Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The study of inherited cardiomyopathies is a rapidly evolving area of cardiology. Increasingly recognized because of improvements in cardiac imaging, clinical genetics, and clinical knowledge, these unique disease entities often present in healthy individuals with dramatic clinical implications, both for the affected individual and their relatives. With an increased risk for associated ventricular arrhythmias (VAs), a clinical priority focuses on risk stratification and prevention of sudden cardiac death (SCD). Detailed clinical assessment, family screening, and the correct identification of patients in whom an implantable cardioverter-defibrillator (ICD) is indicated form the mainstay of this management strategy. At present, the role of primary prevention ICDs in patients with a nonischemic cardiomyopathy (NICM) is less clear compared with that for ischemic cardiomyopathy (ICM). Until recently, clinical practice followed similar considerations as for ICM, with an ICD recommended for those with New York Heart Association (NYHA) class II or III symptoms of heart failure and a left ventricular ejection fraction (LVEF) less than 35%. This was based largely on results from five primary prevention ICD trials, summarized in a meta-analysis by Desai et al., in which NICM patients with an ICD benefited from a significant reduction in all-cause mortality compared with pharmacologic therapy alone. Nevertheless, the randomized controlled DANISH (Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure) trial found no ICD benefit in all-cause mortality for NICM patients (the majority with dilated cardiomyopathy [DCM]). Subgroup analysis revealed significant all-cause mortality reduction in younger patients (<68 years) who received an ICD therapy. Importantly, there was a high rate of patients on both β-blockers and angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (>90% in both arms), along with 58% of patients who had a cardiac resynchronization therapy (CRT) pacemaker, making this study more in line with contemporary practice than older trials. Currently both the European and American guidelines continue to recommend primary prevention ICDs for NICM patients with NYHA class II or III symptoms of heart failure and LVEF of less than 35%, in whom life expectancy exceeds 1 year. , In clinical practice, however, an individualized approach, which recognizes the specific subtype of NICM, would appear to be of great potential importance.
The rapidly evolving field of catheter ablation for VA in NICM is offering real hope for patients who are living with recurrent VA and frequent ICD therapies. Compared with ischemic-related ventricular tachycardia (VT), patients with NICM have distinctly different scar location, topography, and electrophysiologic properties. These differences dictate the methods and success rates of ablation in these conditions. Ischemic-related scar is predictably in keeping with coronary territories and has a predominance to the endocardial surface. NICM scar varies with commonly described intramural anteroseptal and epicardial inferolateral subtypes, along with variations depending on the etiology, such as apical scar in some cases of HCM and scar isolated to the right ventricle (RV) in arrhythmogenic cardiomyopathies (ACM). Excellent long-term VA-free survival and significant reduction in ICD therapies is reported in many of the nonischemic disease subtypes, especially ACM, where catheter ablation should now be considered the treatment of choice for recurrent VT.
This chapter describes the specific inherited cardiomyopathy subtypes, focusing on risk reduction of SCD and the management of associated life-threatening VA in the contemporary era.
DCM is the phenotypic endpoint for numerous subtypes of cardiac diseases. It is defined as dilation and impairment of left ventricular (LV) systolic function, with or without RV involvement. It is the main indication for cardiac transplant in the United States. Specific subtypes of DCM include familial, idiopathic, inflammatory, toxin-induced, and ischemic. Familial DCM is most commonly inherited in an autosomal dominant fashion; however, variable penetrance and expressivity, along with other modes of inheritance, such as autosomal recessive, X-linked recessive, and mitochondrial inheritance, have been described. To date, mutations in over 100 genes encoding sarcomeric, desmosomal, mitochondrial, nuclear membrane, and RNA-binding proteins have all been linked to familial DCM. The most commonly implicated genetic variant is the Titin (TTN) truncating variant. TTN encodes the Titin protein, which regulates sarcomere contraction and signaling. Despite the recognition of an increasing number of disease-causing mutations, genetic testing in suspected familial DCM identifies a pathogenic variant in only 25% to 40% of patients.
The need to treat the phenotype regardless of a proven genotypic diagnosis is imperative. Because our experience in genotype-phenotype correlations has increased, some specific subsets of high-risk patients with either a highly arrhythmogenic tendency or a frequent clinical progression to advanced heart failure can be identified. Early diagnosis of these subtypes using a combination of clinical acumen, clinical investigations, and genotypic characterization is vital for optimal planning for VA management, ICD therapy, and heart failure treatment, including temporary mechanical circulatory support and cardiac transplantation. This chapter will outline some of the high-risk subtypes of familial DCM, with a focus on risk stratification and prevention of SCD along with the treatment of recurrent VA.
The lamin A/C gene ( LMNA ) encodes for membrane filament proteins lamin A and lamin C. These proteins form part of the multimeric structure associated with the nuclear membrane known as the lamina . Defects in their function result in destabilization of the lamina structure within the nucleus and nucleus deformability. Patients with LMNA -related cardiomyopathy form an important, high risk, and increasingly recognized group within the broad spectrum of DCM. In addition to LMNA -related cardiomyopathy, LMNA mutations are associated with a spectrum of neuromuscular and adipose tissue phenotypes, including Emery-Dreifuss muscular dystrophy, familial lipodystrophy, and Charcot-Marie-Tooth disease.
LMNA -related cardiomyopathy characteristically presents with atrioventricular (AV) block followed by VA and DCM with a high rate of progression to end-stage heart failure. Sustained monomorphic VT is common, reported in 35% of patients within 7 years, and frequently occurs before significant symptoms of heart failure and LV dysfunction. The aggressive clinical course of the disease means that early recognition and assessment for treatment and protection from SCD with an ICD, along with early referral for cardiac transplant, are imperative.
Early consideration of ICDs is particularly relevant to the highly arrhythmogenic LMNA -related cardiomyopathy. The American guidelines recognize this by advising a class 2a recommendation for an ICD in patients with NICM secondary to an LMNA -related cardiomyopathy with at least two of the following risk factors: nonsustained VT, LVEF of less than 45%, non-missense mutation, and male sex. Secondary prevention ICD therapy is undisputed, with both the European and American guidelines advising ICD therapy in patients with NICM who have either survived a cardiac arrest or experience hemodynamically compromising VT. ,
Antiarrhythmic drugs have poor efficacy for treating VA in LMNA -related cardiomyopathy. Catheter ablation can be considered for recurrent VT refractory to antiarrhythmic drugs, recurrent ICD therapies, or electrical storm. Nevertheless, the high burden of VA, along with scar size, location, and topography in LMNA -related cardiomyopathy, makes control of VA with catheter ablation challenging. VT circuits are often deep in the intramural myocardium, most commonly located in the basal septum, basal inferior, and periaortic mitral continuity regions of the LV, depicted in Figs. 88.1 and 88.2 . Adjunctive novel forms of ablation may be required to effectively reach this deep substrate, including the use of lower ionic irrigant solutions, such as half-normal saline, transcoronary ethanol ablation, or stereotactic body radiation therapy.
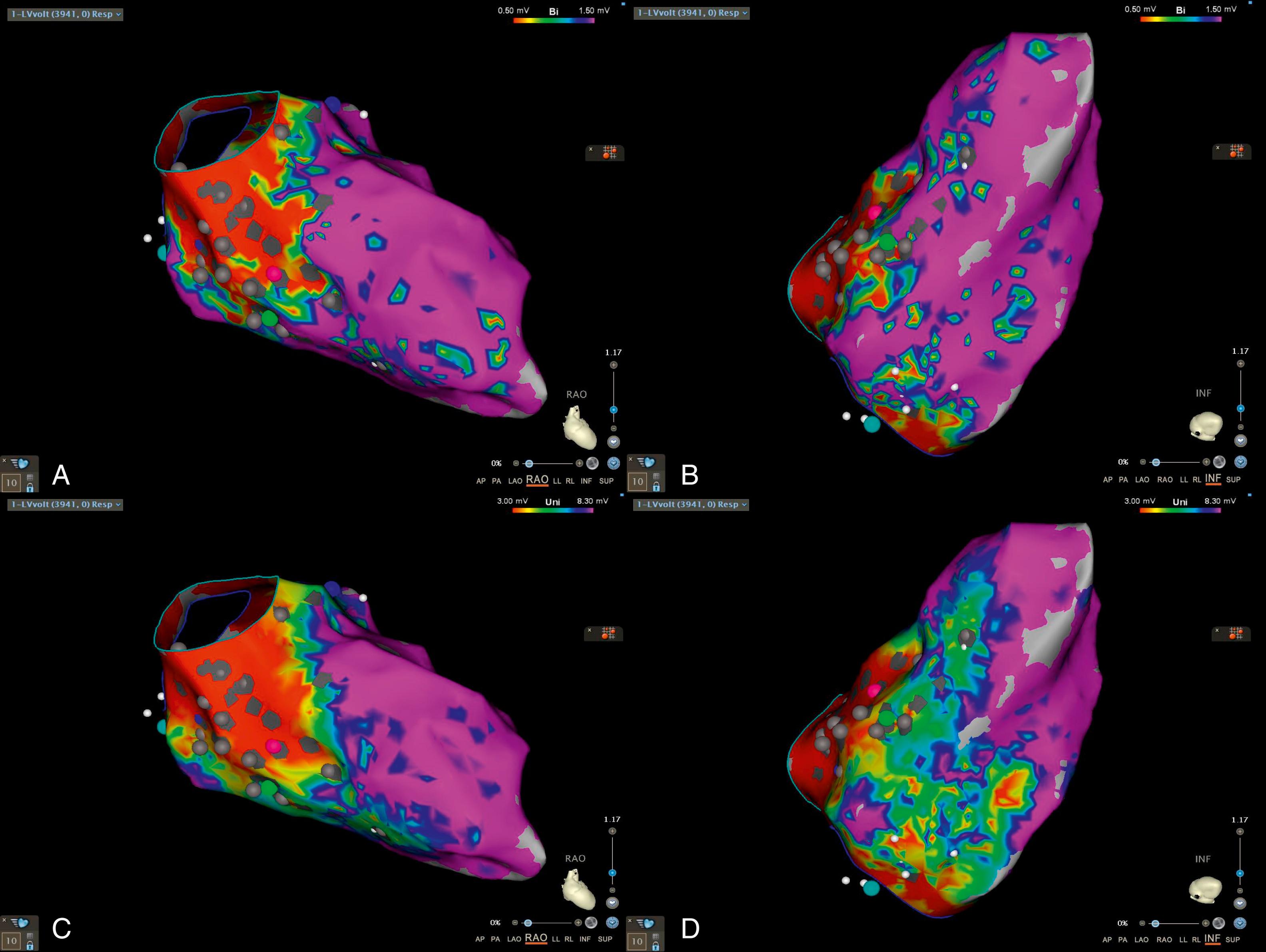
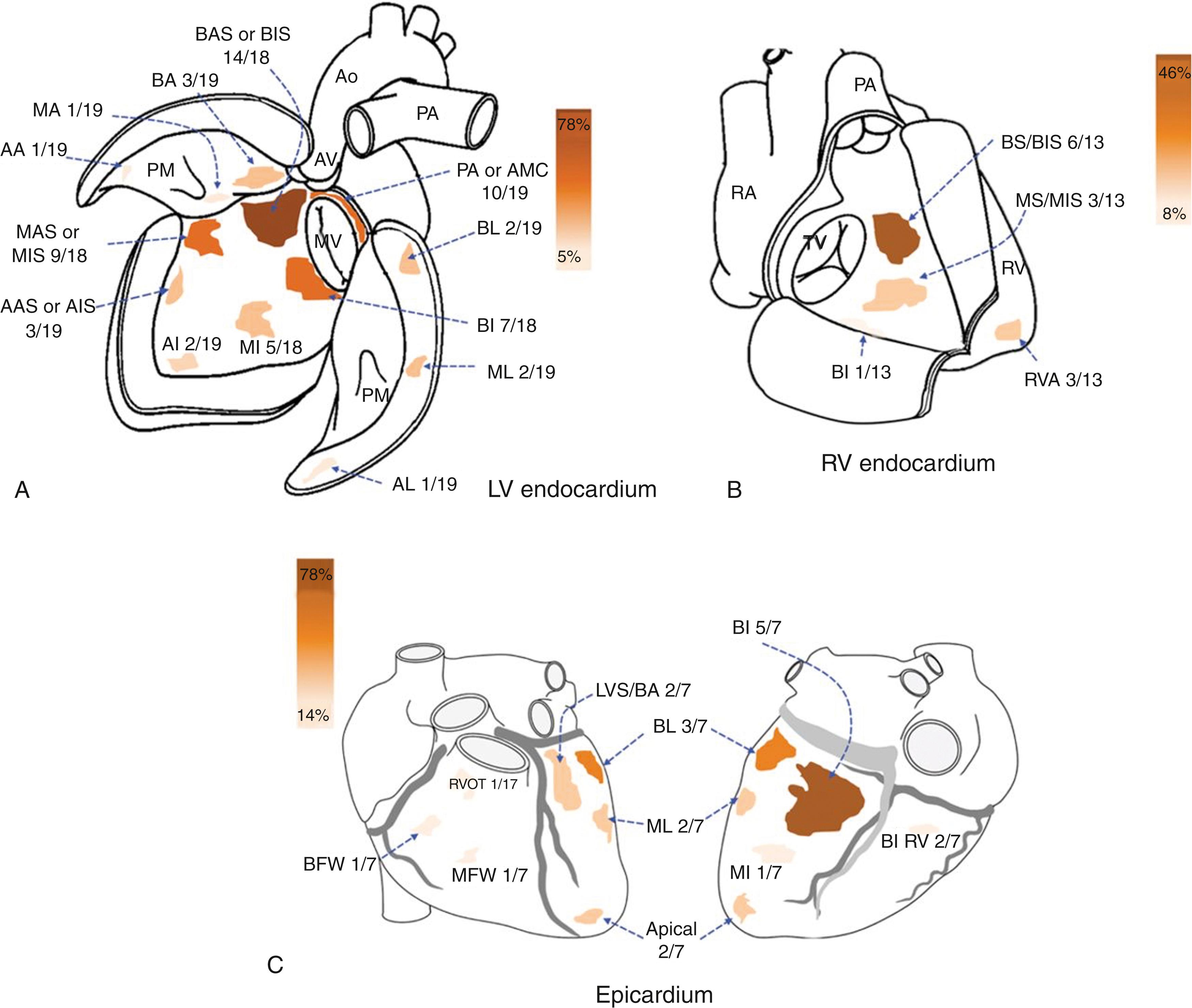
In a multicenter study, Kumar et al. found that, despite ablation in high-volume centers, alongside the use of adjunctive ablation therapies, results are generally poor. Recurrence rates were as high as 91% over a median follow-up of 7 months. From a heart failure and mortality perspective, 44% of patients had either received or were awaiting mechanical circulatory support or cardiac transplant for end-stage heart failure, and 26% of the patients had died during this 7-month median follow-up period. Ablation did achieve a reduction in median shock burden (1.5 shocks before vs. 0 shocks after, P = .1) and mean antiarrhythmic drug use (2.6 ± 1.2 before vs. 1.5 ± 0.9 after, P = .06), confirming a palliative effect of catheter ablation.
LMNA -related cardiomyopathy with VT has a poor prognosis and, unfortunately, the diagnosis is often delayed until the point when refractory VT is occurring, or at the time of postmortem. Given the potential long wait times for heart transplantation, it is important to have a low threshold for early genetic testing when LMNA -related cardiomyopathy is possible. Catheter ablation and antiarrhythmic drugs should essentially be used as a temporizing therapy until transplant.
Phospholamban-related cardiomyopathy is an inherited condition that stems from mutations in the phospholamban gene ( PLN ), resulting in dysfunction of the sarcoplasmic reticulum handling of intracellular calcium. The condition is associated with impaired cardiac contractility and VA, often with an early age of onset. Originally reported in the Netherlands in a population of patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) and DCM, patients with PLN mutation were more likely to suffer from VA and end-stage heart failure than PLN mutation-negative DCM patients. Classically, the electrocardiogram (ECG) demonstrates reduced R wave amplitude in the leads corresponding to areas of late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (CMR), along with T wave inversion, frequent premature ventricular contractions (PVCs), and late potentials on signal-averaged ECG. , In a recent Canadian study, 50 PLN mutation carriers and family members were identified across multiple inherited arrhythmia clinics. Increasing age, frequent PVCs, low amplitude QRS, T wave inversion, and late potentials were predictors of impaired cardiac function, with older patients more likely to have disease phenotype than younger patients.
Initial data suggest that phospholamban-related cardiomyopathy has a highly malignant course. In the Netherlands cohort, 47% experienced appropriate ICD therapy compared with 10% in the DCM/ARVC PLN mutation-negative patients; a family history of SCD was present in 36% of those with a PLN mutation and 16% in those without; and progression to end-stage heart failure requiring cardiac transplant was again 36% in those with versus 16% in those without a mutation. Prompt identification of affected individuals and family members through genetic screening is imperative to guide appropriate risk stratification, optimal heart failure, and VA management, along with potential transplant planning if necessary.
The SCN5A gene encodes the ion conducting α-subunit of the cardiac sodium channel. The channel allows sodium to move into the cell, resulting in the depolarization phase of the action potential and subsequent cardiac contraction. Mutations in SCN5A have been linked to long QT syndrome (see Chapter 96 ), Brugada syndrome (BrS; see Chapter 95 ), and DCM. In DCM, at least 20 mutations of SCN5A have been identified with gain or loss of SCN5A function and, in some cases, both gain and loss of function. Approximately 2% of familial DCM cases are secondary to a mutation on the SCN5A gene. Gain of SCN5A function increases sodium influx into the cell and may lead to arrhythmias through triggered activity in the repolarization phase. Loss of function can lead to slowing of conduction, bradyarrhythmias, and the facilitation of reentrant VT. Theories regarding how SCN5A mutations result in DCM include arrhythmia-induced cardiomyopathy after long-standing frequent PVCs from gain-of-function mutations, conduction defects from loss-of-function mutations, and destabilization of cellular structure through its close interaction with the cytoskeleton and intercalated discs. SCN5A -related DCM displays a similar phenotype to LMNA -related cardiomyopathy with early-onset AV or sinus node disease, VA, and progression to heart failure. Management should be focused on pharmacologic treatment of heart failure, avoidance of sodium channel blockers in most situations, identification of at-risk family members, risk stratification for SCD, and subsequent assessment for ICD therapy, as per the current guidelines previously outlined for NICM in general. Management of VA can involve amiodarone or catheter ablation for drug-refractory VT. Limited data exist regarding ablation, specifically in SCN5A -related cardiomyopathy, but both focal and reentrant mechanisms of VA occur, and these are amenable to mapping and ablation. In some patients, the sodium channel blocker hydroquinidine has been reported to successfully suppress multifocal ectopic Purkinje-related PVCs after failure of first-line antiarrhythmics and catheter ablation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here