Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Leukocytes are regulated by complex homeostatic mechanisms that direct their responses to infection and inflammation.
Leukocytosis often reflects an underlying abnormality; leukopenia, especially neutropenia, places a patient at risk for infection.
Hematopoietic neoplasms have been categorized by a World Health Organization classification according to cell of origin, cytogenetic and molecular abnormalities, immunophenotype, and clinical features.
Acute leukemias are rapidly progressing neoplasms of precursor myeloid or lymphoid cell origin. An understanding of biology is associated with improvements in therapy.
Myeloproliferative neoplasms and myelodysplastic syndromes are heterogeneous disorders of differentiated myeloid cells. They often are initially indolent but ultimately progress.
Non-Hodgkin lymphomas most often arise from mature B cells. They are pathologically and clinically heterogeneous. T-cell lymphomas are less common, also heterogeneous, and often difficult to treat successfully.
Hodgkin lymphoma is a neoplasm of defective B cells. Much of its pathology is due to an associated inflammatory milieu.
Leukocytes, or white blood cells (WBCs), are found within the bone marrow (BM), the peripheral blood, and the tissues. They protect the body from infection and other foreign insults and include granulocytes, lymphocytes (B cells, T cells, and natural killer [NK] cells), monocytes, eosinophils, and basophils. Leukocyte function and homeostasis are highly complex areas of biology that span several disciplines.
Laboratory examination of leukocytes occurs as part of the automated complete blood count (CBC) for almost every patient. The total leukocyte (or WBC) count—as well as the relative and absolute concentrations of neutrophils, lymphocytes, monocytes, eosinophils, and basophils—is determined and compared with normal values for the patient’s age and sex. The absolute concentrations (i.e., the product of the WBC count and the percentage of the respective cell series, such as lymphocytes or neutrophils) are of most value in determining abnormalities. Abnormal results from the automated count are flagged and then reviewed by a skilled laboratory technologist.
These abnormalities include leukocytosis, or increase in the total WBC count, and leukopenia, a decrease in the total WBC count, each compared with normal values for age and sex. The differential count, usually first performed on an automated instrument, quantifies the relative and absolute concentrations of the various forms of leukocytes and the maturation state for neutrophils. An increase in the absolute concentration of cells in each series is termed neutrophilia ( neutrophilic leukocytosis ), lymphocytosis , monocytosis , eosinophilia ( eosinophilic leukocytosis ), and basophilia ( basophilic leukocytosis ). A decrease in the absolute concentration is termed neutropenia , lymphopenia (or lymphocytopenia ), monocytopenia , eosinopenia , and basopenia . Increased numbers of band neutrophils and/or presence of immature neutrophils (metamyelocytes and earlier) are noted and enumerated and often confirmed or modified by a manual differential count.
Nucleated red blood cells (RBCs) are also counted and subtracted from the WBC count by automated or manual method. Abnormalities may occur in both neoplastic conditions (e.g., leukemia or marrow infiltration by metastatic tumor) and nonneoplastic conditions (e.g., infection, drug effect or nutritional deficiency). An increase in any cell type may be clinically important, but a decrease is most important for neutrophils. Lymphocytopenia is important in immunodeficiency syndromes. Isolated monocytopenia, eosinopenia, or basopenia is not usually considered pathologic. Functional neutrophil abnormalities occasionally occur.
Neutrophilic leukocytosis or neutrophilia refers to an absolute concentration of neutrophils in the blood above normal for age. The normal reference interval (established for each laboratory separately) is approximately 1.8 to 7.0 × 10 3 /μL for adults, with a slightly wider range (1.0–8.5 × 10 3 /μL) in young children. The neutrophilias are acquired disorders; key causes are listed in Box 34.1 .
Acute inflammatory—collagen vascular, vasculitis
Acute infectious—bacterial, some viral, fungal, parasitic
Drugs, toxins, metabolic—corticosteroids, growth factors, uremia, ketoacidosis
Tissue necrosis—burns, trauma, MI, RBC hemolysis
Physiologic—stress, exercise, smoking, pregnancy
Neoplastic—carcinomas, sarcomas, myeloproliferative disorders
The primary factors influencing the neutrophil count are (1) the rate of inflow of cells from the bone marrow; (2) the proportion of neutrophils in the marginal granulocyte pool (MGP; cells adhering to vessel walls) and the circulating granulocyte pool (CGP) of the blood—MGP and CGP are approximately equal in size and in equilibrium in health; and (3) the rate of outflow of neutrophils from the blood.
Physiologic leukocytosis is produced by factors or situations that are not related to underlying tissue pathology. Severe exercise, hypoxia, stress, or the injection of epinephrine will result in a decrease in the MGP and a corresponding increase in the CGP, resulting in “pseudoneutrophilia” (i.e., no change in total blood granulocyte pool [TBGP]). This simple redistribution of cells between the CGP and MGP, also known as demargination , is the release and detachment of leukocytes from receptors on vessel luminal walls. Stress of greater severity and presence of endotoxin or corticosteroids also results in an increased inflow of cells to the blood from the marrow maturation/storage pool.
Pathologic leukocytosis is an increased WBC count that occurs as a result of disease, usually in response to infection or tissue damage, and is most often neutrophilia. When attracted to a focus of inflammation, neutrophils leave the blood and migrate in response to chemotactic molecules and gradients ( ; ). There is compensatory flow of neutrophils from the marrow storage compartment into the blood; the result is neutrophilia. Usually, production and storage compartments are able to sustain the increased CGP (neutrophilia) and MGP in the face of the increased flow of neutrophils from the blood into the inflammatory site. The marrow will show granulocytic hyperplasia (increased myeloid to erythroid [M/E] ratio and increased cellularity), with intact maturation. An increase in immature peripheral blood granulocytes is often termed a “shift to the left.” Increased WBCs with left shift and toxic features (see later discussion) may resemble leukemia and is often termed a “leukemoid” reaction ( Fig. 34.1 ).
If the demand for neutrophils is extremely great, as in severe infection, there may be depletion of the marrow storage pool with decreased CGP (neutropenia) and MGP because the supply of cells is insufficient for the demand. In these instances, the marrow will show increased early neutrophil precursors through the myelocyte stage, but decreased metamyelocytes, bands, and neutrophils.
Host factors modify the degree of neutrophilic response, with children responding more intensely than adults. The degree of neutrophilia may be impaired by iron, folate, or cobalamin deficiency, or marrow failure due to other causes.
Pyogenic bacteria especially induce neutrophilia. More virulent agents result in higher neutrophil counts; when the infection is overwhelming, toxic neutropenia with a shift to the left can occur, more commonly in the elderly.
Antibiotics modify the response to infection and, while steroid therapy causes neutrophilia by demargination, it tends to impair the host response to infection.
Neutrophilia, sometimes pronounced, is occasionally seen in patients with primary or metastatic nonhematologic neoplasms, including gastrointestinal and hepatic tumors, Hodgkin lymphoma (HL) and renal cell carcinoma ( ; ).
Neutropenia is a reduction of the absolute neutrophil count (ANC) below ≈1.5–2 × 10 9 /L for white adults and below ≈1.2–1.3 × 10 9 /L for black adults. The ANC is the product of the WBC count and the percentage of mature as well as immature neutrophils in the WBC differential count. “Agranulocytosis” refers to severe neutropenia, usually <0.5 × 10 9 /L, and is often associated with depletion of eosinophils and basophils as well. Severe chronic neutropenia (SCN) refers to patients with ANC <0.5× 10 9 /L for months or years, due to inherited or acquired rare disorders. ( ) If the neutrophil count is less than 1× 10 9 /L, the risk of infection is increased, and greater still if less than 0.5× 10 9 /L.
Mechanisms by which neutropenia occurs include (1) decreased or ineffective production (proliferation or maturation defect); (2) increased removal from the blood (survival defect); (3) altered distribution between CGP and MGP; or (4) combinations of these mechanisms. Neutropenias may be inherited, autoimmune, toxic, or drug associated. Drug-induced neutropenia occurs through several mechanisms and is always an important consideration in the differential diagnosis of leukopenia. A partial list of causes associated with neutropenia are shown in Box 34.2 .
∗ Brief, partial list.
Drugs—cancer chemotherapy, chloramphenicol, sulfas/other antibiotics, phenothiazines, benzodiazepines, antithyroids, anticonvulsants, quinine, quinidine, indomethacin, procainamide, thiazides
Radiation
Toxins—alcohol, benzene compounds
Intrinsic defects—Fanconi, Kostmann, cyclic neutropenia, Chédiak-Higashi
Immune mediated—collagen vascular disorders, RA, AIDS
Hematologic—megaloblastic anemia, myelodysplasia, marrow failure, marrow replacement
Infectious—any overwhelming infection
Others—starvation, hypersplenism
Constitutional disorders associated with neutropenia frequently present in early infancy and are of complex molecular origin ( ; ). Those due to myeloid hypoplasia or a proliferation defect include Fanconi anemia (FA), Kostmann syndrome, Shwachman-Diamond syndrome, and cyclic neutropenia. Those that are due to maturation defects include severe congenital neutropenia (SCN), myelokathexis, and Chédiak-Higashi syndrome.
FA is an inherited BM failure syndrome that usually occurs in childhood and rarely presents in adulthood. This condition is heterogeneous in its clinical manifestations but was classically defined by the presence of aplastic anemia and congenital physical malformations. The aplastic anemia of FA patients appears to be indistinguishable from acquired aplasia. FA patients are also susceptible to hematopoietic and certain solid organ malignancies. Diagnosis is usually made by cytogenetic analysis while looking for chromosome breakage after exposure to diepoxybutane or mitomycin C. The underlying molecular pathology is complex and involves multiple and varied DNA repair and signaling pathways ( ; ; ).
Severe congenital neutropenia (Kostmann syndrome) is a rare severe (ANC typically <200/μL) congenital neutropenia appearing in early infancy, usually with an autosomal-recessive pattern of inheritance. The marrow usually shows the presence of early granulocytes (promyelocyte/myelocyte arrest), but few maturing forms are seen; neutrophil survival is normal. Mutations in ELANE/ELA2 , encoding neutrophil elastase, are present in about 50% of cases and are autosomal dominant; mutations of the anti-apoptotic gene HAX-1 are sometimes present and are recessive. Autosomal-dominant negative mutations in GFL1 or G6PC3 are causative in some cases; most recently, mutations of CSF3R , encoding the granulocyte colony stimulating factor (G-CSF) receptor, have been identified and may be associated with increased risk of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). Additionally, some patients have mutations in GATA2 , with neutropenia and sometimes monocytopenia, and familial tendency to AML/MDS. Screening for these mutations may be important, especially if monocytopenia is also present ( ; ).
Cyclic neutropenia typically presents with recurrent episodes of symptomatic infection (fatigue, mouth ulcers, cervical lymphadenopathy, fever) due to cyclic episodes of severe neutropenia related to periodicity or cycling of neutrophil production. This disease usually presents in childhood but may present in adulthood and is usually not life-threatening. Typically, oscillations of neutrophil and monocyte levels (between near normal levels and very low levels) occur over an approximately 21-day period. Mutations in ELA2 , similar to those seen in Kostmann syndrome, have been identified in many patients ( ).
Chronic familial neutropenia or benign familial neutropenia refers to a lower than normal neutrophil count, as found in some ethnic populations. It may be an incidental and clinically stable finding with no predisposition to infection in many families. However, screening for GATA2 mutations may also be indicated when there is family history of AML/MDS.
Common causes of congenital neutropenia are neutropenia of pregnancy-induced hypertension (PIH; most common) and overwhelming bacterial infection. Typical signs of infection, including a granulocytic left shift, toxic granulation, and Döhle bodies, accompany the latter. However, these changes are not seen in PIH, although its pathophysiology is due to an inflammatory state ( ). Neither usually continues beyond the first week of life. Other causes should be searched for in infants with persistent neutropenia ( ). Neonatal alloimmune neutropenia (NAIN or NAN) is rare and occurs due to maternal alloantibodies against human neutrophil antigens (HNAs) that the mother lacks and that are inherited from the father (Porcelijn, 2018).
Patients with congenital or primary immunodeficiency diseases may exhibit some degree of neutropenia. Males with X-linked agammaglobulinemia (XLA) are often neutropenic; XLA should be considered in the differential diagnosis of chronic neutropenia in an infant. An abnormal nonreceptor tyrosine kinase, associated with signal transduction and differentiation of hematopoietic cells, appears to be involved. This is due to various mutations in the gene BTK ( B ruton’s or B lymphocyte t yrosine k inase) on the long arm of the X chromosome (q22). Other congenital syndromes having a prominent association with neutropenia include selective immunoglobulin (Ig) A deficiency, common variable immunodeficiency, and hyper IgM syndrome ( ).
Certain autoimmune diseases can also be associated with chronic neutropenia. Both rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) show this association (Starkebaum, 2002). The combination of chronic neutropenia and RA is termed Felty syndrome (FS). Patients develop symptoms owing to both RA (e.g., subcutaneous nodules, contractures, erythema, warmth, tenderness, symmetric involvement of large and small joints, musculoskeletal pain) and chronic neutropenia (symptoms of recurrent bacterial and fungal infections). A portion of these cases are associated with large granular lymphocyte (LGL) leukemia that may be indistinguishable from FS ( ). In some cases of FS, a neutrophil-specific antibody may be involved. SLE may present with multisystem involvement, making presenting symptoms highly variable. Alterations in neutrophil proliferation and survival with neutropenia due, at least in part, to immune-mediated destruction, occur in both SLE and FS, and increased neutrophil apoptosis may be noted in SLE.
Autoimmune myelofibrosis is a rare cause of isolated neutropenia ( ). Prognosis of these patients appears to be very good with corticosteroid therapy.
Drugs are the most common cause of acute neutropenia (agranulocytosis) and typically show a lack of granulocytic cells in the marrow. However, progenitor hyperplasia may be present and is often termed promyelocytic hyperplasia or maturation arrest . Most classes of drugs have been implicated, with clozapine, trimethoprim-sulfamethoxazole, and methimazole most commonly associated. Pathogenesis is not well understood for individual cases ( ). Mortality is about 5% and management includes cessation of medication, use of antibiotics, and consideration of G-CSF ( ).
Inhibition of granulopoiesis or myeloid toxicity occurs with chemotherapeutic agents, chlorpromazine and others. These effects tend to be dose dependent and reversible. Important and limiting side effects of cancer chemotherapy are severe neutropenia, with its risk of infection, and severe thrombocytopenia, with risk of bleeding. Anemia is more readily controlled with transfusion.
Radiation damages bone marrow progenitor cells as well as marrow stromal elements. Radiation type, dose, and duration are all factors that determine the extent of bone marrow damage, such as aplasia or hypoplasia. Lymphocytes are most sensitive and are directly killed by exposure. The lymphocyte count correlates with and has been used to assess dose and severity of exposure ( ). In addition, hematopoietic precursors undergoing mitosis are very sensitive to injury and death.
Isolated neutropenia or agranulocytosis is fairly uncommon in adults. When a myelophthisic process—such as metastatic carcinoma, disseminated tuberculosis, or Gaucher disease—infiltrates the BM, the damage is not limited to granulopoiesis but rather affects normoblasts and megakaryocytes as well. Because of the short life span of granulocytes, however, neutropenia is the earliest recognizable effect in the blood. It may take weeks before damage to the erythropoietic tissue becomes manifest because of the usually long life span of erythrocytes. Platelets have a rather short life span; on the other hand, megakaryocytes are more resistant to damage.
Neutropenia due to increased ineffective granulocytopoiesis occurs in megaloblastic anemias and with drugs that have an antifolate effect. In these conditions, anemia and thrombocytopenia are usually associated. The marrow is usually hyperplastic. In megaloblastic anemia due to nutritional deficiency, drug-induced suppression of deoxyribonucleic acid (DNA) synthesis, or an inborn error of metabolism asynchronous nuclear/cytoplasmic maturation (megaloblastic change or megaloblastosis) is identified in marrow progenitor cells. Ineffective granulocytopoiesis may also occur in myelodysplasia.
In starvation, cellularity tends to be decreased, and a morphologic marrow change termed serous fat atrophy or gelatinous transformation of the bone marrow is present. This change shows a loss of hematopoietic cells within the marrow stroma replaced by small or shrunken fat cells expanded by an intercellular, homogeneous, eosinophilic material. BM hypocellularity is typically seen with advanced disease.
Transient neutropenia may occur early in some infections, followed by leukocytosis once the marrow production catches up with the demand. Some bacterial infections—notably, brucellosis and Salmonella infections—are associated with neutropenia. They may have some depressing effects on the marrow as well. Patients with measles and rubella have neutropenia for several days after appearance of the rash. Hepatitis, infectious mononucleosis and influenza may also cause acute neutropenia. Lymphocytosis is present and persists after the neutropenia subsides.
The neutropenia of hypersplenism has been attributed to selective removal of neutrophils by the spleen. It is associated with neutrophilic hyperplasia of the marrow and is corrected by splenectomy. Splenomegaly due to many causes may result in shortened neutrophil survival and neutropenia. These include congestive splenomegaly, FS, Gaucher disease, and lymphoma.
Pseudo-neutropenia may be caused by increased margination of neutrophils in some individuals without a decrease in total granulocyte count. Rather than showing an equal distribution between MGP and CGP, an increased proportion of neutrophils appear to be present in the MGP. Small doses of endotoxin will cause a shift of neutrophils into the MGP from the CGP, resulting in an apparent neutropenia before causing leukocytosis.
In addition to quantitative changes, qualitative morphologic alterations occur in neutrophils. These, too, may be inherited or acquired. Some of these, such as toxic granules or cytoplasmic vacuoles, are acquired and disappear after the stimulus that provoked them is gone. Others are hereditary and persist through life, with or without functional impairment ( Box 34.3 ). These were reviewed by and later by .
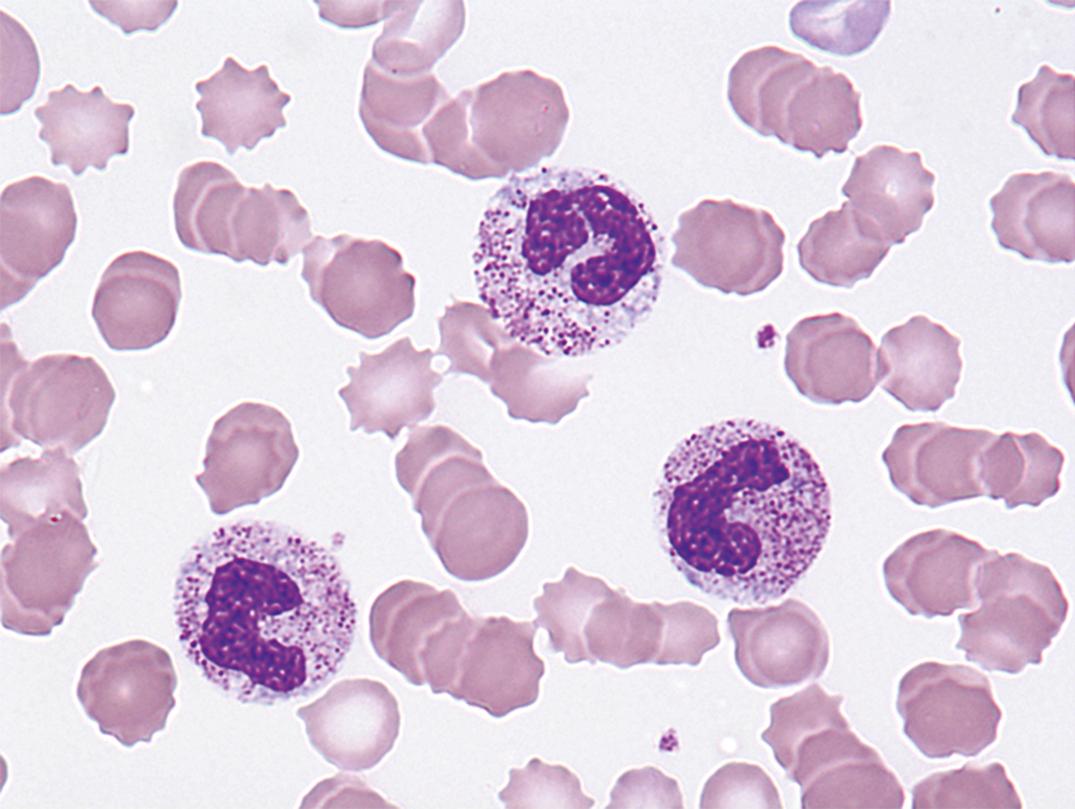
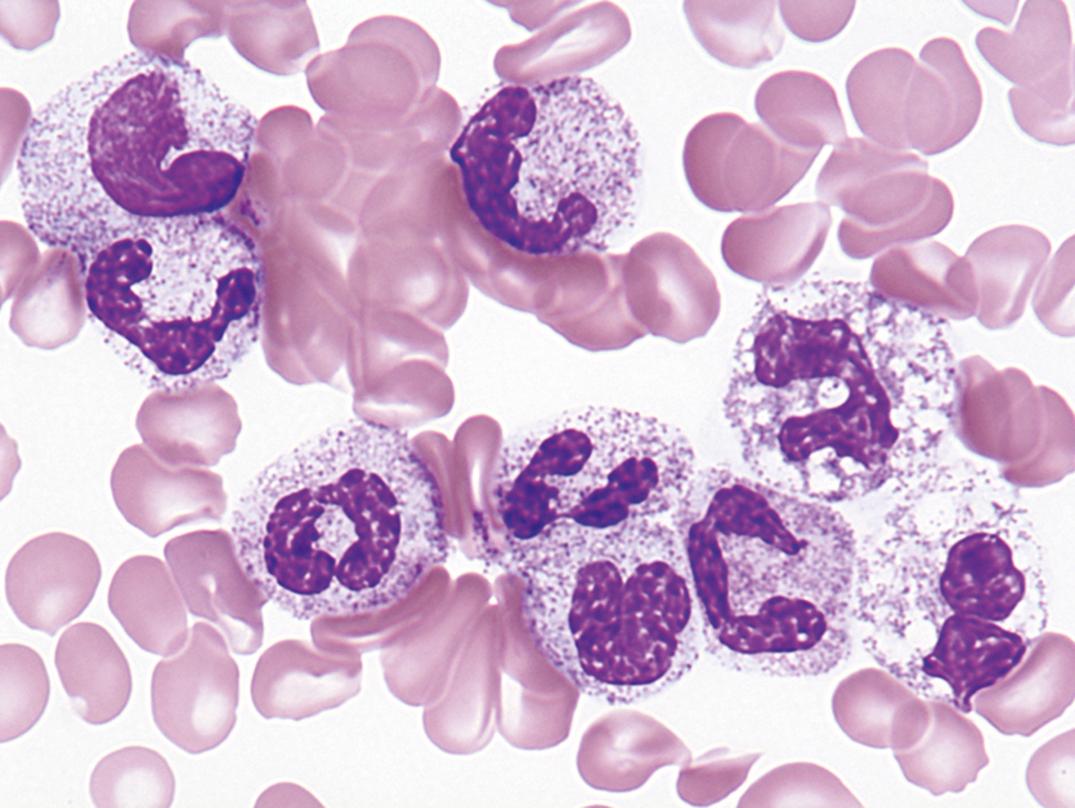
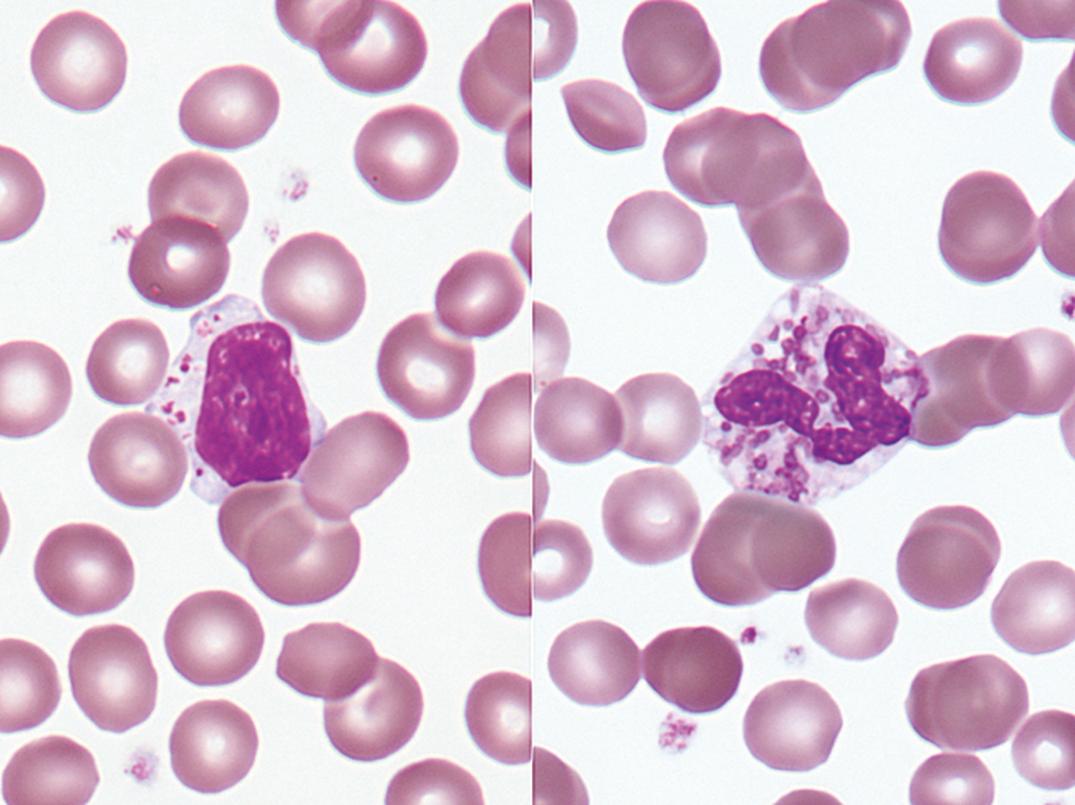
Toxic granulation—azurophilic cytoplasmic granules seen in severe infections, other toxic conditions, and reactive conditions
Cytoplasmic vacuoles—seen in infection, indicating phagocytosis
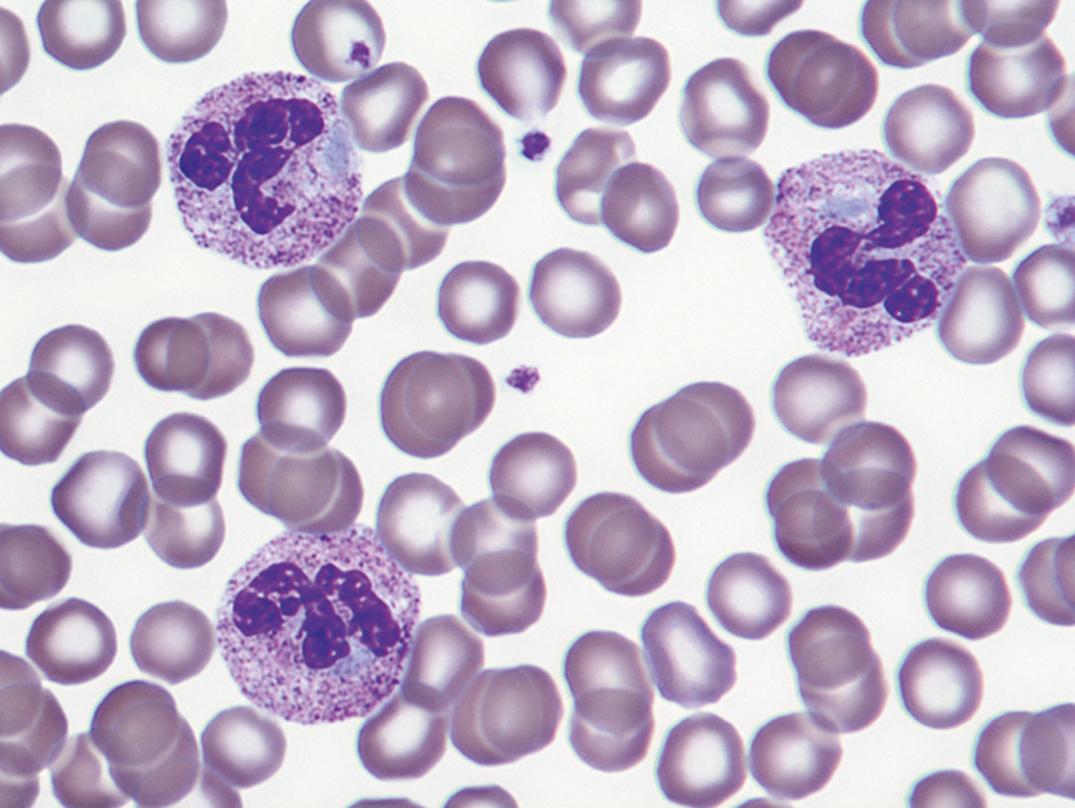
Döhle bodies—pale-blue, oval cytoplasmic remnants of ribosomes seen in infection and other toxic conditions
May-Hegglin anomaly—rare autosomal-dominant condition with pale-blue cytoplasmic ribosomal inclusions resembling Döhle bodies
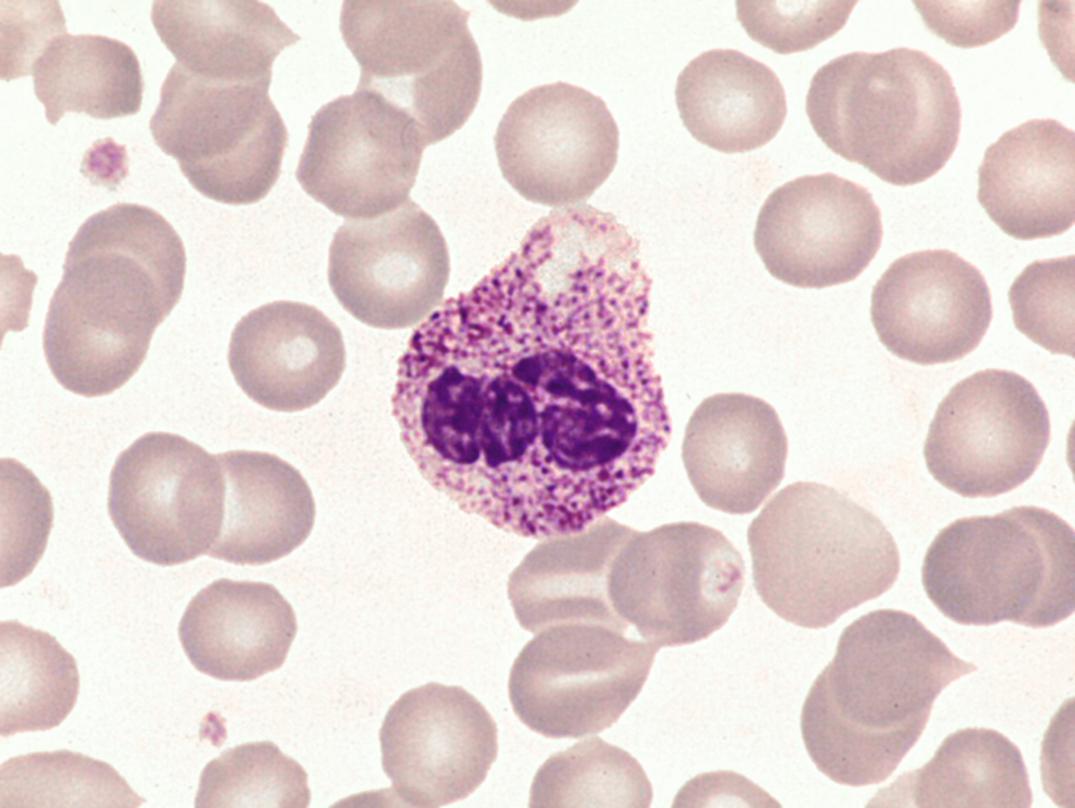
Alder-Reilly anomaly—prominent azurophilic granulation not related to infection
Pelger-Huët anomaly—bilobed or rounded nuclei with pince-nez shape
Chédiak-Higashi syndrome—autosomal-recessive disorder with giant granules, likely representing giant fused lysosomes, and abnormal leukocyte function
It should be noted that disorders of leukocyte function may exist with no structural abnormality detectable with the usual modes of morphologic examination.
Toxic granules are dark-blue to purple cytoplasmic granules in the metamyelocyte, band, or neutrophil stage. They are peroxidase positive and may be numerous or few in number; less peroxidase activity may be seen in toxic than in normal neutrophils. Toxic granulation is noted in infections or other toxic conditions but may also be seen in noninfectious reactive conditions ( Figs. 34.1 and 34.2 ).
These small, oval inclusions in the peripheral cytoplasm of polymorphonuclear neutrophils stain pale blue with Wright’s stain (see Fig. 34.1 ). They are remnants of free ribosomes or rough-surfaced endoplasmic reticulum, persisting from an earlier stage of development.
This is characterized by pale-blue inclusions resembling Döhle bodies in neutrophils, by giant platelets and, in some persons, by thrombocytopenia ( Fig. 34.3 ). The inclusions are larger and more prominent than the Döhle bodies found in infections. They have been described in eosinophils, basophils, and monocytes as well as in neutrophils. The blue staining of the inclusions is due to RNA. Granulocyte function is normal. May-Hegglin is a rare autosomal-dominant condition involving the nonmuscle myosin heavy chain 9 gene ( MYH9 ) linked to chromosome 22q12-13. The mutations appear to alter the assembly and stability of myosin and may be responsible for the underlying pathophysiology and changes seen in leukocyte and platelet structure and function. Varied mutations in MYH9 are responsible for a phenotypic spectrum of illness, including May–Hegglin anomaly, Sebastian, Fechtner, and Epstein platelet syndromes ( ; ).
Dense, prominent, larger than normal azurophilic granulation in all white blood cells was described by Alder in 1939 ( Fig. 34.4 ) ( ). In neutrophils, it may resemble toxic granulation; however, it is unrelated to infection and is not transient. In 1941, Reilly described similar granulocytes in some but not all patients with gargoylism, Hurler syndrome, or, more generally, the genetic mucopolysaccharidoses ( ). Other observations have shown that the heavy granulation in neutrophils can occur as a feature of the genetic mucopolysaccharidoses or independently in otherwise healthy persons ( ).
Occurring more often than the Alder-Reilly anomaly in the genetic mucopolysaccharidoses is a metachromatic inclusion in the lymphocytes surrounded by a clear space ( Fig. 34.5 ). Macrophages in the marrow frequently contain similar granulation. This group of disorders is inherited and is characterized by deficiencies or derangement in various lysosomal enzymes required for degrading mucopolysaccharides. The result is abnormal deposition and storage of mucopolysaccharides in multiple organs. Skeletal abnormalities are prominent.
This hereditary autosomal-dominant condition involves failure of normal segmentation of granulocytic nuclei. Most nuclei are bilobed and rounded, with a characteristic spectacle or pince-nez shape ( Fig. 34.6 ). The chromatin is quite coarse, and these are not normal young band forms. When a large number of band-like neutrophils appear in the differential count in a patient without infection or other cause, careful analysis of the blood films of the patient and family members will occasionally establish the presence of the Pelger-Huët anomaly. The cells are functionally normal, and the underlying abnormality is mutation of the lamin B-receptor gene ( ).
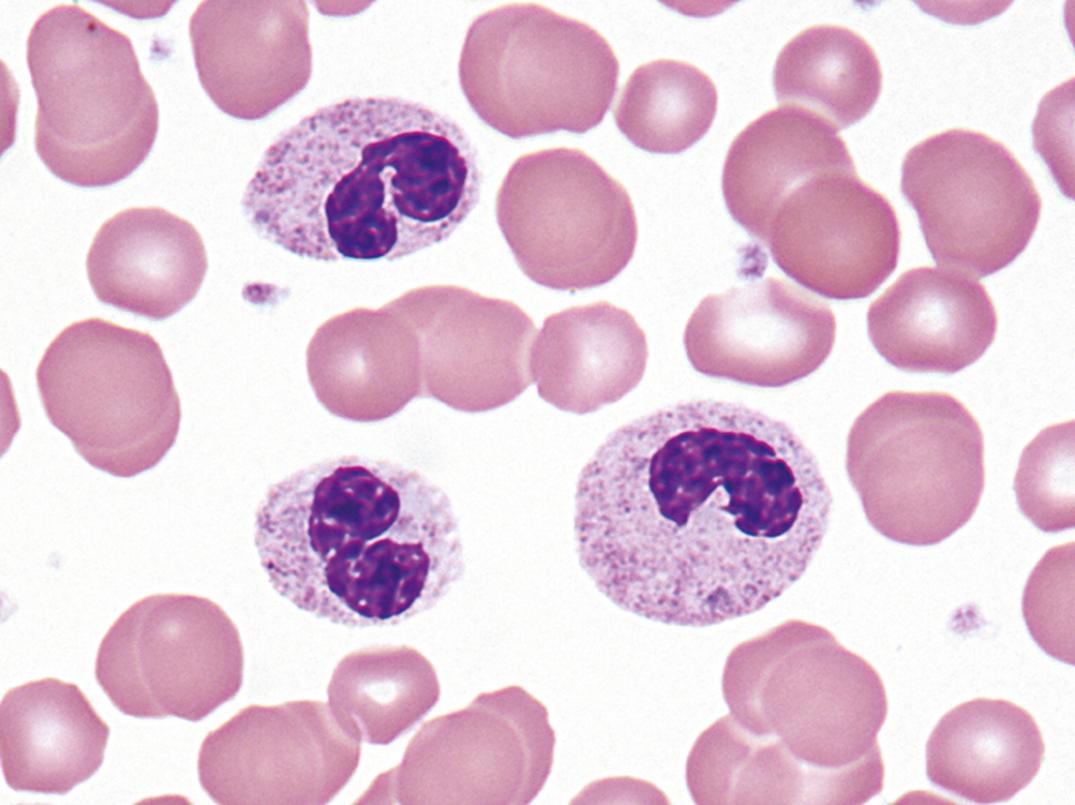
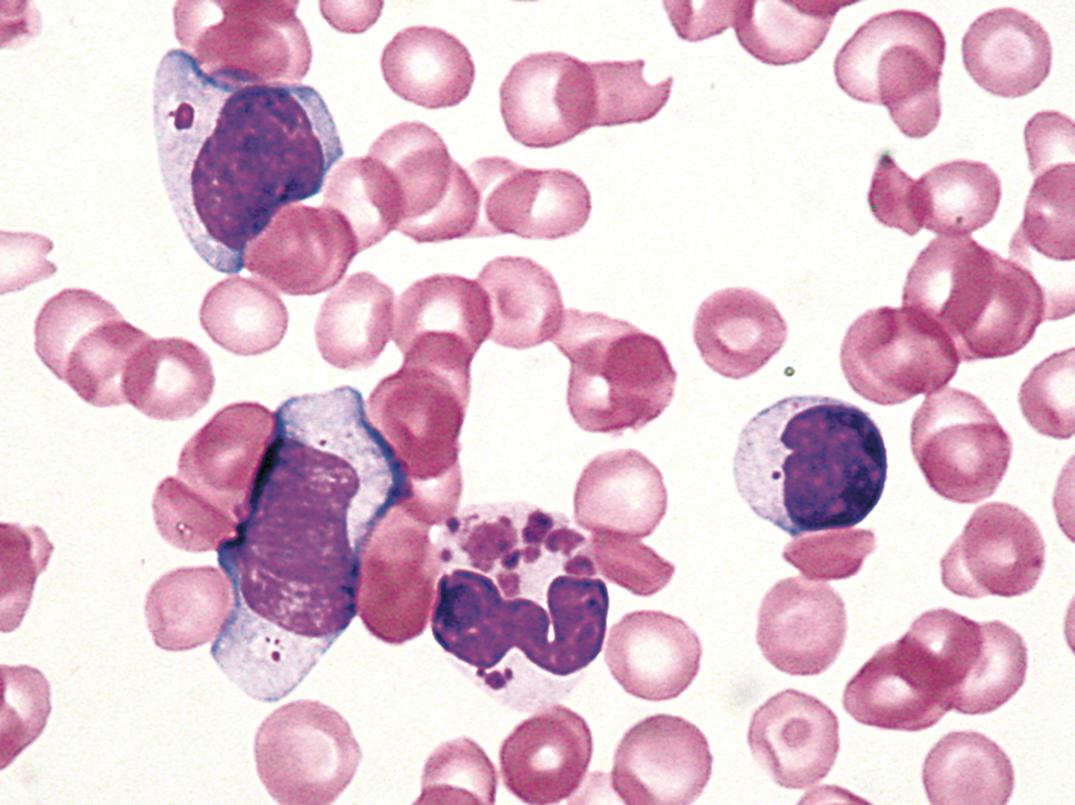
A similar-appearing acquired disorder of nuclear segmentation in granulocytes may be found in cases of granulocytic leukemia, myelodysplastic and some myeloproliferative disorders, in some infections, and after exposure to certain drugs. This is called the pseudo–Pelger-Huët anomaly . In addition to band forms and neutrophils with only two segments, mature cells with round, nonsegmented nuclei and coarse chromatin are common. In contrast to the congenital Pelger-Huët anomaly, ring-shaped and other abnormal nuclei may be seen, and the cytoplasm is usually hypogranular.
Partial oculocutaneous albinism, photophobia, immunodeficiency, abnormally large granules in leukocytes and other granule-containing cells, neurologic defects, and frequent pyogenic infections characterize this rare autosomal-recessive disorder (Introne, 1999). An accelerated lymphoma-like phase occurs, with lymphadenopathy, hepatosplenomegaly, and pancytopenia. Lymphoid infiltrates are widespread, and death ensues at an early age. Granulocytes, monocytes, and lymphocytes contain giant granules ( Fig. 34.7 ), which appear to be abnormal lysosomes. The pathogenesis of this disorder is linked to an abnormality of granule maturation, causing enlargement and apparent fusion of granules and vesicles (such as lysosomes, melanosomes, and platelet dense granules) in all cell types. Leukocyte functional abnormalities exist.
Myelokathexis applies to peripheral neutropenia with the presence of BM neutrophils. WHIM ( w arts, h ypogammaglobulinemia, i nfection, and m yelokathexis) syndrome is a rare autosomal-dominant disease of leukocyte trafficking involving chromosome 2q21. The disease appears to be due to mutations in the chemokine receptor gene CXCR4 and altered leukocyte response to its functional ligand CXCL12. Hematologic changes include severe peripheral neutropenia with lymphopenia also common, but with granulocytic hyperplasia of the BM ( ; ).
Inherited and acquired disorders affecting neutrophils and other leukocytes may result in abnormal function and susceptibility to infections. Deficiencies of humoral factors (antibodies, components of complement) may result in defective chemotaxis or opsonization. As described previously, some inherited disorders, such as the May-Hegglin, Alder-Reilly, and Pelger-Huët anomalies, have altered morphologic appearances but apparently normal granulocytic function. Other inherited conditions, such as the Chédiak-Higashi syndrome and specific granule deficiency (SGD), display alterations in both morphology and function. Inherited conditions such as chronic granulomatous disease (CGD), myeloperoxidase deficiency (MYD), and leukocyte adhesion deficiency (LAD) exhibit functional disorders with essentially normal morphology on Wright’s- and Giemsa-stained films ( ).
CGD ( ) is a rare primary immunodeficiency affecting neutrophils, eosinophils, macrophages, and monocytes. It results from the inability of these phagocytic cells to kill intracellular microorganisms. Patients present with recurrent bacterial and fungal infections (skin, liver, mouth, lymph nodes). These lead to chronic granulomatous lesions (a hallmark of the disease) that may, in turn, obstruct vital organ systems and ultimately lead to death. Symptoms occur early in life, and both autosomal-recessive (≈66%) and X-linked (≈33%) modes of inheritance are known. The disease results from genetic defects in any of the membrane-bound or cytoplasmic components of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.
Primary MYD is a congenital disorder that is not uncommon and is easily identifiable with automated cell counters incorporating measurement of MPO activity in the differential count. Secondary MYD, which can be transient and corrected with treatment of the underlying disease, may occur secondary to conditions such as myeloid neoplasms, drugs, severe infectious diseases, diabetes mellitus, and pregnancy. Primary MYD may be total or partial and does not show the severity of CGD but can increase frequency and severity of infections ( ).
Human eosinophil peroxidase is structurally related to MPO but is not identical and is encoded by a different gene. Deficiency is thought to be extremely rare, is unrelated to MYD, may show autosomal-recessive inheritance, and appears to be without clinical symptoms. Eosinophil peroxidase largely functions by neural signaling ( ).
SGD is an extremely rare condition that presents with multiple bacterial infections, atypical bilobed nuclei within neutrophils, and lack of secondary/specific cytoplasmic granules within neutrophils on Wright’s-stained peripheral blood films. SGD also affects eosinophils. In addition to granule deficiencies and impaired bactericidal activity, neutrophils are defective in chemotaxis, receptor upregulation, and disaggregation ( ).
Proper neutrophil function is dependent on cell–cell adhesion via the integrins, nonadhesive surface molecules in circulating leukocytes, which, upon proper stimulation, become adhesive or develop increased binding properties for their specific ligands ( ). In LAD, defective leukocyte adhesion and migration occur, resulting clinically in recurrent infection and leukocytosis. Delayed separation of the umbilical cord often occurs. Several types (LAD-I, -II, and -III) are described. Some forms of LAD can be diagnosed by flow cytometry, with lack of expression of CD18 or CD15a on neutrophils. Hematopoietic stem cell transplantation is the only cure.
Eosinophilia exists if blood eosinophils exceed ~0.35 to 0.5 × 10 9 /L. Reactive eosinophilia is typically associated with allergic processes and parasitic infections. Classically, the major function of eosinophils appeared to be the release of granule contents or reactive oxygen species to damage the target organism or offending cell. Immunoregulatory and proinflammatory signaling roles also exist; thus, eosinophils are both effector and regulatory cells. Causes of eosinophilia have been reviewed ( ; ; ).
Eosinophil production is stimulated by interleukin-5 (IL-5), migration is influenced by the chemokine eotaxin, and the two factors interact in producing eosinophilia. Causes and conditions associated with eosinophilia are shown in Box 34.4 .
Allergic—urticaria, hay fever, asthma
Inflammatory—eosinophilic fasciitis, Churg-Strauss syndrome
Parasitic—trichinosis, filariasis, schistosomiasis
Nonparasitic infections—systemic fungal, scarlet fever, chlamydial pneumonia of infancy
Respiratory—pulmonary eosinophilic syndromes (Loeffler, tropical pulmonary eosinophilia), Churg-Strauss syndrome
Neoplastic— MPN with translocation of PDGFRA/B or FGFR1 , CML, mastocytosis, Hodgkin lymphoma, T-cell lymphomas, lymphocytic HES
Idiopathic hypereosinophilic syndromes—affecting heart, liver, spleen, CNS, other organs
Others—certain drugs, hematologic and visceral malignancies, GI inflammatory diseases, sarcoidosis, Wiskott-Aldrich syndrome
Eosinophilia is frequent in a variety of hematologic neoplasms, including clonal myeloproliferative diseases (described later in this chapter). It also occurs with a broad spectrum of nonhematopoietic primary or metastatic neoplasms, usually associated with advance-stage disease. Paraneoplastic eosinophilia occurs in Hodgkin lymphoma and peripheral T-cell lymphomas. In some other lymphomas and leukemias of other lineages, it is clonal and part of the neoplastic process, such as B lymphoblastic leukemia with t(5;14) ( IL3-IgH ), while in lymphocytic variant hypereosinophilic syndrome (HES), it may be secondary to an abnormal lymphoid proliferation.
Moderate to severe peripheral blood eosinophilia is most commonly associated with infection by helminthes, including nematodes, trematodes, and cestodes. Eosinophilia is more pronounced if tissues are invaded (e.g., trichinosis) than when parasites inhabit the lumen of a viscus (e.g., tapeworm). Some parasites, such as Taenia solium (cysticercosis) produce little inflammatory response while alive, but trigger an inflammatory response upon degeneration of the organism. Other parasites, such as Trichinella spiralis (trichinosis) incite peripheral eosinophilia upon larval invasion of the muscles and encystment. Leukocytosis and eosinophilia extending over months are seen in toxocariasis ( Toxocara canis or T. catis ) or visceral larva migrans. Cutaneous larva migrans caused by larvae of the dog ( Ancylostoma braziliense ) or cat ( A. caninum ) hookworm also cause eosinophilia.
Loeffler syndrome (simple eosinophilic pneumonia) affects all age groups and is characterized by repeated, transient pulmonary exudates accompanied by fever and clinical symptoms of bronchitis, often producing sputum that contains eosinophils. It may be caused by a variety of exposures, including certain drugs, inhaled antigens, or helminth (see earlier discussion) infestation. The latter occurs during periods of dissemination or migration when the parasites pass from the blood into the alveoli of the lung. Loeffler syndrome may also be idiopathic. Tropical pulmonary eosinophilia is a syndrome of paroxysmal cough and bronchospasm associated with marked eosinophilia and often associated with Wuchereria bancrofti in India, Africa, Southeast Asia, and the South Pacific.
Many drugs are also associated with blood or pulmonary eosinophilia and pulmonary infiltrates. Implicated drugs include: pilocarpine, physostigmine, digitalis, p -aminosalicylic acid, sulfonamides, chlorpromazine, phenytoin, some antidiabetic drugs, some anticancer agents, and many others.
Idiopathic HES ( Fig. 34.8 ) is a heterogeneous syndrome composed of a number of different disorders of varying severity, after myeloproliferative neoplasms with abnormalities of PDGFRA , PDGFRB , and FGFR1 , and CMML with eosinophilia are excluded.
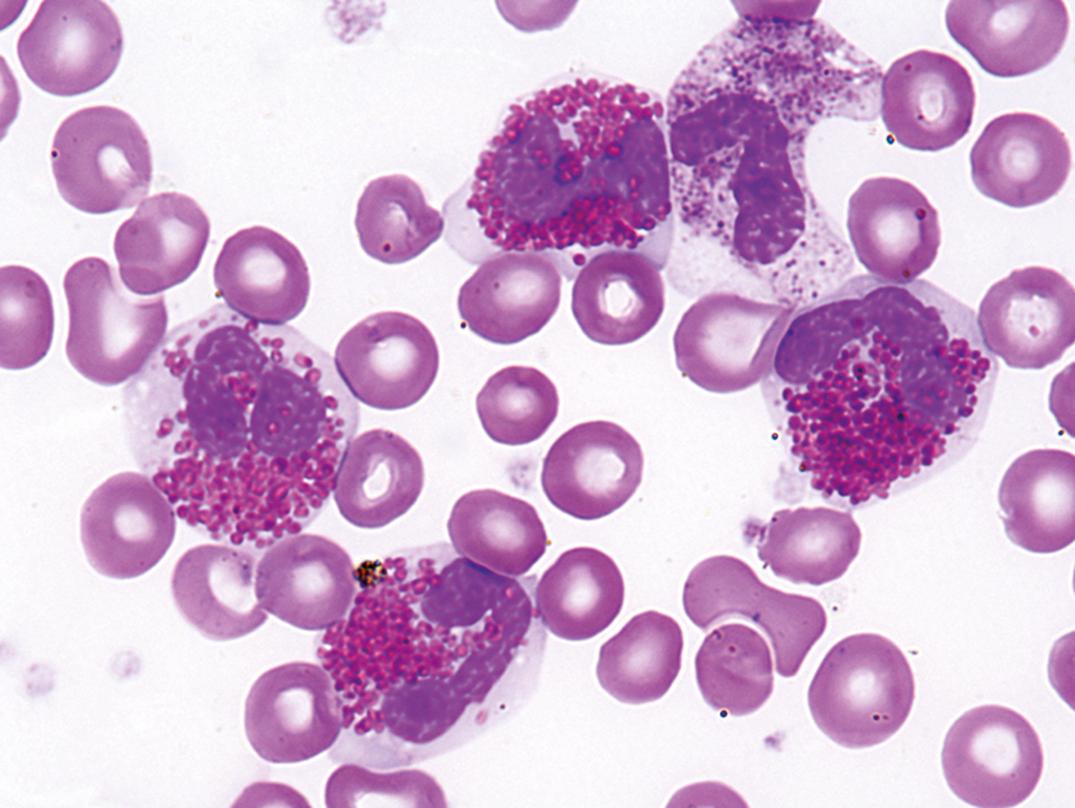
The organ most consistently affected in HES is the heart, with mural thrombi formation, myocarditis, endocardial and myocardial fibrosis, constrictive pericarditis, and fibroplastic endocarditis ( ). Hepatosplenomegaly is common. Other organ systems may be involved, including the central nervous system (CNS) and retina, lungs, skin, gastrointestinal (GI) tract, and kidney.
Basophilia is an increase in the absolute basophil count above 0.2 × 10 9 /L. Causes of basophilia are listed in Box 34.5 . Basophilia is seen most frequently in hypersensitivity and allergic reactions, chronic myeloid leukemia ( Fig. 34.9 ), myeloid metaplasia (extramedullary myelopoiesis), and polycythemia vera. Relative basophilia may be transient following irradiation. Basophilia may be present in hypothyroidism, in chronic hemolytic anemia, and following splenectomy. Reactive basophilia is an uncommon finding overall, sometimes seen in patients combating helminth infections ( ).
Myeloproliferative disease
Allergic—food, drugs, foreign proteins
Infectious—variola, varicella
Chronic hemolytic anemia—especially postsplenectomy
Inflammatory—collagen vascular disease, ulcerative colitis
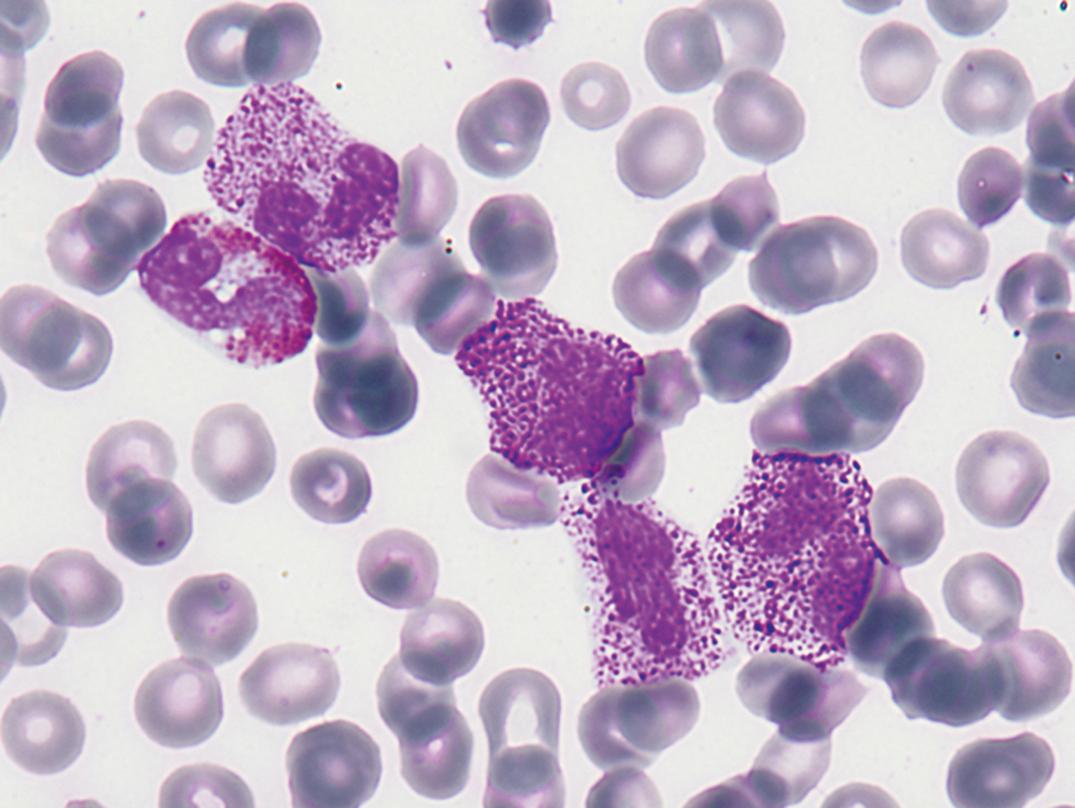
Monocytosis is an increase in monocytes above the upper reference value, especially when greater than 1.0 × 10 9 /L. The most common causes are recovery from neutropenia and indolent infections. Causes and associations of monocytosis are shown in Box 34.6 .
Infectious—tuberculosis, subacute bacterial endocarditis, syphilis, protozoan, rickettsial
Recovery from neutropenia
Hematologic—leukemias, myeloproliferative disorders, lymphomas, multiple myeloma
Inflammatory—collagen vascular disease, chronic ulcerative colitis, sprue, myositis, polyarteritis, temporal arteritis
Others—solid tumor, immune thrombocytopenic purpura, sarcoidosis
Monocytosis is present during the recovery stage from acute infection and from agranulocytosis, in which it is considered a favorable sign. Monocytosis may be present in subacute bacterial endocarditis. In this condition, monocytes may show phagocytosis of other blood cells, red blood cells, and leukocytes. It may be present in mycotic, rickettsial, protozoal, and viral infections.
Infectious disease, however, is an uncommon cause of monocytosis. In a classic study of 160 successive cases of absolute monocytosis ( ), more than half (85) were associated with hematologic neoplasms . These included acute monocytic and granulocytic leukemias, lymphoma (HL most frequently), multiple myeloma, and myeloproliferative disorders.
Monocytopenia is a decrease in circulating monocytes below the lower reference value of 0.2 × 10 9 /L. During therapy with prednisone, monocytes fall during the first few hours after the first dose but return to above original levels by 12 hours. Monocytopenia has been observed in hairy cell leukemia (HCL), in congenital complex immunodeficiency involving monocytopenia and susceptibility to mycobacterial infection (MonoMac syndrome) due to GATA2 mutation, other rare immunodeficiencies, and in B-lymphoblastic leukemia ( ; ; ; ). Monocytopenia is uncommon as an isolated finding.
In these disorders, the pathologist should consider neoplastic conditions, which are covered in the later sections of this chapter, as well as the benign causes, immediately following.
In normal individuals, the absolute numbers of lymphocytes and T cells are highest in young children. The percentage of lymphocytes in the blood is normally up to about 50% for the first 5 years. During the first decade of life, the absolute lymphocyte counts and the absolute number of T cells decrease but remain higher than observed in the adult. By the time of adolescence, the absolute lymphocyte count and absolute number of T cells have leveled off to values observed throughout adulthood. The absolute number of B lymphocytes remains stable during all stages of life ( ; Perkins, 2004). In adolescence and adulthood, lymphocytes constitute about 20% to 40% of all leukocytes, or 1.5 to 4.0 × 10 9 /L cells/L.
The normal CD4/CD8 ratio is between 1.0 and 3.4, but we have seen values as high as 12.0 in reactive conditions. In the neonate, the numbers of total T cells and CD8+ T cells may be similar or decreased compared with adults, whereas CD4+ T cells may be similar or increased.
Lymphocytosis is an increase in the number of lymphocytes in the peripheral blood; reference intervals are ≈1.5 to 4.0 × 10 9 /L in the adult and ≈1.5 to 8.8 × 10 9 /L in the child. Relative lymphocytosis (an increase in the percentage of lymphocytes) is present in various conditions and is especially prominent in disorders with neutropenia. Lymphocytosis is unusual in acute bacterial infections but is commonly associated with viral infection (Epstein-Barr virus [EBV], hepatitis; Box 34.7 ).
Infectious—many viral, pertussis, tuberculosis, toxoplasmosis, rickettsial
Chronic inflammatory—ulcerative colitis, Crohn
Immune mediated—drug sensitivity, vasculitis, graft rejection, Graves, Sjögren
Hematologic—ALL, CLL, lymphoma
Stress—acute, transient
Lymphocytosis is a frequent finding in children with viral infection and may be marked. Lymphocytic leukemoid reactions were previously referred to as acute infectious lymphocytosis (AIL), often lasting 3 to 5 weeks but without other blood changes in most patients. Lymph node enlargement is rare and minimal when present. The spleen and liver are rarely, if ever, enlarged. Lymph node biopsy may show reactive follicular hyperplasia but no characteristic changes. In some cases, there has been an increase in white cells in the cerebrospinal fluid (CSF), with about 40% lymphocytes.
A chronic form of infectious lymphocytosis also occurs in children. The leukocyte count is 10 to 25 × 10 9 /L, with 60% to 80% lymphocytes of normal appearance.
Whooping cough (pertussis), although reduced by routine immunization, still occurs in unimmunized children and is most severe in infants in whom most disease-associated deaths occur. The disease has been resurgent in the United States in recent years in part due to vaccine factors ( ). It also occurs in adults with immunity reduced since childhood vaccination. The etiologic agent is Bordetella pertussis , a highly infectious agent that produces an inflammatory reaction of the entire respiratory tract. The incubation period is approximately 6 to 20 days. Symptoms begin as a head cold, progressing to paroxysms of coughing productive of thick sputum. Paroxysms typically end with a “whooping” sound due to deep inspiration, giving the disease its descriptive name. There is frequently pain over the trachea and bronchi. The disease typically lasts 6 to 12 weeks.
Patients frequently develop significant lymphocytosis, with counts higher than 30 × 10 9 /L recorded. The lymphocytes are small, mature T cells with a normal CD4/CD8 ratio ( ). The lymphocyte count is highest during the first 3 weeks of the illness, decreasing during the fourth and subsequent weeks ( ). The lymphocytosis is at least partially due to the release of lymphocytosis-promoting factor (LPF) or pertussis toxin (PT) from the organism. PT inhibits chemokine signaling through G-coupled protein receptors, reducing lymphocyte retention in bone marrow and spleen, inhibits LFA-1–mediated arrest on lymph node venules, and reduces CD62L expression on circulating leukocytes, inhibiting extravasation ( ). Thus, the lymphocytosis is due to redistribution of lymphocytes into the peripheral circulation without increased lymphopoiesis.
Persistent reactive lymphocytosis is an uncommon event in adults. Significant lymphocytosis should raise suspicion of neoplastic disease, such as chronic lymphocytic leukemia. Coexistence of neutropenia or classic FS suggests large granular lymphocyte (LGL) leukemia. Often, tissue biopsy and/or immunophenotyping of peripheral blood will point to a specific disease or diagnosis. Mononucleosis or lymphocytosis due to other viruses occasionally presents later in life, however.
A rare condition, persistent polyclonal lymphocytosis, has been reported in adults, predominantly in female smokers, and in the postsplenectomy state ( ; ). It is a benign polyclonal B-cell proliferation with binucleated atypical lymphocytes in the blood. However, the possibility of incipient lymphoma has been considered ( ).
Human T lymphotropic virus type 1 (HTLV-1), discovered in 1980, is a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) ( ). It is endemic in areas of Japan, the Caribbean basin, and the southeastern United States. HTLV-2, a second human retrovirus, was first found in a patient with hairy cell leukemia (HCL) ( ). A third human retrovirus, human immunodeficiency virus type 1 (HIV-1), was recognized and confirmed as the cause of acquired immunodeficiency syndrome (AIDS [discussed further later]) in 1983 ( ; Gallo, 1983). Subsequently, a second but distinct type of HIV—now called HIV-2—was identified ( ). More recently, additional retroviruses, HTLV-3/4 and simian foamy retrovirus, have also been found to infect humans ( ).
HIV-1 infects both monocytes/macrophages and T cells (both essential cells for normal immune system function) using cell surface chemokine receptors (CCR5 on the former and CXCR4 on the latter). Infection ultimately causes progressive loss of CD4+ lymphocytes, disrupts normal immune function, and produces immunodeficiency and disease. Disease not only includes many different types of infections, but also an increase in neoplastic disease, including lymphomas. HIV-1 plays an indirect role (through immune dysregulation and not by direct induction) in the development of lymphoma. HIV-2 is less widespread and somewhat less virulent ( ).
HTLV-1 and HTLV-2 also infect and transform T cells: primarily CD4+ cells with HTLV-1 and CD8+ cells with HTLV-2. HTLV-1 infects 15 to 20 million people worldwide, with endemic areas in Japan, the Caribbean, and Africa. It is usually transmitted by breast milk or exposure to blood. Acute infection shows few clinical symptoms, consisting of fever, limited lymphadenopathy, and occasional skin rash. Lymphocytosis occurs, usually less than 20 × 10 9 /L. Most (90%–95%) of patients with antibodies against HTLV-1 are symptom free. After a years-long latency, 3% to 5% manifest ATL or tropical spastic paraparesis ( ).
Infectious mononucleosis (IM) is usually a self-limited infectious disease characterized by sore throat, prolonged malaise, atypical lymphocytosis with the presence of large transformed lymphocytes ( Fig. 34.10 ), lymphadenopathy (most often posterior cervical), and often splenomegaly ( ). In immunocompromised patients, EBV is associated with benign B-cell hyperplasia, malignant lymphoma, and posttransplantation lymphoproliferative disease .
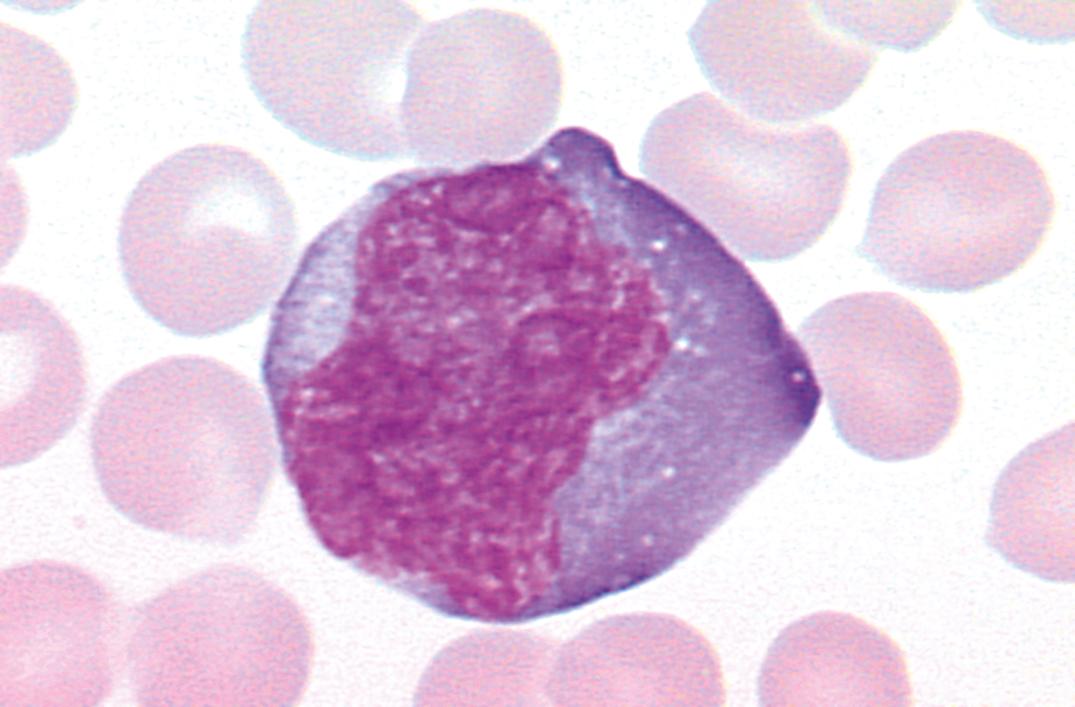
Etiology and Pathophysiology. IM is a disorder that occurs secondary to infection with EBV. When the primary infection occurs in healthy individuals during early childhood, the disease often goes unnoticed. However, when the infection involves healthy adolescent individuals or adults, the resultant disorder is the IM syndrome. In most cases, the virus gains entry into the body through the oropharyngeal epithelial and lymphoid tissues, and the virus appears to infect both tissues. The virus attaches to C3d complement receptors (CD21) on B lymphoid cells and enters into the cells. The EBV then stimulates DNA synthesis in these B cells and induces the formation of several new antigens, including the viral capsid antigen (VCA), the membrane antigen (MA), early antigen (EA)—both diffuse (EA-D) and restricted (EA-R) subtypes—Epstein-Barr nuclear antigens (EBNA), and the lymphocyte-detected membrane antigen (LYDMA) (Harrington, 1988). Thus, the earliest phase of this disease is characterized by an infection of B cells that proliferate, develop neoantigens, circulate, stimulate an immune response, and synthesize immunoglobulin (Sixbey, 1984). VCA, EA, and EBNA are the viral proteins most important for serodiagnosis in immunocompetent patients. Clinical and laboratory features are summarized in Box 34.8 .
Virus enters through oropharyngeal epithelial and lymphoid cells
Virus attaches to CD21 on B cells
Viral antigens—viral capsid antigen (VCA), early antigen (EA), Epstein-Barr nuclear antigen (EBNA)—are produced and elicit antibody production
Immunoglobulin (Ig) M against VCA rises during incubation and prodrome, falls over few weeks to months
IgG against VCA rises during incubation, decreases during convalescence, remains detectable for life
Antibodies to EA rise 2–3 weeks after onset of illness, then fall
Antibodies to EBNA rise during convalescence, detectable for life
T cells activated during second week of illness
CD8-positive cytotoxic T cells kill infected B cells
Natural killer cells kill infected B cells
Some resting memory B cells remain latently infected
2- to 5-week incubation period
Vague onset of symptoms
Fever, sore throat, lymphadenopathy
Adolescents, young adults more often symptomatic than younger children
Leukocytosis with absolute lymphocytosis and atypical lymphocytes
Transient monocytosis
Relative and absolute neutropenia early on
Mild thrombocytopenia in half of cases
Hemolytic anemia in 1%–3% of cases, often with anti-I specificity
Elevated transaminases in 85%–100% of cases, but clinical jaundice rare
Spot test is simple, rapid, specific, based on agglutination of horse red blood cells (RBCs)
Heterophil antibody (HA) test is based on differential absorption of IM-specific HA by beef RBC stroma and guinea pig kidney
Typically, the humoral immune response is characterized by the rise in titer of IgG and IgM viral capsid antibodies during the incubation and early prodrome periods. The titer of IgM capsid antibody starts to fall during the second and third weeks of illness and then diminishes to undetectable levels within the following several months. The IgG viral capsid antibody decreases during convalescence but remains detectable for life. Approximately 2 to 3 weeks after the onset of illness, EBV antibodies against the early antigens appear and then decline over the succeeding 2 months. The titer of the EBV nuclear antigen antibodies rises during the latter portion of convalescence and is apparently detectable throughout life.
The cellular immune response in IM is characterized by proliferation and activation of T cells, usually during the second week of illness, in response to EBV-induced B-cell infection and activation. Because these activated T cells are derived mostly from the cytotoxic/suppressor cell subpopulation, marked suppression and destruction of EBV-infected B cells occur. Indeed, most of the atypical lymphocytes observed in the peripheral blood of patients with IM during the second week of illness possess the CD8 antigen. These cytotoxic/suppressor T cells kill infected B cells and diminish polyclonal antibody production induced by the EBV. In addition to T-cytotoxic cells, the activity of NK cells has a profound effect on limiting the proliferation of EBV-infected B cells in patients with IM.
Most (over 90%) immunocompetent adults have serologic evidence of past EBV infection. EBV has the capacity not only to infect cells (many of which are destroyed by an intact immune system) but to produce a latent infection in resting memory B cells and, in some cases, produce transformation of lymphocytes to malignant lymphoma. However, a majority of latently infected individuals show no disease manifestations throughout their lifetimes.
The resting memory B cells can act as a reservoir of EBV, because viral antigens are not expressed on the lymphocyte surface. In IM, a spectrum of EBV expression patterns (programs) is found in different cells ( ). In type III latency-growth program, cells express a full set of EBV-encoded proteins, including six nuclear proteins (EBNA1–6) and three latent membrane proteins (LMP1, LMP2 and LMP3).
LMP1 has strong immunogenic activity and is present only in acute infection, except in immunocompromised hosts. The type I latency program involves expression of only one nuclear protein, EBNA-1, and is found in some memory B cells. Type IIa latency involves expression of EBNA-1, LMP-1, and LMP-2. Type IIb latency expresses all EBNAs (EBNA1–6) but not LMP-1.
Tumor cells in posttransplant lymphoproliferative disorders (PTLDs) correspond to latency type III, BL cells to latency type I, and cells in EBV+ HL and EBV+ nasopharyngeal carcinoma (NPC) to latency type IIa, with LMP-1 expression. EBV-encoded ribonucleic acids (RNAs) EBER-1 and EBER-2 are reliably expressed at high levels in virtually all latent EBV infections and are detected with high sensitivity by in situ hybridization assays that are currently the standard for EBV identification ( ).
Clinical Features. IM has been observed in patients from 3 months to 70 years of age but is most common in adolescents and young adults. A 2- to 5-week incubation period prior to onset of symptoms usually occurs. Patients usually have vague symptoms. including fever, sore throat with ulcerative pharyngitis, and lymphadenopathy.
Complications. Hemolytic anemia related to anti-I antibodies occurs in 1% to 3% of cases. Mild thrombocytopenia occurs in about half of cases. Splenic rupture may also occur. Neutropenia, pancytopenia, chronic active EBV infection (CAEBV) and EBV hemophagocytic syndrome (EBVHS) occur rarely.
Abnormal liver function tests indicative of hepatitis occur in 85% to 100% of patients with IM. Clinical jaundice is rare, but occasionally jaundice and acute pharyngitis are the only clinical manifestations of IM. Although uncommon, complications may also involve the nervous system, heart, kidney, and lungs.
Approximately one-third of patients with IM carry β-hemolytic streptococci in the pharynx. Thus, one should pay attention to strict clinical, hematologic, and serologic criteria in distinguishing IM from streptococcal pharyngitis.
Hematologic Features. Leukocytes are increased, ranging from 12 to 25 × 10 9 /L. Rarely, counts as high as 80 × 10 9 /L have been recorded. The leukocytosis is usually due to lymphocytosis (60%–90%) composed of a variety of atypical lymphocytes. The total leukocyte count, as a rule, returns to normal within 3 weeks. The atypical lymphocytes have nuclear alterations and an increase in the amount and basophilia of cytoplasm.
Lymphocytes include “monocytoid” lymphocytes, which likely correspond to immunoblasts in lymph nodes (see Fig. 34.10 ). Other atypical lymphocytes, which are more numerous, include plasmacytoid lymphocytes and those with small nuclei but abundant cytoplasm.
Often, the number of monocytes rises transiently. The term mononucleosis refers to an increase in lymphocytes, not in monocytes (“mononuclear” cells include both lymphocytes and monocytes).
Cytologic alterations are not pathognomonic of IM. Similar cells are found in a variety of disorders, including cytomegalovirus mononucleosis, toxoplasmosis, and infectious hepatitis, and usually to a lesser extent in viral pneumonia, varicella, mumps, and viral exanthemas of children.
Neutrophils are relatively and absolutely decreased in most cases during the first week of illness. During this time, a shift to the left may occur, with an increase in band cells and metamyelocytes. Toxic granules and Döhle bodies may be seen. The eosinophils are within normal limits.
The BM from patients with IM usually shows increased cellularity. Numbers of lymphocytes, macrophages, plasma cells, megakaryocytes, and erythroid cells are increased. The neutrophilic series appears decreased. About half of cases may have collections of mononuclear cells forming loose granulomas.
The spot test for IM is based on the principle that horse erythrocytes are more sensitive than sheep erythrocytes in testing for IM. A positive test for IM shows agglutination of horse erythrocytes by serum absorbed with guinea pig kidney but not by serum absorbed with beef erythrocyte stroma. The spot test has proved to be a simple, rapid, highly specific, and sensitive test for the heterophil antibodies of IM. False-positive tests occur but are very rare. The spot test is still in use, as are other similarly performing immunoassays and latex-based detection tests for heterophil antibody. False-negative tests occur particularly in young children who produce heterophil antibodies (IgM) in limited amounts. In heterophil-negative IM, the diagnosis may be substantiated by assay for antibody to EBV.
As previously mentioned, several antibodies are produced by the host in response to a variety of EBV antigens (see Box 34.8 ). Antibody to the viral capsid antigen arises within the first 2 weeks of onset. This antibody, measured by an immunofluorescent or other method, is used for determining exposure to EBV. Assaying for the presence of EBV antibody is usually limited to the few cases of heterophil-negative IM.
In addition to heterophil and EBV antibodies, patients with IM frequently produce antibodies to a wide variety of antigens. Antibodies against human erythrocytes, leukocytes, and platelets have been described. Patients with IM have an increased frequency of cold agglutinins. Positive tests for rheumatoid factor and antinuclear factor have been reported.
In immunocompromised patients, serologic tests are of limited value; direct detection methods are considered more reliable. Determination of EBV viral load by polymerase chain reaction (PCR) appears to be one of the better tests for this patient population (Hess, 2004).
Differential Diagnosis. Clinical, hematologic, and serologic features of IM permit an accurate diagnosis to be made in more than 90% of cases. When the heterophil test is negative, one must consider several possibilities. The patient could still have EBV antibody–positive but heterophil-negative IM. Cytomegalovirus infection, however, is the most common cause of heterophil-negative mononucleosis. Other possibilities include toxoplasmosis, infectious hepatitis, human herpesvirus 6, HIV-1 and HIV-2, and ingestion of drugs ( p -aminosalicylic acid, phenytoin [Dilantin], and diaminodiphenylsulfone).
Course. Classic IM is a benign disorder, with complications occurring in less than 5% of patients. The disorder usually resolves in 3 to 4 weeks. Fatalities are extremely rare but tend to occur in X-linked lymphoproliferative disease (XLP). EBV infection can produce any one of three severe complications in XLP: fulminant IM, life-threatening lymphoproliferative disease and B-cell lymphoma, and dysgammaglobulinemia. Although classically associated with EBV infection, XLP can occur in patients seronegative for EBV. In addition to XLP, EBV-associated lymphoproliferative disorders can occur in individuals with congenital and acquired immunodeficiencies.
Some individuals infected with cytomegalovirus develop a syndrome similar to IM ( ). This disorder can occur following massive blood transfusion (posttransfusion mononucleosis) or spontaneously (cytomegalovirus mononucleosis).
Leukocytosis is characteristic with absolute lymphocytosis. Usually, 20% or more of the leukocytes are atypical lymphocytes. BM aspirates have shown increased numbers of normal lymphocytes and atypical lymphocytes. Abnormal liver function test results are the most frequent abnormal laboratory finding. In a small percentage of patients, titers of cold agglutinins, rheumatoid factor, or antinuclear antibodies may be increased. No rise in heterophil, EBV, or Toxoplasma gondii antibodies occurs. The diagnosis is usually made by isolating the cytomegalovirus from urine, saliva, blood, or tissue biopsy, or by serology.
Toxoplasma gondii, a protozoan parasite, can produce in both young and old a disease (toxoplasmosis) similar to IM. Diagnosis is critical in congenitally infected fetuses and newborns, women infected during pregnancy, immunocompromised patients, and patients with chorioretinitis.
Only a small proportion (≈10%) of immunocompetent individuals are symptomatic. These patients typically present with lymphadenopathy, commonly cervical. Some patients may also present with fever, headache, sore throat, hepatosplenomegaly, chorioretinitis, and an increased number of atypical lymphocytes in the peripheral blood. In immunocompromised patients, disease commonly involves the CNS and eyes but may also involve the lungs and heart.
The histopathology of lymph nodes is usually distinctive, with scattered epithelioid histiocytes that often blur the germinal center/mantle zone border, and with sinus monocytoid B-cell hyperplasia ( Fig. 34.11 ). Morphology correlates closely with elevated Toxoplasma antibody titers (Dorfman, 1973). BM biopsy specimens have no specific pathologic lesion.
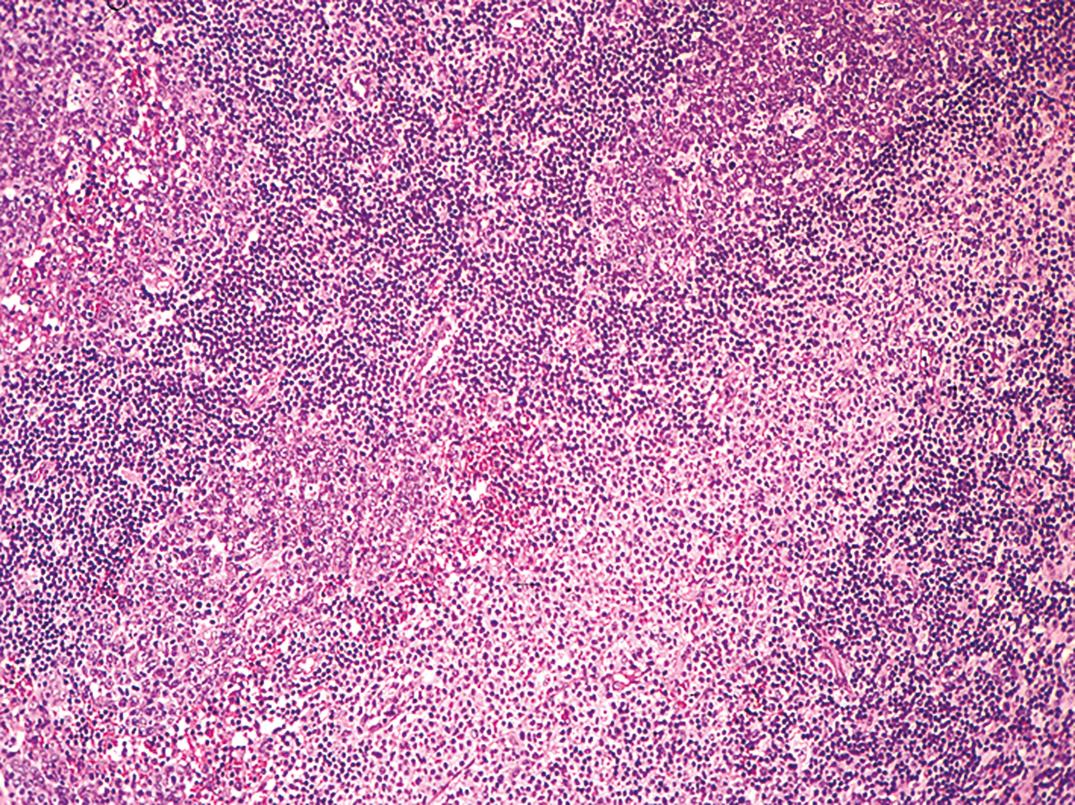
The diagnosis is established by demonstrating an elevation of Toxoplasma antibodies in immunocompetent patients. In immunocompromised patients, serologic tests are not sensitive, and direct detection of the organism from blood, from body fluids, or in tissue is necessary for definitive diagnosis ( ; ).
Autoimmune lymphoproliferative syndrome (ALPS) is an uncommon cause of lymphadenopathy, splenomegaly, and autoimmune cytopenias, with onset in childhood that is caused by an inheritable genetic defect, a mutation in one of the genes encoding FAS, or CD95, or occasionally a caspase gene. FAS is a key component of apoptosis. The disease has variable penetrance and is characterized by chronic lymphoid hyperplasia with presence of CD4/CD8 double negative T cells, and the symptoms mentioned earlier ( ).
Other causes of benign or reactive lymphadenopathy include autoimmune disease and infection, Castleman disease, Rosai-Dorfman disease (sinus histiocytosis with massive lymph node hyperplasia), Kikuchi histiocytic necrotizing lymphadenitis, Kawasaki disease, and others.
Lymphocytopenia is present when the absolute lymphocyte count is below ≈1.8 × 10 9 /L in adults and below ≈2.0 × 10 9 /L in children. Normally, about 80% of circulating peripheral blood lymphocytes are CD3+ T cells, and a majority (≈65%) of these cells are CD4+ helper T cells. A number of immunologic deficiency disorders that are genetically determined have lymphocytopenia, along with various other immunologic defects of humoral or cell-mediated immunity. Lymphocytopenia in these disorders is due to impaired lymphopoiesis. Increased levels of adrenocortical hormones, administration of chemotherapeutic drugs, or irradiation will result in lymphocytopenia. Impaired drainage of the intestinal lymphatics with loss of lymphocytes into the intestines due to a number of causes has been implicated as a mechanism for lymphocytopenia. In advanced cases of non-Hodgkin lymphoma (NHL) and HL, as well as in terminal cases of carcinoma, lymphocytopenia is often observed. Causes and conditions associated with lymphocytopenia include those listed in Box 34.9 .
Destructive—radiation, chemotherapy, corticosteroids
Debilitative—starvation, aplastic anemia, terminal cancer, collagen vascular disease, renal failure
Infectious—viral hepatitis, influenza, typhoid fever, TB
AIDS associated—HIV cytopathic effect, nutritional imbalance, drug effect
Congenital immunodeficiency—Wiskott-Aldrich syndrome
Abnormal lymphatic circulation—intestinal lymphangiectasia, obstruction, thoracic duct drainage/rupture, CHF
Acquired immunodeficiency syndrome (AIDS), once a progressively fatal infectious disorder with characteristic clinical, hematologic, and serologic abnormalities, can now be controlled in most patients with antiretroviral therapy (ART).
Etiology. AIDS is a disorder secondary to infection with HIV-1 and HIV-2—RNA retroviruses that are cytotropic for CD4+ T cells and for other cells, including macrophages, monocytes, megakaryocytes, and CNS microglial cells. Viral entry into a cell results from interaction with the CD4 receptor and with chemokine coreceptors that determine target cell tropism ( ). HIV is responsible for both direct killing of infected cells and indirect killing of neighboring cells, utilizing both apoptotic-dependent and apoptotic-independent mechanisms of cell destruction ( ).
HIV infection is spread by contamination with secretions, excretions, blood, and tissues that contain the virus. Because of the cytotropic effect of the AIDS viruses for CD4+ cells, there is a marked decrease in the number of T-helper cells and an imbalance in T-suppressor/cytotoxic cells in the blood and lymphoid tissues of the body. As a result, a profound cellular immune depression occurs, characterized by infection with a variety of opportunistic organisms. Initially, B cells are not involved and immunoglobulin levels are normal or increased. However, as the disease progresses, the frequency of malignancy in these patients is increased. AIDS-associated cancers include Kaposi sarcoma, NHL, and cervical cancer (all AIDS-defining cancers), as well as HL and anogenital cancers (Bellan, 2003; Mbulaiteye, 2003). Although the cause of these malignancies is undoubtedly multifactorial, recent studies suggest that HIV may possess oncogenic potential ( ). In addition, the functions of monocytes and NK cells are abnormal.
Hematologic Features. The most common hematologic abnormality in patients with AIDS is anemia of chronic disease and lymphopenia (80%–85% of cases), particularly of the T-helper/inducer (CD4) subset. Thrombocytopenia occurs in approximately 30% of cases and neutropenia in 40%, often with a left shift; the former is often immune mediated (Hoxie, 1995).
The peripheral blood film usually displays atypical lymphocytes that have a plasmacytoid appearance. Monocytes are often large, with a fine nuclear chromatin and cytoplasmic vacuoles. Immune-mediated anemia, thrombocytopenia, and neutropenia in AIDS have also been described.
The BM is usually normocellular to hypercellular (Castella, 1985). Often, increased numbers of immature myeloid precursor cells, macrophages laden with iron, and plasma cells occur. Defects occur in BM progenitor cells, such as colony-forming unit-granulocyte, monocyte (CFU-GM); colony-forming unit-granulocyte, erythrocyte, monocyte, megakaryocyte, pluripotential stem cell (CFU-GEMM); colony-forming unit-megakaryocyte (CFU-MK); and burst-forming unit-erythrocyte (BFU-E). Many drugs used to treat AIDS and its infectious complications are also myelosuppressive. Suppression of the marrow is, in many patients, multifactorial, resulting from an interaction of direct HIV cytopathic effect, dysregulation of the immune system and of apoptosis, possible complicating infection and/or nutritional imbalance, and drug effect. The most clinically valuable information of BM biopsy is usually the identification of infection or malignancy in the marrow. The disease course has been converted to a relatively indolent chronic course in those who receive and adhere to ART, but AIDS remains a tragic illness in disadvantaged populations and in those not treated.
Plasma cells are not normally present in circulating blood. They are increased in a variety of chronic infections, in allergic states, in the presence of neoplasms, and in other conditions in which the serum γ-globulin concentration is elevated. Plasma cells have also been recorded in the blood of patients with viral disorders, including rubella, measles, chickenpox, and mumps. They are moderately increased in cutaneous exanthemas, IM, syphilis, subacute bacterial endocarditis, sarcoidosis, and collagen disease. Rarely, bacterial sepsis may show a peripheral plasmacytosis mimicking plasma cell leukemia. Their increase is usually linked with increases in lymphocytes, monocytes, and eosinophils. Causes and conditions associated with plasmacytosis include those listed in Box 34.10 .
Viral—infectious mononucleosis, measles, rubella, HIV
Bacterial—tuberculosis, syphilis, streptococcus, staphylococcus
Parasitic—malaria, trichinosis
Inflammatory—SLE, RA, inflammatory bowel disease, alcoholic liver disease
Neoplastic—plasma cell leukemia, myeloma
Immune stimulation—immune complex disease (serum sickness), drug sensitivity, transfusion
Trauma
In the marrow, an average of 1% to 2% of plasma cells are present in adults. An increase beyond 4% is significant; lower values are found in children. Increases of up to 20% of plasma cells may be found in a variety of conditions other than multiple myeloma, including metastatic carcinoma, chronic granulomatous infection, conditions linked with hypersensitivity, and following administration of cytotoxic drugs. They are often increased in aplastic anemia, but this is probably just a relative increase. On the other hand, they are decreased or absent in agammaglobulinemia.
A leukemoid reaction is an excessive leukocytic response in the peripheral blood. It includes leukocytosis of 50 × 10 9 /L or higher with a shift to the left; lower counts, even below normal, with considerable numbers of immature granulocytes; and similar quantitative or qualitative changes in lymphocytes or monocytes. Depending on the predominant cell, leukemoid reactions may be neutrophilic, eosinophilic, lymphocytic, or monocytic.
A rare family with chronic congenital neutrophilia has been described with a mutation of CSF3R (T617N), activated GCSF receptor, and increased response to GCSF ( ).
Excessive neutrophilia may occur in many situations, including hemolysis, hemorrhage, malignancy with bone involvement, HL, myelofibrosis, infection (especially tuberculosis), severe burns, eclampsia, and certain toxins.
Examination of the blood is usually more helpful than marrow examination. Leukemoid reactions lack the characteristic differential count that is seen in CML, including the myelocyte “peak,” eosinophilia, and basophilia ( Fig. 34.12 ).

Cells as immature as eosinophilic myelocytes rarely appear in the blood in reactive eosinophilia, in which the leukocyte count may exceed 50 × 10 9 /L (see Fig. 34.8 ). Eosinophilic leukemoid reactions usually occur in children and are frequently caused by parasitic infection. Hypereosinophilia is discussed later in this chapter.
In patients with or without anemia, circulating normoblasts frequently are accompanied by a neutrophilic leukemoid reaction; this, then, is a leukoerythroblastotic reaction. A moderate anemia with normoblasts in the peripheral blood is fairly common in metastatic carcinoma involving BM. Leukoerythroblastosis may also be associated with marrow infection and/or fibrosis, and may be seen in benign conditions such as GI bleeding and hemolytic anemia.
Extremely high counts of normal-appearing lymphocytes may occur in infectious lymphocytosis and in pertussis (see earlier discussion). When atypical lymphocytes are strikingly increased or immature (which may occur in conditions such as IM), the distinction from leukemia may be difficult.
Examination of the marrow may be useful because lymphocytes are minimally increased, if at all, in most leukemoid reactions in contrast to leukemia. Flow cytometric studies of peripheral blood and/or BM would reveal a nonclonal population of lymphocytes with a normal combination of cell surface markers in benign proliferations.
The myeloproliferative neoplasms recognized by the WHO are chronic myeloid leukemia, BCR-ABL1 positive (CML); chronic neutrophilic leukemia (CNL); polycythemia vera (PV); primary myelofibrosis (PMF); essential thrombocythemia (ET); chronic eosinophilic leukemia, not otherwise specified (NOS); and myeloproliferative neoplasm, unclassifiable ( ). These are clonal proliferations of a pluripotential stem cell that can differentiate along granulocytic, erythroid, and megakaryocytic lines. Each has a chronic course that may terminate as acute leukemia, myelofibrosis, or a coagulopathy. One, CML, has a characteristic 9;22 translocation; another, PV, exhibits a mutation of JAK2 V617F that is shared with approximately 50% each of ET and PMF, most of the remainder of which carry calreticulin or other mutations. Key features of the major disorders and of myelodysplastic/myeloproliferative diseases are summarized in Table 34.1 .
| Disorder | Demographics | Laboratory Features, Morphology | Cytogenetics | Prognosis |
|---|---|---|---|---|
| CML | Middle-aged | BCR-ABL1 present | t(9:22)(q34;q11) BCR-ABL1 | Dependent on response to TKI |
| CNL PV |
Older adults Middle-aged, M > F |
Neutrophilia > 25K/μL Major criteria: Hb >16.5 g/dL in M or >16.0 g/dL in F; JAK2 V617F (or Exon 12); hypercellular marrow with panmyelosis Minor criterion:↓EPO; all three major criteria, or first 2 major plus minor criterion |
CSF3R mutation JAK2 V617F (or Exon 12) negative t(9;22) |
Indolent 10–20 years |
| PMF | >50 years | Major criteria: megakaryocyte proliferation/atypia with marrow fibrosis; CML, PV, MDS ruled out; JAK2 V617F, CALR , MPL mutation or other clonal marker Minor criteria: leukoerythroblastosis, ↑LD, anemia, leukocytosis ≥11 × 10 9 /L splenomegaly; all 3 major plus at least one minor criterion |
JAK2 V617F, CALR , MPL ; TET2, ASXL1, EZH2, CBL, IDH1/IDH2, TP53, SF3B1, and SRSF2 mutations; +8, +9, del(20q), del(13q), del(1p), negative t(9;22) |
Dependent on phase: ≈10 years in early prefibrotic phase; ≈5 years in fibrotic phase |
| ET | 5th decade (M = F), second peak in 30’ s (F > M) | Major criteria: platelet count >450 K; BM with proliferation of mature megakaryocytes; PV, PMF, CML, MDS ruled out; JAK2 V617F, CALR , or MPL mutation Minor criterion: clonal marker or absence of reactive thrombocytosis N ote : All four criteria must be met. |
JAK2 V617F in 50%; CALR 30%; MPL 3% del(13q22), +8, +9 seen in 5%–10% of cases; negative t(9;22) | Stable for many years (most cases) |
| CMML | Median, 65–75 years, M > F | Monocytosis >1000 with monocytes accounting ≥10% of WBC, cytopenias, myeloid dysplasias, <20% blasts | +8, –7, 12p abn in 20%–40% of cases; negative t(9;22) | 20–40 months with progression to AML in 15%–30% |
| JMML | Younger than 3 years, M > F | Monocytosis >1000, blasts <20%, plus 2 of ↑Hb F, splenomegaly, immature grans, clonal abn, GM-CSF hypersensitivity, hyperphosphorylation of STAT5 | Monosomy 7, negative t(9;22) | Poor; possible benefit from BMT |
| Myeloid/lymphoid neoplasms w/↑eos and PDGFR/FGFR1/PCM1-JAK2 | M 25–55 years | PB w/inc eos, BM eos with mast cells | FIP1L1-PDGFRA, PDGFRB, FGFR1, PCM1-JAK2 | Variable response to TKI |
| Mastocytosis | All ages | Mononuclear w/central nuclei, variable basophilic granules (often not seen in fixed tissue); dense aggregates of spindle cells | KIT D816V | Cutaneous, indolent; systemic, variable; leukemia, aggressive |
| CEL NOS, idiopathic HES | Usually adult M; any age or sex | PB ≥1.5 K eos/μL, <20% blasts | +8, i(17q) 8p11 w/various partners |
Indolent, 80% 5-year survival |
The leukocyte count is usually over 5 × 10 9 /L and may exceed 30 × 10 9 /L. The differential count is characteristic. There is a complete spectrum of granulocytic cells, from a few myeloblasts to mature neutrophils, with myelocyte and neutrophil “peaks” (see Fig. 34.12 ). Myeloblasts account for less than 10% of the cells. The relative percentage of neutrophil myelocytes increases as the total leukocyte count increases. Basophilia is consistently present and eosinophilia is almost always noted, along with the presence of eosinophil myelocytes. Monocytes are also absolutely increased in most patients.
A synthetic tyrosine kinase inhibitor (TKI; imatinib mesylate [Gleevec]) specifically targets the BCR-ABL1 protein and results in long-term remission for most patients ( ). Treatment failures occur due to secondary mutations; newer-generation TKIs are often helpful ( ; ). In the past, treatment (busulfan or hydroxyurea) controlled the disease only in the chronic phase.
Prior to TKI therapy and in patients who fail treatment, the disease changes after a variable period into a more aggressive or accelerated phase. This is characterized by one or more features of progressive myeloproliferation: increased blood or marrow blasts of 10% to 19%; peripheral blood basophilia >20%; persistent thrombocytopenia unrelated to therapy <10 × 10 9 /L; thrombocytosis >100 × 10 9 /L; increasing leukocytosis and splenomegaly unresponsive to therapy; or cytogenetic clonal evolution. Granulocytic dysplasia, increased small dysplastic megakaryocytes and reticulin fibrosis are also suggestive.
Blast phase is essentially a progression to acute leukemia and is defined by >20% blasts in the blood or BM and large aggregates of blasts in the marrow or in extramedullary locations. Blast lineage is myeloid in 70% of cases and may include any myeloid cell types (neutrophilic, eosinophilic, basophilic, monocytic, erythroid, or megakaryocytic), although Auer rods are rarely found. In approximately one-third of cases, however, the appearance is that of acute lymphoblastic leukemia (ALL). Usually, these are of precursor B-lineage. Occasional cases are precursor T-lineage. Myeloid antigens are often coexpressed, and bilineal myeloid/lymphoid cases are rarely found. Occasionally, a patient will initially present in blast phase.
CNL is a rare myeloproliferative disorder characterized by persistent and unexplained neutrophilia of >25 × 10 9 /L mature granulocytes resembling reactive neutrophilia (Elliott, 2001; Bain, 2008). It typically affects older adults and presents with splenomegaly and sometimes hepatomegaly, often with mucocutaneous bleeding, pruritus, or gout. There may be a left shift with bands present and toxic granulation. BM findings include hypercellularity with granulocytic hyperplasia showing a myeloid/erythroid ratio of up to 20:1 and a predominance of mature neutrophils to myelocytes. Erythroid cellularity and megakaryocytes may be increased, but dysplasia is not present. Cytogenetics are usually (90%) normal, with some cases showing abnormalities, including +8, +9, del(20q), and del(11q). Cases with variant Ph′-positive chromosome and neutrophilia are considered CML.
Mutations of CSF3R (T618I and T615A) have been associated with CNL, often together with ASXL1 or SETBP1 mutations ( ; ; ). JAK2 mutation has been reported in some cases. Reactive processes due to occult malignancy or other causes of inflammation may induce marked neutrophilia mimicking CNL ( ).
Polycythemia vera is a clonal stem cell proliferation affecting primarily the erythroid series, characterized by excessive proliferation of erythroid and usually granulocytic and megakaryocytic elements in the marrow (panmyelosis). It is genetically characterized by mutation of Janus 2 kinase, JAK2 V617F , or another functionally similar mutation such as JAK2 exon 12 mutation ( ). JAK2 mutation occurs in essentially all patients with PV but is also present in approximately 50% of patients with thrombocythemia and primary myelofibrosis. The disease is manifested in the blood by an absolute increase in red cell mass, leukocytosis, and thrombocytosis. Serum and urine erythropoietin are decreased. The production of erythrocytes is autonomous with endogenous erythroid colonies (EECs) growing in vitro without erythropoietin. There is initially a proliferative phase and eventually a spent phase with iron depletion and associated anemia, marrow fibrosis, increased splenomegaly, and extramedullary hematopoiesis.
The disease is slightly more frequent in men than in women. It usually begins in middle age. Affected patients exhibit ruddy cyanosis, and splenomegaly is present in two-thirds. Thrombotic or hemorrhagic phenomena occur in about half of patients, and thrombosis is most common. Myocardial infarction, cerebral thrombosis, splenic or pulmonary infarcts, and thrombophlebitis account for the most frequent thrombotic episodes; upper GI bleeding, often from peptic ulcer, is the most common bleeding problem. Pruritus, especially after bathing, is common.
Blood. Erythrocytes exceed 6 to 12 × 10 12 /L, and the hemoglobin is >18.5 g/dL (males) or >16.5 g/dL (females). Mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC) are normal or low. The erythrocytes become hypochromic and microcytic if chronic blood loss has occurred. Macrocytes, polychromatic cells, and normoblasts may be found but are not a prominent feature of the disease. Red cell production is increased. Red cell destruction is normal during the period of erythrocytosis. Later in the disease, as splenomegaly develops, red cell survival diminishes. The total blood volume is increased, primarily because of increased red cell mass, and the plasma volume may also be elevated to a lesser degree. Blood viscosity is high, and it may be difficult to prepare good blood films. The erythrocyte sedimentation rate (ESR) is reduced.
The platelet count is increased in about two-thirds of patients, often to levels exceeding 1000 × 10 9 /L. In 80% of untreated patients, functional platelet abnormalities are present with decreased aggregation in response to adenosine diphosphate (ADP) and epinephrine. No consistent abnormality of secondary hemostasis is present.
Moderate neutrophilic leukocytosis in the range of 10 to 30 × 10 9 /L is common. Immature granulocytes are seen in about one-half of cases, and basophils are often absolutely increased. The neutrophil alkaline phosphatase (NAP) is markedly elevated in 80% of patients.
The arterial oxygen saturation is normal. Hyperuricemia appears in many patients with PV as a result of increased nucleic acid metabolism. In some patients, secondary gout or renal uric acid stones occur.
Marrow. The marrow is moderately to markedly hypercellular, with prominent normoblastic erythroid hyperplasia in panmyelosis. Megakaryocytes show an increase in and clustering of variable small and large megakaryocytes associated with an increase in dilated sinuses and frequently cluster around sinusoids ( Fig. 34.13 ). Increased reticulin is often present, and storage iron is decreased or absent in 95% of cases.
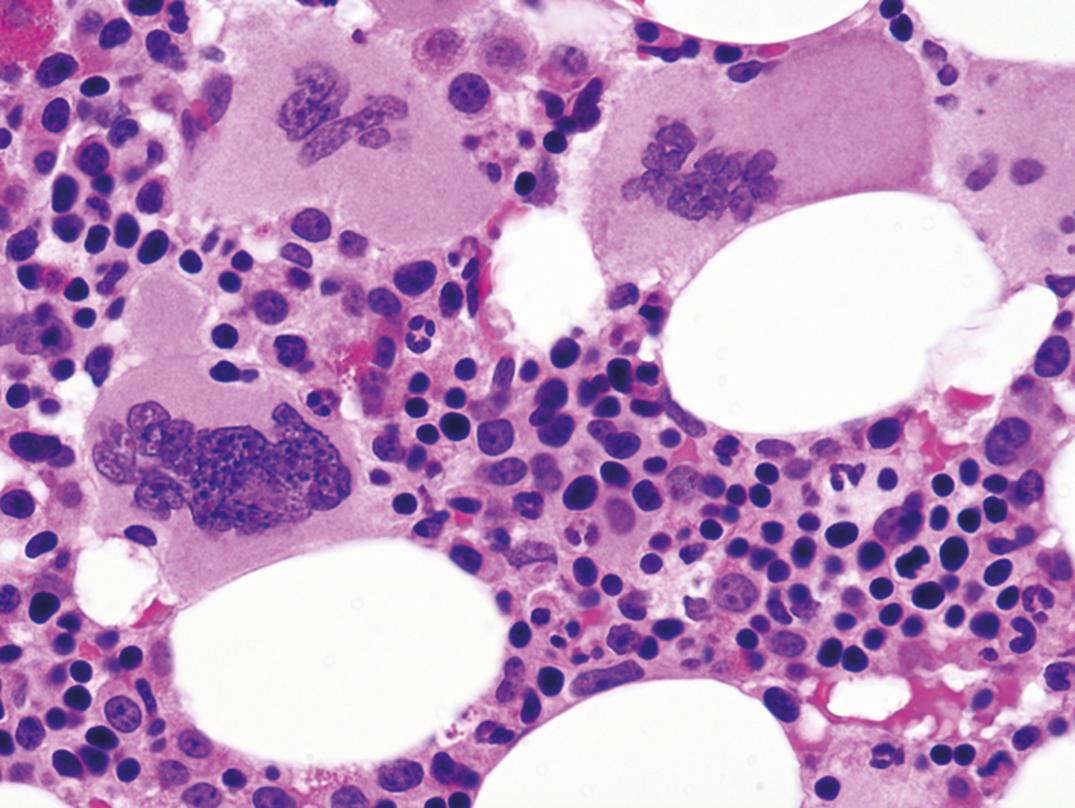
Criteria of the WHO for the diagnosis of PV ( ):
Major Criteria
Elevated Hb >16.5 (males) or 16 (females) g/dL or hematocrit or other evidence of increased red cell volume.
Bone marrow biopsy showing hypercellularity with panmyelosis with granulocytic, erythroid, and megakaryocytic proliferation with pleomorphic megakaryocytes.
Presence of JAK2 V617F or similar mutation such as JAK2 exon 12 mutation.
Minor Criterion
Low serum erythropoietin level
Major criterion number 2 (bone marrow biopsy) may not be required in cases with persistent erythrocytosis (hemoglobin levels > 18.5 g/dL in males or 16.5 g/dL in females) if major criterion number 3 and the minor criterion are present.
In addition to JAK2 V617F or exon 12 mutations, there is emerging information on other genetic abnormalities, including mutations of TET2 , expression of STAT5B, and varied signaling abnormalities ( ; ; ; ).
PV is a chronic disease; patients usually live 10 to 20 years under good control. Phlebotomy, chlorambucil, radioactive phosphorus ( 32 P), and hydroxycarbamide (hydroxyurea) have been used to control the manifestations of the disease.
In about 20% to 40% of patients, the spent or postpolycythemic phase occurs with progressive anemia, gradual splenic enlargement, and further elevation of the leukocyte count, with more immature granulocytes and more circulating nucleated red cells. Many erythrocytes become oval, and teardrop cells (dacryocytes) become prominent. BM aspiration becomes impossible because of myelofibrosis, and splenomegaly increases, owing to extramedullary hematopoiesis. Manifestations at this stage of the disease are indistinguishable from those of myelofibrosis with myeloid metaplasia. Another late complication of PV is acute leukemia or MDS associated with 32 P and alkylators but not with hydroxyurea ( ).
This is a chronic, progressive clonal panmyelosis characterized by megakaryocytic and often granulocytic hyperplasia with varying degrees of reactive fibrosis of the marrow and extramedullary hematopoiesis ( ). Typically, leukoerythroblastic anemia occurs with marked red cell abnormalities, circulating normoblasts, immature granulocytes, and atypical platelets. Chronic idiopathic myelofibrosis (CIM) is an uncommon disease with an incidence one-third that of CML. It occurs typically in persons over the age of 50 years and has an insidious onset, with weight loss, anemia, and abdominal discomfort due to the large spleen. Often, the liver is enlarged as well, and the patient may be slightly jaundiced. Radiography shows patchy osteosclerosis in up to half of patients, but osteoporosis may also be seen.
A prefibrotic stage in 20% to 30% of cases is characterized by mild normocytic anemia with poikilocytosis, including dacryocytes, nucleated RBCs, thrombocytosis, and mild leukocytosis with some immature forms. The marrow is hypercellular and contains abnormal megakaryocytes, which cluster around sinuses and trabeculae. The histopathology of the BM is dominated by atypical, enlarged, and immature megakaryocytes with cloud-like immature nuclei, and small megakaryocytes ( ; ). Fibrosis may be minimal initially. Intrasinusoidal hematopoiesis is often present ( Fig. 34.14 ).
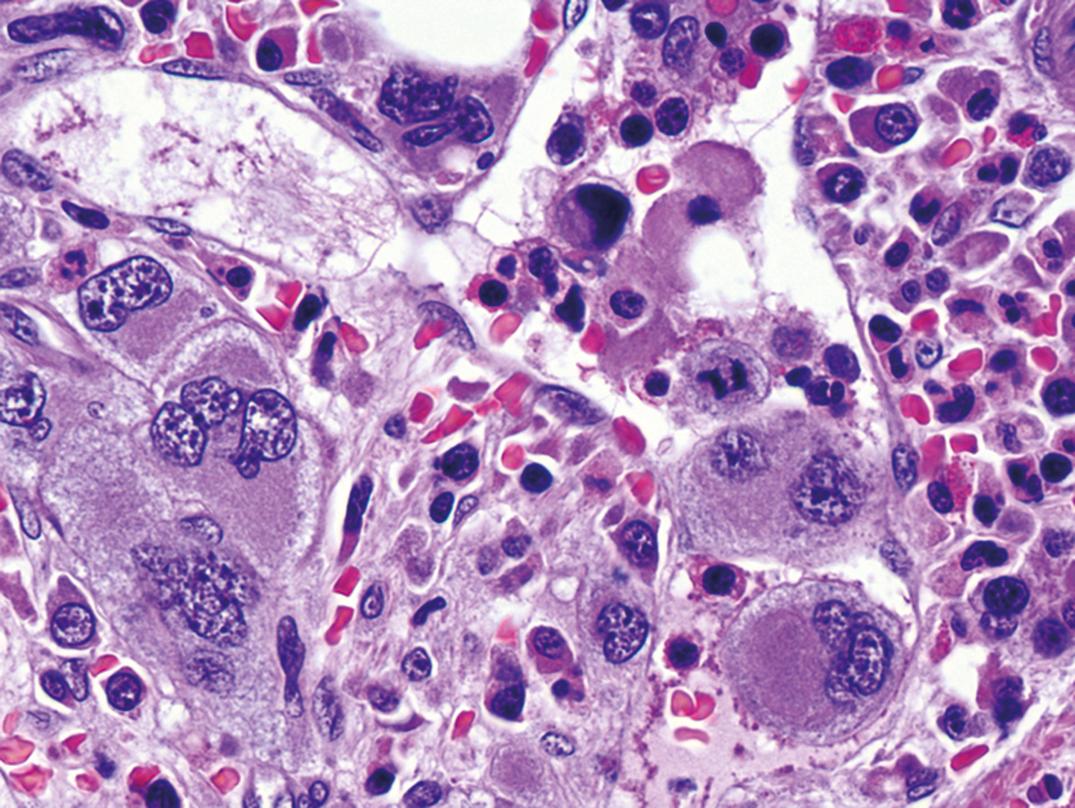
Characteristic findings in the fibrotic stage include moderate normochromic, normocytic anemia (often with some hypochromic cells and basophilic stippling), moderate anisocytosis, and marked poikilocytosis, including prominent teardrop forms (dacryocytes) and elliptocytes. Normoblasts are often increased out of proportion to the degree of anemia with slight reticulocytosis. The anemia may have a complicated origin, with components of marrow failure, ineffective erythropoiesis, and hemolysis. Splenomegaly due to extramedullary hematopoiesis is typical.
The leukocyte count is moderately increased; immature neutrophils and occasionally myeloblasts are present. Basophils are often increased. Platelets are normal or decreased in number (rarely increased) and often atypical, with distinct clear “zones.” Micromegakaryocytes the size of lymphocytes with both nucleus and cytoplasm or small megakaryoblasts may usually be found if searched for; on rare occasions, they are present in considerable numbers.
In vitro blood cell culture shows increased colonies (CFU-GM) similar to those in CML. Serum GM-CSF is high, serum uric acid is frequently increased, and cobalamin is normal or elevated.
It is usually impossible to aspirate marrow; thus, biopsy is necessary. The marrow is fibrotic, with residual islands of atypical megakaryocytes, erythroid, and granulocytic precursors. The fibrosis is of loose connective tissue with scanty collagen, but reticulin fibers are abundant. Foci of osteoid and/or new bone formation in endophytic plaques may be found, and bony trabeculae may be irregularly thickened (myelosclerosis). The marrow may show a mixture of hyperplasia and fibrosis in one sample or may vary in different sites of the body. Megakaryocytes with abnormal nuclei cluster or form sheets that may be found in sinuses with other marrow precursors. Extramedullary hematopoiesis is found in the spleen and liver.
Although the proliferating megakaryocytes are neoplastic, the stromal proliferation is reactive because of inappropriate release of megakaryocyte/platelet-derived growth factors, including platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β, basic fibroblast growth factor (bFGF) and calmodulin (Reilly, 1998).
Approximately 50% to 60% of patients show JAK2 V617F mutation; 30% show mutation of CALR (calreticulin) ( ). A small number, up to 5%, have an MPL W515K/L gene mutation. Testing for these is important for prognostication. TET2 may be a predisposing mutation and modifying mutations include ASXL1, EZH2, CBL, IDH1/IDH2, TP53 and SRSF2. Chromosomal studies show absence of BCR-ABL1 , but variable cytogenetic changes; +8, +9, del(20q), del(13q)(q12-22), partial trisomy 1q, del(5q) and der(6)t(1;6)(q21-23;p21.3). Additional cytogenetic abnormalities may be associated with cytotoxic therapy and/or progression of disease.
The natural course is increasing fibrosis with progressive anemia and enlargement of the spleen. Hemolysis frequently becomes an increasing element in the anemia, and infection may be a serious problem. Portal hypertension occurs in 10% to 20% of cases and may result in bleeding esophageal varices. Hypertension may be due to portal vein thrombosis or intrahepatic obstruction as a result of myeloid metaplasia coupled with increased portal blood flow.
The median survival has been about 5 years, considerably less than that of PV. However, some patients live longer—in those patients, the terminal event is frequently acute leukemia. Therapy has been largely palliative but JAK kinase inhibitors may alter the disease course.
Occasionally, patients exhibit cytopenias and marrow fibrosis due to other causes, some of which may show favorable response to specific therapies. These include metastatic tumors, primary hematopoietic tumors such as HCL or plasma cell dyscrasia, damage from radiation, and, rarely, autoimmune myelofibrosis. Malnourished children with vitamin D deficiency (rickets) sometimes show myelofibrosis. Solvent exposure may be a factor; benzene has been most often suspected and is a known inducer of AML at threshold levels of exposure. Current environmental exposure appears much lower than levels likely to be associated with hematologic malignancies ( ; Courage , 2001). Peritrabecular marrow fibrosis occurs in primary hyperparathyroidism or secondary hyperparathyroidism of chronic renal failure.
Thrombocythemia is a clonal myeloproliferative neoplasm primarily affecting the megakaryocytic lineage with the principal manifestation of sustained thrombocytosis. Of ET cases, 50% to 60% carry a JAK2 V617F mutation, 30% of cases have CALR mutation and 3% MPL . It most often occurs in the fifth decade, with equal sex distribution, with another peak of incidence in females in their 30s ( ).
Patients often (50%) present with asymptomatic thrombocytosis, but up to half of patients present with hemorrhage or thrombosis. The latter occurs as arterial or venous thrombosis or as microvascular occlusion resulting in transient ischemic attacks (TIAs) or digital ischemia. Characteristic recurrent, spontaneous mucosal hemorrhages are most common in the GI tract or the upper airway. Hemorrhages are occasionally preceded or accompanied by thrombosis. Mild splenomegaly occurs in 50% of cases.
Blood. The most striking feature is the marked increase in platelets (≥450 × 10 9 /L; usually >1000 × 10 9 /L), often with abnormal and giant forms and usually accompanied by fragments of megakaryocytes. Neutrophilic leukocytosis is sometimes present. Hypochromic microcytic anemia due to chronic blood loss is present in many cases. Platelet function defects in thrombocythemia are frequently demonstrable. The most typical finding is decreased aggregation in response to epinephrine.
Marrow. The marrow shows increased and enlarged megakaryocytes with mature cytoplasm and multilobulated nuclei and a tendency to cluster in a normal or only slightly hypercellular BM ( ) ( Fig. 34.15 ). Megakaryocyte nuclei are not described as atypical but often are distinctive and resemble other myeloproliferative neoplasms (MPNs). Erythroid proliferation may be present secondary to blood loss. Biopsy features may be indistinguishable from those of PV, but usually with less erythroid prominence. Splenic extramedullary hematopoiesis may be present.
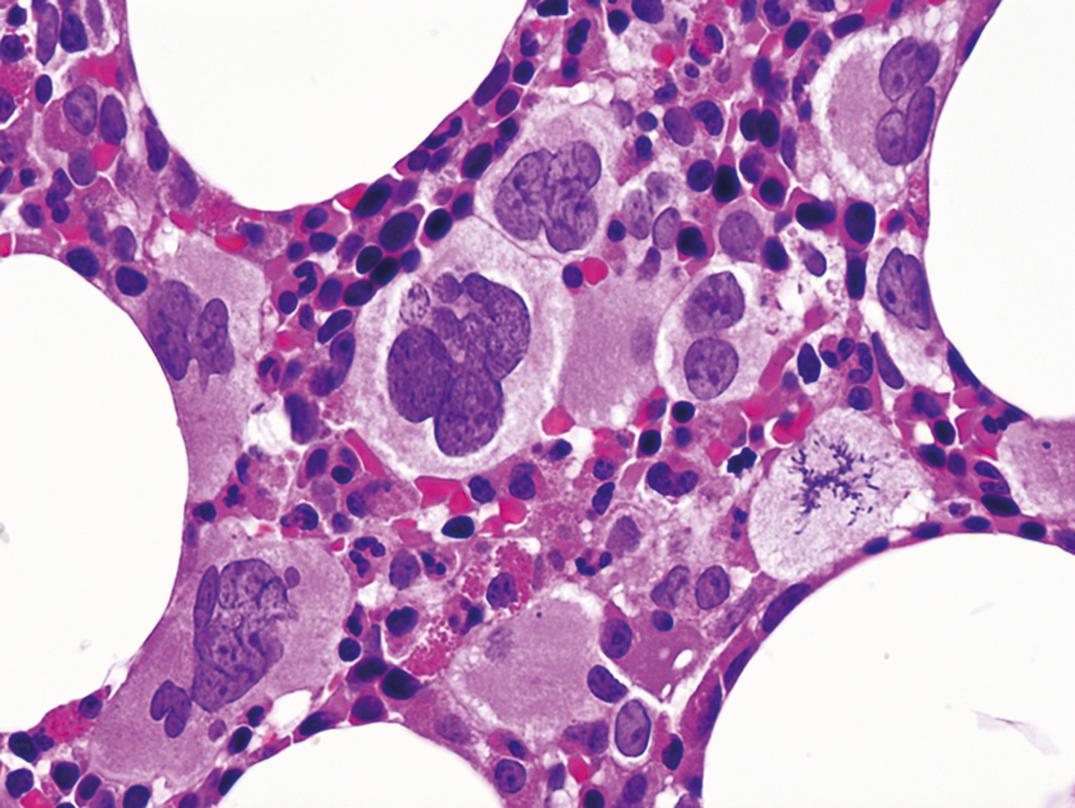
Criteria of the WHO for the diagnosis of thrombocythemia ( ):
Major criteria
Sustained platelet count ≥450 × 10 9 /L.
BM biopsy showing proliferation of mainly megakaryocytes with enlarged mature nuclei, no increase or left shift of granulopoiesis or erythropoiesis.
Not meeting WHO criteria for polycythemia vera, PMF, BCR-ABL1 –positive CML, MDS, or other myeloid neoplasm.
Demonstration of JAK2 V617F, CALR , or MPL mutation.
Minor criterion
Demonstration of a clonal marker or absence of evidence for reactive thrombocytosis.
The diagnosis of ET requires that either all major criteria or the first 3 major criteria plus the minor criterion are met.
From 40% to 50% of cases carry a JAK2 V617F mutation. Most other cases carry a mutation of calreticulin ( CALR ) that is mutually exclusive to JAK2 mutation and carries reduced or no risk of transformation to PV ( ) . BCR-ABL1 translocations must be ruled out to exclude CML. Some cases show +8, abnormalities of 9q or del(20q). Mutations seen in PMF are seen in reduced frequency in ET ( ; ), although this raises the possibility of early PMF mimicking ET.
Most cases are stable for many years, but a small proportion may merge into other chronic myeloproliferative neoplasms or, rarely, develop into acute leukemia. Current treatment recommendations are based on clinical risk stratification to guide cytoreduction, platelet inhibition and anti-coagulation ( ).
These are cases with features of MPN but do not meet criteria for a specific entity or have intermediate features ( ). Examples of MPN-U include prefibrotic/early PMF, masked/prepolycythemic PV, and early-phase ET, as well as cases with features of MPN but presenting with some dysplasia or increased blasts, and those with convincing evidence of MPN that is obscured, however, by a concurrent inflammatory or neoplastic condition. BCR-ABL1 and other specific cytogenetic abnormalities are absent. This category overlaps with myelodysplastic/myeloproliferative disease, unclassifiable. Often, with follow-up, characteristic findings develop that allow definitive diagnosis.
CEL is a clonal proliferation of eosinophils with chronic increase in the blood and often organ damage due to tissue infiltration. The blood contains >1.5 × 10 9 /L mostly mature eosinophils and often increased but fewer than 20% blasts in the blood or marrow. A working conference on eosinophil disorders has recommended that the term hypereosinophilia ( HE ) be used for these cases, subcategorized as HE FA for familial cases, HE US for cases of undetermined significance, HE N for those due to clonal neoplasms and HE R for reactive eosinophilias ( ) . A lymphocyte variant of HE is associated with clonal T cells showing abnormal antigen expressions ( ). Some other neoplasms, including T-cell lymphoma and HL, are often causes of secondary eosinophilia due to cytokine production (IL2, IL3, IL5, GM-CSF). When associated with a defined MPN, the eosinophilia is considered a component of that disease and not termed CEL . MPNs with eosinophilia and PDGFRA , PDGFRB , or FGFR1 gene abnormalities are classified separately.
Some cases of CEL have clonal cytogenetic abnormalities; +8, i(17q) or 8p11 translocations, including t(8;13)(p11;q12), t(8;9)(p11;q32-34), and t(6;8)(q27;p11). The 8p11 translocation may also occur in AML, precursor B- or precursor T-ALL. Other abnormalities, including JAK2 V617F mutation and trisomy 10, may occur. The clinical outcome of CEL and HES is usually indolent, with 5-year survival of about 80%.
Mastocytosis is a clonal neoplastic proliferation of mast cells involving skin, BM, lymph nodes, and/or spleen ( ; ), with variable degrees of malignancy. Subtypes are described based on distribution of disease and clinical manifestations. Symptoms are complex and arise from secretion of vasoactive amines, including histamine and serotonin, as well as from mass effects.
Mastocytosis is associated with mutations of the KIT proto-oncogene that encodes the tyrosine kinase receptor for stem cell factor (SCF), most frequently, KIT D816V with CD117 expression. Morphologically, increased mast cells are found in involved organs, often with associated fibrosis. Mast cells normally are mononuclear cells with central nuclei, clumped chromatin, and dense basophilic granules. They are frequently difficult to identify, however, as mast cell granules are not well preserved in fixed tissue and cells may appear like bland clear cells, histiocytes, fibroblasts, or others. Mast cells may be identified in tissue using Giemsa or toluidine blue stain and immunohistochemistry. Mast cell tryptase is the most specific marker, but cells also express CD117, as well as CD45, CD33, CD68, CD2, and CD25. Mast cells lack myeloperoxidase but often express chloroacetate esterase. Diagnostic testing should include immunologic assays (immunohistochemistry and/or flow cytometry) for CD117, CD2, CD25, and tryptase, as well as assays for KIT D816V mutation in marrow, blood, or other extracutaneous organs for systemic disease.
Urticaria with melanin pigmentation is common with skin involvement and is referred to as urticaria pigmentosa ( UP ) or maculopapular cutaneous mastocytosis ( MPCM ) . This form occurs in both children and adults. In children, lesions are often large and papular or are associated with blistering in children younger than 3 years old. It is often self-limited or may spontaneously regress. Histology shows aggregates of spindle-shaped mast cells filling the papillary dermis and extending into the reticular dermis ( ). A diffuse cutaneous variant is referred to as diffuse cutaneous mastocytosis and a localized variant as mastocytoma of skin .
Systemic mastocytosis is defined by involvement of at least one extracutaneous organ with or without skin involvement. It presents with variable cutaneous and constitutional symptoms, including urticaria, pruritus and dermatographism, symptoms of systemic histamine release, bone pain, arthralgia and fractures, splenomegaly, hepatomegaly, or lymphadenopathy.
BM involvement is sometimes difficult to identify, with paratrabecular and perivascular aggregates of increased lymphocytes, eosinophils, neutrophils, mast cells, and fibroblasts. Marrow biopsy of most cases shows multifocal, sharply demarcated compact aggregates of mast cells. These may be spindle shaped and lacking in apparent granules on hematoxylin and eosin (H&E) stain, or may consist of mixed infiltrates of mast cells, lymphocytes, eosinophils, histiocytes, and fibroblasts, with a peripheral rim or central core of lymphocytes. Compact infiltrates of hypergranular mast cells also occur and are referred to as tryptase-positive round cell infiltrates , or TROCI. The latter may also be seen in basophilic leukemia or AML. Mast cells are frequently increased in hematologic neoplasms such as HCL, lymphoplasmacytic lymphoma, and myeloid neoplasm and are most apparent around BM particles on aspirate smears. Reactive mast cells do not typically show compact aggregates.
In addition, systemic mastocytosis (SM) not infrequently presents simultaneously with or in close temporal relationship with other hematopoietic neoplasms, including acute leukemia, myeloproliferative and/or myelodysplastic neoplasms, and, occasionally, other hematopoietic neoplasia. SM is sometimes detected only after treatment for an acute process ( Figs. 34.16 and 34.17 ). These cases are referred to as systemic mastocytosis with associated hematologic neoplasm ( SM-AHN ) . The associated hematologic neoplasm (AHN) is categorized by criteria for the same disorder without SM.
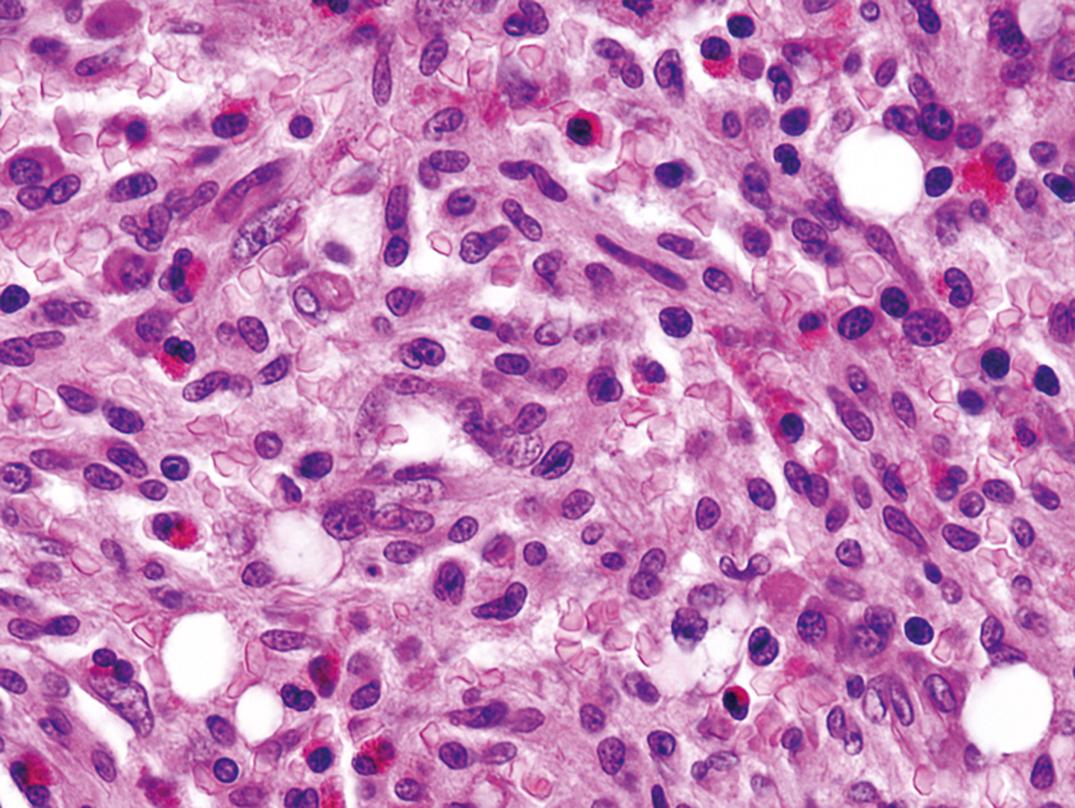
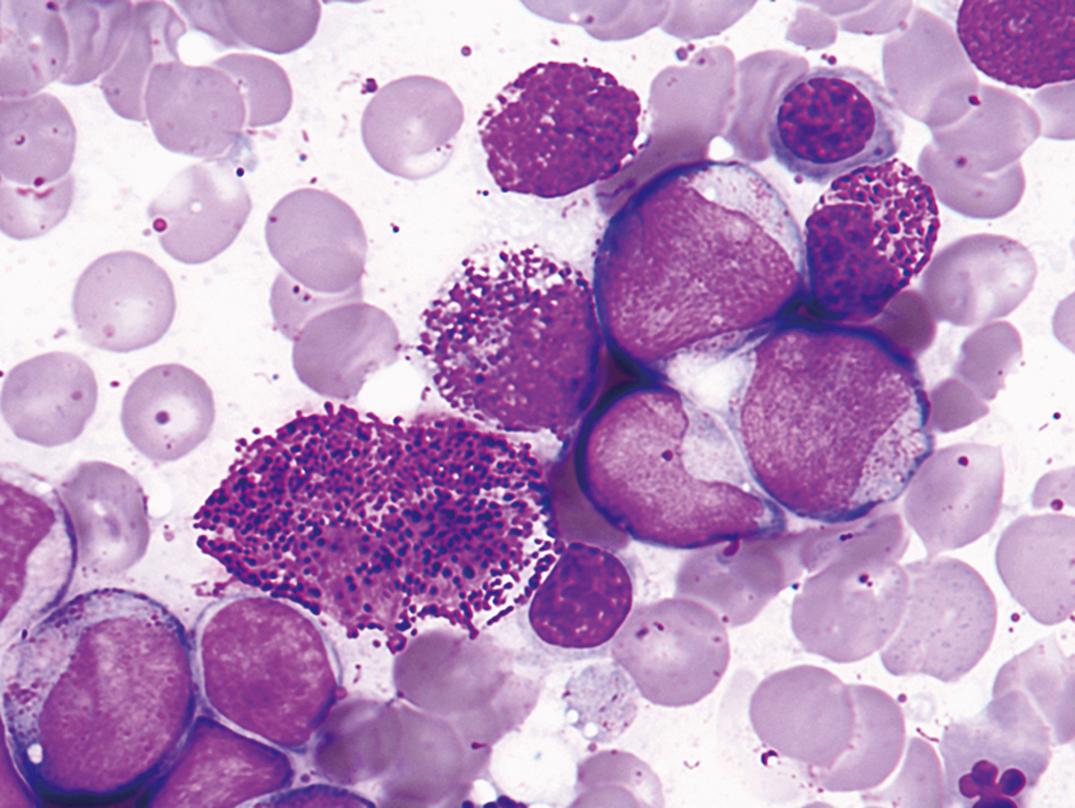
Mast cell leukemia also occurs and is defined by the presence of atypical mast cells constituting more than 20% of a marrow aspirate. More than 10% of the circulating cells are usually mast cells ( ). Chronic forms and a prephase with 5% to 29% mast cells in the BM occur. Myelomastic leukemia (MML) is a related entity in which mastocytic differentiation in advanced myeloid neoplasms occurs without SM ( ).
Mast cell sarcoma refers to localized, destructive extramedullary tumors of highly atypical mast cells, occurring in the larynx, bowel, meninges, bone, and skin. Extracutaneous mastocytoma of mature granulated mast cells occurs rarely in the lung.
The outcome is highly variable, with cutaneous disease usually indolent. In children, it usually regresses. Systemic disease without skin involvement is more aggressive than with skin disease, and mast cell leukemia and sarcoma are highly aggressive.
This disease category contains a group of myeloid/lymphoid neoplasms associated with eosinophilia that have genetic abnormalities resulting in abnormal tyrosine kinases that may be successfully treated with TKIs, the first two of which are responsive to imatinib and/or similar TKIs ( ; ).
MPN with rearrangement of PDGFRA is most often associated with a FIP1L1-PDGFRA fusion protein resulting from a cryptic deletion at 4q12. The disease usually presents as CEL with peripheral eosinophil counts >1.5 × 10 9 /L, with multisystem involvement and organ damage. BM eosinophilia is often accompanied by increased mast cells that are considered part of the disease. It usually occurs in men between 25 and 55 years but may occur in others as well. Presentation also occurs as AML and as T-lymphoblastic lymphoma/leukemia (T-LBL). Diagnosis and appropriate therapy depend on identification of the genetic abnormality by reverse transcriptase polymerase chain reaction (RT-PCR) or by cytogenetic fluorescent in situ hybridization (FISH) assay for deletion of the CHIC2 gene, or use of a break-apart probe encompassing FIP1L1 and PDGFRA . Other genetic variants have been described and are generally imatinib sensitive ( ).
Rearrangement of PDGFRB at 5q32, usually due to t(5;12)(q32;p13.2) with production of ETV6-PDGFRB , resembles chronic myelomonocytic leukemia (CMML) with eosinophilia. Variant translocations involving PDGFRB may be associated with other morphologies, including CEL, MPN, and others. This disease occurs most often in middle age, but with a wide age range and a 2:1 M/F ratio. Diagnosis of MPN with PDGFRB (rather than a morphologic diagnosis as MPN, NOS, or CMML) is imperative because it is sensitive to TKI therapy ( ) . Diagnostic testing is by cytogenetics to detect t(5;12) and/or by RT-PCR. Secondary trisomy 21 is frequent.
Myeloid and lymphoid neoplasms involving FGFR1 at chromosome region 8p11 are heterogeneous and may present as CEL, AML, T- or, less often, B-lymphoblastic leukemia/lymphoma, or mixed phenotype acute leukemia (MPAL). Eosinophilia is present in 90% of cases overall. Although an abnormal tyrosine kinase is produced, it is not imatinib sensitive. The genetic abnormalities are most often translocations and karyotyping may be diagnostic. It was formerly called 8p11 stem cell syndrome or a similar name. Prognosis is currently poor ( ; ) . Hematologic neoplasms associated with t(8;9) (p22;p24.1) resulting in PCM1-JAK2 share characteristic features, often including eosinophilia and left shifted neutrophils in the peripheral blood. These cases may have the hematologic features of CEL, MDS/MPN, AML, B- or T-ALL. Prognosis is quite variable.
Myeloproliferative diseases, in general, are neoplasms in which proliferation of hematopoietic cells outpaces apoptosis, and cellular elements in the blood are increased while the morphology of hematopoiesis is near normal. Myelodysplastic diseases or syndromes are disorders in which apoptosis predominates, hematopoiesis is ineffective, and cytopenias occur. The myelodysplastic/myeloproliferative disorders show features of both, with variable increases in cells, as well as cytopenias and morphologic dysplasia. These include chronic myelomonocytic leukemia (CMML), atypical chronic myeloid leukemia (aCML), BCR-ABL1 negative, juvenile myelomonocytic leukemia (JMML), myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, and myelodysplastic/myeloproliferative neoplasm, unclassifiable.
MDS occurs primarily in persons over age 50 years and usually presents as an anemia refractory to hematinics (iron, folate, B 12 ), with or without neutropenia and thrombocytopenia. Liver, spleen, or lymph nodes are not usually enlarged. The marrow is hypercellular, with abnormal maturation in one or more of the three hematopoietic cell lines, and blast cells are often increased. These disorders have also been called dysmyelopoietic syndromes or “preleukemias” because of the high proportion of cases that ultimately progress to overt acute leukemia ( , ; ). A scoring system for predicting survival and risk of acute leukemic transformation has been developed based on percentage of blasts, cytogenetics, and extent of cytopenias. In general, a blast count >5% elevates risk and >10% increases risk further; complex chromosomal abnormalities or chromosome 7 abnormalities are high risk; del(5q), isolated del(2q), -Y, and normal cytogenetics are low risk; other cytogenetic findings are intermediate risk; and more than one cytopenia increases risk.
Molecular genetic analyses suggest that MDS occurs due to initial oncogenic driver mutations and that progression occurs with accumulating mutations. Most patients have 2 to 3 driver mutations occurring in genes of RNA splicing ( SF3B1 , SRSF2 , U2AF1 , ZRSR2 ), DNA methylation ( TET2 , DNMT3A , IDH1/2 ), chromatin modification ( ASXL1 , EZH2 ), transcription regulation ( RUNX1 ), DNA repair ( TP53 ), signal transduction ( CBL , NRAS , KRAS ) and/or cohesin (STAG2) ( ; ; ). Abnormalities of the “spliceosome” and of DNA methylation lead to epigenetic disruption.
Dyserythropoiesis resembles megaloblastic change and includes nuclear fragmentation or karyorrhexis, multinuclearity, nuclear budding or bridging, basophilic stippling, and ring sideroblasts. These features may also be seen in toxicity due to drugs, chemotherapy, heavy metals, alcohol, and other toxins ( Figs. 34.18 and 34.19 ). Erythroid cells may be decreased or increased in number. Erythrocytic abnormalities in the blood film include presence of oval macrocytes, anisochromia, basophilic stippling, dacryocytes, and reticulocytopenia.
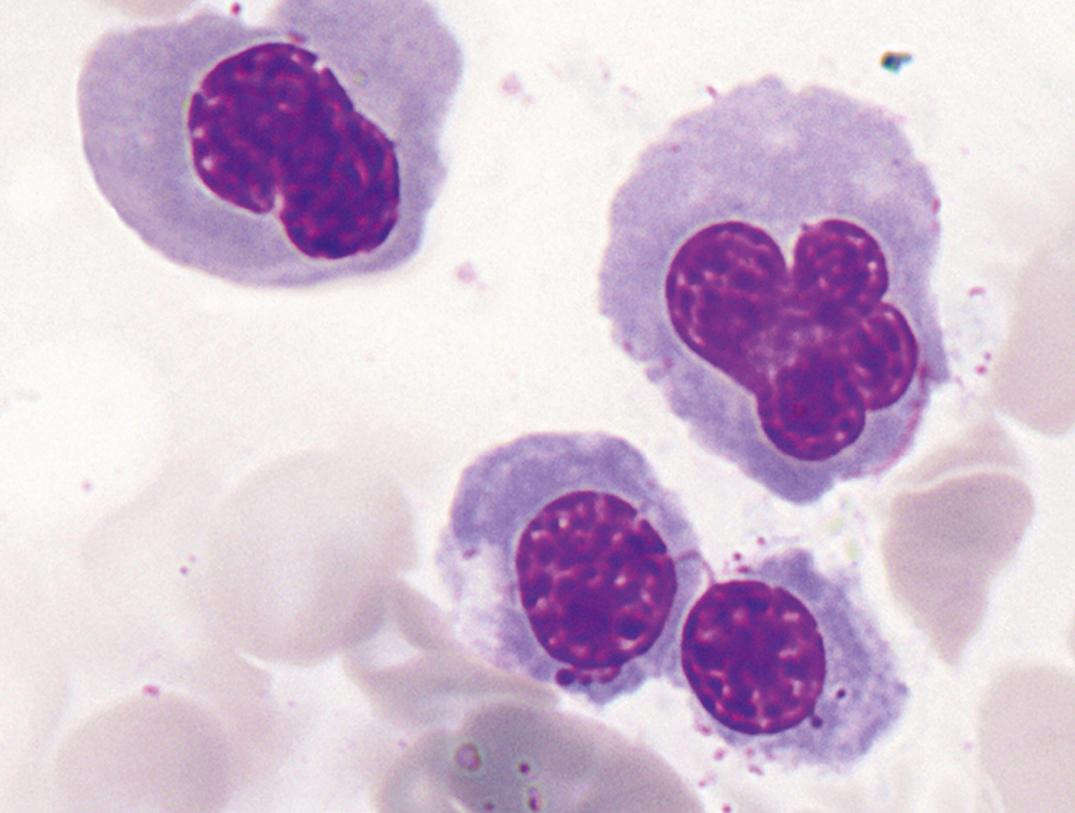
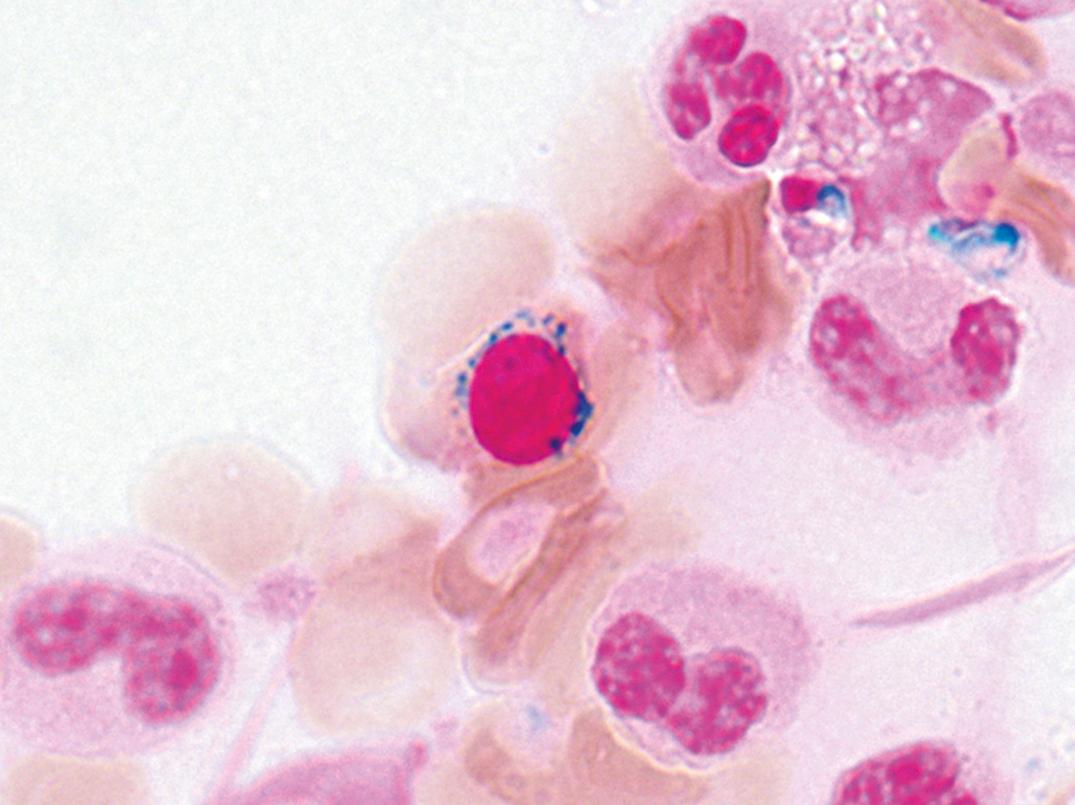
Dysgranulopoiesis includes nuclear/cytoplasmic asynchrony, hypogranulation, nuclear hyposegmentation with increased chromatin condensation, occasionally abnormal large azurophilic granules, inappropriately prominent nucleoli, or other abnormalities. Neutrophil precursors and monocytes often resemble one another. Neutrophils with mature chromatin but with decreased granulation and bilobed or unilobed nuclei resemble the cells of Pelger-Huët anomaly and are termed pseudo–Pelger-Huët cells ( Figs. 34.20 and 34.21 ).
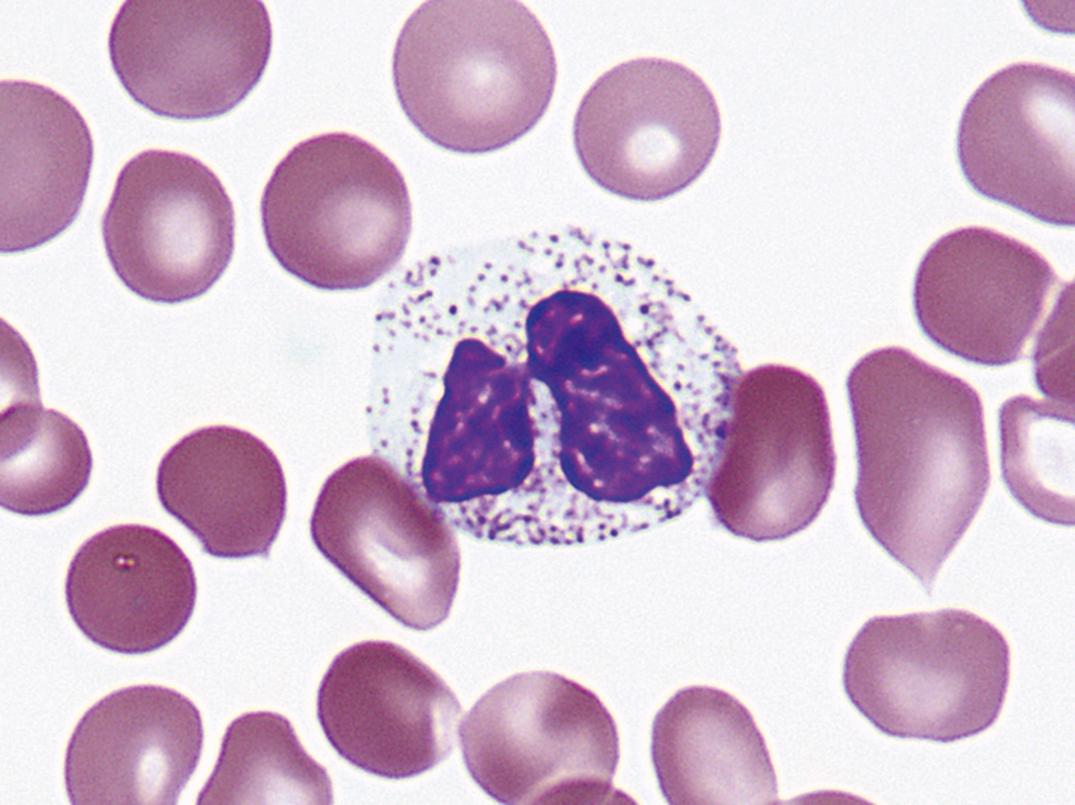
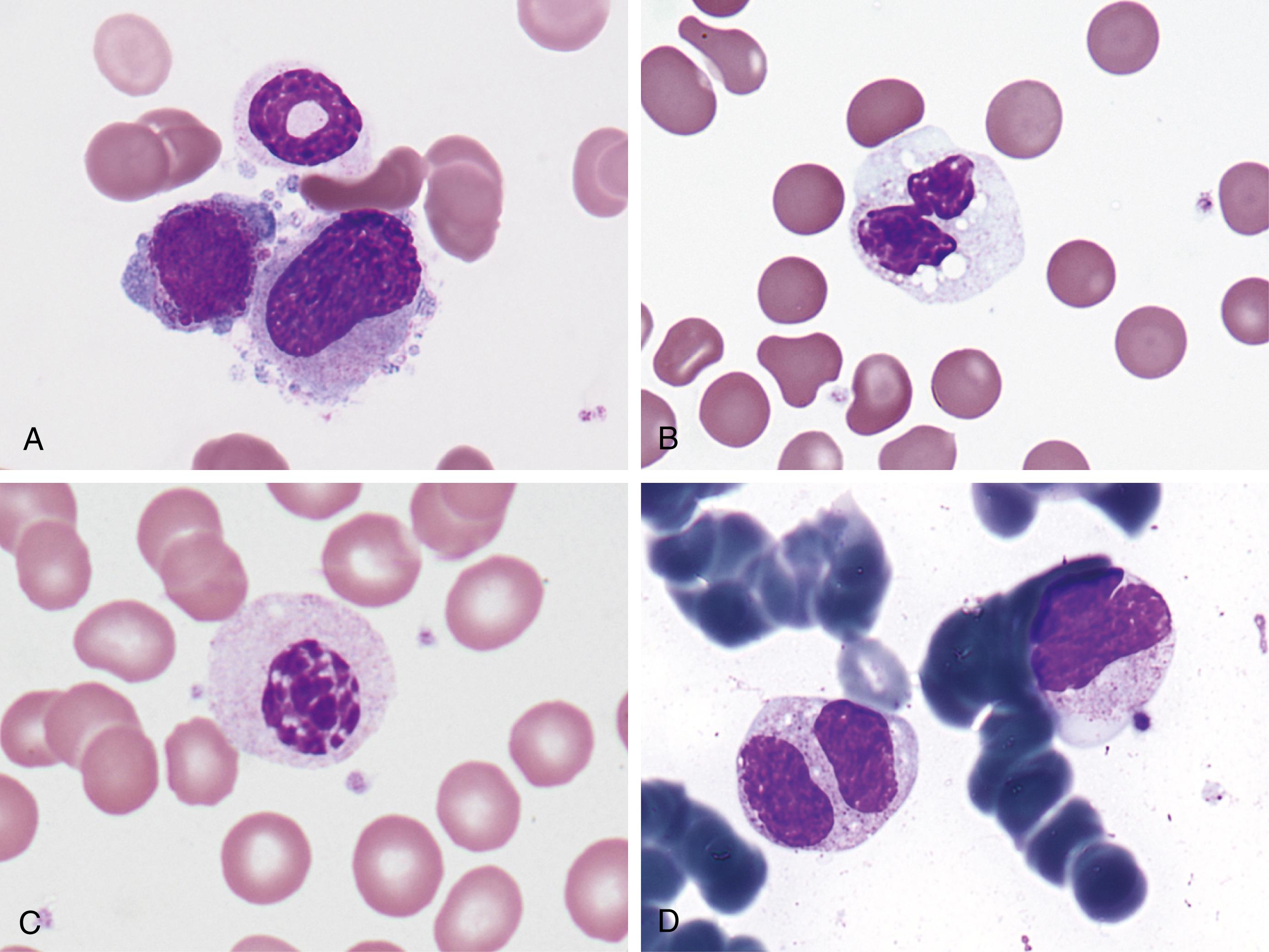
Dysmegakaryocytopoiesis includes large megakaryocytes with unsegmented nuclei, micro megakaryocytes, and megakaryocytes with two or more small, unconnected nuclei ( Fig. 34.22 ). Megakaryocytes may be decreased in number. In the blood film, giant hypogranular platelets are frequent, and micro megakaryocytes are seen rarely.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here