Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Access the accompanying videos for this chapter online. Available on ExpertConsult.com .
Access the accompanying videos for this chapter online. Available on ExpertConsult.com .
Approximately 8% of adolescents (12 to 19 years of age) in the United States suffer from severe obesity, with a significant proportion affected by obesity-associated comorbidities. Nonoperative strategies to treat obesity include lifestyle and behavioral modifications. However, these approaches rarely result in durable weight loss or resolution of comorbidities. Bariatric surgery, on the other hand, promotes sustainable weight loss, improves comorbidities, and augments quality of life. When associated comorbidities are present, current indications for adolescent bariatric surgery include a body mass index (BMI) greater than or equal to 35 kg/m 2 for older children and greater than or equal to 120% of the 95th percentile for children younger than 18 years of age. Independent of associated comorbidities, adolescents with a BMI greater than or equal to 40 kg/m 2 (or if younger than age 18, ≥140% of the 95th percentile of BMI for age) should also be considered if nonoperative management is unsuccessful. While Roux-en-Y gastric bypass (RYGB) was the gold standard operation for many years, the pendulum has shifted to favor vertical sleeve gastrectomy (VSG). This paradigm shift is multifactorial. First, unlike the RYGB, the VSG results in no anatomic defects for potential internal herniation. Second, as the VSG does not bypass the duodenal mucosa, a principal site for iron and micronutrient absorption, the risk of malabsorption is theoretically lower. Lastly, weight loss in adolescents appears similar between those who undergo RYGB and VSG at least 3 years after surgery. Two populations that may benefit from RYGB are children with syndromic (e.g., Prader-Willi syndrome) or hypothalamic-related obesity as case series suggest that RYGB may outperform VSG and adjustable gastric bands for long-term weight loss in these patients. As RYGB is now rarely offered at most adolescent bariatric centers, the remainder of this chapter focuses on the VSG.
The workup for obese adolescents is individualized and largely based on the review of systems and physical examination. In the broadest sense, adolescent obesity can manifest itself as dysfunction in any number of organ systems. It is incumbent on the multidisciplinary bariatric team to perform a comprehensive assessment of obesity-related comorbidities during the preoperative workup. Comorbidities that often require further preoperative workup and optimization include gastroesophageal reflux, obstructive sleep apnea (OSA), steatohepatitis, and type 2 diabetes mellitus. Dietary and lifestyle modifications are key elements to the preoperative phase of care as well. While weight loss during this initial phase is not a prerequisite for bariatric surgery, it is encouraged. In general, for adolescents who continue to gain weight during the preoperative medically supervised weight management and education phase, surgery should be deferred until such time as weight maintenance (plateau) or weight loss is observed. Some data have suggested a correlation between preoperative weight loss and the postoperative results. For patients who meet operative criteria, have successfully modeled positive lifestyle changes as instructed by the multidisciplinary team, have maintained or lost weight, and have comorbid conditions optimized for surgery, the bariatric procedure can be scheduled. The perioperative phase of care is driven by an enhanced recovery after surgery (ERAS) protocol. The goals of the ERAS protocol used at our institution are to maintain a euvolemic state, optimize multimodal analgesia, minimize postoperative nausea and vomiting, and promote early oral intake ( Table 25-1 ). Applying these ERAS principles, we focus on improving patient comfort, decreasing complications, and shortening the length of hospitalization.
| Bariatric ERAS Metrics |
Preoperative
|
Intraoperative
|
Postoperative
|
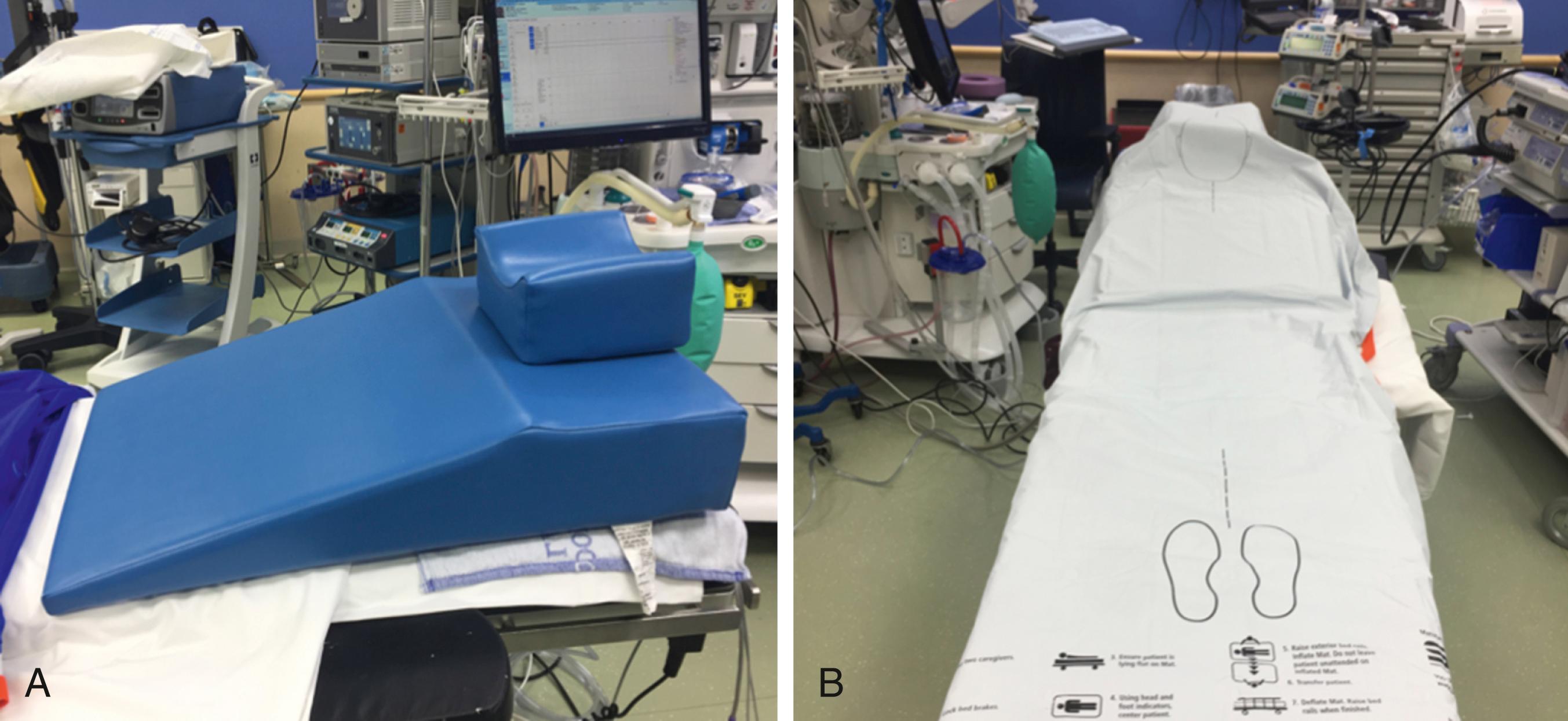
In preparing the operating room for a VSG, both a hover mat–type device (e.g., Prevalon Mobile Air Transfer System [MATS, SAGE Products, Cary, IL]) and a wedged pillow (e.g., Troop pillow, Mercury Medical, Clearwater, FL) are placed on the operating room table ( Fig. 25-1 ). This pillow is helpful to position the head, neck, and shoulders in a 30-degree ramped position to optimize laryngoscopic viewing for tracheal intubation. The MATS device assists with the patient’s transfer back to the stretcher at the end of the operation but prior to extubation. This device protects operating room staff from painful musculoskeletal strain and injuries that have occurred with unaided attempts to transfer patients who are undergoing a bariatric operation.
After intubation, the Troop pillow is removed and the patient is then positioned with both arms abducted and all pressure points padded. Care is taken to secure the thighs and lower legs with multiple straps. An upper body convection warming device (e.g., Bair hugger, 3M, St. Paul, MN) is then placed. Two standing platforms are positioned on either side of the patient and two monitors are angled near the patient’s shoulders to promote ergonomic operating. Standard 32-cm adult-length instruments are usually sufficient to perform the operation.
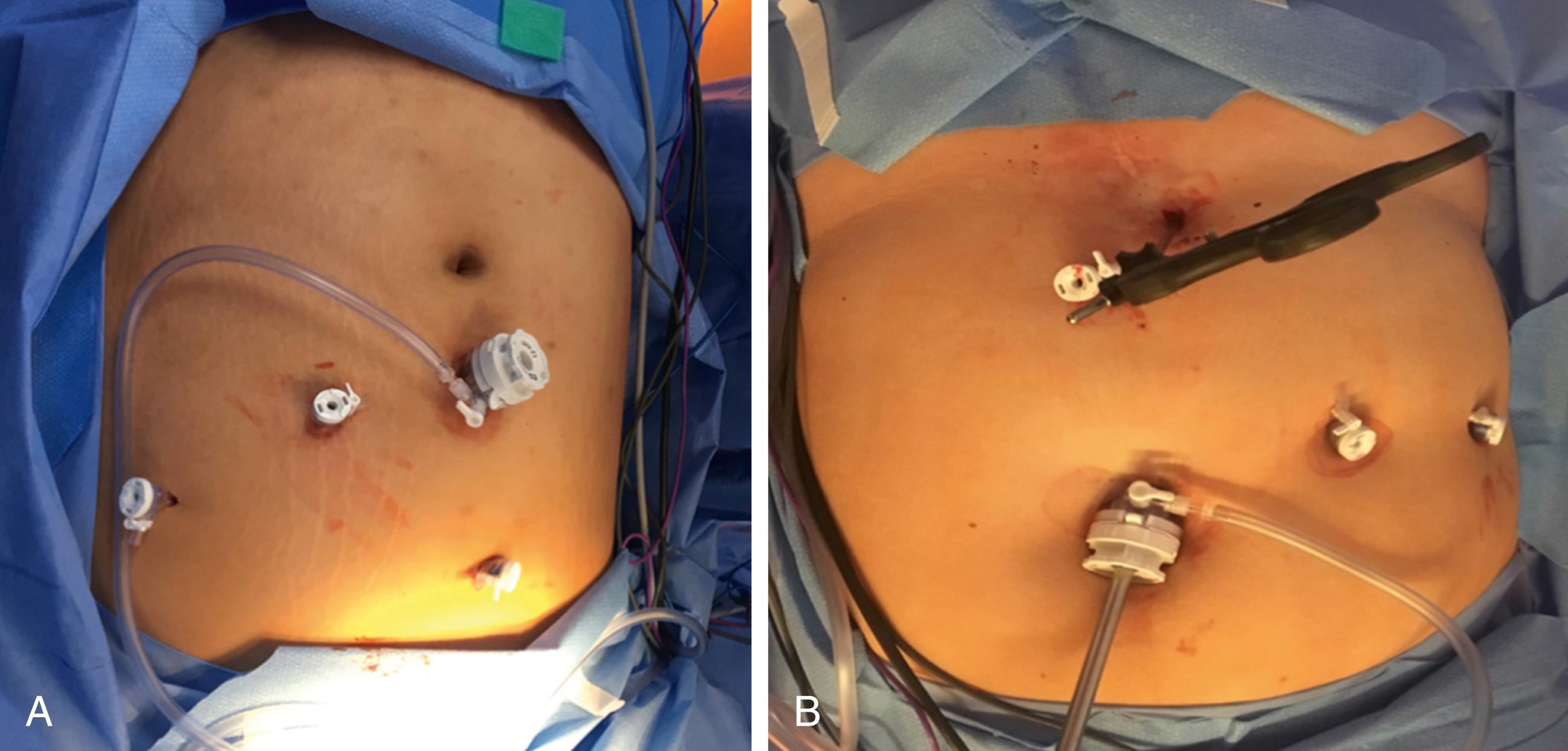
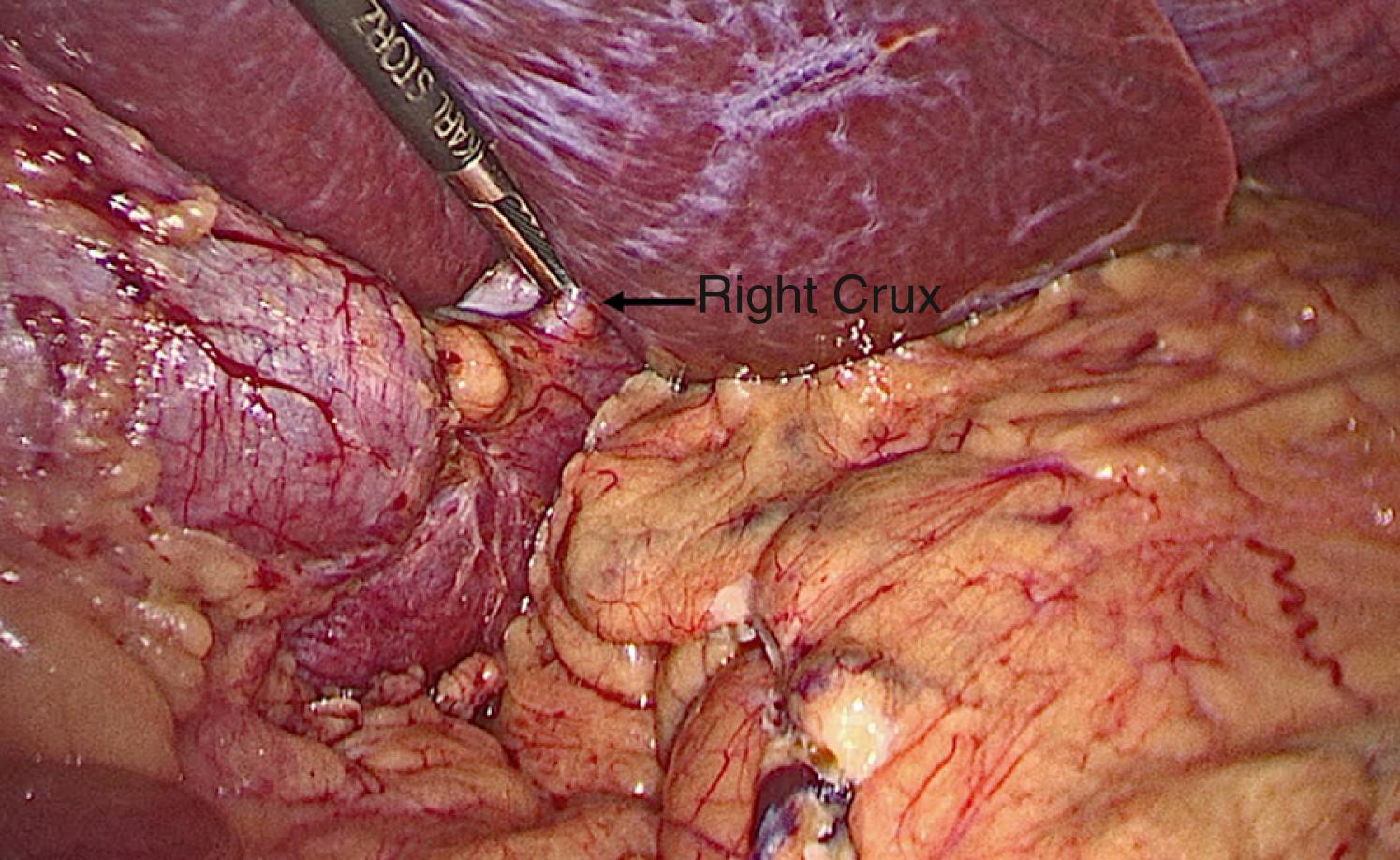
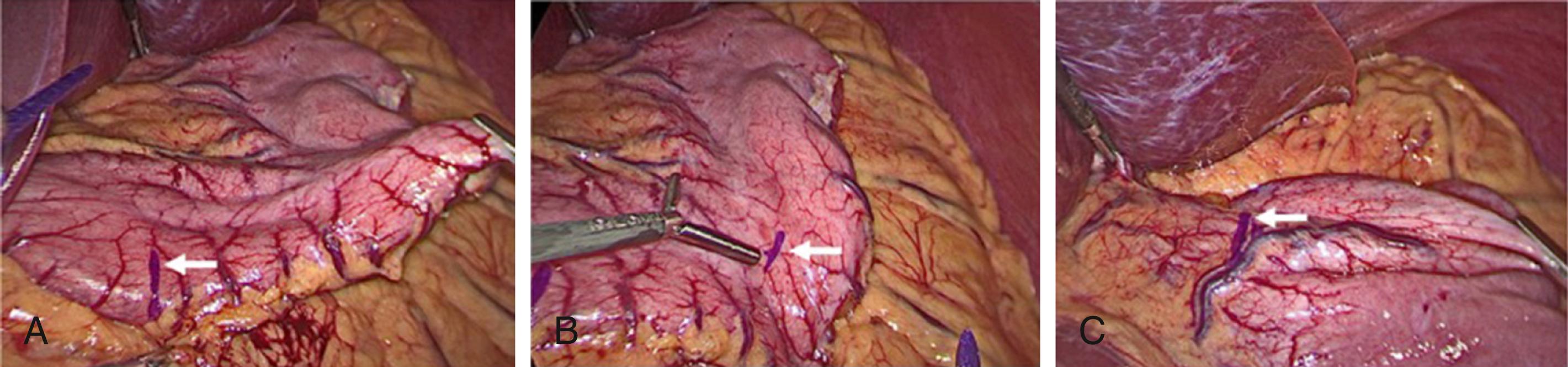
The operation can be performed with a single surgeon (patient’s right side) and an assistant (patient’s left side). Access to the abdominal cavity can safely be achieved with a bladeless, direct-viewing, 12-mm trocar and cannula with a 10-mm 0-degree laparoscope positioned in the center of the cannula (VersaOne, Medtronic, North Haven, CT). The camera port is placed through a midline transverse incision made approximately 15 cm below the xiphoid process. After accessing the abdominal cavity, the 0-degree telescope is exchanged for one with a 30-degree angle. The abdominal cavity is insufflated with high-flow carbon dioxide to a pressure of 15 mm Hg and the patient is placed in the Trendelenburg position. Three 5-mm ports are then inserted under visualization. The first is positioned in the left upper quadrant along the anterior axillary line and serves as an assist port. The second left upper quadrant port is placed along the midclavicular line and serves as a working port. And the last one is inserted adjacent to the falciform ligament in the right upper quadrant and serves as the second working port. Next, a locking grasper is introduced through a subxiphoid stab incision ( Fig. 25-2 ). After placement of the grasper underneath the left lateral segment of the liver, it is locked onto the right crus, resulting in retraction of the liver anterosuperiorly ( Fig. 25-3 ). Alternatively, a Nathanson retractor (Cook Medical, Bloomington, IN) can be used. However, we find this to be time consuming without providing better visualization in most cases. One benefit of preoperative weight loss is a reduction in hepatic steatosis, which in turn reduces the size of the liver and assists with visualization.
As 60% of obese adolescents who undergo a bariatric operation have nonalcoholic fatty liver disease, we routinely perform liver biopsies at the beginning of each case. Under direct visualization, a Core Biopsy Needle Monopty (JIT4You, Irvine, CA) is percutaneously introduced below the right costal margin. Avoiding the gallbladder, two biopsies are obtained. Hemostasis at the biopsy sites is achieved with Kleppinger bipolar forceps (Millennium Surgical, Narberth, PA).
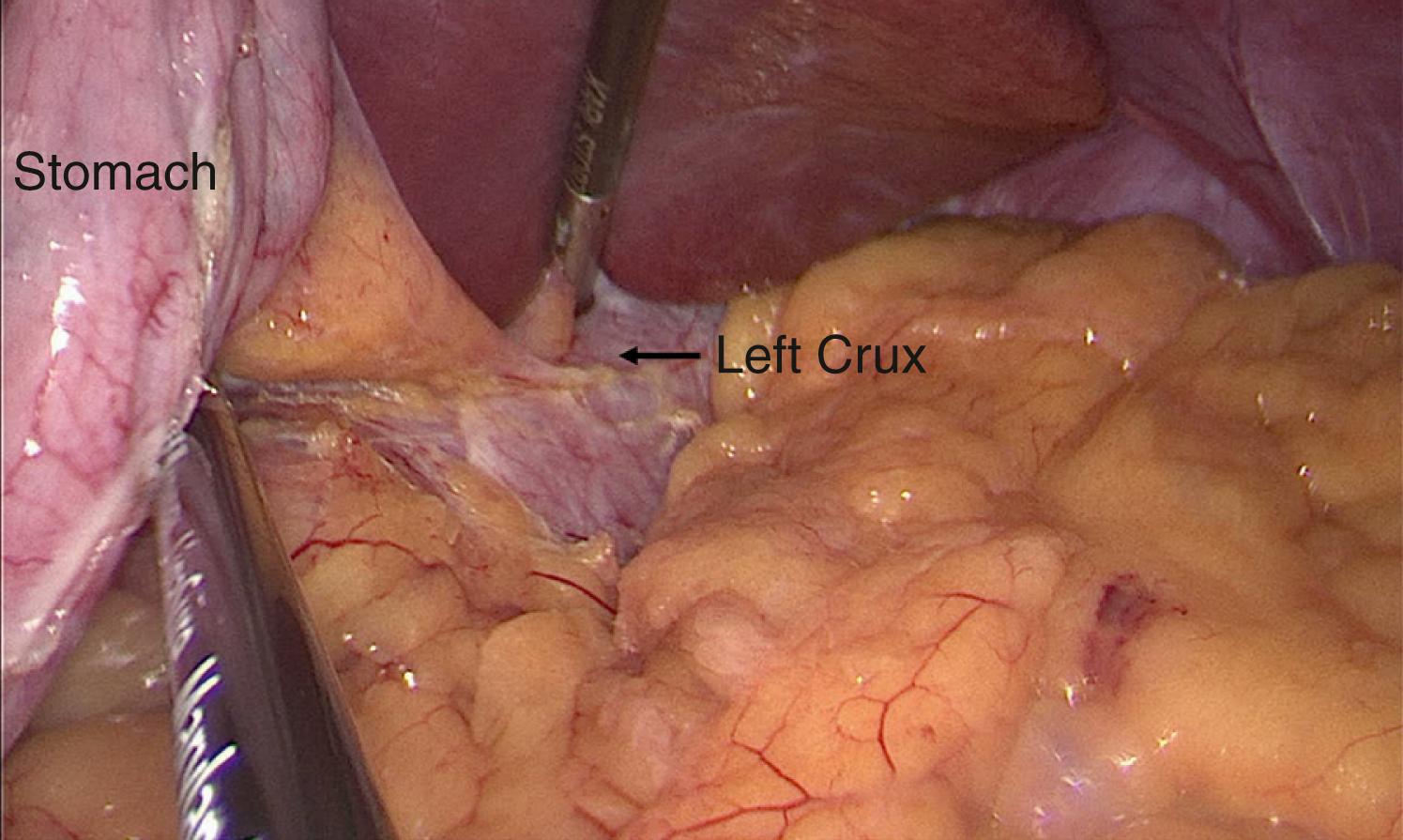
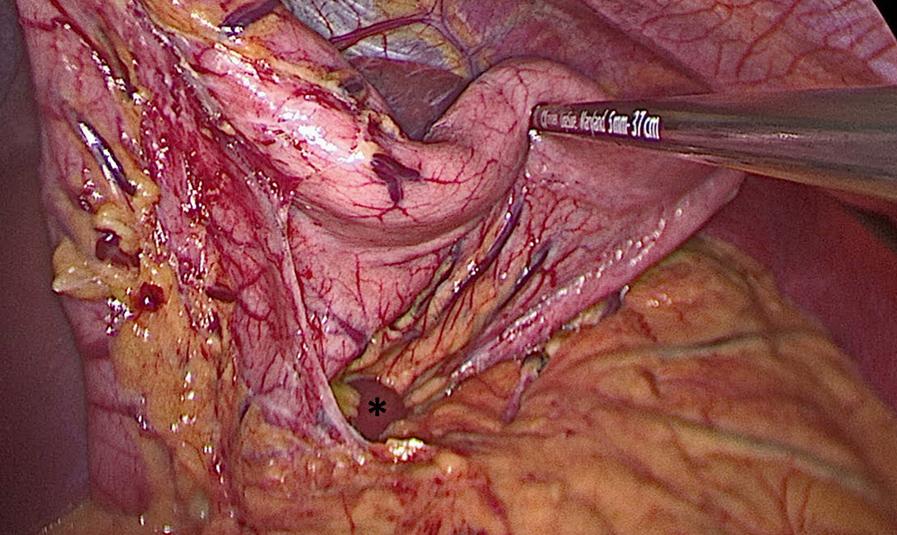
Next, we perform the partial gastrectomy. With a 5-mm Maryland Jaw Tip LigaSure (Medtronic, Boulder, CO), we access the lesser sac by dividing the gastrocolic ligament. The dissection begins 6 cm proximal to the pylorus and extends along the greater curvature of the stomach in the direction of the spleen. After ligating the short gastric vessels, we carefully dissect the fundus off the left crus of the diaphragm, which requires a combination of blunt dissection and bipolar cauterization ( Fig. 25-4 ). We then lift the stomach anteriorly and ligate any posterior attachments ( Fig. 25-5 ). Complete mobilization of the stomach is critical to avoid unrecognized gastric wall redundancy (particularly of the posterior fundus). If the stomach is not freed from all posterior attachments, it may lead to retained stomach not incorporated in the staple line. The 10-mm 30-degree laparoscope is then exchanged for a 5-mm 30-degree laparoscope, which is introduced through the left upper quadrant working port. The tip of a permanent marker is held by a round-tipped grasper and inserted through the 12-mm port to mark the anticipated staple line. With retraction of the stomach to the patient’s left, we mark three points along the anterior surface of the stomach—6 cm from the pylorus along the greater curvature of the stomach, 3 cm from the incisura angularis on the antrum, and 1 cm from the gastroesophageal junction adjacent to the angle of His ( Fig. 25-6 ). Next, an 18 Fr orogastric tube (or bougie dilator) is inserted into the stomach and guided along the lesser curvature to ensure lumen patency prior to stapling.
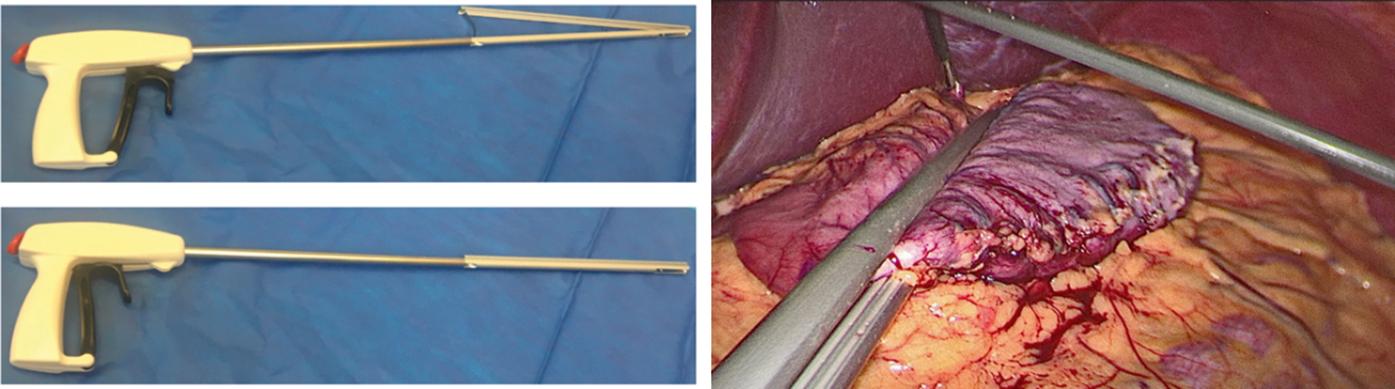
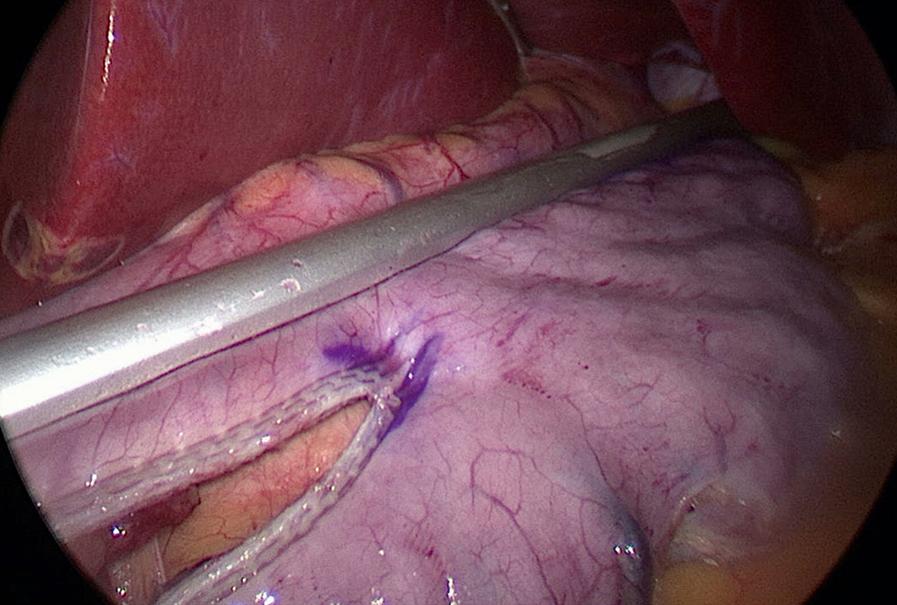
A second 12-mm port is then placed 5 cm to the right of the umbilicus and a Standard Clamp (Standard Bariatrics, Cincinnati, OH, Fig. 25-7 ) is introduced through it and positioned onto the stomach. The clamp is adjusted such that the markings on the stomach lie along the lateral edge and the orogastric tube moves freely along the lesser curve (see Fig. 25-7 ). After clamping down on the stomach, rotation of the Standard Clamp counterclockwise allows visualization of the posterior aspect of the stomach and the clamp is repositioned as needed to incorporate any redundant stomach wall, particularly the fundus that should be included in the resection. An EndoGIA stapler (Medtronic, North Haven, CT) is then introduced through the 12-mm midline port. The first staple load along the antrum is a 60-mm Tri-Staple purple load (inner to outer row, 3 mm, 3.5 mm, 4 mm, respectively, Medtronic, North Haven, CT) to compensate for the thickened antrum ( Fig. 25-8 ). Subsequent staple loads are 60-mm Tri-Staple tan loads (inner to outer row, 2 mm, 2.5 mm, 3 mm, respectively). One purple and three tan 60-mm loads are routinely used to complete the gastrectomy. Occasionally, an additional tan load is required. After completing the gastrectomy, the orogastric tube and Standard Clamp are removed, and the entire staple line is carefully inspected for irregularities and bleeding ( Fig. 25-9 ). Some surgeons perform either an air leak test or a methylene blue (by orogastric tube) leak test at this point.
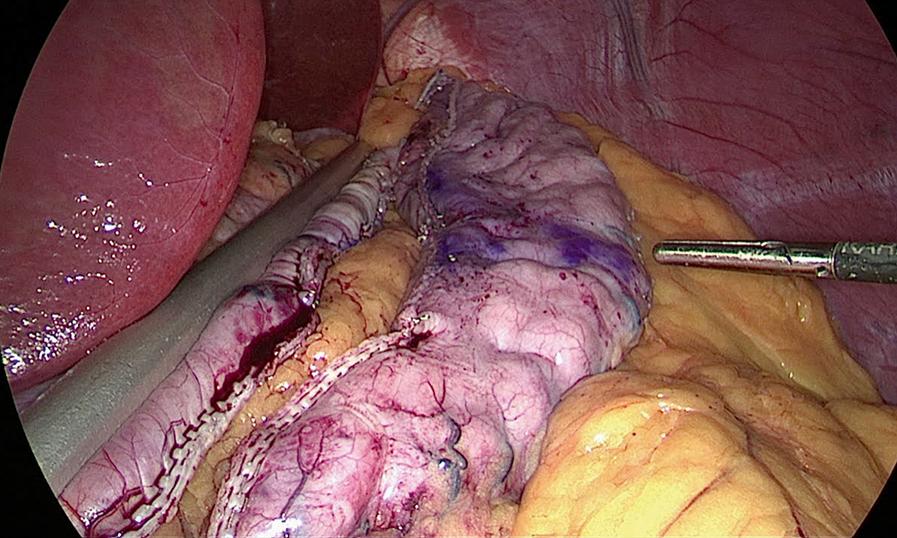
The fascia at the midline 12-mm port is spread with a Kelley clamp to accommodate the gastrectomy. After the specimen has been removed with the Kelley clamp, some surgeons place a closed-suction operative drain. Next, the fascia is closed at the enlarged port site used for extraction of the stomach using a Carter-Thompson laparoscopic port closure system (CooperSurgical Medical Devices, Trumbull, CT). The skin is then closed with 4-0 Monocryl.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here