Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The functional unit of the kidney is the nephron. Because glomerular filtration is the initiating phase of all nephron functions, quantitative or qualitative assessment of the glomerular filtration rate (GFR), or some variable that bears a reasonably constant relationship to it, together with assessment of the integrity of the filtration barrier, generally provide the most useful indices to assess the severity and progress of kidney damage.
This chapter will describe tests that have proved the most practical and useful for screening for and diagnosing impaired kidney function in clinical laboratories and for assessing the severity and monitoring the course and management of chronic kidney disease (CKD) and acute kidney injury (AKI). Diagnosis and classification of CKD is primarily based upon measures of GFR and proteinuria/albuminuria and of AKI upon serum creatinine and urine output. This chapter describes the contemporary use of kidney function tests to facilitate diagnosis and classification, including their biochemistry and physiology, analytical procedures, and clinical utility.
Each kidney contains between 0.4 and 1.2 million nephrons. In nearly all types of renal disease, impaired function of the kidneys is attributed to a diminished number of functioning nephrons rather than to compromised function of individual nephrons. Glomerular filtration is the initiating phase of all nephron functions. Consequently, quantitative or qualitative assessment of the glomerular filtration rate (GFR)—or some variable that bears a reasonably constant relationship to it—and assessment of the integrity of the filtration barrier generally provide the most useful indices for physicians to assess the severity and progress of kidney damage.
Defects or changes in particular functions of nephrons can also be identified and evaluated. For example, assessment of the maximum concentrating capacity of the kidneys gives an estimate of vasopressin (antidiuretic hormone or ADH)-controlled reabsorption of solute-free water in the distal portion of the tubule. Pinpoint defects, caused by genetic deficiencies of specific tubular transport systems or ion channels that give rise to characteristic biochemical disorders, are considered in Chapter 49 .
This chapter will describe tests that have proved the most practical and useful for screening and diagnosing impaired kidney function in clinical laboratories and for monitoring the course and management of progressive chronic kidney disease (CKD). In general, where blood markers are discussed, either heparinized plasma or serum can be used for most of these tests; however, the term serum is mainly used throughout this chapter. The classification systems for both CKD and acute kidney injury (AKI) are described in detail in Chapter 49 . Classification of CKD is primarily based upon measures of GFR and proteinuria/albuminuria and of AKI upon serum creatinine and urine output. This chapter describes the use of kidney function tests to facilitate diagnosis and classification, including their biochemistry and physiology, analytical procedures, and clinical utility. The chapter starts with the commonly used urine reagent strip tests, then describes the individual analytes often measured for assessment of renal function, and concludes with a description of ways to determine the GFR.
Examination of the urine is often the first step in the assessment of a patient suspected of having, or confirmed as having, deterioration in kidney function. The appearance (color and odor) of urine itself can be helpful; a darkening from the pale normal straw color indicates more concentrated urine or the presence of another pigment. Hemoglobin and myoglobin can give a pink-red-brown coloration, depending on the concentration. Turbidity in a fresh sample may indicate infection but also may be due to fat particles in a patient with nephrotic syndrome or, rarely, chyluria due to lymphatic obstruction. Excessive foaming of urine when shaken suggests proteinuria. Very unusual colors may be seen in response to treatment with some medications or in certain diseases (e.g., black urine in alkaptonuria, red urine darkening on standing in porphyria). Urine is often evaluated via point-of-care testing either using reagent strips or via microscopic examination.
Over the years, many tests of renal significance have been adapted for use on strips of cellulose, pads of cellulose, or strips of plastic that have been coated or impregnated with reagents for the analyte in question. This type of analytical test is commonly known as a “dipstick” test. A reagent strip may contain reagents for just one test per stick or reagents for multiple tests on a single stick. With this type of test, the different methods detect substances that may overflow from plasma into the urine, such as glucose, ketones, bilirubin diglucuronate, and urobilinogen, changes in the concentration of which reflect a change in organ systems in the body. These tests can also detect changes in constituents that are linked to alterations brought about directly by pathology affecting the kidney or urinary tract. These include tests for urine protein or albumin, pH, nitrite, specific gravity, and hemoglobin. Urine samples for reagent strip testing should be collected in sterile containers, and testing should be performed on the fresh urine unless delayed measurement has been validated. As with all laboratory tests, the preanalytical factors of timing of collection, patient preparation, and sample handling must be considered. Reagent strips should be used only if they have been stored properly desiccated because they can deteriorate in a matter of hours otherwise. Specific attention to timing and assessment of color change must be monitored; timing can significantly differ between analytes (e.g., from 30 seconds to 2 minutes). Reagent strips are available from a number of different manufacturers: concentrations associated with the semiquantitative results determined from the color charts supplied with the strips are not always comparable between manufacturers, which can make direct comparison of performance difficult.
In addition to manual reading of reagent strips, automated readers have come into routine use, including devices designed for use at the point of care and within the laboratory. These automated instruments may have additional features such as cell and other particle counting facilities based on flow cytometry or automated microscopy. ,
The use of an automated reader can reduce interoperator variability and improve diagnostic accuracy, as well as perform calculations based on the result (e.g., calculate a protein-to-creatinine ratio). Additionally, some automated readers provide results on a continuous scale as opposed to the semiquantitative results that are obtained from visual reading of reagent strips. The manual and automated methods use the same reagent strips (strips and readers must come from the same manufacturer); therefore the understanding of testing methodology and limitations are the same, irrespective of the reading mechanism. However, with laboratory-based analysis the stability of the sample for each of the tests must be considered. A more recent approach is the reading of the reagent strip using a smartphone application allowing home testing with standardized reading, capture, and transmission of the results.
Examples of the method principles and comments on the performance of tests pertinent to the assessment of kidney function are given in the following sections.
Proteinuria is a cardinal sign of kidney disease. Although not all patients with CKD have proteinuria, it is a common finding in those patients, and its presence suggests a poorer prognosis in a concentration-dependent manner. Use of a reagent strip assay has been considered an important initial test in any patient suspected of having renal disease. Among patients with suspected or proven CKD, including reflux nephropathy, early glomerulonephritis, and those with hypertension or previously detected asymptomatic hematuria, annual urinalysis for proteinuria has been accepted as a useful way of identifying patients at risk of progressive kidney disease. Often the findings of proteinuria and/or hematuria are incidental when urine is being subjected to testing by multi-test reagent strip devices for another purpose ( Fig. 34.1 ). Currently no proven role has been identified for reagent strip protein analysis in the screening of unselected populations. , For many years, reagent strip testing for proteinuria was deemed inadequate for the detection of CKD among patients with diabetes, in whom urinary albumin loss should be measured specifically (see later); growing consensus suggests that reagent strip testing is also inadequate in other clinical scenarios. Clinical interpretation of urinary protein measurements, including by reagent strip devices, is discussed later in this chapter.

The reagent strip test for total protein includes a cellulose test pad impregnated with tetrabromophenol blue and a citrate buffer (pH 3). The reaction is based on the “protein error of indicators” phenomenon by which certain chemical indicators demonstrate one color in the presence of protein and another in its absence. Thus tetrabromophenol blue is green in the presence of protein at pH 3 but yellow in its absence. The test has a lower detection limit of 150 to 300 mg/L, depending on the type and proportions of protein present. The reagent is most sensitive to albumin and less sensitive to globulins, Bence Jones protein, mucoproteins, and hemoglobin. Indeed, it is confusing to note that one commercial application of this principle has been manufactured as Albustix (Siemens Medical Solutions, Tarrytown, NY), although the chemical principle underlying this test is identical to those of devices that claim to measure “protein.”
A creatinine test pad (using the peroxidase-like activity of transition metal creatinine complexes) has been added to some reagent strip systems which, with the appropriate automated device, can enable a protein-to-creatinine ratio (PCR) to be reported, thereby reducing the within-subject variation seen with random urine collections. An evaluation of one such device (Multistix PRO 10LS, Siemens) read semiquantitatively on the Clinitek Status® automated strip reader (Siemens) in a renal outpatient setting concluded that the test was suitable for ruling out significant proteinuria (>300 mg/day).
Reagent strip methods are available for the more specific detection of albumin at low concentrations (i.e., in the approximate range 20 to 200 mg/L). Both colorimetric and immunologic methods for detecting albumin have been described for use with urine samples, the former based on binding of bis (3′,3″-diiodo-4′,4″-hydroxy-5′,5″-dinitrophenyl)-3,4,5,6-tetrabromosulfonephthalein (DIDNTB) at pH 1.5. Examples include the colorimetric Clinitek Microalbumin 2 reagent strips read on a Clinitek Status analyzer (both Siemens) which produces semiquantitative albumin and albumin-to-creatinine ratio (ACR) results and the immunologic Micral reagent strip (Roche Diagnostics) which provides a visual result for albumin alone. Other point-of-care devices exist that provide a similar service, although using technology other than the usual reagent strips—for example, the DCA Vantage (Siemens) and the Afinion (Abbott Laboratories, Maidenhead, Berkshire, UK) devices that are capable of reporting fully quantitative ACR results. Reasonable analytical performance has been demonstrated. Novel multiplex immunoassay approaches using complementary metal oxide semiconductor sensor technology have also been reported: outputs may be linked to a personal computer or smartphone.
Diagnostic performance has been reported in several settings : sensitivity of the Clinitek semiquantitative device for albuminuria detection does not achieve the 95% clinical sensitivity level deemed acceptable by the American Association for Clinical Chemistry and the American Diabetes Association, whereas the fully quantitative DCA device achieves this criterion. , ,
The presence of hemoglobin in the urine may be due to glomerular, tubulointerstitial, or postrenal disease, although the latter two causes are the more common. The presence of blood in the urine can be detected by using a phase contrast microscope to determine the presence of red cells in the urine sediment or by using a reagent strip test. Chemical detection of hemoglobin in urine depends on the peroxidase activity of the protein employing a peroxide substrate and an oxygen acceptor.
For this test, the reagent pad is impregnated with buffered tetramethyl benzidine (TMB) and an organic peroxide. The method depends on detection of the peroxidase activity of hemoglobin, which catalyzes the reaction of cumene hydroperoxide and TMB. The color change ranges from orange through pale to dark green, and red cells or free hemoglobin are detected together with myoglobin. Two reagent pads are used for the low hemoglobin level; if intact red cells are present, the low-level pad will have a speckled appearance, with a solid color indicating hemolyzed red cells. The presence of intact red cells is consistent with a source of bleeding in the urinary tract although the survival of intact red cells is dependent on the tonicity of the urine. Free hemoglobin can be seen with intravascular hemolysis and bleeding in the renal tract. The detection limit for free hemoglobin is 150 to 600 μg/L and for intact red cells is 5 to 20 cells/μL. The test is equally sensitive to hemoglobin and to myoglobin and thus a negative result also excludes myoglobinuria. Water must not be used as a negative control with this test because of the matrix requirements of the assay: a false-positive result will be obtained.
The presence of free hemoglobin or red cells in the urine commonly indicates the presence of renal or bladder disease. Hematuria can be present in a range of kidney diseases, including glomerular nephritis, polycystic kidney disease, sickle cell disease, vasculitis, and a range of infections. A spectrum of urologic diseases may also give rise to hematuria, including bladder, prostate, and pelvic and/or ureteral malignancy, kidney stones, trauma, bladder damage and ureteral stricture. The presence of persistent hematuria is a marker of potential CKD and risk of renal function decline. At present, reagent strips are the only routine method for identifying hematuria. While requests for urine ACR are recommended for investigation and staging of CKD, the use of reagent strips for hemoglobin in this context is less common.
Glucose appears in urine when the tubular maximal resorptive capacity is exceeded; typically this occurs at a blood glucose concentration of approximately 180 mg/dL (10 mmol/L), although this is highly variable. The test can therefore provide an indication of the presence of hyperglycemia during the time the urine was produced. However, while a positive result for urine glucose supports a diagnosis of diabetes mellitus and indicates the need for further investigation, it plays no role in the diagnostic criteria for the disease. Due to variability in the tubular resorption of both water and glucose, urine glucose cannot be used to estimate plasma glucose concentration.
For glucose measurements, the reagent pad is impregnated with glucose oxidase, peroxidase, potassium iodide, and a blue dye. The reaction employs glucose oxidase and peroxidase to produce hydrogen peroxide, which is subsequently reduced with concurrent oxidation of potassium iodide to release iodine. The free iodine blends with the background color to produce a range of colors from green to dark brown. The sensitivity of this method varies between manufacturers, operators, urine samples, and time of reading but is in the range of 18 to 72 mg/dL (1 to 4 mmol/L).
The presence of leukocyte esterase is indicative of pyuria (i.e., the presence of white blood cells in the urine; significant pyuria is normally defined as a urinary white cell count ≥10 leukocytes/mL). The detection of nitrite is indicative of the presence of bacteria that degrade nitrate excreted in the urine. The combination of the two tests is valuable in patients with urinary tract infection (UTI). The absence of both constituents is a valuable test to “rule out” UTI, thereby reducing the number of samples sent to the laboratory for additional tests. , The nitrite test may be less helpful in young children in whom the urine remains in the bladder for less time, thereby limiting the time for nitrite production.
The reagent strip test for leukocytes uses an absorbent cellulose pad impregnated with a buffered mixture of derivatized pyrrole amino acid ester and diazonium salt. Granulocytic leukocytes contain esterases, which catalyze the hydrolysis of the derivatized pyrrole amino acid ester to liberate 3-hydroxy-5-phenylpyrrole. This pyrrole then reacts with a diazonium salt to produce a purple product. The test is claimed to have a detection limit of 5 to 15 cells/μL in urine and the darkest color block is equivalent to 500 cells/μL or greater. A decrease in the true test result may occur in samples with increased glucose concentration, high specific gravity, or in the presence of cephalexin, cephalothin, tetracycline, or high concentrations of oxalic acid.
To measure nitrite, the reagent pad is impregnated with p -arsanilic acid and tetrahydro-benzo(h)quinolin-3-ol. The reaction is based on arsanilic acid in the presence of nitrite converting to a diazonium salt, which couples with the quinolol to produce a pink color. The detection limit of the test is 61 to 103 μg/dL (13 to 23 μmol/L) nitrite in urine with a normal specific gravity. The test is less sensitive with urine that has a high specific gravity. At urine ascorbic acid concentrations above approximately 25 mg/dL (1.4 mmol/L), a false-negative result may occur at low nitrite values (81 μg/dL [13 μmol/L] or less). The test measures only nitrite and is claimed to detect populations of bacteria at a level of 10 5 /mL or more.
The specific gravity test correlates with osmolality and can provide a bedside indication of the concentration of urine. The test device for specific gravity consists of an absorbent cellulose pad impregnated with bromothymol blue, poly methyl vinyl ether, and/or maleic anhydride and sodium hydroxide. The test depends on the apparent pK a change of the pretreated polyelectrolyte in relation to ionic strength; the hydrogen ions released are detected by the pH indicator. The color changes from a dark blue at low specific gravity (1.000) to yellow-green at a specific gravity of 1.030. While specific gravity performed on routine clinical samples using a laboratory analyzer shows a high correlation with urine osmolality, a urine reagent strip result, with either automated or triplicate visual reading showed only limited correlation with the laboratory test. Laboratory-based testing showed different responses to different solutes, especially with devices that detect ionic species, and led to an underestimation of specific gravity in the presence of glucose. Caution with interpretation should also be considered for urine collected after intravenous administration of iodine-containing radiopaque compounds for radiologic studies. These could give extraordinarily high values. Glucose and protein may also contribute substantial increments to the density of urine, and semiquantitative determination of these substances is necessary for valid interpretation or correction of urine specific gravity measurements. Diabetic patients with uncontrolled hyperglycemia and glucosuria may have high urine specific gravity even when the normal renal concentrating function is seriously impaired. For assessment of urine concentration, measurement of osmolality is the preferred method.
Measurement of urine pH can be helpful in the assessment of patients with renal tubular acidosis and in stone formers, although evaluation using a pH electrode may be more informative. To measure the pH of a sample, the test pad is impregnated with indicators—one example being a mixture of methyl red and bromothymol blue. Methyl red in a diluted form is red at pH values below 4.2 and yellow at values above 6.2. Bromothymol blue is yellow at pH values below 6.0 and blue at values above 7.6. At pHs within these values, the indicators give shades of orange and green, respectively. Thus the reagent blocks are compared with a color chart where the lowest pH block at 5.0 is orange in color and the highest at 8.5 is blue. It is important to recognize that the pH of urine and the color of the testing strip can change after collection; therefore careful adherence to the recommended procedure is important. It is important to be aware that reagent strip technology for urine pH carries considerable measurement variability.
Microscopic examination of the sediment obtained from the centrifugation of a fresh urine sample shows the presence of a few cells (erythrocytes, leukocytes, and cells derived from the kidney and urinary tract), casts (composed predominantly of Tamm-Horsfall glycoprotein [THG], also called uromodulin), and possibly fat or pigmented particles. An increase in red cells or casts implies hematuria; white cells or casts imply the presence of white cells in the tubules. Inflammation of the upper urinary tract may result in polymorphonuclear leukocytes and various types of casts; in lower urinary tract inflammation the casts are not present. In acute glomerulonephritis, hematuria may lead to coloration of the urine and the presence of large numbers of red cells and white cells; as the duration of the disease increases, the amount of sediment diminishes. Nephrologists will often describe urine as having either an “active urinary sediment,” or as “bland.” Having an active urinary sediment means that red blood cells, white blood cells, and casts associated with active kidney inflammation can be detected when urine is examined under the microscope.
Flow cytometry and flow imaging systems have been developed for the characterization of erythrocytes in the differential diagnosis of hematuria and as a means of improving the recognition of other particulate material in urine. This form of analysis can be used to discriminate between and quantify the particulate matter in a defined volume of urine, bringing the added benefit of better standardization of the technique. Thus the flow imaging method can identify red blood cells, white blood cells, white blood cell clumps, hyaline casts, pathologic casts, squamous epithelial cells, nonsquamous epithelial cells, yeast, crystals, and sperm. As stated earlier these have been combined with automated readers, and these systems have the potential to replace urinary microscopy because they offer better discriminatory power and quantitation while providing closer agreement between laboratories. The principles of flow cytometry are described in Chapter 28 . A flow imaging analyzer, such as the Iris iQ200 (Iris Diagnostics, Chatsworth, CA), analyzes unspun urine by aspirating sample through a flowcell positioned in a microscope. A digital camera captures the images, and neural network-based particle image recognition software is used to identify and count the particles present from five hundred 884- × 680-μm fields with 0.68-μm resolution. The number of particles for the volume scanned is then calculated. Flow image analysis has been shown to be more accurate than the flow cytometric approach while helping to improve workflow and save valuable technologist time by reducing the number of manual microscopic examinations required. , However, it has been suggested that manual microscopy may still be required for the assessment of crystals.
Higher-molecular-weight proteins are retained within the circulation by the glomerular filter, and lower-molecular-weight proteins are freely filtered, reabsorbed, and catabolized within the tubular cells. Consequently, the appearance of notable amounts of protein in the urine suggests renal disease. The association between kidney disease and proteinuria dates to at least as far back as the early 19th century, when Bright first described albuminous nephritis. While the term “proteinuria” is generally applied to the total amount of all types of proteins in the urine, it can be further classified as either glomerular or tubular, depending on the pattern of proteinuria observed (see Chapter 49 ). A third category, overflow proteinuria, is also recognized in which filtration of excessive amounts of low-molecular-weight protein exceeds the tubular capacity for reabsorption ( Table 34.1 ). Examples of the latter include Bence Jones proteinuria and myoglobinuria. Proteinuria is a potent risk marker for progressive kidney disease and reduction of protein excretion is a therapeutic target. This section considers the analytical approach and the rationale used in the quantitation of urinary proteins, with emphasis on total protein and albumin measurement.
| Type of Proteinuria | Causes | Examples of Proteins Seen |
|---|---|---|
| Glomerular | Increased glomerular permeability | Progressively increasing excretion of higher molecular weight proteins as permeability increases (e.g., albumin, IgG) |
| Tubular | Proximal tubular damage: decreased tubular reabsorptive capacity and/or release of intracellular components (e.g., due to nephrotoxic drugs) | α 1 -Microglobulin β 2 -Microglobulin Retinol binding protein Enzymuria (e.g., N -acetyl-β-d-glucosaminidase, alkaline phosphatase, α-glutathione-S-transferase) |
| Decreased nephron number: increased filtered load per nephron | As above | |
| Distal tubular damage | Tamm-Horsfall glycoprotein π-Glutathione-S-transferase |
|
| Overflow | Increased plasma concentration of relatively freely filtered protein | Bence Jones protein Lysozyme Myoglobin |
There is debate regarding the appropriate urine sample to use for the investigation of urinary protein loss. It is generally recognized that a 24-hour sample is the definitive means of demonstrating and quantifying the presence of proteinuria. However, it is widely accepted that this is a difficult procedure to control effectively, and inaccuracies in urinary collection may contribute to errors in estimation of protein losses. For most purposes, spot urine ACRs (or PCRs) are used in place of 24-hour collections. A key advantage of a spot sample is patient convenience, and therefore compliance, and avoidance of errors due to over or under collection. Overnight, first void in the morning (early morning urine [EMU]), second void in the morning, or random sample collections have also been used. An EMU sample is preferred because it correlates well with 24-hour protein loss and is unaffected by orthostatic (postural) proteinuria. However, a random urine sample is acceptable if no EMU sample is available with a low result excluding proteinuria. Because creatinine excretion in the urine is fairly constant throughout the 24-hour period, measurement of the ACR or PCR allows the use of a spot sample with correction for variations in urinary concentration in an individual but with the addition of some between-person variability due to the rate of creatinine production (see later).
Several authors have recommended use of the PCR on a spot sample based on its excellent diagnostic performance and the good correlation that has been demonstrated with the 24-hour collection. , Ginsberg and coworkers studied this issue in detail and found little variation in the ratio during the daytime, indicating that the first void and either of the two subsequent collections can give a reliable indication of the 24-hour urine protein loss. Newman and associates demonstrated a significant reduction in the within-subject variation (CV I ) in the PCR compared with the protein concentration in random urines collected throughout the day (a mean reduction from 96% to 39%). In a systematic review, random urine PCR was shown to perform better as a test for ruling out significant proteinuria than as a rule-in test; the authors suggested that positive PCR results may still require confirmation with a 24-hour collection.
A body of literature also supports the use of ACR as a suitable alternative to timed measurement of urine albumin loss. However, a recent meta-analysis of the diagnostic performance of urinary ACR and urinary albumin concentration in random urine samples from patients with diabetes, both compared against albumin loss as measured in a 24-hour collection, found no advantage to using the ACR compared to albumin concentration in the spot sample.
If required, daily albumin or protein loss (in mg) can be estimated by multiplying the ACR or PCR (measured in mg/mmol) by a factor of 10 because, although daily excretion of creatinine depends on muscle mass, an average value of 10 mmol creatinine per day can be assumed. , Some authors have suggested that adjusting ACR for variations in creatinine excretion, by taking into account the influence of age, gender, and race, improves the ability of ACR to estimate albuminuria (see later). Equations have been developed to enable estimation of ACR when only PCR is known; these may be useful when retrospectively evaluating clinical and research studies. Multiple forms of result presentation may occur due to the use of varying sample types, units, and reporting of ratios and analyte concentrations; it is essential to ensure that the correct reference interval or clinical decision point is applied to results and that there is awareness of the relevant uncertainties when using decision points from one sample type to another (e.g., when switching from EMU to a random spot urine or 24-hour sample).
The diagnosis and staging of CKD include a measurement of proteinuria and the recommended sample types are an EMU for albumin creatinine ratio (preferably) or protein creatinine ratio (if ACR not available). A timed sample and calculation of albumin or protein excretion rate is suggested if a more accurate estimate is required. Adherence to relevant guidelines is vital for correct application of the diagnostic criteria.
Establishing the diagnosis of albuminuria or proteinuria has both prognostic and management implications. In the setting of diabetes, the best possible metabolic control should be achieved before patients are examined for albuminuria, and patients should not be screened during other transitory illnesses. Screening should commence 5 years after diagnosis in patients with type 1 diabetes mellitus and at diagnosis in patients with type 2 diabetes. Patients demonstrating an ACR ≥3.0 mg/mmol b
b Readers should note that the units of expression used for ACR and PCR differ within the United States from those used outside the United States. US guidelines express albuminuria or proteinuria as mg/g creatinine, whereas other guidelines use mg/mmol creatinine. A conversion factor of 0.1136 can be used to convert ACR or PCR results in mg/g to mg/mmol (e.g., 200 mg/g = 23 mg/mmol, 30 mg/g = 3.4 mg/mmol).
should have urine samples sent to the laboratory on two other occasions (ideally within 2 months) for albumin estimation (see Fig. 34.1 ). Patients demonstrating increased ACRs in one or both of these additional samples have persistent albuminuria. This recommendation is suggested to avoid errors due to short–term clinical changes in the patient and a response to the very high CV I in urine albumin and urine protein (for more discussion on biological variability, see Chapter 8 ). It is important to consider other causes of increased albumin loss including (1) upright posture (orthostatic proteinuria), , (2) menstrual contamination, (3) vaginal discharge, (4) uncontrolled hypertension, (5) symptomatic UTI, (6) heart failure, and (7) strenuous exercise. Diabetic nephropathy is uncommon in patients who have had type 1 diabetes for less than 5 years and other causes of kidney disease should be considered.
Samples for urinary albumin (or total protein) measurement may be analyzed fresh, stored at 4 °C for up to 1 week, or stored at −70 °C for longer periods. Freezing at −20 °C appears to result in loss of measurable albumin and is not recommended. For analysis, stored samples should be allowed to reach room temperature and thoroughly mixed prior to testing.
Numerous methods can be used for the measurement of protein in urine including (1) the original Lowry method, (2) turbidimetry after mixing with trichloroacetic or sulfosalicylic acid, (3) turbidimetry with benzethonium chloride (benzyl dimethyl {2-[2-( p -1,1,3,3-tetramethyl butylphenoxy)ethoxy]ethyl} ammonium chloride, and dye binding with (4) Coomassie Brilliant Blue, (5) pyrogallol red molybdate, and (6) pyrocatecholviolet-molybdate, which is used in dry-slide applications.
Total protein measurement is more difficult in urine than in serum. The concentration of urinary protein is normally low (100 to 200 mg/L); large sample-to-sample variation in the amount and composition of proteins is common. The concentration of nonprotein potentially interfering substances is high relative to the protein concentration and is highly variable, and the inorganic ion content is high. All these factors affect the precision and accuracy of the various methods.
Concerns about different responses to different proteins have led to many variants of the published methods. Because of the need for automation, the benzethonium chloride and dye-binding methods have become the most popular in current clinical use. The analytical range of the turbidimetric assays has been of concern, with the equivalent to an “antigen excess” equivalence point being apparent when high protein concentrations give a lower signal because an inadequate amount of denaturant is present relative to protein. This limitation can be overcome by monitoring the rate of turbidity formation.
As with urine reagent strip analysis, turbidimetric and dye binding methods do not provide equal analytical specificity and sensitivity for all proteins. Most methods tend to react more strongly with albumin than with globulin and other nonalbumin proteins, , , although incorporating sodium dodecyl sulfate (SDS) in pyrogallol red reagent is claimed to reduce this issue. Additionally, between-method variation in response to low-molecular-weight proteins is quite marked, and this may contribute to differences at low total urine protein concentrations. Significant interferences include aminoglycosides, nonvisible hematuria, and infused modified gelatin solutions used as plasma expanders, all of which may falsely increase measured urinary total protein concentration.
A key difficulty with urine protein assays lies with the different analytical specificity of the assays and consequently the definition of the measurand. As different methods are responding to different properties of proteins and the abundance of these properties varies between the proteins found in urine, the “quantity intended to be measured” is not the same for different assays. In this setting, metrological traceability and standardization of the different assays is not possible. For those reasons, a reference measurement procedure is not listed by Joint Committee for Traceability in Laboratory Medicine (JCTLM), and a standardized reference material for urinary total protein is not available. The variety of methods in use means that significant between-laboratory variation is inevitable. This variation tends to diminish at higher concentrations of urinary total protein, presumably in part as albumin becomes the predominant protein and thus reduces the relative between-sample variation. In addition to the methodologic differences between various protein assays, calibration differences have been found to be one of the major determinants of intermethod variability. , , Improvements in assay comparability are possible with the use of a “urine protein” calibrator but nonlinearity of the material in different assays, and variation in sample protein composition limits this approach. These methodological problems are a major reason why urine albumin is a preferred analyte for assessment of proteinuria.
Urinary albumin has been measured using immunoassay since the 1960s when the first such assays became available. Urinary albumin is predominantly measured using quantitative immunoturbidimetric or nephelometric approaches capable of detecting albumin at low concentrations. Commercial assays typically have lower limits of quantitation of between 2 and 5 mg/L. Considerable disagreement has been reported between different commercial urinary albumin assays. Due to the wide range of concentrations that may be observed for urine albumin (greater than 100-fold) immunologic light scattering methods are falsely low due to a high-dose hook or prozone effect. Solutions to such issues include re-testing samples with high total protein concentration and the use of antigen addition protocols.
Dry chemistry systems have been developed for the quantitation of albumin in urine. , For example, in one such device, the urine albumin laterally flows along a porous matrix through an area containing gold particle-labeled antibodies to albumin. In the presence of albumin these antibody molecules are neutralized and pass through a portion of the matrix containing immobilized albumin to a detection zone, where they appear as a pink coloration. Point-of-care testing devices for urinary albumin have been discussed earlier (see “Reagent Strip [‘Dipstick’] Testing”).
As for total protein, no reference measurement procedure or higher-order reference material is currently listed on the JCTLM database. A candidate liquid chromatography tandem mass spectrometry (LC/MS-MS) reference measurement procedure has been developed. A high purity primary reference calibrator (CRM2925) has been released by the National Institute of Standards and Technology (NIST) allowing the development of a recognized traceability chain. , To date, most urinary albumin assays have been standardized against a serum-based calibrant (ERM-DA-470k/IFCC) distributed by the Institute for Reference Materials and Measurements of the European Commission, as has been recommended by Kidney Disease Improving Global Outcomes (KDIGO). In a comparison of 17 commercial assays the average bias compared with the candidate reference method was generally within ±20% at 30 mg/L and ±10% at 300 mg/L, with more recent data from the College of American Pathologists showing major methods within ±15% at 40 and 180 mg/L and a negative bias of up to 26% at 16 mg/L. The differences between results from these assays are due to a combination of calibration, nonlinearity, imprecision, and some differences in analytical specificity. However, because bias is the major error component, large improvements can be achieved by using appropriate reference materials and reference methods. Although intuitively standardization issues should be more easily addressed than those for total protein measurement, albumin is known to undergo polymerization and fragmentation on storage, when freeze-thawed, and when lyophilized. ,
There is no consistent definition of proteinuria. The upper limit of the reference interval for urinary total protein loss varies between 150 and 300 mg/day, depending on the laboratory. Given average daily creatinine excretion of about 10 mmol (1.13 g), an upper limit of normal protein loss of 150 mg/day is equivalent to a urinary PCR of approximately 15 mg/mmol (130 mg/g). The protein in the urine of healthy individuals is made up of albumin (<30 mg/day) and some smaller proteins, together with proteins secreted by the tubules, of which THG predominates (for more discussion on THG, see Chapter 49 ). Typical concentrations of proteins found in urine are listed in Table 34.2 .
| Protein | Mr (kDa) | Free Plasma Concentration (g/L) | Diameter (nm) | pI | Glomerular Sieving Coefficient | Filtered Load (mg/L) a | Urinary Concentration (mg/L) b | % Reabsorbed |
|---|---|---|---|---|---|---|---|---|
| IgG | 150 | 10 | 5.5 | 7.3 | 0.0001 | 1 | 0.1 | 99 |
| Albumin | 66 | 40 | 3.5 | 4.7 | 0.0002 | 8 | 5 | 99 |
| α 1 -Microglobulin | 31 | 0.025 | 2.9 | 4.5 | ∼0.3 | 7.5 | 5 | 99 |
| Retinol-binding protein | 22 | 0.025 | 2.1 | 4.5 | ∼0.7 | 17.5 | 0.1 | 99 |
| Cystatin C | 12.8 | 0.001 | 3.0 | 9.2 | ∼0.7 | 0.7 | 0.1 | 99 |
| β 2 -Microglobulin | 11.8 | 0.015 | 1.6 | 5.6 | 0.7 | 1.1 | 0.1 | 99 |
| β-Trace protein , | 23–30 | 0.0004 | n/a | 7.4–8.4 | n/a | n/a | n/a | n/a |
Proteinuria is often detected at the point of care using urine reagent strip devices and “clinical” proteinuria has sometimes been defined as equivalent to a color change of “+” or greater on the relevant pad on the strip. This equates to approximately 300 mg/L of total protein or a PCR of 50 mg/mmol, or protein loss of approximately 500 mg/day (assuming an average urine volume of 1.5 L/day). Indeed the limits for proteinuria and albuminuria are best described as clinical decision points because they are generally described or defined by expert groups rather than as the product of formal reference interval studies. For example, although formal reference interval studies have been performed for urine albumin and ACR, the results have been shown to be highly dependent on age, gender, blood pressure, body mass index, and triglyceride concentration. Furthermore, urine albumin and the ACR have highly skewed distributions, with the median less than 1/5th the value of the 97.5th centile, a situation where a large uncertainty would be expected in an estimate of the upper reference interval.
Among patients with diabetes, the classification of diabetic nephropathy has traditionally been based on concentrations of albuminuria (commonly expressed as an ACR). Exact definitions of albuminuria differ among national societies and by gender. For a variety of reasons that will be discussed, this concept has now been simplified and extended to nondiabetic kidney disease. In the international classification of CKD, three categories of albuminuria, expressed as an ACR, are recognized: A1 normal to mildly increased, less than 3.0 mg/mmol (approximately equivalent to <30 mg/g); A2 moderately increased 3 to 30 mg/mmol (approximately equivalent to 30 to 300 mg/g) and; A3 severely increased greater than 30 mg/mmol (approximately equivalent to ≥300 mg/g). This classification has been broadly accepted in other national guidelines, although in Australasia gender-specific cut-points of less than 2.5 mg/mmol for men and less than 3.5 mg/mmol for women have been retained. , The gender differences relate to differences in average creatinine excretion between men and women; however, these differences are small when compared against the considerable within-subject variation (see later).
The KDIGO categories are approximately equivalent to what would have formerly been considered normoalbuminuria, microalbuminuria, and macroalbuminuria (sometimes referred to as “clinical” or “significant” proteinuria), respectively. Microalbuminuria is a term that has been widely used to describe an increase in urinary albumin loss above the healthy, nondiabetic reference interval, but at a level that is not generally detectable by less sensitive clinical tests such as reagent strips designed to measure total protein. The term “microalbuminuria” is somewhat misleading, in that the albumin being measured is identical in form to that circulating in plasma, and the so-called microalbuminuric range refers to increased, not “micro-,” albumin losses. Current guidelines do not support the continuing use of this term, , and the term albuminuria will be used in this chapter.
The rate of albumin loss is affected by a variety of physiologic factors (e.g., exercise, posture, time of day), and the variability is such that reliance cannot be placed upon a single estimation. A variety of estimates of the within-person biological variability (CV I ) of urinary albumin loss have been reported, ranging from 4 to 103% (median 33%) when expressed as an ACR. In most studies the CV I for ACR was lower than other measures of albuminuria. , Howey and colleagues reported that the variability was lowest in EMU samples and could be reduced by expressing the concentration relative to creatinine concentration. It should be noted that reports of the biological variability of total proteinuria are similar to those of albumin; indeed the biological variability of ACR explains most of the variability in PCR. Recent studies of the cubilin gene suggest that genetically determined variability in the tubular handling of albumin may contribute to individual variability.
Creatinine excretion is affected by a variety of nonrenal influences including age and gender; therefore it follows that different cut-offs for ACR (and PCR) may be required in different individuals. , For example, as creatinine excretion falls with age, ACR rises in the absence of any change in the rate of albumin loss. While it has been suggested that age-related decision points could be used, the lower creatinine is itself a risk predictor and carries some of the prognostic information in the ACR ; such limits are not generally used in clinical practice. Similarly, extremes of body muscularity can affect the ACR and PCR, leading to low-range false positive results for subjects with very low muscle mass and vice versa. At the population level systematic differences in creatinine excretion with age and sex can affect prevalence estimates and at the individual level extremes of muscularity can affect diagnosis.
The physiology and pathophysiology of renal protein handling and the clinical significance of proteinuria in specific clinical situations are discussed in more detail in Chapter 49 ; more general considerations follow here. Proteinuria may be detected and measured using reagent strip devices or laboratory measurements of either total protein or albumin.
Like most analytes in urine, protein excretion displays considerable biological variability. Because standard urine reagent strips rely on estimation of protein concentration, this in turn depends on hydration or how concentrated the urine sample is. Together with the uncertainty of the measurement process itself, these tests can give only a rough indication of the presence or absence of pathologic proteinuria. Ralston and associates observed poor specificity for reagent strip analysis in detecting protein loss of 300 mg/day in a rheumatology out-patient population. A pooled analysis of six studies undertaken in obstetric patients reported positive and negative likelihood ratios for “+” protein or greater on reagent strip analysis for predicting 300 mg/day proteinuria as 0.6 (95% CI: 0.5 to 0.8) and 3.5 (1.7 to 7.3), respectively, suggesting that reagent strips are not good at ruling in or ruling out significant proteinuria during pregnancy. KDIGO and the National Institute for Health and Care Excellence (NICE) advise against the use of reagent strips to detect proteinuria. , An evidence-based systematic review undertaken by the US National Academy of Clinical Biochemistry (NACB) recommended against the use of reagent strips when undertaking proteinuria screening. Academy reviewers found no evidence that the use of urine reagent strip testing for proteinuria at the point of care improved patient outcomes; they described significant evidence of a high false-negative rate and poor negative predictive value compared with laboratory testing. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI), NACB, and NICE concur that these devices may give false-negative results when the urine is dilute. , , Most authors agree that positive tests require confirmation by laboratory measurement of the PCR or ACR on an early morning or random urine sample. A suitable protocol for the further investigation of patients found to have proteinuria at screening is given in Fig. 34.1 .
In the setting of preeclampsia, proteinuria is generally defined as greater than 300 mg/day or a PCR greater than 30 mg/mmol. A recent large study has identified ACR as the preferred test of proteinuria for preeclampsia diagnosis. However, PCR was suitable as a rule out test and ACR, along with PCR, has been acceptable in guidelines for this purpose.
Most commonly, proteinuria reflects albuminuria. Several groups have suggested that urinary total protein measurement can be replaced by urine albumin measurement. Strong evidence has linked urinary albumin loss to cardiovascular mortality and kidney disease progression in diabetes. More recently the UK Biobank study demonstrated significant positive associations of ACR with the presence of atrial fibrillation, coronary artery disease, heart failure, hemorrhagic stroke, lipid-lowering medication, and type 2 diabetes. Other evidence suggests that urinary albumin is a sensitive test to enable detection of glomerular pathology associated with some other systemic diseases including hypertension and systemic sclerosis. , In health, relatively small amounts of albumin (less than 30 mg/day) are lost in the urine. Because of this, and because total protein assays are imprecise at low concentrations, relatively large increases in urine albumin loss can occur without a significant measurable increase in urinary total protein. In a methodologic study of urine from patients attending renal transplant, general nephrology and medicine and obstetric clinics, Newman and colleagues observed increased albumin losses (defined here as ≥25 mg/day) in 63% of samples that were classified as not having increased total protein loss (defined here as ≥250 mg/day). Changes in albumin loss may also reflect overall changes in vascular permeability and therefore may not indicate explicit deterioration in renal function. Concern has been expressed that replacing urinary total protein measurement with albumin measurement may cause tubular proteinuria to be missed. , Counter to this, it is known that in most situations, tubular proteinuria is also accompanied by albuminuria and, furthermore, that an increased PCR in the presence of normal albumin loss may represent a false positive signal.
Increased urinary albumin loss has been considered a clinically important indicator of deteriorating renal function in diabetic subjects for many years. , Both European and US diabetes societies recommend regular screening of urinary albumin loss when monitoring type 1 and type 2 diabetes. , This recommendation came as a consequence of the widespread availability of sensitive assays for urinary albumin measurement and effective treatments, validated in large multinational trials, along with detailed cost-benefit analyses in the vanguard of evidence-based medicine. Because large numbers of clinical studies have been performed, guidelines for albuminuria screening in diabetes are now well established. , , , , The management of albuminuria in the setting of diabetic nephropathy is discussed in Chapter 49 .
Increased urinary albumin loss is common among the general population and is not solely attributable to the presence of diabetes. Data from the US Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance System suggested that the population prevalence of albuminuria was 10.1% in 2016, most of which was present in people without decreased GFR. Similar estimates have been reported from population surveys in Australia (6.8%), the Netherlands (7.2%), and China (9.4%). In the Netherlands study, after individuals with diabetes and hypertension were excluded, the population prevalence was 6.6%. Among patients with risk factors for CKD, prevalence was notably higher. For example, in the NHANES III study, 28.8% of people with diabetes and 16.0% of those with hypertension had albuminuria.
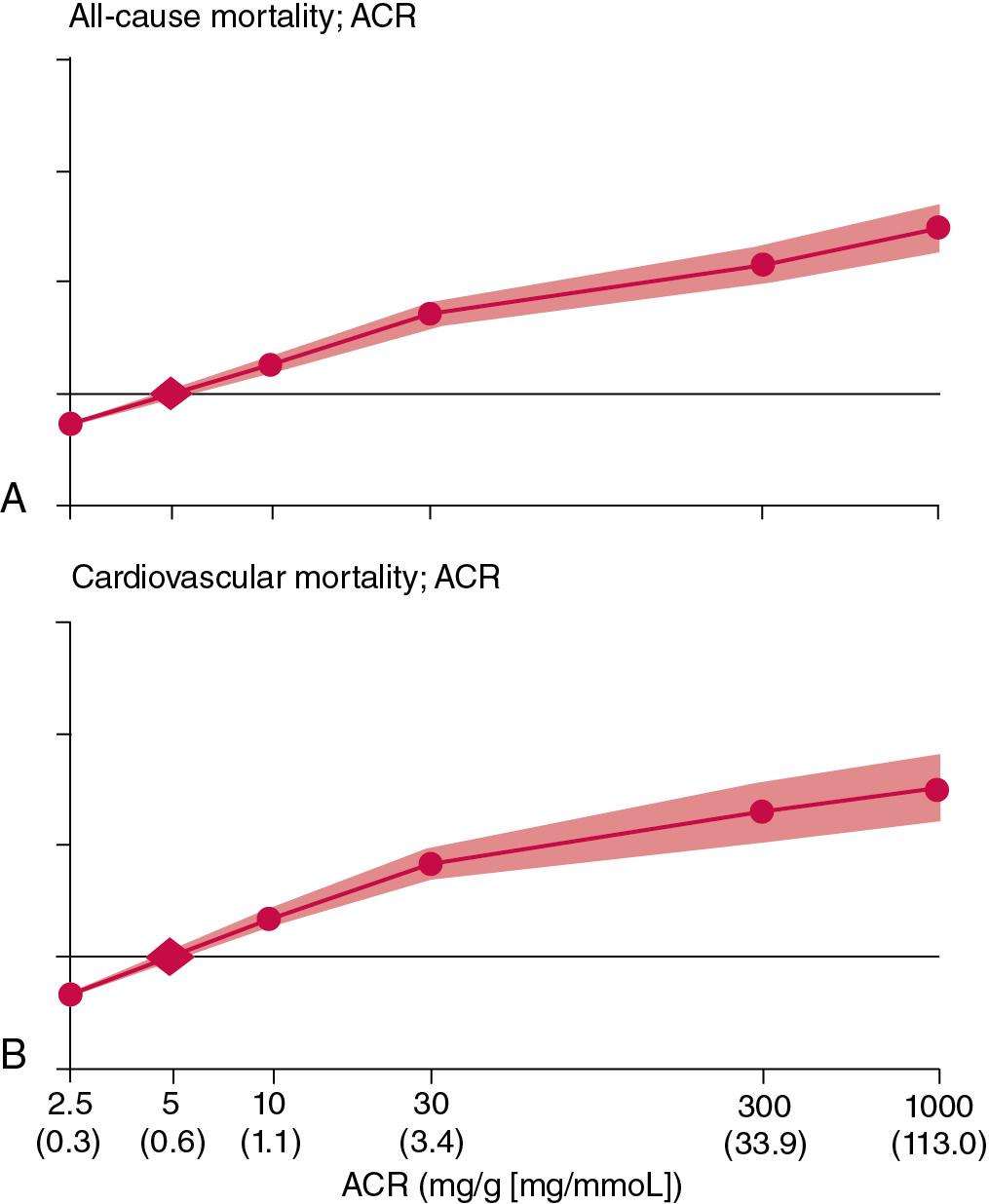
Strong evidence links albuminuria to cardiovascular and noncardiovascular morbidity and mortality in nondiabetic individuals, , including in the general population, among older people, people with kidney disease, and among others with hypertension. Albuminuria also predicts the development and progression of kidney disease, the requirement for renal replacement therapy, and the risk of AKI. Some of the most powerful data comes from meta-analyses undertaken by the CKD Prognosis Consortium demonstrating continuous associations between increasing ACR and subsequent risk of all-cause and cardiovascular mortality, kidney failure, AKI and CKD progression in the general population and in populations with increased risk for cardiovascular disease; these associations extend down to concentrations of albuminuria previously considered normal ( Fig. 34.2 ). These data have provided a powerful stimulus to the classification of kidney disease based on concentrations of albuminuria. In contrast to the situation among the diabetic population, however, little information is currently available concerning the risks and benefits of intervention (e.g., with angiotensin-converting-enzyme [ACE] inhibitors or angiotensin receptor blockers [ARBs] in albuminuric nondiabetic individuals).
The presence of immunoglobulin light chains (Bence Jones proteins, 22 kDa) in the urine is an important indication of the presence of myeloma and, in approximately 20% of cases, may occur in the absence of a paraprotein band in the serum. The pathologic significance of these proteins is considered in greater detail in Chapter 49 . A variety of tests have been used for the detection of Bence Jones protein, including the classic heat test and the Bradshaw test. To date, electrophoresis supplemented by immunofixation has been the most reliable approach. , Quantitation of Bence Jones protein excretion may be required when patients with light chain only myeloma are monitored. This has generally been achieved in timed urine collections by using a combination of electrophoresis and densitometry. Recently, immunoassays for kappa and lambda serum free light chains are playing an increasing role in this setting (see Chapter 49 ).
Myoglobin is a small (17.8 kDa), heme-containing protein normally catabolized by endocytosis and proteolysis in the proximal tubule following glomerular filtration. Typically, less than 5% of filtered protein appears in the urine. However, following rhabdomyolysis, large amounts of myoglobin are released into the plasma, saturating the tubular reabsorptive mechanism. This results in the appearance of notable quantities of myoglobin in the urine (which may color the urine red-brown). Myoglobin is directly toxic to the renal tubules and can cause acute tubular necrosis with AKI. Myoglobin will give a positive reaction with hemoglobin reagent strip tests and basic methods of detection relied on this principle following removal of hemoglobin (e.g., by ammonium sulfate precipitation or filtration with 20,000 Mr cut-off), although false-negative and false-positive results were common. Urinary myoglobin is better measured by immunochemical means, although preconcentration of urine may be required. However, for most purposes, evidence that rhabdomyolysis has occurred is better provided by an increase in serum creatine kinase activity not attributable to a cardiac source. There is no strong evidence demonstrating that urinary myoglobin measurement provides useful prognostic information in this setting.
The integrity of the renal tubule can be assessed indirectly through measurement of functional change and detection of tissue damage. The most common approach has been the measurement of urinary concentrations of low molecular weight proteins using immunoassay technology. These are normally freely filtered at the glomerulus and then are reabsorbed and catabolized within the proximal tubule. Consequently, the appearance of notable quantities of these proteins in the urine reflects failure of tubular reabsorptive mechanisms (see Table 34.1 ). There has been considerable study of urinary β 2 -microglobulin and retinol binding protein (RBP) in this context; however, both markers are hampered by pH-dependent instability. , Alpha 1 -microglobulin (Mr 31 kDa), also referred to as protein HC because of its human complex-forming capacity with IgA, is synthesized by the liver and the free form is readily filtered at the glomerulus; it is also widely used as a marker of tubular damage. For the identification of tubular damage, urinary RBP may be more sensitive than α 1 -microglobulin, but the higher concentration and excellent stability of the latter in human urine ex vivo facilitates its use as a marker of tubular damage in clinical studies. , Cystatin C, generally measured in serum as a marker of GFR (see later), can also be measured in urine as a marker of proximal tubular damage. THG, located in the thick ascending limb of the loop of Henle, has been measured in urine as a marker of more distal tubular damage, and more recently has been investigated as a predictive factor for tubular function and progression of CKD among other factors. ,
Tubular damage results in the release of intracellular components into the urinary tract, and the measurement of these components reflects the functional integrity of the tubule. A large number of enzymes have been measured in urine. N -acetyl-β- d -glucosaminidase (NAG) is stable in urine and has been used as a marker of tubular integrity in a variety of settings associated with renal injury. , However, although it is a sensitive marker of kidney damage, it has not generally been shown to provide a unique benefit over other markers of tubular proteinuria. Measurement of α - and π-glutathione-S-transferase (EC 2.5.1.18) isoenzymes has been proposed to discriminate between proximal and distal tubular damage, respectively, but the role of these markers in clinical practice has yet to be established. An evaluation of several of these biomarkers in patients presenting to hospital with nonoliguric acute tubular necrosis suggested that urinary cystatin C and α 1 -microglobulin were the best predictors of an unfavorable outcome as reflected by the requirement for renal replacement therapy.
AKI is characterized by an abrupt decline in kidney function (see Chapter 49 ). Current definitions of AKI are based upon recognition of changes in serum creatinine concentration, despite its many limitations in this setting, or reduction in urine output. The need for better biomarkers of AKI has long been appreciated, and the last few years has seen many newer biomarkers emerge as a result of proteomic discovery studies. Paramount among these have been plasma and urinary neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1). , More recently, interleukin-18 (IL-18), L- fatty acid binding protein (L-FABP), calprotectin, and the product of tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7) have been proposed as biomarkers , In contrast to serum creatinine concentration, changes in concentrations of these newer biomarkers appear to occur more rapidly (within hours) of onset of AKI. This is a rapidly expanding area and the following comments are limited to some general observations regarding NGAL and KIM-1. The reader is referred to a comprehensive review of the biochemistry and clinical performance of these markers. Renal expression of NGAL is increased following kidney injury and, in an emergency care setting, urinary NGAL has been found to be a better predictor of the presence of AKI than either urinary NAG, α 1 -microglobulin, or serum creatinine concentration. KIM-1 is a type-1 transmembrane protein that normally is not present in urine but is expressed on the proximal tubule apical membrane in response to injury. Most reports have demonstrated reasonable short- and long-term stability of NGAL and KIM-1 in urine at 4 and −80 °C respectively. , Between-day CV I estimates of urinary biomarkers in patients with CKD have been reported: NGAL (86.3%), KIM-1 (71.6%), NAG (59.5%), TIMP-2 (67.9%), and IL-18 (94.6%). Variability can be reduced by correcting for urinary creatinine concentration. Age influences the reference interval for urinary KIM-1 and both age and gender that for urinary NGAL concentration, in addition to leukocytouria and CKD. In the clinical setting, urine is not always easily obtained; measurement of plasma concentrations of several of the above biomarkers may have higher discriminative power for risk stratification and early diagnosis of AKI than urine biomarkers. , ,
As glomerular damage increases, the permeability of the membrane also increases, with an increasing proportion of higher-molecular-weight proteins appearing in the urine. The protein selectivity index expresses the relative clearance of proteins with differing Mr (e.g., transferrin and IgG) to assess the selectivity of the membrane and to provide an assessment of glomerular damage; however, this test is not widely used in clinical practice. A commercial semiautomated SDS-agarose gel electrophoresis system (Hydragel, Sebia Electrophoresis, Norcross, GA) has been introduced for qualitative analysis of urinary proteins. This separates proteins on the basis of their molecular size, enabling visualization of glomerular, tubular, and mixed patterns of proteinuria. Panels of protein measurements, including albumin, α 1 -microglobulin, IgG, and α 2 -macroglobulin, have been employed in the differential diagnosis of prerenal and postrenal disease. This general strategy was extended with the inclusion of reagent strip tests for hematuria, leukocyturia, and proteinuria in the development of an expert system achieving 98% concordance with clinical diagnosis.
Immunoassay is the preferred method for the accurate and sensitive quantitation of individual proteins (see Chapter 26 ). A variety of approaches have been used, including (1) immunodiffusion immunoassay, (2) electroimmunoassay, (3) light-scattering assay with particle enhancement, and (4) labeled immunometric assays, but light-scattering immunoassay, with turbidimetric or nephelometric detection of immunoaggregate formation, is the most popular approach.
Automated measurement of NGAL in urine and plasma (The NGAL Test, BIOPORTO Diagnostics, Denmark) has been described, and a routine method for urine on a specific automated immunoassay platform is available (Abbott Laboratories). Measurement in whole blood using a point of care testing device (Triage Biosite, Inverness Medical Innovations) is also available. Substantial intermethod variability exists between NGAL assays, most likely due to a combination of standardization and epitope-recognition issues. For example, some assays may preferentially detect the dimeric form of NGAL, which is produced by neutrophils, compared to the monomeric form resulting from renal damage. KIM-1 measurement has been described using enzyme-linked immunosorbent assay (Quantikine DKM100, R&D Systems, www.RnDSystems.com ) and electrochemiluminescent immunoassay (Meso Scale Discovery, Rockville, MD).
When considering urine marker assays for possible routine clinical use in the setting of AKI prediction, diagnosis, or staging, a key factor is rapid result availability in a cost-effective manner. Therefore assays that can be applied to routine chemistry or immunoassay platforms, or point of care devices, are a requirement.
Creatine, the immediate precursor of creatinine, is synthesized in the kidneys, liver, and pancreas by two enzymatically mediated reactions. In the first, transamidation of arginine and glycine forms guanidinoacetic acid; in the second, methylation of guanidinoacetic acid occurs with S-adenosylmethionine as the methyl donor. Creatine is then transported in blood to other organs such as muscle and brain, where it is phosphorylated to phosphocreatine, a high-energy compound.
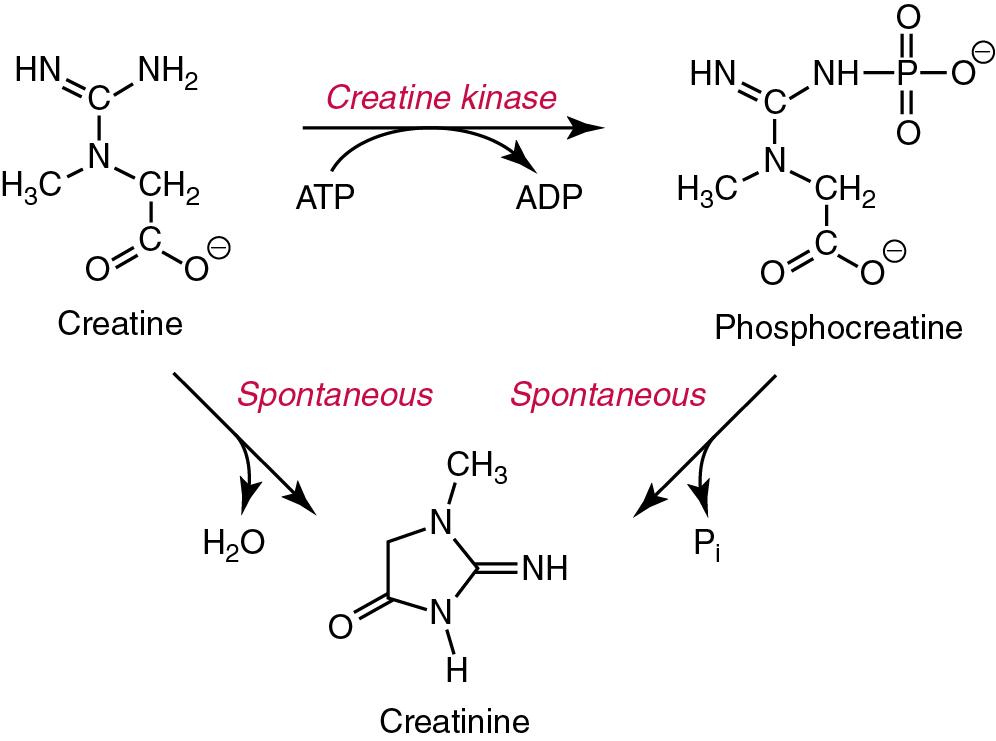
Interconversion of phosphocreatine and creatine is a particular feature of the metabolic processes of muscle contraction. A proportion of free creatine in muscle (thought to be between 1 and 2%/day) spontaneously and irreversibly converts to its anhydride waste product, creatinine. Thus the amount of creatinine produced each day in an individual is fairly constant and is related to the muscle mass. In health, the blood concentration of creatinine is also fairly constant although it may be influenced by diet (see later). Creatinine (Mr approximately 113 Da) is present in all body fluids and secretions and is freely filtered by the glomerulus. Although it is not reabsorbed to any great extent by the renal tubules, a small but notable tubular secretion is present, as well as concentration-related losses in the gut. Creatinine production also decreases as the circulating concentration of creatinine increases; several mechanisms for this have been proposed, including feedback inhibition of the production of creatine, reconversion of creatinine to creatine, and conversion to other metabolites.
Creatinine in serum, plasma, or diluted urine is stable for at least 7 days at 4 °C, and serum creatinine is stable during long-term frozen storage (at −20 °C and below) and after repeated thawing and refreezing. However, it should be noted that delayed separation (beyond 14 hours) of serum from erythrocytes leads to a significant increase in apparent serum creatinine concentration using some kinetic Jaffe (but not enzymatic) assays, possibly as the result of release of non-creatinine chromogens from the red cells (see later). , Posture, length of tourniquet application, and fist clenching are not associated with changes in creatinine measurements. Bacterial contamination, which can occur in samples stored for long periods of time, has been reported to falsely lower creatinine values measured using the Jaffe reaction, purportedly as the result of bacterial production of a substance that retards the reaction. Creatinine concentration increases in blood after meals containing cooked meat or fish, because of the conversion of creatine to creatinine. Ideally blood for serum creatinine measurement should be obtained in the fasting state. While the effect is dependent on the amount and type of meat consumed and time of sampling, the effect can be to increase the creatinine concentration by 25%, with consequent similar reduction in estimates of GFR based on the result. Use of creatine ethyl ester, a component of some nutritional supplements, has also been shown to cause an increase in serum creatinine concentration following its conversion to creatinine within the gastrointestinal tract.
Serum creatinine is measured in virtually all clinical laboratories as a test of kidney function. Most laboratories use adaptations of the same assay for measurements in both serum and urine. Both chemical and enzymatic methods are used to measure creatinine in body fluids.
Most chemical methods for measuring creatinine are based primarily on the reaction with alkaline picrate. In this reaction, first described by Jaffe in 1886, creatinine reacts with picrate ion in an alkaline medium to yield an orange-red complex.
The Jaffe reaction is not specific for creatinine. Many compounds have been reported to produce a Jaffe-like chromogen, including protein, , glucose, ascorbic acid, ketone bodies, pyruvate, guanidine, hemoglobin F, blood-substitute products, streptomycin, acetaminophen, aspirin, metamizole, and cephalosporins. The degree of interference from these compounds is dependent on the precise reaction conditions chosen. It is therefore important to be aware that different versions of the Jaffe reaction used by different manufacturers will respond in variable ways to interferences, that is, there are many Jaffe assays, with different performance characteristics (see later for more details). Among the interferences, the effects of ketones and ketoacids are probably of the greatest significance clinically, although the effect is method dependent. Acetoacetate interference varies from a negligible increase to an increase of 3.5 mg/dL (309 μmol/L) in the apparent creatinine concentration at an acetoacetate concentration of 82 mg/dL (8 mmol/L). Bilirubin is typically a negative interferent with the Jaffe reaction, although positive interference can also occur. The addition of buffering ions, such as borate and phosphate, together with surfactant, have been used to minimize the effects of bilirubin interference. A popular maneuver in this context has been the addition of ferricyanide (O’Leary method), which oxidizes bilirubin to biliverdin, hence reducing its interference. , Noncreatinine chromogens do not generally interfere with urinary creatinine measurements.
The most common approach to improving specificity of the Jaffe reaction has come from the use of a kinetic measurement approach in combination with careful choice of reactant concentrations and the time of reading the change in optical absorption. Although manual methods have traditionally been equilibrium methods, with 10 to 15 minutes allowed for color development at room temperature, kinetic assays were developed in a quest both for specificity and for faster and automated analyses. Early studies of interferences in kinetic methods identified two types of noncreatinine chromogens: those whose rates of adduct formation were very rapid in the first 20 seconds after mixing of reagent and sample (e.g., acetoacetate) and those whose rates did not become rapid until 80 to 100 seconds after mixing (e.g., protein). The “window” between 20 and 80 seconds, therefore was a period in which the rate signal being observed could be attributed predominantly to the creatinine-picrate reaction (some investigators found 60 seconds as the upper limit of this window.) Thus improved specificity in kinetic assays was achieved by selecting times for rate measurements 20 to 80 seconds after initiation of the reaction (mixing). This approach has been implemented on various automated instruments and kinetic assays are now the most widely used approach to creatinine measurement. Extensive literature exists on the choice of reactant concentrations and reading interval, and on the choice of wavelength and reaction temperature. Brief comments follow.
The Jaffe reaction is pseudo-first order with respect to picrate up to 30 mmol/L picrate, with most methods employing a concentration between 3 and 16 mmol/L. At concentrations greater than 6 mmol/L the rate of color development becomes nonlinear, so a two-point fixed interval rather than a multiple data point approach is required.
The initial rate of reaction is pseudo-first order with respect to hydroxide concentrations above 0.5 mmol/L; however, at 500 mmol/L degradation of the Jaffe complex is increased. Furthermore, at hydroxide concentrations greater than 200 mmol/L the blank absorbance increases notably.
Although the absorbance maximum of the Jaffe reaction is between 490 and 500 nm, improved method linearity and reduced blank values have been reported at other wavelengths, the choice varying with hydroxide concentration.
The rate of Jaffe complex formation and the absorptivity of the complex are temperature dependent, with measurable differences being observed even between 25 and 37 °C. Consequently, temperature control is an important component of assay reproducibility.
The effect of bilirubin on absorbance in the assay environment commences after addition of the sample and before the addition of the picrate reagent. This has been exploited by measuring the rate of change in absorbance prior to picrate addition and subtracting this from the rate of absorption after addition. Good resistance from bilirubin interference has been demonstrated using this approach.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here