Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Between 1953 and 1956, a strong association between donor leukocyte chimerism and acquired donor-specific tolerance was demonstrated in studies of mouse models. Bone marrow transplantation a dozen years later in immune-deficient and cytoablated human recipients was a logical extension.
In contrast, when clinical organ transplantation was precociously accomplished between 1959 and 1962 in the presumed absence of leukocyte chimerism, it promptly became dogma that organ alloengraftment involved mechanisms other than the chimerism-dependent ones of the mouse models.
We begin this chapter by reviewing the steps that led to this intellectually crippling error. The rest of the chapter describes the way the error was identified in the 1990s and how the resulting fresh insight can be applied in flexible protocols that minimize the dependence of organ recipients on chronic immunosuppression.
The modern era of transplantation usually is dated to the demonstration in 1943 by Medawar and Gibson that tissue rejection is an immune reaction. Two key observations in 1953 by Billingham, Brent, and Medawar revealed a vulnerability in the host immune defense. First, it was possible to engraft splenic leukocytes or bone marrow cells in gestational and newborn mouse recipients who were too immunologically immature to reject the donor cells ( Fig. 88-1 , outer circle). The second finding was that the recipients endowed with donor leukocyte chimerism could freely accept skin from mice of the cell donor strain, but from no other strain (donor-specific tolerance).
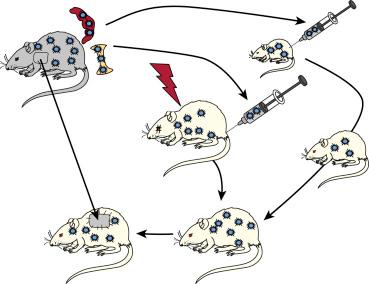
The association of tolerance with leukocyte chimerism was reinforced in 1955 when similar results were produced in adult mice whose normal immune responsiveness had been weakened with supralethal total body irradiation (TBI) in advance of donor bone marrow cell infusion (see Fig. 88-1 , inner circle). The two kinds of mouse experiments presaged the clinical use of bone marrow transplantation for the treatment of immune deficiencies, hematological disorders, and an array of malignant diseases. For surgeons the additional possibility was obvious of engrafting donor hematolymphopoietic cells as a preparatory step for organ transplantation.
Clinical bone marrow transplantation for any therapeutic objective was forestalled, however, with the recognition that the engraftment of immune competent donor leukocytes caused graft-versus-host disease (GVHD) unless the donor and recipient had a good histocompatibility match. Because histocompatibility (human leukocyte antigen [HLA]) antigens were yet to be identified, human bone marrow transplantation was delayed until 1968. When long-surviving bone marrow recipients were finally produced, the patients were obvious analogues of the tolerant mice.
By 1968, thousands of human kidneys had been successfully transplanted, as well as the first livers and hearts ( Table 88-1 ). None of these patients had detectable leukocyte chimerism, nor had any been given an infusion of donor leukocytes. Consequently, it was not surprising that GVHD had not been seen. Another difference from bone marrow recipients was the assumption that all organ transplant survivors were destined for lifetime dependence on daily immunosuppression ( Table 88-2 ). In contrast, the stipulated objective in bone marrow recipients was complete liberation from antirejection drugs by 1 year.
| Organ | City | Date | Physician/Surgeon | Reference |
|---|---|---|---|---|
| Kidney | Boston | January 24, 1959 | Merrill/Murray | 7, 20 |
| Liver | Denver | July 23, 1967 | Starzl | 18 |
| Heart | Cape Town, South Africa | January 2, 1968 | Barnard | 19 |
| Bone Marrow | Minneapolis Madison, WI |
August 25, 1968 September 27, 1968 |
Gatti/Good Bach |
16, 17 |
| Feature Marrow | Organ Transplantation | Bone Marrow Transplantation |
|---|---|---|
| Host cytoablation | No | Yes ∗ |
| HLA matching | Not essential | Critical |
| Principal complication | Rejection | Graft-versus-host disease |
| Immunosuppression-free | Uncommon | Common |
| Term for engraftment | Acceptance | Tolerance |
∗ Three decades later, it was realized that this therapeutic step accounted for all of the other major differences between the two kinds of transplantation.
With the presumed absence of leukocyte chimerism, the possibility that organ engraftment was related to the tolerance of the mouse models, or (after 1968) to the analogous human bone marrow recipients, was dismissed out of hand. There were, however, perplexing exceptions to the starkly different profiles of organ and bone marrow transplantation.
The first exceptions were the first two successful kidney transplantations in the world (in any species). The operations were performed in January and June 1959 by Joseph Murray and colleagues in Boston and then by a Paris team led by Jean Hamburger. The two fraternal twin recipients had been conditioned in advance with sublethal doses of TBI. No leukocyte infusions were given. The kidneys functioned for 20 and 26 years, respectively, without resorting to chronic posttransplant immunosuppression.
Although these results were never duplicated, there were five more “successes” between 1959 and early 1962, defined as such by patient and kidney survival of at least 1 year. These allografts ultimately were destroyed, however, by rejection ( Table 88-3 ). Advances in immunosuppressive drug development now caused abandonment of pretransplant irradiation. It is noteworthy, however, that TBI has been recently revived as part of modern-day protocols.
| Physician | Site | Date | Donor Relationship | Outcome |
|---|---|---|---|---|
| Joseph E. Murray | Boston, Massachusetts | January 24, 1959 | Fraternal twin | 20 Years † |
| Jean Hamburger ∗ | Paris, France | June 29, 1959 | Fraternal twin | 26 Years † |
| Rene Küss ∗ | Paris, France | June 22, 1960 | Unrelated | 18 Months † |
| Jean Hamburger ∗ | Paris, France | December 19, 1960 | Mother | 22 Months † |
| Rene Küss ∗ | Paris, France | March 12, 1961 | Unrelated | 18 Months † |
| Ralph Shackman | London, England | March 26, 1961 | Brother | 3 Years |
| Jean Hamburger ∗ | Paris, France | February 12, 1962 | Cousin | 15 Years ‡ |
| Joseph E. Murray | Boston, Massachusetts | April 1962 | Unrelated | 17 Months § |
| Thomas E. Starzl | Denver, Colorado | 1962-1963 | Mixed series | 50 Years |
∗ Kuss and Hamburger described periodic administration of adrenal cortical steroids in these patients.
† Patient death occurred at listed time.
‡ Patient underwent successful retransplantation in the 1970s.
§ First success with drugs-only immunosuppression (no radiation).
After the antileukemia drugs, 6-mercaptopurine (6-MP) and its imidazole derivative, azathioprine, were shown to be immunosuppressive in rodent skin allograft models. It also was demonstrated that kidney and liver allograft survival could be significantly prolonged in outbred (mongrel) dogs. Although only about 5% of the animals survived for as long as 100 days, a small subset of this 5% did not reject their grafts after drug treatment was stopped. This was most frequently seen in canine liver recipients at the University of Colorado ( Fig. 88-2 ).

The first round of canine kidney transplant studies had been done between 1959 and 1961 in London, Richmond, Boston, and Minneapolis (summarized and annotated in references ). Although truly long survival of the animals had been rare, a clinical kidney transplant trial with drugs-only immunosuppression was undertaken at the Peter Bent Brigham Hospital in Boston. Doses of the 6-MP or azathioprine were given daily along with other myelotoxic drugs. Only 1 of the first 12 renal recipients survived more than 6 months. Moreover, the exceptional patient lost his graft to rejection after 17 months.
At the nadir of the resulting pessimism, an algorithmic strategy for the combined use of azathioprine and prednisone was developed in dog transplant models at the University of Colorado in 1962 and promptly applied in human kidney and liver recipients. As expected, rejection of both organs that developed under azathioprine monotherapy was highly reversible with the addition of large doses of prednisone. The second key observation, best exemplified by human kidney recipients, was that the reversal frequently was succeeded by a declining need for treatment.
Daily azathioprine doses were given to the Denver kidney recipients for at least 1 week before as well as after transplantation, adding prednisone only for treatment of the rejections that occurred in almost every case. Although the surviving recipients were still on immunosuppression when first reported in 1963, “tolerance” in the article’s title denoted their declining need for such treatment. The word tolerance ultimately proved to be apposite. Nine (19.6%) of 46 kidneys donated by genetically related family members between the autumn of 1962 and early 1964 functioned for the next 40 years.
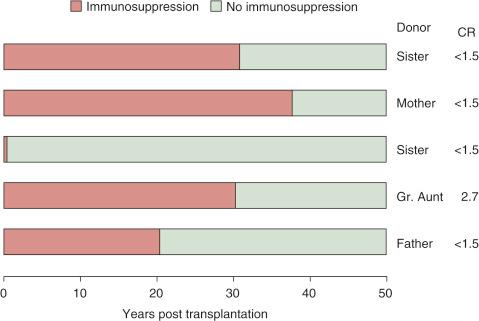
Moreover, 7 of the 9 40-year survivors had been off all immunosuppression for 4 to 39 years when reported in 2003. One of the 7 was shot to death in a love triangle at 40 years. Five of the other 6 lived on to pass the half-century milestone in early 2014. Now off immunosuppression for 13 to 49 years ( Fig. 88-3 ), they bear the longest-surviving kidney allografts in the world. Their donors were a mother, sisters, great-aunt, and father. The sisters were the only donors later shown to have a perfect HLA match.
Although these early kidney transplant results in Colorado had been encouraging, two formal changes in management were made in December 1963. First, the pretransplant administration of azathioprine depicted in Figure 88-4 , left , was deemphasized because of immunosuppression-related infections that had delayed or prevented operation (see Fig. 88-4 , right ).
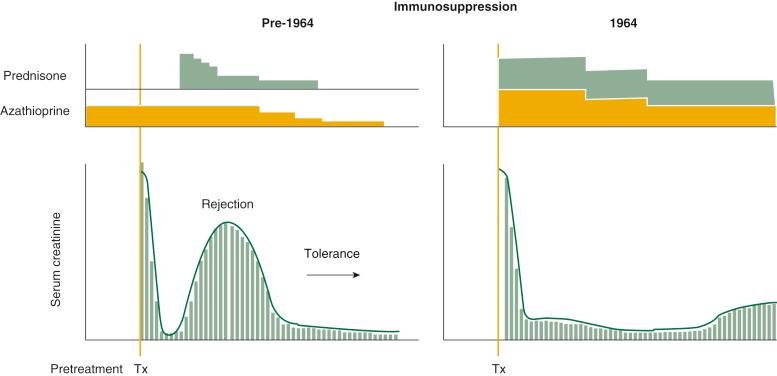
The second change was the routine administration of large doses of prednisone from the time of transplantation (see Fig. 88-4 , right ) rather than in response to rejection (see Fig. 88-4 , left ). This second modification was prompted by a nearly 20% rate of graft losses to nonreversible rejections with the incremental drug strategy. Moreover, the acute loss rate had approached 35% when the kidney donors (including cadavers) were genetically unrelated.
The heavy prophylactic immunosuppression reduced the early graft losses. Inexplicably, however, no similar cluster of drug-free kidney recipients was ever produced again, anywhere in the world. Nearly 30 years later, after elucidation of the mechanisms of alloengraftment, it was realized that the changes in timing and dosage of immunosuppression, both in Colorado and elsewhere, were responsible.
In retrospect, an antitolerance effect of strong posttransplant immunosuppression also was evident in our historical liver transplant experience. In 2006, 35 liver recipients, most treated in Colorado before the Denver team moved to Pittsburgh, had reached or passed their twenty-fifth transplant anniversary ( Fig. 88-5 ). By then, many had been off immunosuppression for long periods or permanently.
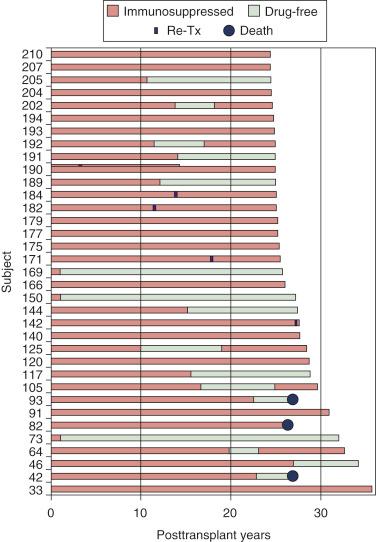
Patients 73, 150, and 169 were of special interest because they had stopped immunosuppression 1 year after their liver replacements. As of May 2014, they have been drug-free for 40.6, 35.9, and 34.4 years, respectively. These and a large proportion of the other drug-free liver recipients had been treated with weak azathioprine-based immunosuppression.
More immunosuppression-free liver recipients were produced during two brief periods after cyclosporine replaced azathioprine as the baseline drug and was replaced in turn by tacrolimus. Both new drugs were introduced with the incremental strategy employed in 1962-1963 for the original Colorado kidney recipients (i.e., prednisone or other agents were added only in the event of rejection).
Just as had occurred with azathioprine, a drift soon occurred away from primary monotherapy with each of the new drugs (see Fig. 88-4 , left ) to the use of heavy prophylactic immunosuppression with multiple drugs as the worldwide standard of care (see Fig. 88-4 , right ). Drug-free liver recipients were seen no more.
In 1966 it was demonstrated in France and promptly confirmed in England and elsewhere that liver engraftment occurs with no treatment at all in about 20% of outbred pigs. Importantly, these pigs were shown by Calne et al to accept the skin or kidneys of the same donor. It was subsequently reported that spontaneous liver engraftment is the routine outcome in a limited number of rat models and in 80% of all inbred mouse strain combinations. Heart and kidney allografts also have been shown to self-induce engraftment without treatment, albeit in a much shorter list of mouse strain combinations.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here