycerides of the chylomicron and releases free fatty acids. Lipoprotein lipase is bound to the capillary endothelial cells in muscle, adipose, and breast tissues. The activity of lipoprotein lipase is regulated based on energy needs. For example, in the fasting state, lipoprotein lipase activity increases in heart muscle and decreases in adipose tissue. In addition, in the postpartum state, breast lipoprotein lipase activity increases 10-fold to promote milk production. Because of the action of lipoprotein lipase, the circulating chylomicrons become progressively smaller, and triglyceride-poor chylomicron remnants are removed from the circulation in the liver, where apo E is the ligand for the hepatic low-density lipoprotein receptor–related protein.
The cholesterol concentration in the blood is controlled primarily by the low-density lipoprotein (LDL) pathway. Approximately 70% of total plasma cholesterol is LDL. The LDL receptor—located on the surface of all cells—facilitates the internalization of lipoproteins. Approximately 75% of LDL is taken up by hepatocytes. The number of LDL receptors on each cell is in flux and is tightly regulated to keep the intracellular cholesterol concentration constant. Sterol regulatory element–binding protein (SREBP) mediates LDL receptor synthesis. Thus, when a cell's cholesterol content is in positive balance, LDL receptor expression is downregulated by decreased expression of SREBP. In addition, with increased cellular cholesterol, the cholesterol synthetic enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase is also downregulated. When a cell's cholesterol content is in negative balance, increased expression of SREBP leads to increased numbers of LDL receptors and enhanced cholesterol uptake from the circulation.
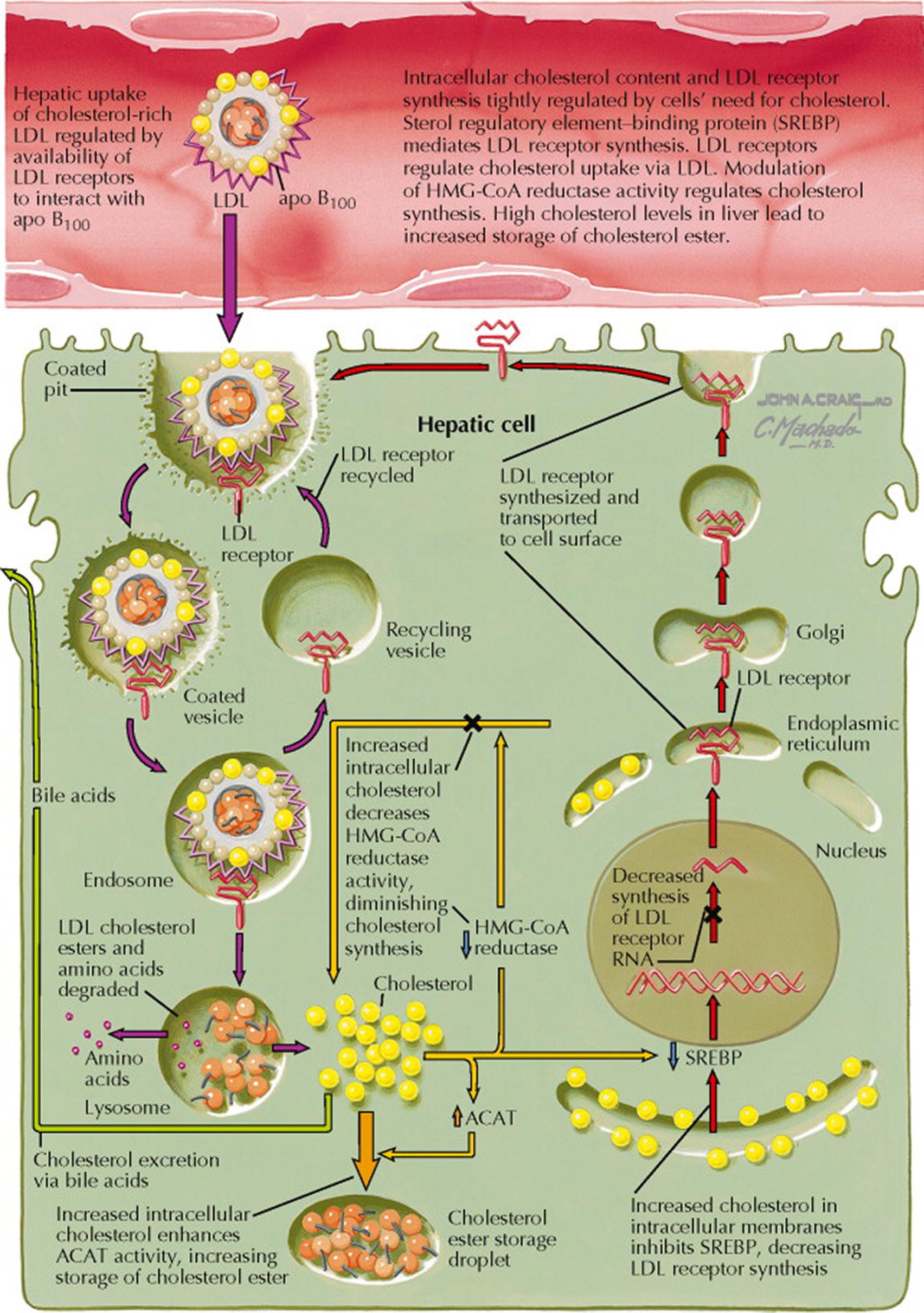
The LDL receptor binds lipoproteins that contain the apolipoproteins (apo) apo B 100 and apo E (e.g., LDL, chylomicron remnants, very low-density lipoprotein [VLDL], and VLDL remnants). The lipoprotein–LDL receptor complex localizes to an area of the cell membrane referred to as the “coated pit.” The coated pit contains clathrin that facilitates the clustering of LDL receptors to an area of the cell membrane that can invaginate to form an intracellular vesicle (endosome). As the endosome becomes more acidic, the LDL receptor and lipoprotein dissociate and the lipoproteins are degraded in lysosomes. The free LDL receptor returns to the cell surface in a recycling vesicle. The intracellular pool of cholesterol and cholesterol esters in the hepatocyte is dynamic. Increased intracellular cholesterol enhances acyl-CoA:cholesterol acyltransferase (ACAT) activity, increasing the esterification and storage of cholesterol. In turn, cholesterol ester hydrolase can generate free cholesterol.
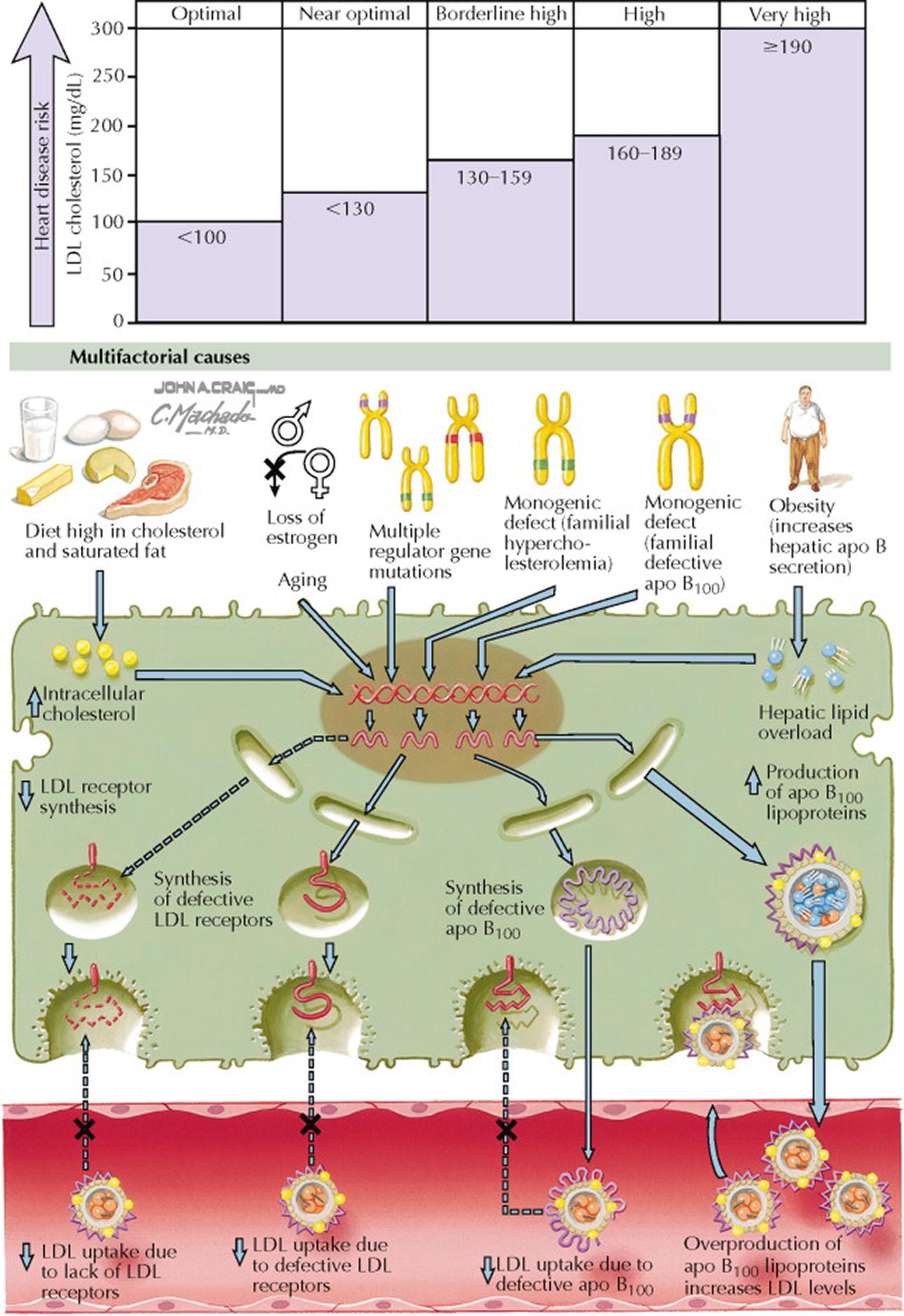
The guidelines from the 2002 National Cholesterol Education Program suggest the following cutoffs for plasma total cholesterol concentrations: less than 200 mg/dL, desirable; between 200 and 240 mg/dL, borderline high; and greater than 240 mg/dL, high. Increased blood concentrations of cholesterol are related to increased production or secretion into the circulation or to decreased clearance or removal from the circulation (or both).
Familial hypercholesterolemia (FH) is an autosomal dominant disorder that increases susceptibility to coronary heart disease (CHD). FH is caused by mutations in the gene encoding the LDL receptor, leading to two- to threefold increased plasma cholesterol concentrations in heterozygous individuals (prevalence of one in 500) and a three- to sixfold increase above the upper limit of the reference range in homozygous individuals. These patients may have characteristic physical findings (see Plates 7-6 and 7-7 ). Familial defective apo B 100 is a disorder caused by mutations in the gene encoding apo B 100 . Defective apo B 100 apolipoprotein binding to the LDL receptor results in a high plasma LDL concentration and an increased CHD risk.
Type III hyperlipoproteinemia (familial dysbetalipoproteinemia) is an autosomal recessive disorder characterized by moderate to severe hypercholesterolemia and hypertriglyceridemia and is the result of mutations in the gene encoding apo E, with resultant defective lipoprotein binding to the LDL receptor (see Plate 7-9 ).
Elevated plasma lipoprotein(a) (Lp[a]) is a disorder characterized by increased concentrations of modified LDL particles in the plasma, in which the apo B 100 protein of LDL is covalently bonded to Lp(a). Lp(a) has structural similarity to plasminogen and can interfere with fibrinolysis. Increased plasma Lp(a) is associated with increased CHD risk.
Polygenic hypercholesterolemia refers to combinations of multiple genetic and environmental factors that contribute to hypercholesterolemia. Polygenic hypercholesterolemia is diagnosed by exclusion of other primary genetic causes, absence of tendon xanthomas, and documentation that hypercholesterolemia is present in fewer than 10% of first-degree relatives.
High-density lipoproteins (HDLs) are small particles that contain 50% lipid (phospholipid, cholesteryl esters, free cholesterol, triglyceride) and 50% protein. The main apolipoproteins (apo) are apo AI (65%), apo AII (25%), and smaller amounts of apo C and apo E. The two major subclasses of HDL are HDL 2 and HDL 3 . HDL 1 is a minor subclass and is associated with apo E. HDLs function to redistribute lipids among cells and lipoproteins by a process referred to as reverse cholesterol transport , in which HDL acquires cholesterol from cells and transports it either to other cells or to the liver.
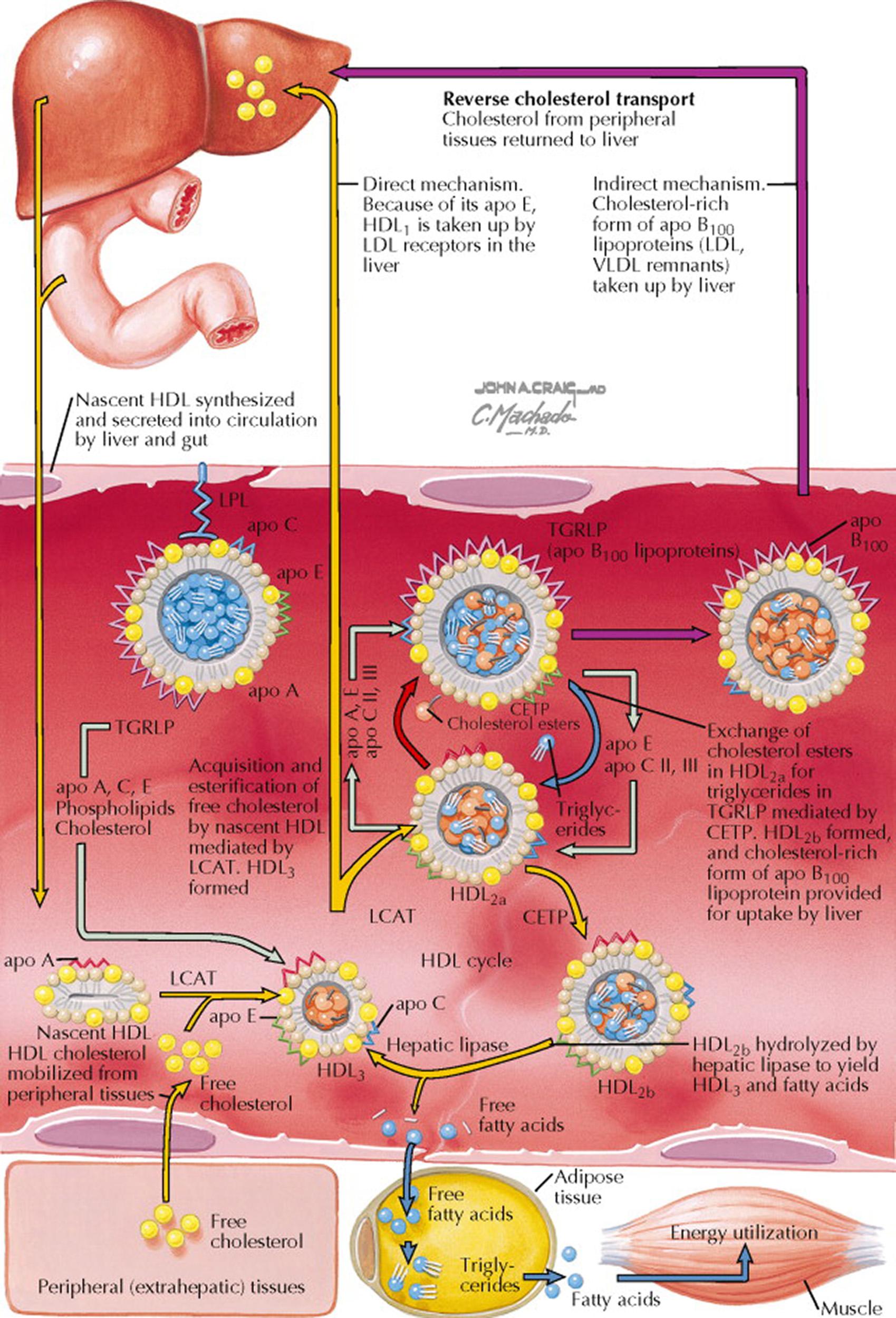
The steps in the formation and metabolism of HDL include the following: small nascent or precursor HDL disks composed of apo AI and phospholipid are synthesized in the liver and small intestine; precursor HDL disks accept free cholesterol from cells or from other lipoproteins (triglyceride-rich lipoproteins [TGRL] and chylomicron and very low-density lipoprotein [VLDL] remnants); and HDL free cholesterol is esterified by the apo AI–activated enzyme, lecithin–cholesterol acyltransferase (LCAT). The esterified cholesterol increases its hydrophobicity, and it moves away from the surface of the disk to form a cholesteryl ester–rich core and changes the HDL shape from a disk to a sphere. The spherical, mature HDL 2 particles function to remove excess cholesterol, and as they enlarge, the particle is termed HDL 3 . HDL acquires cholesterol by aqueous transfer from cells (passive desorption) or by transport that is facilitated by cell surface–binding proteins. Several cell surface proteins facilitate the efflux of free cholesterol. For example, ABCA1 binds apo AI and facilitates the transfer of free cholesterol and phospholipids onto HDL. Mutations in the gene that encodes ABCA1 can prevent this transfer process, resulting in a lipid disorder called Tangier disease (see Plate 7-8 ).
Because of its apo E, HDL 1 is taken up by LDL receptors in the liver. In addition, cholesteryl ester transfer protein (CETP) transfers cholesteryl esters (in exchange for triglycerides) from HDL 2 to TGRL (e.g., VLDL, LDL, and remnants), which are then delivered to the liver. An additional pathway of cholesterol redistribution from HDL is via scavenger receptor B1 (SR-B1) facilitation of selective uptake of cholesteryl esters by the adrenal glands, gonads, and liver. The HDL 2 particles that have been partially depleted of cholesteryl esters and enriched with triglycerides by CETP can be converted back to HDL 3 by the action of hepatic lipase that hydrolyzes the triglycerides.
Reverse cholesterol transport—with a redistribution of cholesterol from cells with excess (e.g., arterial walls) to cells requiring cholesterol or to the liver for excretion—is antiatherogenic. There is an inverse relationship between plasma HDL concentration and cardiovascular risk. In addition to reverse cholesterol transport, HDL has other antiatherogenic properties. For example, the HDL-associated enzyme paraoxonase serves to inhibit oxidation of LDL. In addition, HDL and apo AI stabilize the erythrocyte cell membrane and prevent transbilayer diffusion of anionic lipids, a step that is required for prothrombin activation and thrombus formation.
Several alterations in the HDL pathway can result in low or high plasma HDL concentrations. For example, mutations in the gene encoding apo AI can decrease HDL formation because of lack of LCAT activation; resultant plasma concentrations are less than 10 mg/dL (reference ranges: low, less than 40 mg/dL; normal, 40 to 60 mg/dL; desirable, greater than 60 mg/dL). Increased plasma HDL concentrations are found in individuals with CETP deficiency because of the decreased transfer of cholesteryl esters from HDL to apo B-containing lipoproteins. CETP deficiency homozygotes have HDL concentrations greater than 100 mg/dL.
Cholesterol has a chief role in the function of cell membranes and serves as a precursor of steroid hormones. However, when blood concentrations of low-density lipoprotein (LDL) cholesterol exceed certain levels, it is termed hypercholesterolemia . Hypercholesterolemia can predispose to atherosclerosis and increase the risk for vascular disease (e.g., coronary heart disease [CHD], cerebrovascular disease, and peripheral vascular disease).
The National Heart Lung and Blood Institute of the National Institutes of Health has published a series of three guidelines (1988, 1993, 2002), each updating the existing recommendations for clinical management of high blood cholesterol. The Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults is termed the Adult Treatment Panel III (ATP III) and is the most recent version from the National Cholesterol Education Program (NCEP). ATP III set cutoffs for blood LDL cholesterol concentrations on the basis of cardiovascular risk. For example, a plasma LDL concentration less than 100 mg/dL is considered optimal. As plasma LDL levels increase, cardiovascular risk increases. The presence of risk factors (e.g., cigarette smoking, hypertension, low plasma concentration of high-density lipoprotein [HDL] cholesterol, family history of premature CHD, age [men, ≥45 years; women, ≥55 years]) should modify the target for LDL cholesterol. For example, the presence of CHD or CHD risk equivalents (e.g., diabetes mellitus) decreases the LDL cholesterol goal to less than 100 mg/dL; if there are two or more risk factors, the LDL cholesterol goal should be less than 130 mg/dL; if there is zero to one risk factor, the LDL cholesterol goal should be less than 160 mg/dL. These targets may be modified when NCEP ATP IV guidelines are published in the fall of 2011.
Many factors contribute to high plasma LDL concentrations. For example, diets high in saturated fats and cholesterol lead to increased blood cholesterol concentrations. Although the level of cholesterol in the blood is controlled at multiple sites, the primary regulator is the LDL receptor pathway. LDL receptors are present on the cell surface of most cells and mediate the uptake of lipoproteins that contain the apolipoproteins (apo) apo B 100 and apo E (e.g., LDL, chylomicron remnants, very low-density lipoproteins [VLDL], VLDL remnants, and HDL 1 ).
Familial hypercholesterolemia is a relatively common disorder caused by mutations in the gene that encodes the LDL receptor. Decreased synthesis or synthesis of defective LDL receptors leads to increased plasma concentrations of LDL cholesterol (three- and sixfold increased above the reference range in heterozygotes and homozygotes, respectively) (see Plates 7-6 and 7-7 ).
Mutations in the gene that encodes apo B 100 are relatively common and lead to defective binding of LDL cholesterol to the LDL receptor. The clinical findings and the blood lipid profile are similar to those of familial hypercholesterolemia (see Plates 7-6 and 7-7 ).
Familial hyperapobetalipoproteinemia (with overproduction of apo B 100 ) and familial combined hyperlipidemia are both inherited in an autosomal dominant fashion. Although the genetic defects underlying these conditions have yet to be identified (probably multiple genetic defects), they are relatively common disorders and are associated with elevations of plasma LDL cholesterol and triglyceride concentrations and increased susceptibility to CHD. Other associations include moderate decrease in plasma HDL cholesterol concentrations, fasting hyperglycemia, obesity, and hyperuricemia. Familial combined hyperlipidemia should be suspected in individuals with moderate hypercholesterolemia in combination with moderate hypertriglyceridemia in the setting of a family history of premature CHD. Xanthomas are not seen in individuals with familial hyperapobetalipoproteinemia or with familial combined hyperlipidemia.
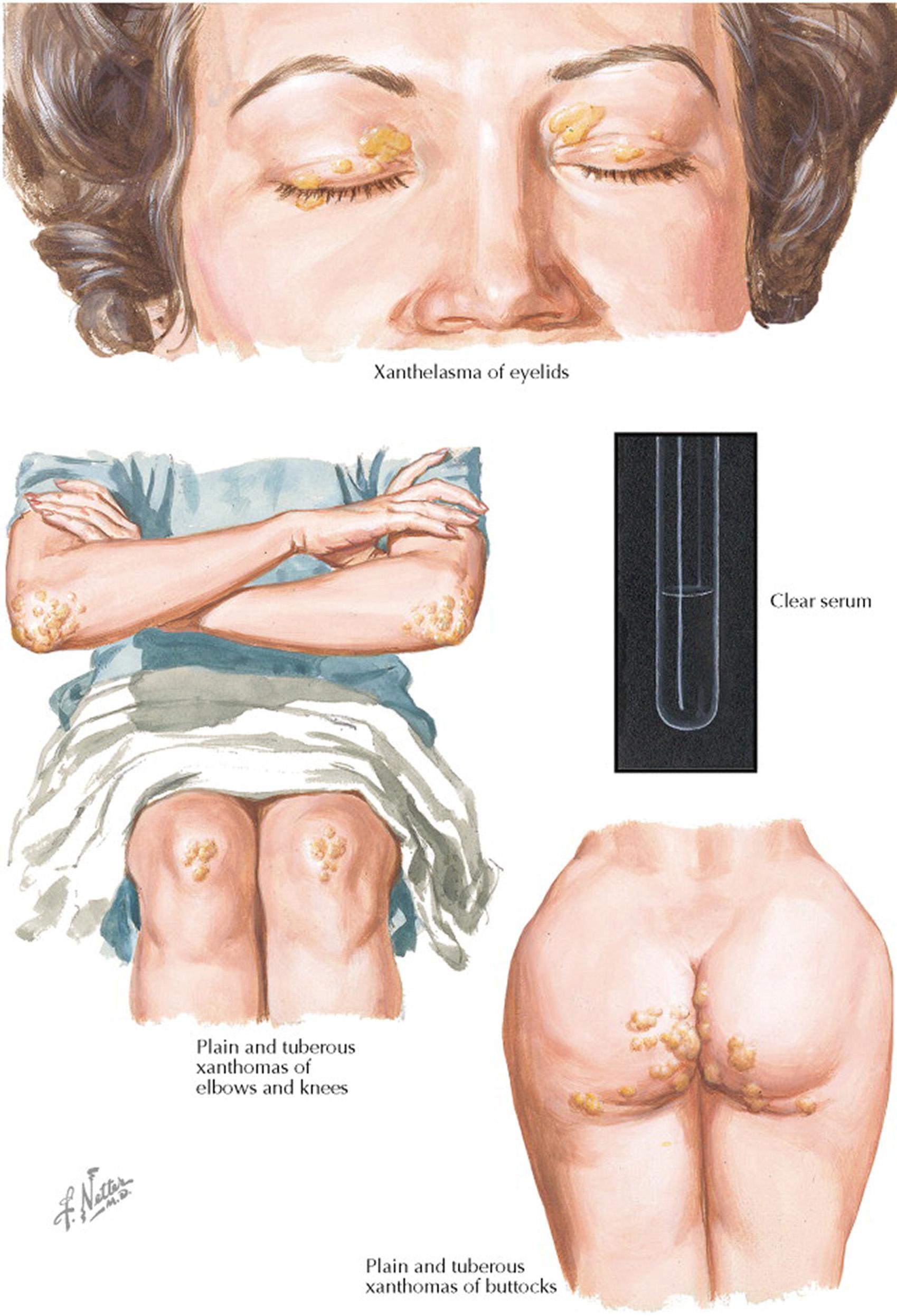
Severe hypercholesterolemia can lead to cutaneous and tendinous xanthomas. These cutaneous protuberances represent the accumulation of large (10–20 μm in diameter), cholesterol-filled macrophages. The high concentrations of low-density lipoprotein (LDL) cholesterol in the blood are taken up by the nonsaturable scavenger receptors on macrophages. Xanthelasma of the eyelids is frequently accompanied by premature arcus corneae (i.e., in persons younger than 40 years). Plain and tuberous xanthomas are most frequently found over the elbows, knees, and buttocks, possibly related to continuous irritation by garments. Tuberous xanthomas are seen most frequently in individuals with homozygous mutations in the gene that encodes the LDL receptor (see the following text).
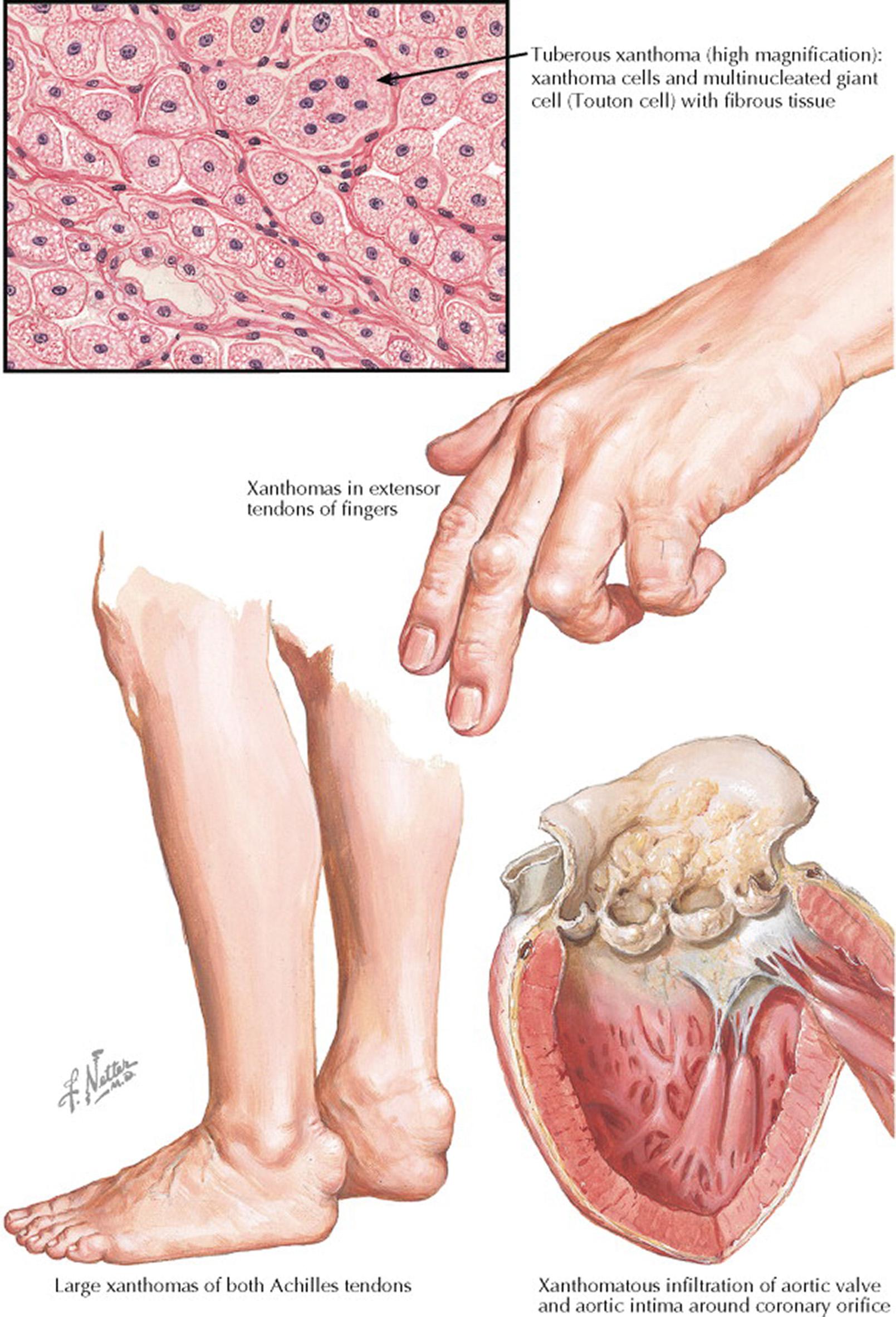
The characteristic lesions of tendinous xanthoma are actually part of the tendon, from which they cannot be mechanically separated. The nodules are found in the extensor tendons of the hands, Achilles tendons, and patellar tendons. This type of nodular lesion may be easily confused with the nodules of rheumatoid arthritis, but it can readily be distinguished because a xanthoma is not painful, and patients with rheumatoid arthritis lack the marked increased in blood LDL cholesterol concentrations that are seen in patients with xanthomas.
In the first phase, atherosclerotic lesions consist of cushionlike elevations of lipid-filled macrophages (foam cells) beneath the intima. Later, they become sclerotic (see Plates 7-12 and 7-13 ). The atheroma of the arterial intima is the most dangerous feature of familial hypercholesterolemic xanthomatosis because of its frequent occurrence in the coronary vessels, which may cause angina and myocardial infarction at an early age.
Hypercholesterolemic xanthomatosis is a manifestation of either familial hypercholesterolemia (FH) (an autosomal dominant disorder caused by mutations in the gene that encodes the LDL receptor) or familial defective apolipoprotein apo B 100 (caused by mutations in the gene that encodes apo B 100 ).
FH is a monogenic disorder caused by mutations in the gene that encodes the LDL receptor. Thus, LDL cholesterol is not effectively cleared from the circulation, and plasma concentrations of LDL cholesterol are increased. There is increased uptake of LDL cholesterol by the macrophage scavenger receptors, with marked lipid accumulation in the macrophages (foam cells). More than 900 different mutations in the LDL receptor have been identified to cause FH. The types of mutations in the gene that encodes the LDL receptor include mutations that cause the following: decreased LDL receptor synthesis, decreased intracellular transport of the LDL receptor from the endoplasmic reticulum to the Golgi apparatus, defective binding of LDL cholesterol to the LDL receptor, and a defect in the internalization of the LDL receptor after binding LDL cholesterol. Thus, the impact of the LDL receptor mutation on plasma LDL cholesterol concentrations and coronary heart disease (CHD) risk is very dependent on the specific mutation. In addition, individuals with homozygous LDL receptor mutations are much more severely affected than heterozygous individuals.
Heterozygous FH is a relatively common disorder, affecting one in 500 persons, and its manifestations are present from birth. In individuals with heterozygous FH, the plasma total cholesterol concentrations are typically more than 300 mg/dL, and the LDL cholesterol concentrations are more than 250 mg/dL. Plasma triglycerides are not elevated in this condition. Approximately 75% of patients with heterozygous FH have xanthelasma and tendon xanthomas. Also, premature CHD and heart valvular disease occurring before age 45 years are common. Heterozygous FH should be suspected in individuals with high plasma concentrations of LDL cholesterol, normal plasma triglyceride concentrations, tendon xanthomas, and a family history of premature CHD. The diagnosis of heterozygous FH is made on clinical grounds. Because of the large number of potential mutations, germline mutation testing for abnormalities in the gene that encodes the LDL receptor is not routinely done.
Fortunately, homozygous FH is rare. These individuals come to clinical attention either because of a family history of premature CHD or the appearance of xanthomas at a young age (i.e., younger than 10 years). Typical plasma total cholesterol concentrations range from 600 to 1000 mg/dL; plasma LDL cholesterol concentrations range from 550 to 950 mg/dL. Tuberous xanthomas usually develop before age 6 years and are unique to homozygous FH; these individuals also develop the xanthelasma and tendon xanthomas that are common in individuals who are heterozygous for mutations in the LDL receptor gene. Symptomatic CHD can occur before age 10 years, and fatal myocardial infarction usually occurs before age 20 years if the hypercholesterolemia is not treated. Aortic valvular disease (e.g., aortic stenosis) is more common (occurring in ∼50% of affected individuals) and is more severe in homozygous FH than in heterozygous FH. The diagnosis of homozygous FH should be suspected when the plasma LDL cholesterol concentration is more than 500 mg/dL.
Treatment of individuals with heterozygous FH includes a low-cholesterol diet and pharmacologic therapy with a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor. Some patients may require the addition of a bile acid sequestrant or an intestinal cholesterol absorption inhibitor.
Treatment of individuals with homozygous FH is problematic. Because of very little residual LDL cholesterol binding, pharmacologic therapy with HMG-CoA reductase inhibitors, bile acid sequestrants, and intestinal cholesterol absorption inhibitors is suboptimally effective. The most effective therapy involves the periodic (i.e., every 1–3 weeks) selective removal of LDL cholesterol by extracorporeal apheresis.
Familial defective apo B 100 is relatively common disorder affecting in one in 500 persons that is caused by a mutation in the gene encoding apo B 100 . To date, most affected patients have the same single point mutation at nucleotide number 3500. Apo B 100 is the ligand that binds LDL cholesterol to the LDL receptor; thus, biochemical and clinical phenotypes are very similar to those in individuals with LDL receptor mutations. These individuals have isolated elevations in plasma LDL cholesterol concentrations, xanthelasma, tendon xanthomas, and premature CHD. In general, the clinical presentations of heterozygous and homozygous familial defective apo B 100 are less severe than those of the heterozygous and homozygous forms of FH, respectively. Clinically, familial defective apo B 100 cannot be distinguished from FH; germline mutation testing is the only method currently available to make this distinction.
Treatment of familial defective apo B 100 is similar to that of heterozygous FH, with emphasis on a low-cholesterol diet and pharmacologic therapy with HMG-CoA reductase inhibitors, bile acid sequestrants, and intestinal cholesterol absorption inhibitors.
Tendon xanthomas and premature CHD can also occur independently of an abnormality in LDL cholesterol metabolism. Sitosterolemia is an autosomal recessive disorder resulting from mutations in the genes encoding the adenosine triphosphate–binding cassettes G5 and G8 that normally limit plant sterol absorption. There is a resultant increase in gastrointestinal absorption of cholesterol and plant sterols. The plant sterols and LDL cholesterol accumulate in the plasma and peripheral tissues, leading to premature CHD and tendon xanthomas. Plasma levels of LDL cholesterol are high. Gas-liquid chromatography shows high levels of plant sterols.
Cerebrotendinous xanthomatosis (CTX) is an autosomal recessive lipid storage disease and a form of leukodystrophy. CTX is caused by a block in bile acid synthesis because of absent 27-hydroxylase (caused by mutations in CYP27A1 ), resulting in an accumulation of cholesterol and cholestanol in all tissues. The plasma lipid levels in individuals with CTX are normal. Xanthomas develop in the central nervous system, tendons, skin, bones, and lungs. Because of the associated defects in synthesis and maintenance of the myelin sheath of nerves, CTX has dominant effects on the central nervous system with resultant cerebellar ataxia and pyramidal tract signs.
Two familial syndromes are characterized by severe deficiency or absence of specific lipoproteins: abetalipoproteinemia and Tangier disease.
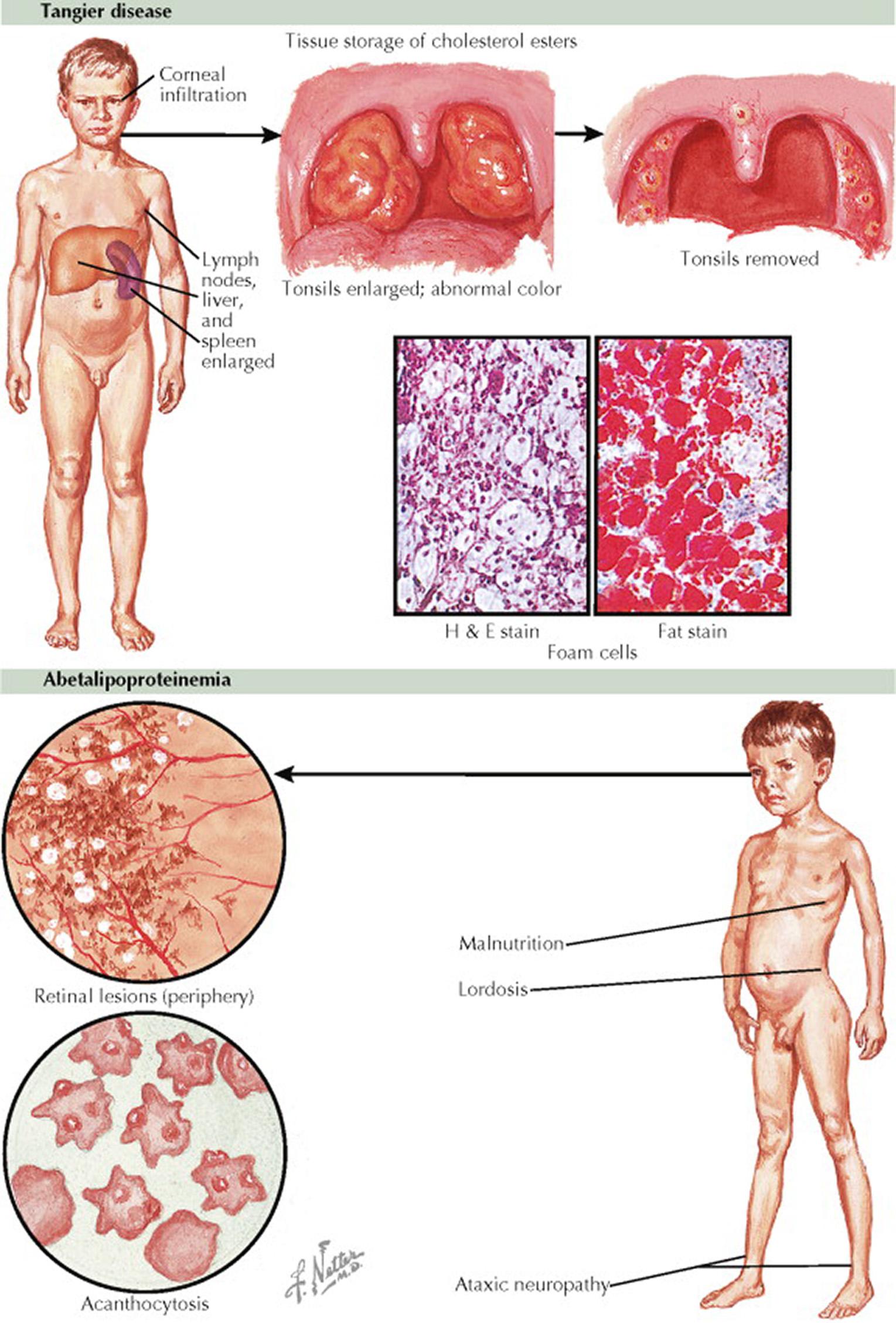
Abetalipoproteinemia (OMIM 200100) is a rare autosomal recessive disorder that usually presents in infancy with fat malabsorption, hypocholesterolemia, and acanthocytosis. Later in life, deficiencies in fat-soluble vitamins result in atypical retinitis pigmentosa, posterior column neuropathy, and myopathy. Abetalipoproteinemia is caused by mutations in the gene encoding the large subunit of microsomal triglyceride transfer protein, resulting in abnormal production and secretion of apolipoprotein B (apo B) and apo B–containing lipoproteins. Microsomal triglyceride transfer protein is key in the transfer of triglycerides and phospholipids into the lumen of the endoplasmic reticulum of the enterocyte for the assembly of very low-density lipoprotein (VLDL), a step required for normal hepatic secretion of apo B 100 . Insufficient lipidation of these nascent particles prevents synthesis and secretion of chylomicrons and VLDL by the intestine and liver. This defect results in gastrointestinal fat malabsorption and extremely low plasma concentrations of cholesterol and VLDL triglycerides and absent betalipoprotein.
The absence of apo B results in steatorrhea, symptoms associated with deficiency of fat-soluble vitamins (vitamins A, D, E, and K), neurologic manifestations (e.g., retinitis pigmentosa, peripheral neuropathy, ataxia, lordosis caused by muscular weakness, sensory motor neuropathy, mental retardation), and acanthocytosis (crenated appearance of erythrocytes). In individuals who are homozygous for mutations in the disease-causing gene, there may be deficient adrenocortical glucocorticoid production. The neurologic manifestations may dominate the clinical presentation with early onset (e.g., age 1–2 years) of generalized weakness, distal muscular atrophy, loss of proprioception, posterior column degeneration with sensory neuropathy, and cerebellar atrophy with ataxia and nystagmus. Children with abetalipoproteinemia appear malnourished and have growth retardation. Some patients may have hepatic steatosis and cirrhosis, which can result from treatment with medium-chain triglycerides. In one patient who underwent liver transplantation for hepatic cirrhosis, the serum lipoprotein profile normalized but gastrointestinal fat malabsorption persisted.
Laboratory studies show the absence of plasma apo B–containing proteins and extremely low levels of total cholesterol (<50 mg/dL). Early diagnosis and treatment are key to avoid growth retardation and neuroretinal complications. Treatment includes a lipid-poor diet (e.g., 5 g/d in children) to treat digestive intolerance and allow normal absorption of carbohydrates and proteins, provision of dietary essential fatty acids in the form of vegetable oils, and high doses of fat-soluble vitamins (vitamins A, D, E, and K).
Tangier disease (OMIM 205400) is an autosomal dominant disorder that results in low serum concentrations of high-density lipoprotein (HDL) cholesterol. Tangier disease was originally described and named on the basis of a kindred living on Tangier Island in Chesapeake Bay. Tangier disease is caused by mutations in the adenosine triphosphate–binding cassette transporter-1 gene ( ABCA1 ), which encodes the cholesterol efflux regulatory protein. ABCA1 is critical for intracellular cholesterol transport, the impairment of which results in decreased cholesterol efflux onto nascent HDL particles, leading to lipid-depleted particles that are then rapidly catabolized. Thus, the inability of newly synthesized apolipoproteins to acquire cellular lipids by the ABCA1 pathway leads to their rapid degradation and an overaccumulation of cholesterol in macrophages. ABCA1 has a critical role in modulating flux of tissue cholesterol and phospholipids into the reverse cholesterol transport pathway. The impaired HDL-mediated cholesterol efflux from macrophages leads to massive accumulation of cholesteryl esters (foam cells) throughout the body and resultant hepatosplenomegaly. These individuals frequently develop premature coronary disease. Individuals with homozygous mutations in ABCA1 have absent plasma HDL, and heterozygotes have HDL concentrations about 50% of those in individuals with two normal alleles.
Findings on physical examination include orange tonsils (caused by cholesterol deposits), corneal opacities, hepatosplenomegaly, and peripheral neuropathy. Findings from laboratory studies show absent HDL cholesterol and low total cholesterol concentrations. Currently, there is no disease-specific treatment for Tangier disease.
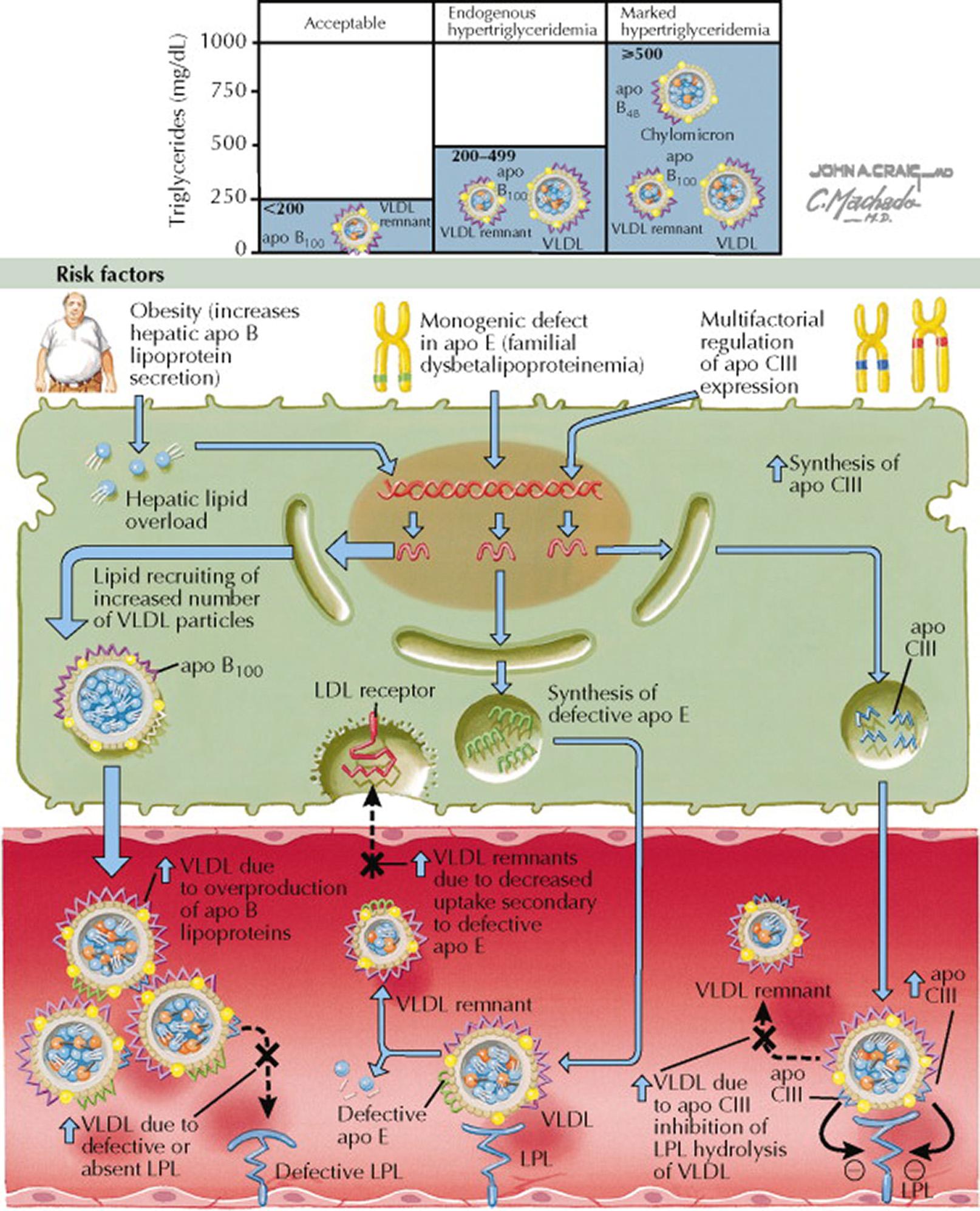
Based on coronary risk, serum triglyceride concentrations can be stratified as follows: normal, less than 150 mg/dL; borderline high, 150 to 199 mg/dL; high, 200 to 499 mg/dL; and very high, 500 mg/dL or greater. Serum triglyceride concentrations greater than 199 mg/dL are termed hypertriglyceridemia and are associated with an increased risk of cardiovascular disease. Hypertriglyceridemia can be caused by or exacerbated by obesity, poorly controlled diabetes mellitus, nephrotic syndrome, hypothyroidism, and orally administered estrogen therapy.
Hypertriglyceridemia results from the accumulation of triglyceride-rich lipoproteins (e.g., very low-density lipoproteins [VLDL], VLDL remnants, and chylomicrons) in blood. Hypertriglyceridemia is associated with variable degrees of hypercholesterolemia because the triglyceride-rich lipoproteins also transport cholesterol. Triglycerides in chylomicrons and VLDL are hydrolyzed by lipoprotein lipase (LPL), and the free fatty acid molecules are used as an energy source. LPL also facilitates the transfer of cholesterol to high-density lipoprotein (HDL) cholesterol. Thus, when LPL activity is deficient, hypertriglyceridemia and low blood HDL cholesterol concentrations occur.
Disorders in lipid metabolism can be categorized by the Fredrickson hyperlipoproteinemia phenotype classification, which is based on the pattern of lipoproteins on electrophoresis or ultracentrifugation:
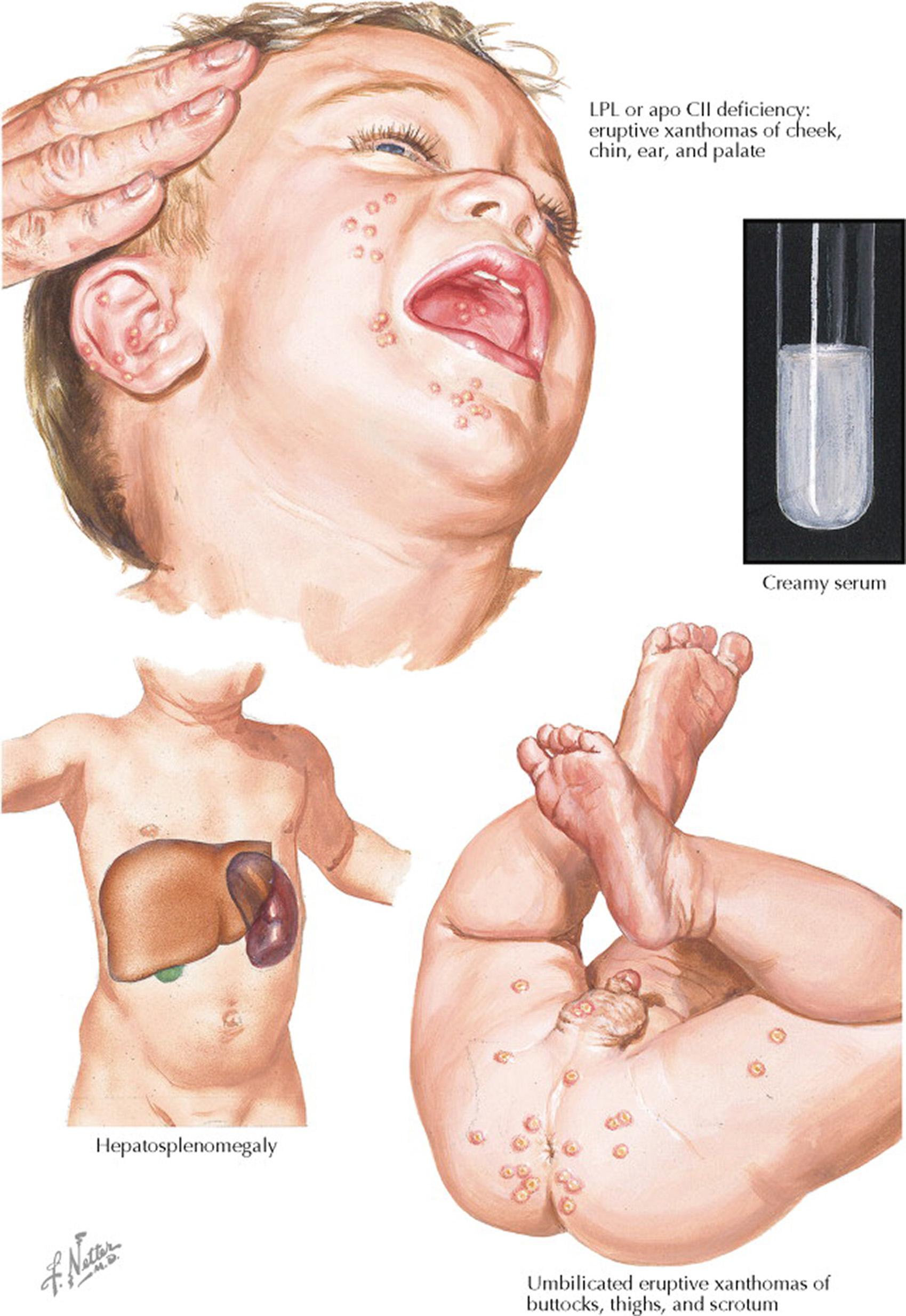
Phenotype I (LPL deficiency): increased serum chylomicron concentration and markedly increased serum triglyceride concentration. The serum has a creamy top layer.
Phenotype IIa (familial hypercholesterolemia): increased serum LDL cholesterol and total cholesterol concentrations. The serum is clear.
Phenotype IIb: increased serum concentrations of LDL and VLDL cholesterol. The serum is clear.
Phenotype III (familial dysbetalipoproteinemia): increased serum concentrations of VLDL remnants and chylomicrons. The serum is turbid.
Phenotype IV (familial hypertriglyceridemia): increased serum concentrations of VLDL. The serum is turbid.
Phenotype V (mixed hypertriglyceridemia): increased serum concentrations of chylomicrons and VLDL. The serum has a creamy top layer and a turbid bottom layer.
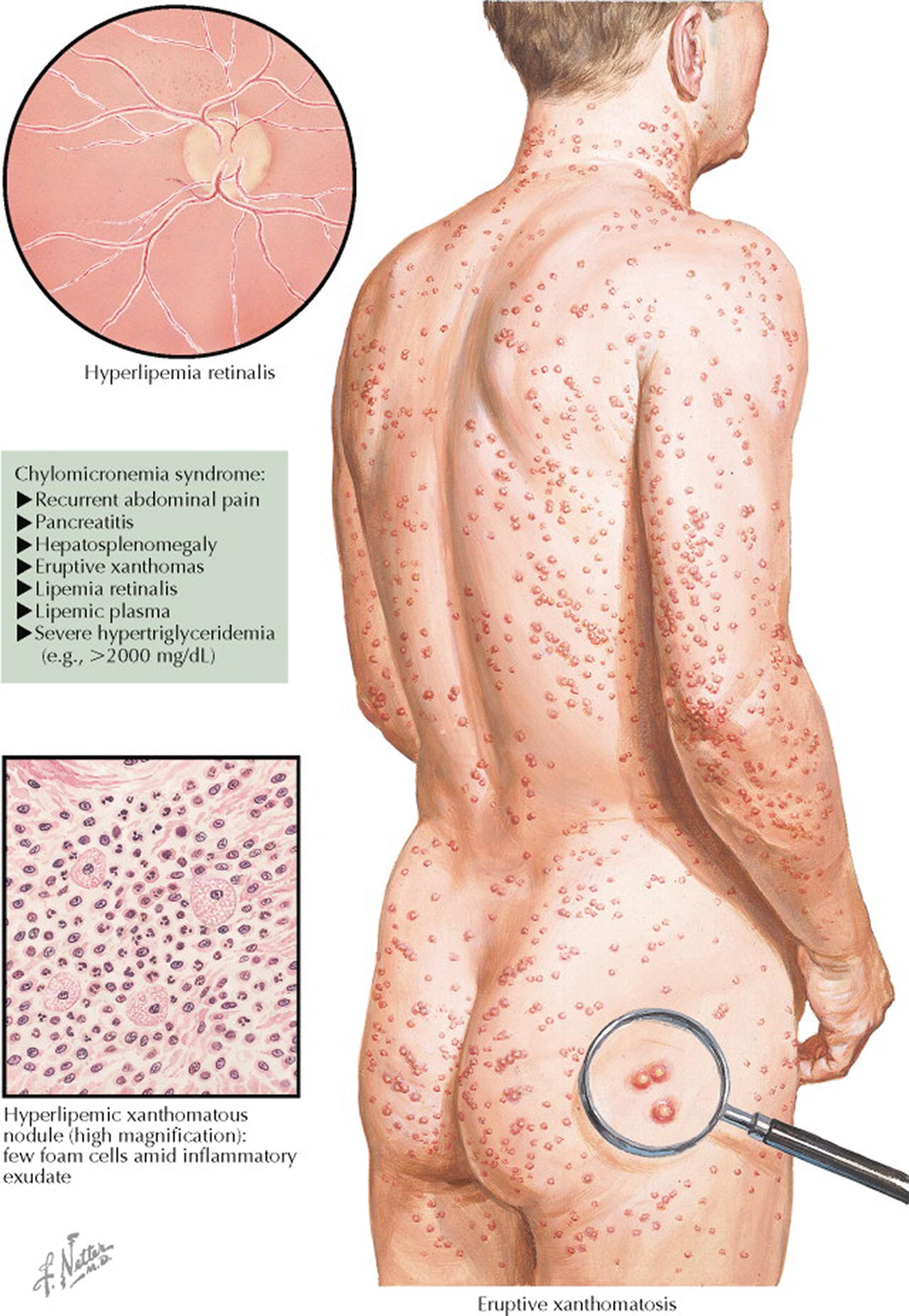
The type I hyperlipoproteinemia phenotype is caused by rare recessive disorders and is associated with complete absence of either LPL activity or apolipoprotein (apo) CII (the ligand for LPL on chylomicrons and VLDL). Severe hypertriglyceridemia results because the clearance of triglyceride-rich lipoproteins from plasma is blocked. Chylomicronemia syndrome is a frequent finding in patients with type I hyperlipoproteinemia (see Plates 7-10 and 7-11 ).
Type IIa hyperlipoproteinemia is familial hypercholesterolemia and is associated with LDL receptor deficiency, resulting in markedly increased serum concentrations of LDL (see Plates 7-6 and 7-7 ). Type IIb hyperlipoproteinemia is combined hyperlipidemia caused by decreased LDL receptor availability or function and increased apo B, resulting in increased blood levels of LDL cholesterol and VLDL (see Plate 7-5 ).
Familial dysbetalipoproteinemia, also termed type III hyperlipoproteinemia, is associated with specific isoforms of the APOE gene; however, other genetic and environmental factors probably contribute to disease development and severity. Apo E is required for receptor-mediated clearance of chylomicron and VLDL remnants. The most common APOE genotype is APOE*E3/APOE*E3 . The E2 isoform has lower affinity for the LDL receptor than the E3 isoform, which leads to poor clearance of VLDL and chylomicron remnants that contain the E2 isoform. Familial dysbetalipoproteinemia occurs when individuals are homozygous for the E2 allele. This defect leads to premature coronary heart disease and peripheral vascular disease. Tuberoeruptive xanthomas may be evident on physical examination (see Plates 7-6 and 7-7 ).
Familial hypertriglyceridemia (type IV hyperlipoproteinemia phenotype) is an autosomal dominant disorder caused by inactivating mutations in the gene encoding LPL and is associated with moderately increased serum triglyceride concentrations (200–500 mg/dL), normal serum LDL cholesterol concentrations, and low serum HDL cholesterol concentrations. The degree of hypertriglyceridemia can be aggravated by exogenous agents (e.g., orally administered estrogen replacement therapy). Familial hypertriglyceridemia is usually associated with obesity, insulin resistance, hyperglycemia, and hypertension.
Mixed hypertriglyceridemia (type V hyperlipoproteinemia phenotype) is characterized by triglyceride levels above the 99th percentile of normal. The plasma supernatant is creamy, and there are increased concentrations of chylomicrons and VLDL. The clinical manifestations include hepatosplenomegaly and eruptive xanthomas.
Familial combined hyperlipidemia is a genetically heterogenous disorder caused by overproduction of hepatically derived apolipoprotein B 100 associated with VLDL. Affected patients typically present with hypercholesterolemia and hypertriglyceridemia.
The C apolipoproteins also regulate triglyceride metabolism. Apo CI and apo CIII modulate the uptake of triglyceride-rich lipoproteins (chylomicron remnants, VLDL) by interfering with the ability of apo E to mediate binding to lipoprotein receptor pathways.
Hypertriglyceridemia is usually asymptomatic. However, serum triglyceride concentrations higher than 1000 mg/dL may result in chylomicronemia syndrome. Signs and symptoms associated with chylomicronemia syndrome include abdominal pain, pancreatitis, eruptive xanthoma, flushing with alcohol intake, memory loss, and lipemia retinalis. The acute pancreatitis can be life threatening, and the patients most commonly affected are those with poorly controlled diabetes mellitus or alcoholism. Serum triglyceride values higher than 1000 mg/dL result in opalescent serum caused by an increase in very low-density lipoprotein (VLDL). At markedly increased levels, the serum may be milky because of hyperchylomicronemia.
Triglycerides in chylomicrons and VLDL are hydrolyzed by lipoprotein lipase (LPL), and the free fatty acid molecules are used as an energy source in muscle for triglyceride synthesis or for storage in adipocytes or formation of hepatic VLDL. LPL is synthesized by adipocytes, myocytes, and macrophages. LPL attaches to heparan sulfate proteoglycans on the surface of capillary endothelial cells, where it interacts with circulating chylomicrons and VLDL. Apolipoprotein (apo) CII is a cofactor for LPL. Mutations that inactivate LPL or apo CII result in severe hypertriglyceridemia (see following text).
Hepatic lipase is synthesized by hepatocytes and is found in capillary endothelial cells of the liver, adrenals, and gonads. Hepatic lipase—activated by androgens and suppressed by estrogens—functions to release lipids from lipoproteins by hydrolyzing triglycerides in the processing of chylomicron remnants and also to convert high-density lipoprotein (HDL) cholesterol from HDL 2 to HDL 3 by removing phospholipid and triglyceride from HDL 2 . Thus, when the hepatic lipase activity is high, serum concentrations of total HDL cholesterol levels are low. Unlike LPL, apo CII is not a cofactor for hepatic lipase. Defects or deficiencies in hepatic lipase result in an accumulation of remnant lipoproteins and HDL 2 .
Mutations in the gene encoding LPL can result in deficient LPL activity, a rare autosomal recessive disorder that manifests with severe hypertriglyceridemia because the clearance of triglyceride-rich lipoproteins from the plasma is blocked. Individuals heterozygous for a mutation in the LPL gene (approximate frequency of one in 500) may have LPL activity that is 50% of normal, leading to mild hypertriglyceridemia.
Normally, chylomicrons are cleared from plasma within 8 hours of eating. In persons with complete LPL deficiency, the chylomicrons can take days to be cleared after a single meal. Chylomicronemia syndrome results when there are massive accumulations of these lipoproteins in the blood. LPL deficiency is usually diagnosed in infancy or childhood when individuals present with chylomicronemia syndrome. Manifestations of chylomicronemia syndrome include recurrent abdominal pain, pancreatitis, hepatosplenomegaly caused by the accumulation of triglycerides in reticuloendothelial cells, eruptive xanthomas, lipemia retinalis, lipemic plasma, neurologic manifestations, dyspnea, and severe hypertriglyceridemia (>2000 mg/dL). The pancreatitis resulting from chemical irritation by fatty acids and lysolecithin can be life threatening. Chylomicrons are usually present whenever the triglyceride concentration is higher than 1000 mg/dL in a fasting blood sample. The serum appears creamy. Because of the effect on blood volume, severe hypertriglyceridemia can lead to measurement errors in serum electrolytes. For example, if serum is not cleared of triglyceride-rich lipoproteins by centrifugation, serum sodium may appear low (pseudohyponatremia).
LPL deficiency should be suspected in infants and children with recurrent abdominal pain and pancreatitis. Eruptive xanthomas are usually present in this setting, especially when serum triglyceride concentrations are higher than 2000 mg/dL. LPL deficiency can be confirmed with the heparin infusion test. Heparin displaces LPL from heparan sulfate proteoglycans on the surface of capillary endothelial cells, and LPL activity can be assayed in plasma.
When patients with LPL deficiency present with pancreatitis, the initial treatment should include a fat-free diet. Long term, patients with LPL deficiency should be treated with a fat-restricted diet, with fat accounting for less than 10% of total calories. The therapeutic goal is to maintain serum triglyceride concentrations at less than 1000 mg/dL. Pharmacologic options are limited for patients with LPL deficiency (see following text).
Apo CII deficiency is another rare autosomal recessive disorder that can cause the chylomicronemia syndrome. The clinical presentation is identical to that of LPL deficiency. The lack of apo CII, an activating cofactor for LPL, results in a functional LDL deficiency. Apo CII deficiency can be confirmed by the absence of apo CII on electrophoresis of plasma apolipoproteins. The treatment of apo CII deficiency is identical to that of LPL deficiency.
Individuals with familial hypertriglyceridemia overproduce VLDL triglycerides, resulting in serum triglyceride concentrations in the range of 200 to 500 mg/dL and normal LDL cholesterol concentrations. The hypertriglyceridemia typically occurs in concert with low serum HDL cholesterol levels and obesity. Because of the relative mild degree of hypertriglyceridemia and the lack of associated symptomatology, most affected patients are not diagnosed until adulthood. The degree of hypertriglyceridemia is usually less than 1000 mg/dL unless aggravated by alcohol use, orally administered estrogen, or hypothyroidism. Treatment of individuals with familial hypertriglyceridemia includes avoidance of alcohol and orally administered estrogens, as well as implementation of some of the nonpharmacologic and pharmacologic approaches outlined in the following text.
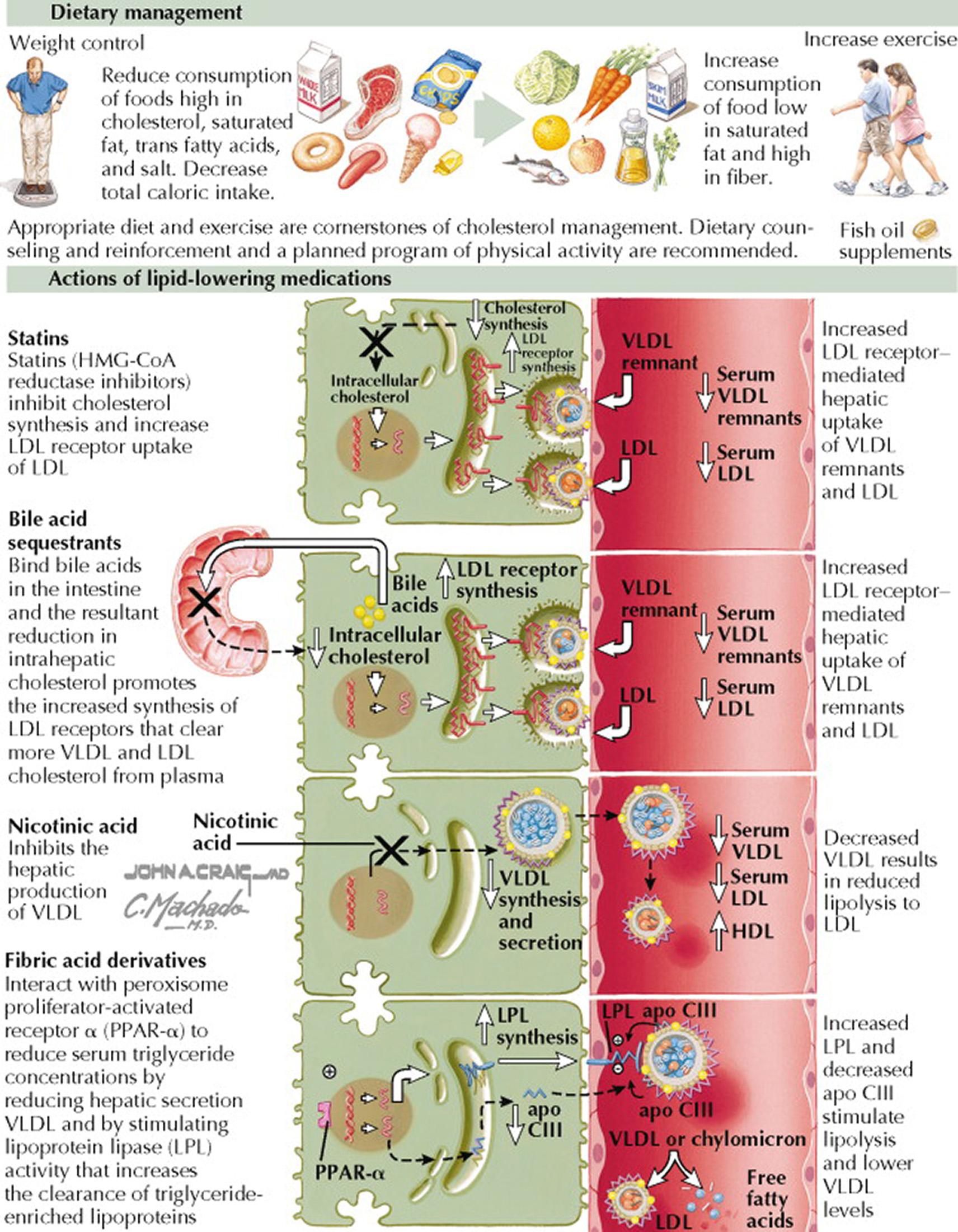
Hypertriglyceridemia promotes atherosclerosis, and treatment should be considered when serum triglyceride concentrations are higher than 200 mg/dL. Nonpharmacologic treatment options include weight loss in obese patients, a regular isotonic exercise program, improved glycemic control in patients with diabetes mellitus, limitation of alcohol intake, and avoidance of free carbohydrates in the diet. Pharmacologic therapy is indicated when hypertriglyceridemia persists despite nonpharmacologic interventions.
When serum LDL cholesterol concentrations are elevated in concert with serum triglycerides, a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor may lower the blood triglyceride concentration, as well as the LDL cholesterol concentration. When the main lipid profile anomaly is hypertriglyceridemia, it can be treated with a fibric acid derivative (i.e., fenofibrate or gemfibrozil), nicotinic acid, or omega-3 fatty acids (e.g., fish oil at doses >3 g/d). Fish oil decreases VLDL production and can lower serum triglyceride concentrations by as much as 50%. Nicotinic acid may cause hyperglycemia and should be avoided in patients with hyperglycemia or impaired glucose tolerance. Gemfibrozil may increase the risk for HMG-CoA reductase inhibitor–related myositis and should be avoided in these patients. Orlistat may be helpful in patients with type V hyperlipoproteinemia and very high serum triglyceride levels that are refractory to the aforementioned therapies because it inhibits gastrointestinal fat absorption and decreases intestinal chylomicron synthesis.
When the serum triglyceride concentration is very high (≥500 mg/dL), the first goal is to avoid pancreatitis. Prompt institution of pharmacologic therapy with nicotinic acid or a fibrate is indicated. For example, gemfibrozil can lower serum triglyceride concentrations in this setting by as much as 70%.
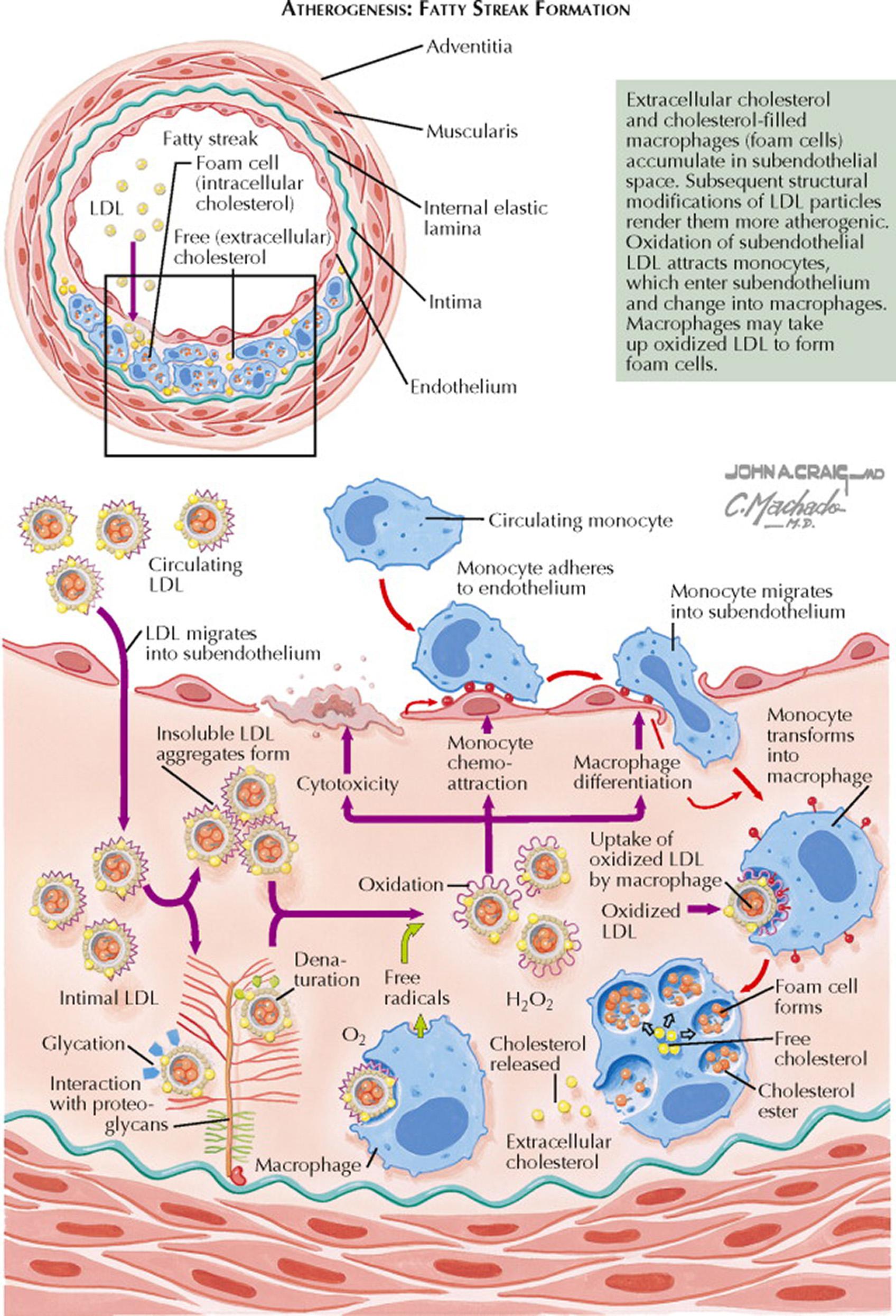
Atherogenesis starts in the arterial wall and eventually may lead to vascular disease (coronary heart disease [CHD], peripheral vascular disease, or cerebrovascular disease). Occlusive arterial disease resulting from atherosclerosis is a leading cause of disability and death. Atherosclerosis, a result of a chronic inflammatory response to vascular injury, is the buildup of plaque in the walls of arteries that is composed of lipoproteins, inflammatory cells, extracellular matrix, vascular smooth muscle cells, and calcium. The sites of atherosclerosis are typically those parts of the arterial vascular tree associated with increased turbulent blood flow (bifurcations and curvatures). Typical locations for symptomatic atherosclerotic lesions are the proximal left anterior descending coronary artery, proximal renal arteries, and carotid bifurcations. These sites have an upregulation of proinflammatory adhesion molecules for inflammatory cells (e.g., monocytes and T cells). Risk factors for endothelial dysfunction and injury include increased serum concentrations of low-density lipoprotein (LDL) cholesterol, decreased serum concentrations of high-density lipoprotein (HDL) cholesterol, increased oxidant stress (e.g., cigarette smoking, hypertension, diabetes mellitus), and aging. The serum concentration of LDL cholesterol is a strong predictor of CHD and atherosclerosis; more than 70% of individuals with premature CHD have hyperlipidemia. Total serum cholesterol concentrations less than 160 mg/dL markedly decrease CHD risk.
Atherogenesis is a slow process that occurs over years. The clinical manifestations of atherosclerosis may be chronic (e.g., stable angina pectoris or intermittent claudication) or acute (e.g., myocardial infarction, stroke). However, most atheromata produce no symptoms. The normal arterial wall is composed of the endothelial cell layer, intima and subendothelial space, internal elastic lamina, media (muscularis layer formed by smooth muscle cells), and adventitia (loose connective tissue). The initial events in atherosclerosis involve movement of electronegative LDL cholesterol and other apolipoprotein (apo) B 100 –containing lipoproteins (e.g., very low-density lipoprotein, lipoprotein[a]) from the blood into the subendothelial space where they are retained because of a charge-mediated interaction with the positively charged proteoglycans. Small LDL particles penetrate the endothelial barrier more effectively than large LDL particles. LDL cholesterol in the subendothelial space becomes oxidized. The presence of oxidized LDL promotes synthesis of monocyte chemoattractant protein 1 and other chemoattractants by endothelial and smooth muscle cells. Circulating monocytes then attach to the surface of endothelial cells and subsequently migrate between these cells to enter the subendothelial space, where they differentiate into macrophages. The activated macrophage releases mitogens and chemoattractants, which recruit more macrophages and smooth muscle cells. The macrophages take up the oxidized LDL cholesterol in an unregulated fashion by scavenger receptors. The internalization of oxidized LDL cholesterol leads to formation of lipid peroxides and facilitates the accumulation of cholesterol esters, resulting in foam cell formation. As foam cells accumulate, they form a visible atherosclerotic lesion—the fatty streak. Fatty streaks, the initial lesion of atherosclerosis, can be seen in infants and young children. The fatty streak may resolve (based on HDL cholesterol reverse cholesterol transport) or may mature into a fibrous plaque that extends into the vessel lumen.
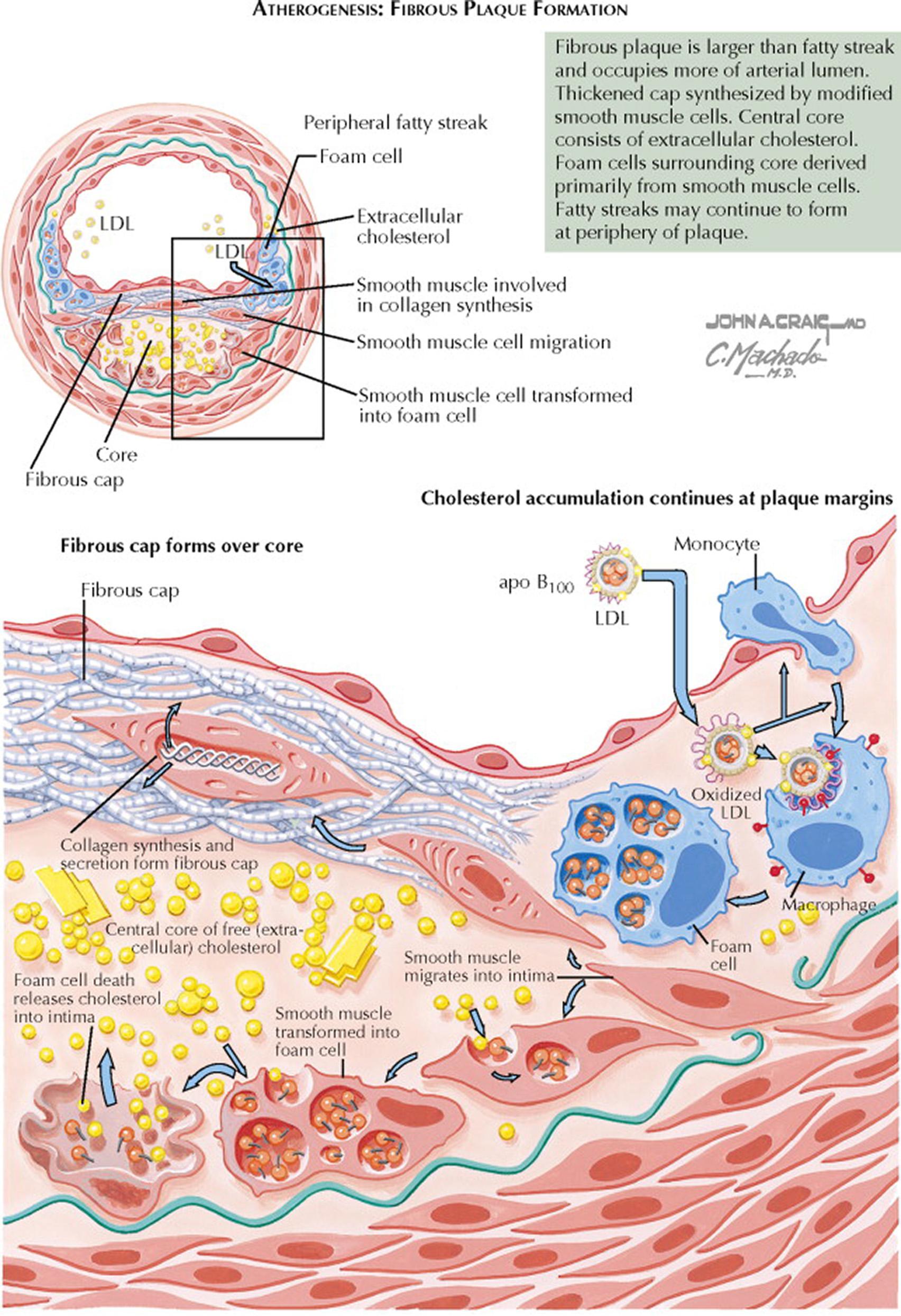
As fibrous plaques enlarge and age, foam cells necrose and release oxidized LDL, intracellular enzymes, and oxygen free radicals that can damage the vessel wall. Oxidized LDL induces apoptosis of endothelial cells and vascular smooth muscle cells. The extracellular cholesterol deposition and continued inflammatory response promote smooth muscle cell proliferation and migration and collagen synthesis. Smooth muscle cells become the main cell type, lying in parallel layers with proteoglycan and basement membrane in between. Continued inflammation results in the recruitment of increased numbers of macrophages and lymphocytes that release proteolytic enzymes, cytokines, chemokines, and growth factors. Focal necrosis develops, and free cholesterol forms the central lipid core of the fibrous plaque. Some smooth muscle cells accumulate lipid to become foam cells. Cycles of accumulation of mononuclear cells, migration and proliferation of smooth muscle cells, and formation of fibrous tissue lead to a continuous restructuring of the atherosclerotic lesion. A fibrous cap develops that overlies the core of lipid and necrotic tissue.
With progression of an atherosclerotic lesion, new microvessels arise from the arterial vasa vasorum. The microvessels provide a portal of entry for monocytes and lymphocytes into the developing plaque. The microvessels are fragile and are prone to rupture, resulting in small focal hemorrhages within the plaque. Calcification may occur as a late event in fibrous plaques, and the elasticity of the arterial wall becomes limited. Bone-related proteins (e.g., osteopontin and osteocalcin) can be found in atherosclerotic plaques. Coronary calcification is a marker of atherosclerosis that can be quantified with the use of cardiac computed tomography (CT), and it is proportional to the extent and severity of atherosclerotic disease. Cardiac CT is a noninvasive tool to assess the presence of coronary artery disease. Increased cardiac CT calcium scores indicate higher risk for CHD in both asymptomatic and symptomatic individuals and can be used to guide management decisions. For example, aggressive preventive medical therapy (see Plate 7-17 ) and risk factor modification (see Plate 7-14 ) should be considered for asymptomatic individuals with high cardiac CT calcium scores.
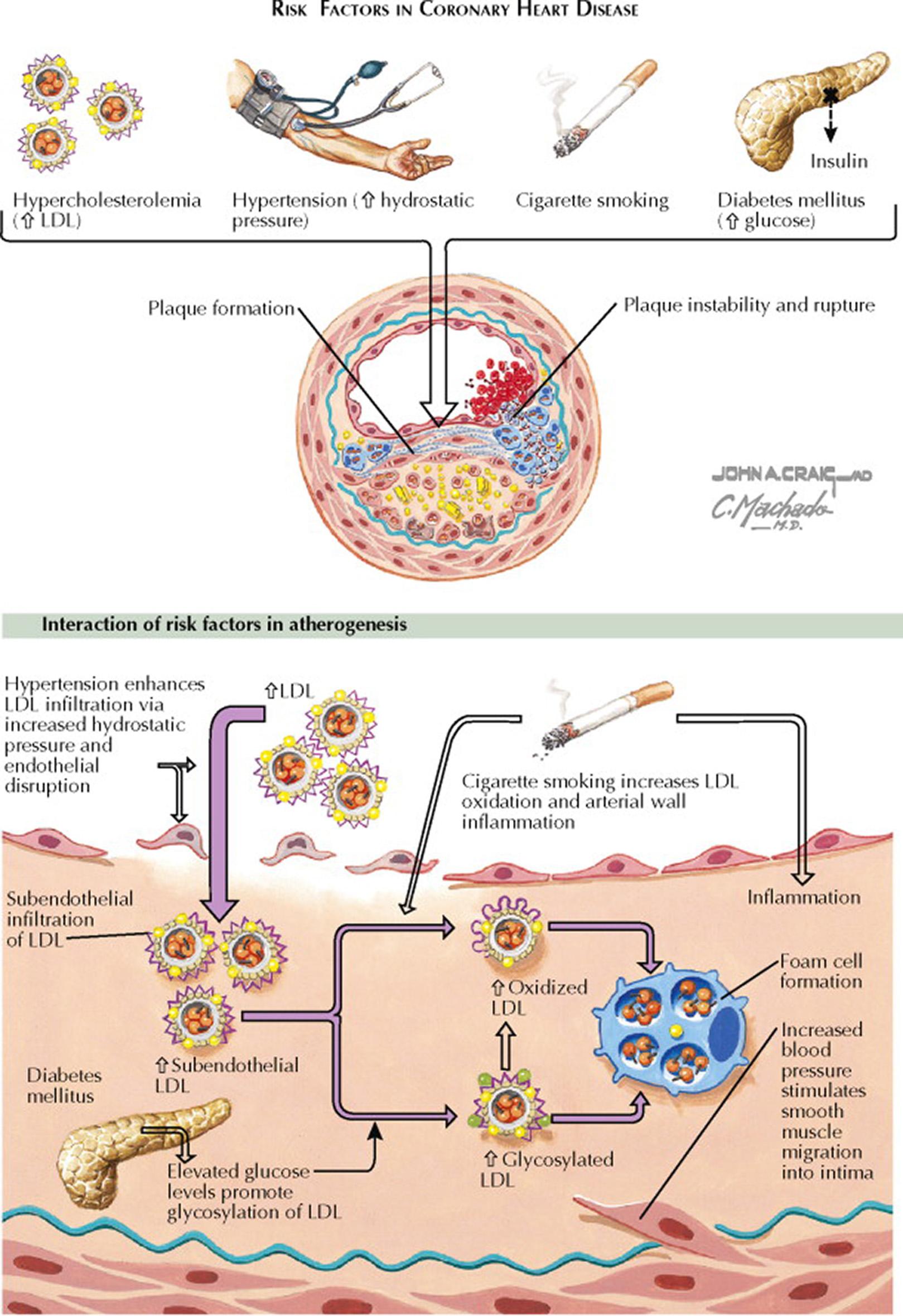
The plaque can progress to a complicated lesion, where the surface endothelial cells may be lost, and the fibrous cap ruptures to expose the subendothelial space. Platelets adhere to the exposed surface, and thrombus formation is initiated. Platelets release their granules, which contain cytokines, growth factors, and thrombin, resulting in further proliferation and migration of smooth muscle cells and monocytes. A large thrombus may form in unstable ruptured plaques where blood dissects into the artery wall. The plaque rupture and thrombosis can lead to acute ischemic syndromes and sudden cardiac death. Plaque rupture is responsible for approximately 75% of fatal coronary thrombi; these plaques tend to have thin fibrous caps, increased macrophage content, and large lipid cores.
The main modifiable cardiovascular risk factors are hypercholesterolemia with increased low-density lipoprotein (LDL) cholesterol, hypertension, cigarette smoking, and diabetes mellitus.
The Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults is termed the Adult Treatment Panel III (ATP III) and is the most recent version from the National Cholesterol Education Program (NCEP). ATP III guidelines recommend lipid screening (total cholesterol, triglycerides, LDL cholesterol, and high-density lipoprotein [HDL] cholesterol) every 5 years in all adults older than 20 years. The 10-year risk for developing coronary heart disease (CHD) can be determined based on data from the Framingham database. The risk factors included in the Framingham calculation are age, total cholesterol, HDL cholesterol, systolic blood pressure, treatment of hypertension, and cigarette smoking. An online risk calculator is found at http://hp2010.nhlbihin.net/atpiii/calculator.asp . The intensity of treatment of hypercholesterolemia should be personalized on the basis of CHD risk. ATP III set cutoffs for blood LDL cholesterol concentrations on the basis of cardiovascular risk. For example, a plasma LDL cholesterol concentration less than 100 mg/dL is considered optimal. As plasma LDL cholesterol levels increase, cardiovascular risk increases. The presence of risk factors (cigarette smoking, hypertension, low plasma concentration of HDL cholesterol, family history of premature CHD, older age [men, ≥45 years; women, ≥55 years]) should modify the target LDL cholesterol concentration. For example, the presence of CHD or CHD risk equivalents (e.g., diabetes mellitus) decreases the LDL cholesterol goal to less than 100 mg/dL; if there are two or more risk factors, the LDL cholesterol goal should be less than 130 mg/dL; if there is zero to 1 risk factor, the LDL cholesterol goal should be less than 160 mg/dL. These targets may be modified when NCEP ATP IV guidelines are published in the fall of 2011.
Although LDL-lowering treatments do not markedly regress known obstructing coronary artery lesions, they do markedly decrease coronary events. Thus, the benefit of lipid lowering in patients with known CHD may not be plaque regression but rather plaque stabilization. In addition, the consistent benefit of LDL lowering by 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) may depend not only on their effects on LDL cholesterol but also on their direct influence on plaque biology.
The cause-and-effect relationship between hypertension and CHD risk is well established. Normalization of blood pressure by nonpharmacologic measures (e.g., weight reduction, sodium-restricted diet, regular isotonic exercise) and pharmacologic measures reduces the risk of stroke, heart failure, and CHD events. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) determined that starting at a blood pressure of 115/75 mm Hg, cardiovascular risk doubles for each incremental increase of 20/10 mm Hg. JNC7 recommends that prehypertensive individuals (systolic blood pressure, 120–139 mm Hg or diastolic blood pressure, 80–89 mm Hg) engage in health-promoting lifestyle modifications to prevent the progressive increase in blood pressure and the development of cardiovascular disease.
Most individuals with diabetes mellitus die of atherosclerosis complications. The increased prevalence of atherosclerosis in individuals with diabetes is partly caused by the presence of small and dense LDL cholesterol, low serum HDL cholesterol concentrations, and increased serum triglyceride concentrations. Increased blood glucose levels also promote glycosylation of LDL cholesterol.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here