Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
| Definition |
|
| Prevalence |
|
| Genetic risk |
|
| Cognitive symptoms |
|
| Clinical features |
|
| Treatment |
|
| Top differential diagnoses |
|
An 85-year-old man came to see us for slowly progressing memory loss over the last few years. When asked the last time he was remembering normally, his family was unsure, with some family members thinking it might have been five years ago or more. He had mild difficulty with some instrumental activities of daily living, such as needing some assistance with bill paying and filling his pillbox. His testing showed mainly problems with memory, including encoding, recall, and recognition of items, as well as some difficulty with executive function in the timed generation of words to specific letters and the Trailmaking Test Part B alternating number-letter sequencing task. In addition to some nonspecific age-related atrophy, his magnetic resonance imaging (MRI) scan was notable for severe hippocampal atrophy. We made a diagnosis of probable Alzheimer’s disease dementia, mild stage, and started him on a cholinesterase inhibitor. He and his family were interested in clinical trials. During a follow-up visit we began the screening process for the trial including, per protocol, scheduling an amyloid positron emission tomography (PET) scan. The PET scan was negative, indicating sparse to no amyloid plaques, inconsistent with a pathologic diagnosis of Alzheimer’s disease.
In the age of amyloid and tau imaging, about 10% to 20% of patients that we and other memory centers diagnose with the syndrome of progressive amnestic dysfunction (also called probable Alzheimer’s disease, dementia of the Alzheimer’s type, and Alzheimer’s clinical syndrome) will end up having a pathology other than Alzheimer’s disease. Although some of these patients will end up with Lewy body or vascular pathology, we now know of another pathology that causes progressive amnestic dysfunction, mimicking Alzheimer’s disease.
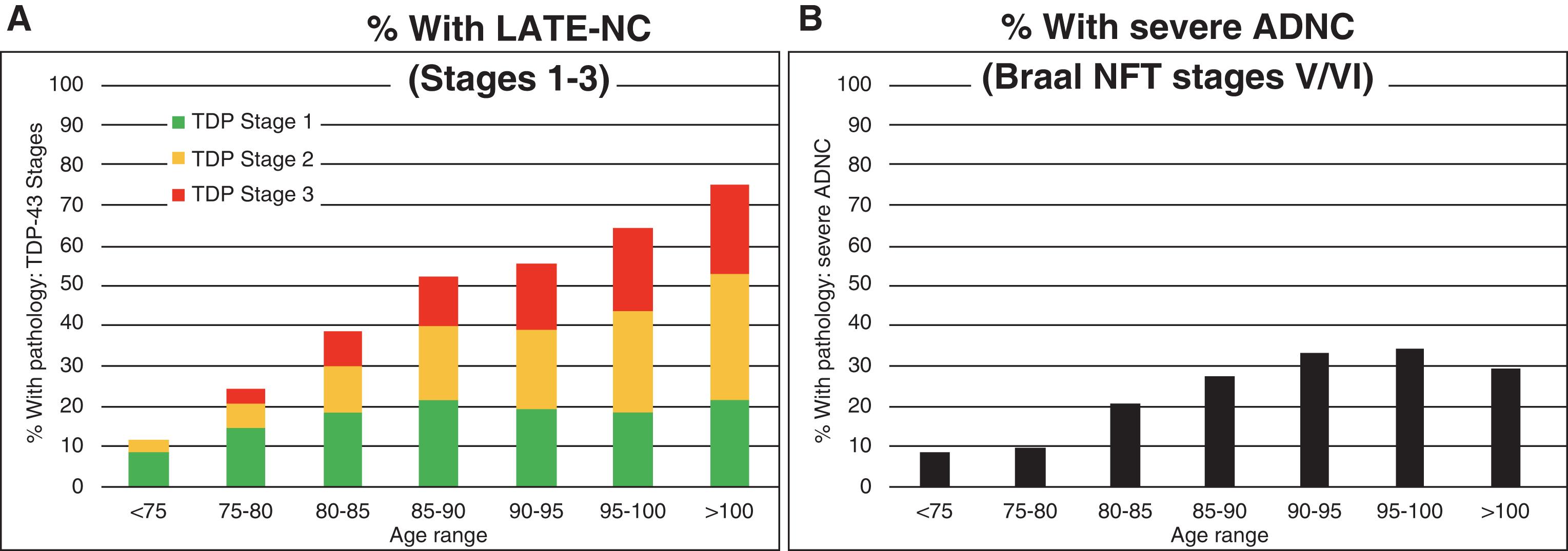
Limbic-predominant age-related TDP-43 encephalopathy (LATE) is a new name for a disease that frequently mimics Alzheimer’s disease and is now recognized to be more prevalent than previously thought. Older names for this disease include “hippocampal sclerosis,” “hippocampal sclerosis of aging,” “hippocampal sclerosis dementia,” “cerebral age-related TDP-43 with sclerosis,” and “TDP-43 pathologies in the elderly.” LATE is commonly observed in individuals older than age 80 years. In fact, the prevalence of LATE pathology is quite high and increases proportionally with age—unlike Alzheimer’s disease pathology, which peaks in the ninth decade and actually decreases over age 100 ( ) ( Fig. 6.1 ). Furthermore, using a statistical approach typically used to evaluate the impact of risk factors on disease prevalence in a population, an analysis of the attributable risk of dementia in individuals with advanced age (mean 89.7 years) found that LATE was the second most common neurodegenerative disease (after Alzheimer’s) and the third most common cause of dementia (after Alzheimer’s and vascular disease) ( ) ( Table 6.1 ). Because our population is aging, we expect that LATE will become an even more important disease in the future (see Fig. 4.1 ). Women and men appear to be equally likely to have LATE pathology at any given age but, because women live longer than men, there are more women with LATE than men. No racial or ethnic differences for LATE have been reported. Retrospective studies suggest that individuals who have only LATE pathology progress more slowly than those with Alzheimer’s pathology, but that progression is swiftest when both pathologies are present ( ). Note that multiple pathologies are common in individuals with dementia in their 80s and 90s. Most patients with LATE will therefore also have some Alzheimer’s, vascular, or Lewy body pathology as well.
| Neuropathological Indices | Fraction Attributable % (95% CI) a |
|---|---|
| ADNC | 39.4 (31.5–47.4) |
| Vascular disease pathology b | 24.8 (17.3–32.1) |
| LATE-NC | 17.3 (13.1–22.0) |
| α -Synucleinopathy/Lewy body pathology | 11.9 (8.4–15.6) |
a 95% CIs were derived using bootstrapping.
b Vascular pathologies included cerebral amyloid angiopathy, atherosclerosis, arteriolosclerosis, and gross infarcts.
There are no published clinical diagnostic criteria for LATE. The diagnosis will typically be made in individuals over the age of 80, who are slowly progressing over years, with memory problems predominating (including rapid forgetting of information), and structural imaging studies showing relatively isolated (and perhaps severe) hippocampal atrophy. The diagnosis of LATE is supported by negative biomarkers for other neurodegenerative diseases, such as negative amyloid imaging, negative tau imaging, negative dopamine transporter scan, and normal amyloid and phosphorylated tau in the cerebrospinal fluid (CSF).
Nonphosphorylated transactive response DNA binding protein of 43 kDa (TDP-43) is normally found in neuronal cell nuclei ( Fig. 6.2 ). In the pathologic state, phosphorylated TDP-43 is found in neuronal cytoplasmic inclusions in the setting of widespread neuronal loss and frequent gliosis in the hippocampal CA1 subfield, subiculum, and amygdala ( ). Although the hippocampal sclerosis that frequently accompanies LATE may be unilateral, TDP-43 pathology is almost always observed bilaterally. TDP-43 inclusions are composed of bundles of 10–20 nm diameter straight filaments frequently accompanied by electron dense granules ( ).
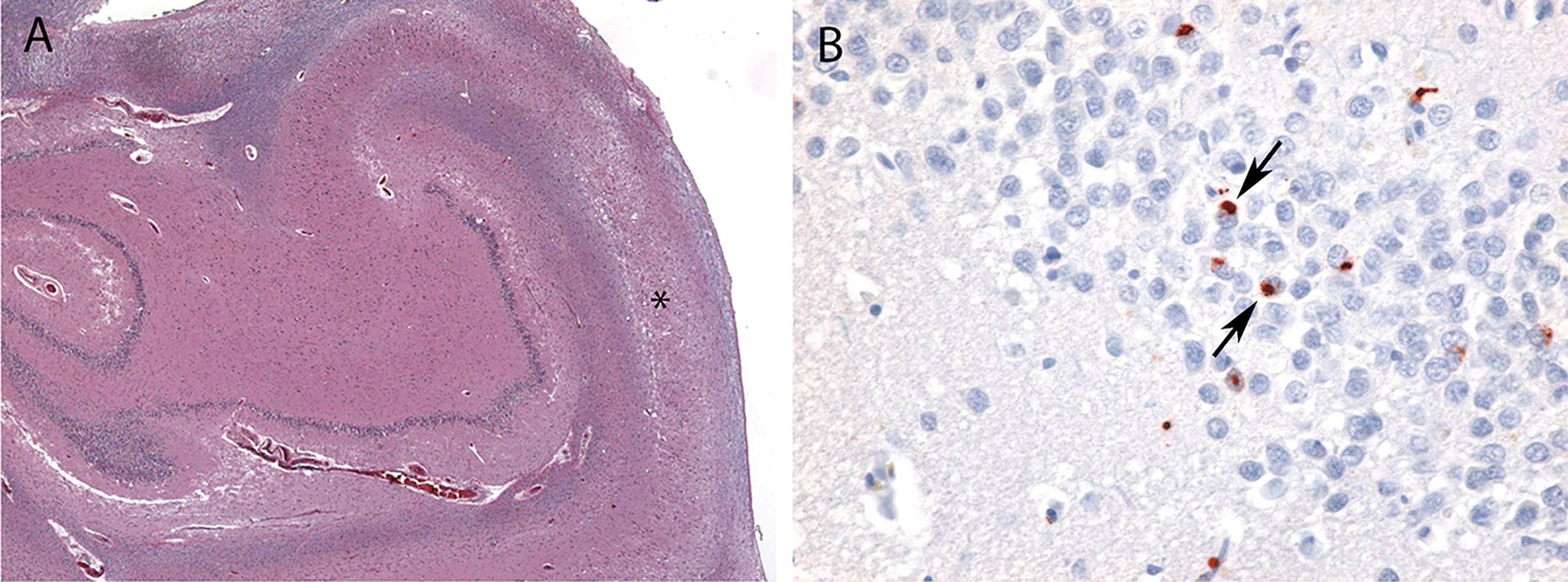
In the 2019 LATE neuropathologic change staging system, Stage 1 involves amygdala only, Stage 2 involves amygdala plus hippocampus, and Stage 3 involves amygdala, hippocampus, plus middle frontal gyrus ( ). Note that although hippocampal sclerosis is often observed in LATE, it is neither necessary nor sufficient for a diagnosis of LATE neuropathologic change. LATE pathology has also been described in other neocortical regions, as well as olfactory bulb, basal ganglia, and brainstem.
Five genes have been reported to confer increased risk for the development of LATE pathology: ATP-binding cassette sub-family member 9 (ABCC9) on chromosome 12p, apolipoprotein E (APOE) on chromosome 19q, granulin (GRN) on chromosome 17q, potassium channel subfamily M regulatory beta subunit 2 (KCNMB2) on chromosome 3q, and transmembrane protein 106B (TMEM106B) on chromosome 7p ( ). Of note, granulin and TMEM106B are also associated with increased risk for TDP-43 associated frontotemporal lobar degeneration leading to either behavioral variant frontotemporal dementia (see Chapter 10 ) or primary progressive aphasia (often semantic variant, see Chapter 9 ). The apolipoprotein E ε4 allele is also associated with increased risk for the pathology of Alzheimer’s disease (see Chapter 4 ), dementia with Lewy bodies (see Chapter 8 ), and chronic traumatic encephalopathy (see Chapter 15 ). There are no other known risk factors for LATE.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here