Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
* Acknowledgments: We especially appreciate the work of the many surgeons who dedicated time and effort to collaborating with the Dartmouth Biomedical Engineering Center Implant Retrieval Laboratory by sending retrieved devices. Without their commitment to this enterprise of continuing study and improvement, none of this work would be possible. We also gratefully acknowledge the leadership and teachings of Professor John P. Collier, Director of the Dartmouth Biomedical Engineering Center (DBEC) for Orthopaedics. He has been the leader and an integral part of each investigation carried out at the DBEC laboratory over the past three decades. We also thank Dr. Michael B. Mayor for his ongoing dedication to the study and documentation of implant retrievals. The studies on which this chapter is built are combined efforts by all the faculty, researchers, and graduate students of the DBEC.
Wherever there exists relative motion between two materials in contact with one another, there will be wear. It is the inevitable outcome of the mechanical, and occasionally chemical, interaction between surface asperities on two articulating bodies. Wear in total knees is no different; debris will be generated and volume will be lost from at least one of the contacting materials. The important clinical questions therefore concern the amount of debris produced, the size of the particles, and volume loss from the bulk that is sufficient to adversely affect the macroscopic mechanical environment. Answering these questions will give an indication of the potential biologic and biomechanical implications for the patient.
It is impossible to generate a meaningful answer to these questions without first understanding the system and clinical history of wear in total knee arthroplasty. This chapter will therefore introduce the field and the context in which the current literature should be considered through general observations taken from knee wear studies. Three such observations are presented to help guide the reader in more fully appreciating the clinical wear literature:
Wear and damage can be distinct phenomena in knee devices.
Quantitative measurement of true wear in knees is challenging because good reference data are difficult to obtain, more difficult than for hips.
Design and system selection can have as much of an effect on wear as the materials selected for the bearing surfaces.
Damage and wear are terms often used interchangeably in the evaluation of tibial insert performance, but there is an important distinction in knees. Polyethylene wear is frequently cited as the cause of device failure and revision by surgeons who contribute devices to retrieval laboratories. Wear refers to the quantified loss of material from a surface and can occur through abrasion, adhesion, or fatigue. Abrasive or adhesive wear, occurring on the backside and articular surfaces of tibial inserts, results in fine particle generation. Fatigue failure (cracking or delamination) is often secondary to oxidation and can result in large amounts of material removal, potentially altering the kinematic function of the device or causing catastrophic failure. Because the clinical impact of each process (abrasion or adhesion versus fatigue) can be different, understanding and maintaining a distinction of the terms is important.
Deformation of tibial inserts by scratching and pitting, which often reflect damage but not wear, can be visually striking, yet represent only superficial surface changes in the polyethylene. This type of damage is almost ubiquitous on clinical retrievals and can result from third-body debris such as cement, residual bone fragments, or loose porous-coating beads. Scratched and pitted surfaces look damaged, but the damage may be only cosmetic ( Fig. 24.1 ).
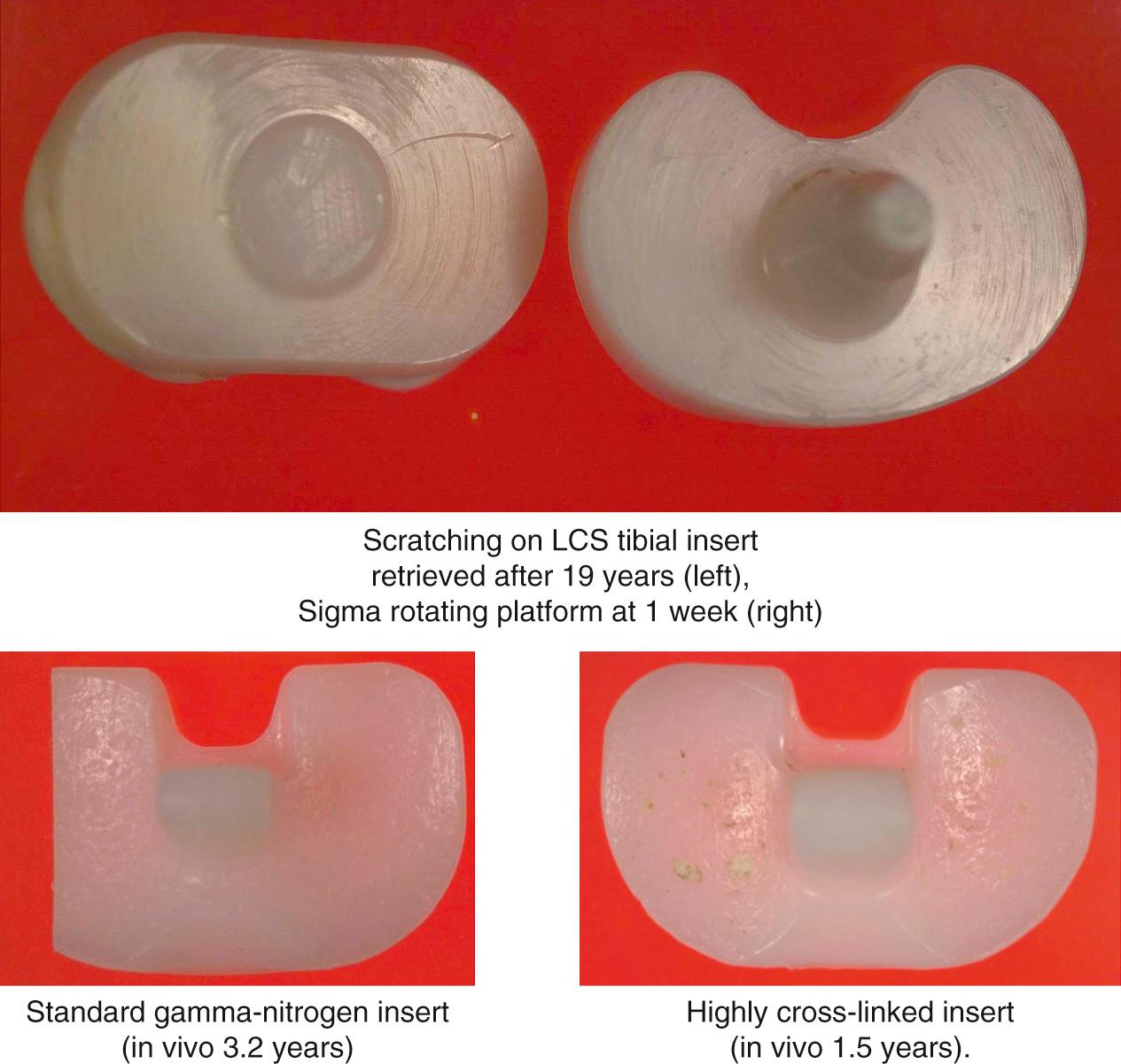
Scratched and pitted surfaces have been reproduced in laboratory simulations of the knee environment in the absence of material removal. Atwood et al. demonstrated reproducible scratching and pitting of polymer pins in an oscillating pin-on-flat experiment. Of note, the damage and characteristic skipping marks observed in retrieval studies were not only reproduced on the polyethylene pins, but clinically comparable scratching was apparent on the cobalt-chromium-molybdenum (CoCrMo) counterface. This damage occurred when bone cement and bone debris from cutting were introduced into the articulation.
The ability of a softer material to scratch a harder material is documented elsewhere in the tribology literature. In knees, the result is a scratch running in the direction of the predominant motion. It is well documented that counterface roughness and scratching can have dramatic effects on the wear rates of polymer bearings, depending on the degree of crossing motion encountered by the system. Therefore, in vivo scratching of metallic femoral components is important to monitor when evaluating tibial wear.
Fatigue failure occurs in all types of gamma-sterilized tibial inserts, regardless of design, material, or method of fabrication (molded or machined; Fig. 24.2 ). The incidence of fatigue failure increases with time in vivo as oxidation and cycles of use increase. Higher initial oxidation means fewer cycles before failure. Hence, devices that were gamma-sterilized in air failed after shorter in vivo durations if they were first stored on the shelf. More recent findings have shown that even gamma barrier–sterilized inserts eventually oxidize and therefore are subject to fatigue over the longer term ( Fig. 24.3 ).
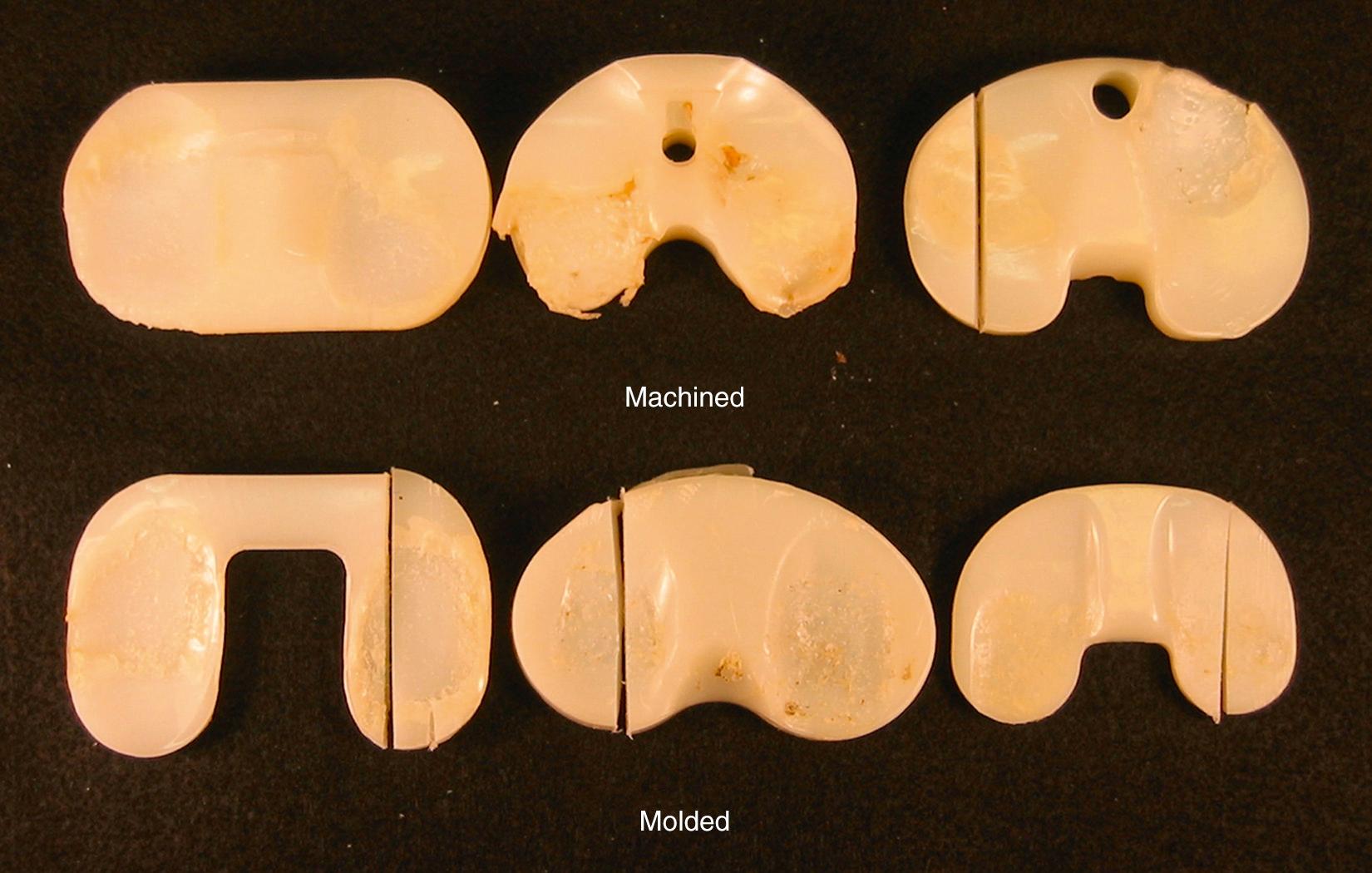
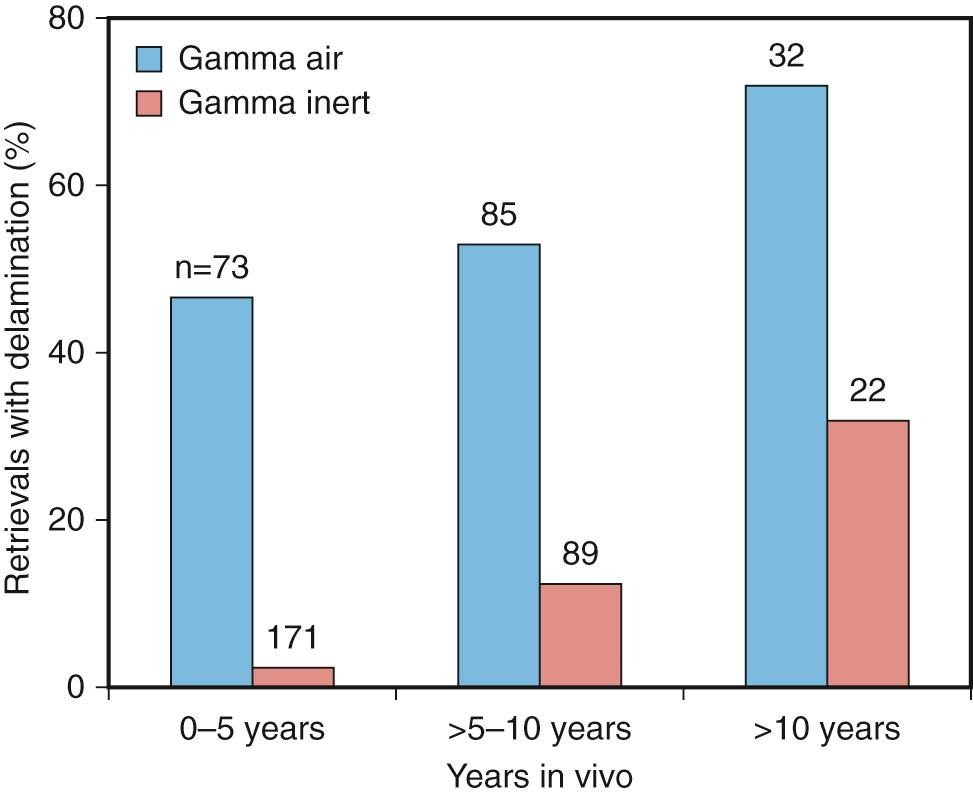
From a clinical perspective, it is difficult to predict when a device will fail because of fatigue mechanisms. Whereas most industrial fatigue life calculations only need to account for the distribution of stress and cycles over a period of time, the orthopedic engineer needs to be cognizant of the changes in material properties and the magnitude of stresses as a function of time. It is difficult, if not impossible, to gauge this for an individual patient, and fatigue failure is therefore identified as being more likely to happen once a component reaches a threshold oxidation value. Although perhaps an oversimplification of the complex chemical-mechanical system, using such threshold values has helped researchers predict failure for long-duration retrievals.
Retrieved gamma air– and gamma barrier–sterilized tibial bearings show articular surface fatigue failure. This fatigue failure is driven by the oxidation of ultra–high-molecular-weight polyethylene (UHMWPE) and the resulting decrease in mechanical properties. It is important to note that the debris released from these types of failed surfaces is not necessarily biologically active. Analyses of the functional biologic activity of wear debris have demonstrated a much stronger biologic response to debris in the submicrometer size range. Debris from a device that fails through fatigue failure is orders of magnitude larger, often several millimeters in size.
The mechanism of UHMWPE debris generation has been well characterized in the literature. † It is generally understood that oxidation, and therefore reduction of mechanical properties, happens at a faster rate below the exposed surface of a tibial bearing. This is independent of whether the material is exposed to oxygen on the shelf or to oxidizing species in the body. In most knee designs, complete conformity is avoided to allow for femoral translation and rotation relative to the tibia. As a result, the nonconforming tibiofemoral contact sets up a stress environment in which the maximum contact stress is developed below the contact surface. Although the maximum subsurface oxidation and subsurface stress are not related to each other, they exist at approximately the same depth. This coincidental colocation allows for a mechanical situation in which cracks can nucleate and propagate to the surface from a depth of about 1 mm. If the cracks propagate immediately to the surface, 1-mm-dimension polyethylene pieces will spall off the device. If the cracks first propagate parallel to the surface before turning toward the articular region, even larger debris will be generated.
† References .
Current generation cross-linked UHMWPE formulations are designed to be resistant to oxidation and thereby avoid the colocation of applied stress and degraded material that has commonly led to fatigue failure of earlier tibial bearing materials. As will be covered later in this chapter, current retrieval studies are focused on monitoring these new bearing materials to investigate the effects of long-term exposure to the in vivo environment.
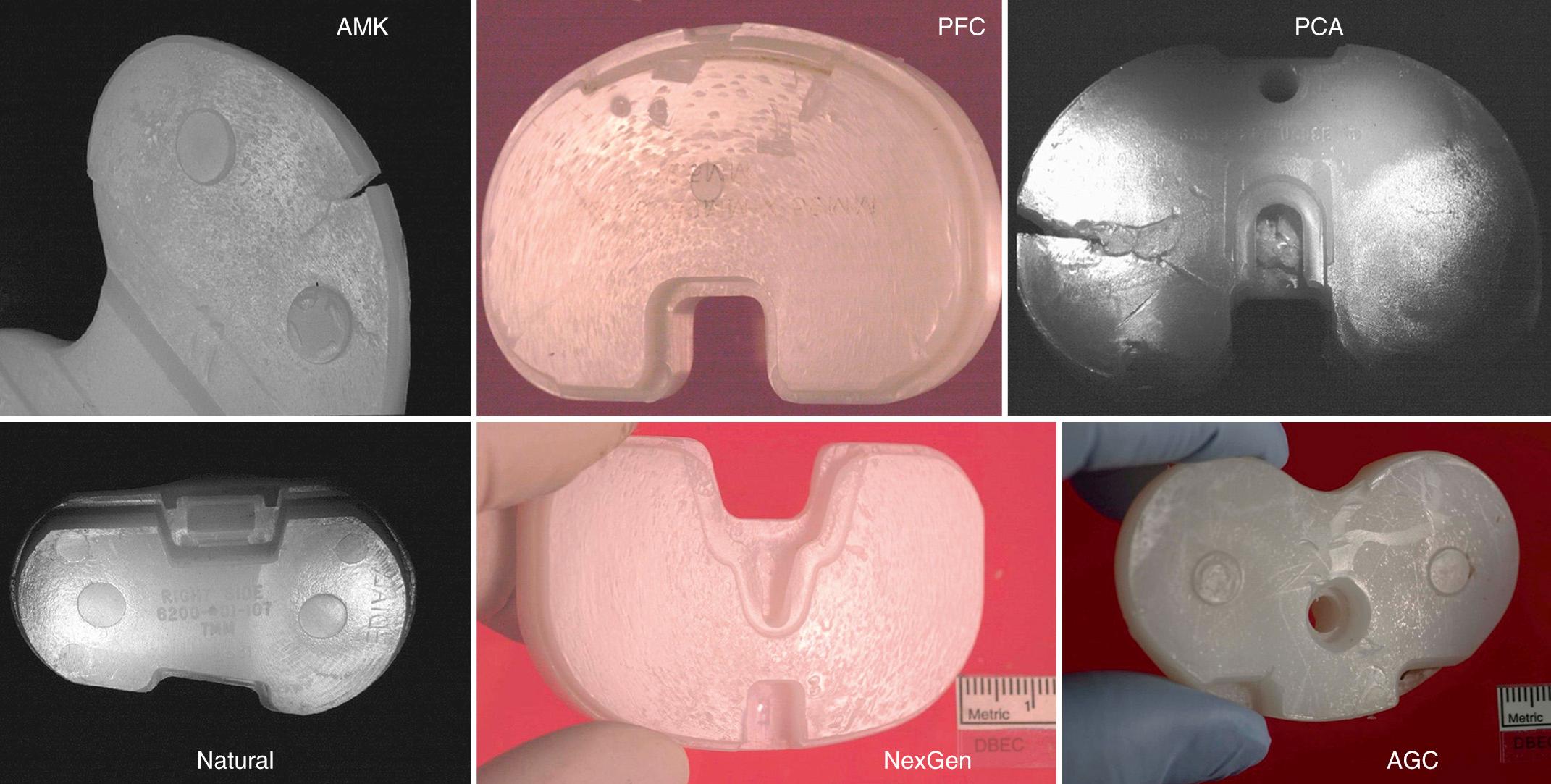
Adhesive and abrasive wear is the process whereby small particles of bearing material are generated at the contact surface and become fine debris. This fine-particle generation is driven by abrasion and adhesion rather than by oxidative degradation. Adhesive and abrasive wear is an inevitable result of all intended articulations ( Fig. 24.4 ); however, it can be easily masked by contact fatigue failure whereby large flakes of material are delaminated and removed.

Fine particulate debris from adhesive/abrasive wear has historically been associated with osteolytic response in hip arthroplasty. In hips, where adhesive/abrasive wear predominates, wear rates of 0.1 mm/year are thought to be sufficient to cause osteolysis. The principal mechanism leading to bearing failure in knees has historically been contact fatigue damage on the articular surface.
However, because contact fatigue failure is addressed by efforts to minimize oxidative degradation of the polyethylene, more attention is being focused on the backside wear of knee inserts. It has been recognized that in modular knee bearings, backside wear mechanisms of abrasion and burnishing can produce small debris particles of the size implicated as the cause of osteolysis. Other studies have documented the effect of backside wear on the locking mechanisms for different modular knee systems.
Adhesive and abrasive wear has been extensively studied over the last three decades, and a thorough discussion of the proposed mechanism is beyond the scope of this chapter. Currently the most widely accepted theory of adhesive and abrasive wear is that the stresses induced during multidirectional articulation orient the polyethylene molecules and, through strain hardening, improve wear behavior in one direction. If stresses are applied to this oriented material through crossing motion, as observed in the hip and to a lesser extent in the knee, the directionally oriented polymer wears quickly because of what is termed a strain-softening effect .
Cross-linking has been known to reduce wear in vitro and in vivo. It is theorized that the primary mechanism for this phenomenon is a reduction of the plastic deformation of the near-surface polyethylene, as well as the strengthening of intermolecular bonds. For example, Edidin et al. have shown that cross-linked polyethylene possesses a shallower plastic deformation zone. Muratoglu et al. have shown wear resistance to be highly correlated with cross-link density, and Wang has modeled the phenomenon with similar results.
Adhesive and abrasive wear is also common on the backside of fixed-bearing modular knees of many designs and types of locking mechanism ( Fig. 24.5 ). With adhesive and abrasive wear, appearances can be deceiving, and this highlights the importance of the distinction between damage and wear ( Fig. 24.6 ). It is therefore imperative that the researcher quantify material removal, rather than simply qualify the appearance of a surface.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here