Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital anomalies of the kidney and urinary tract (CAKUT) are common birth defects that account for 40%-50% of pediatric end-stage kidney disease (ESKD) worldwide. These comprise a wide range of malformations that may be sporadic or inherited. Nephron number can vary up to 10-fold in humans and, although ~40% of variation in creatinine clearance (a proxy for functioning nephrons) is due to inherited alleles, the remaining variation is unaccounted, suggesting that environmental factors, particularly gestational malnutrition, can also “program” long-term metabolic consequences. This chapter addresses the key events that specify normal human kidney development and describes structural and functional consequences of developmental errors. The diagnosis and clinical management of CAKUT is also reviewed.
The mammalian kidney is derived from intermediate mesoderm (IM), a primitive tissue that forms on either side of the aorta ( Fig. 2.1 ). Three pairs of excretory organs of increasingly advanced design develop in a craniocaudal and overlapping temporal sequence. (1) The pronephros originates on day 22 of gestation, as the mesonephric duct emerges via epithelialization of the mesoderm. Primitive tubules join this duct and disappear by day 25. (2) The mesonephros is comprised of excretory units that resemble primitive nephrons and produce small amounts of urine that contribute to the amniotic fluid. By week 16, these regress in females and give rise to elements of the genital duct system in males. (3) The metanephros (definitive kidney) initiates around day 28–32, when a single epithelial outgrowth from the mesonephric duct known as the ureteric bud (UB) invades neighboring metanephric mesenchyme (MM) (see Fig. 2.1 A). The MM is a condensation of IM-derived undifferentiated cells that release inductive molecular signals required for initial UB outgrowth and subsequent branching propagation, as well as inhibitory signals that restrict the site of UB outgrowth to a single location. In turn, signals that emanate from the UB instruct the conversion of undifferentiated MM to epithelial structures. Repetitive bi-directional inductive signals help prevent ectopic branching events and drive the UB to arborize into the collecting system. Concomitantly, the MM proliferates and differentiates to form epithelial tubules (mesenchymal-to-epithelial transition) that span from glomerulus to distal convoluted tubule. Thus, these two synchronized processes, nephrogenesis (MM-derived) and branching morphogenesis (UB-derived), shape human kidney development, a process thought to be complete by week 36.
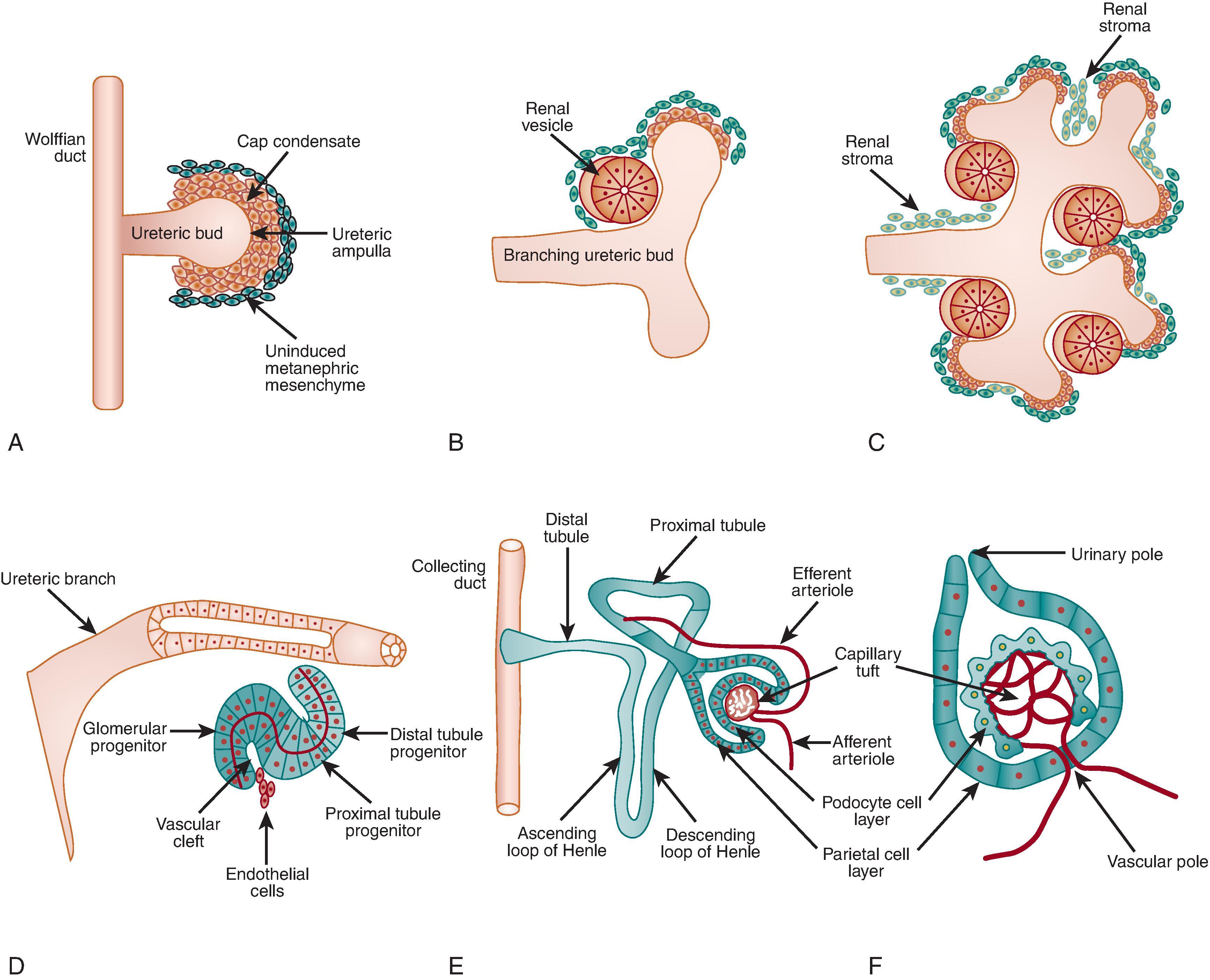
Active UB branching within the kidney’s periphery (the nephrogenic zone) ultimately gives rise to the collecting duct system. (Note that the kidney calyces, sinus, and ureter are also derived from UB epithelia). The number of repeating branching events is a key determinant of final nephron number as UB tips induce discrete subsets of MM cells to undergo nephrogenesis (see Fig. 2.1 C). In humans, branching morphogenesis is thought to be complete by week 20–22 of gestation. Thereafter, collecting duct development occurs by extension of peripheral branch segments and new nephrons form predominantly around the tips of terminal collecting duct branches.
Distinct peripheral (cortical) and central (medullary) domains of the developing kidney are established between gestational weeks 22 and 34. The kidney cortex, comprising 70% of total kidney volume at birth, becomes organized as a relatively compact, circumferential layer at the periphery, while the medulla (30% of total volume) has a modified pyramidal shape with a broad base adjacent to the cortex. These well-differentiated zones can be discerned on fetal ultrasound by week 20 to 25. The apex of the cone is formed by convergence of collecting ducts in the inner medulla known as the papilla. Distinct morphologic differences also emerge between medullary and cortical collecting ducts. The former organize into elongated, relatively unbranched linear arrays, which converge centrally in a region devoid of glomeruli. In contrast, developing cortical collecting ducts continue to induce MM at the nephrogenic zone. The most central segments of the collecting duct system, formed from the first several generations of UB branching, undergo remodeling by combination of growth, apoptosis, and tubule dilatation to form the pelvis and calyces.
As above, the UB arises from the mesonephric duct in response to MM-derived inductive signals. Failure of UB induction results in kidney agenesis, while erroneous outgrowth of multiple UBs can result in kidney malformations including collecting system and/or ureter duplication. The precise position at which the UB is induced is critical to the nature of UB-MM reciprocal interactions, and ectopic positioning can be associated with malformations (kidney dysplasia) or compromise the integrity of the ureterovesical junction.
Signals transmitted from the UB induce adjacent MM to undergo a mesenchymal-to-epithelial transformation (MET). Initially, mesenchyme cells condense around the ampulla of the advancing UB (see Fig. 2.1 A), then form an oval mass called a pretubular aggregate (see Fig. 2.1 B). An internal cavity forms within this developing renal vesicle, which is comprised of multipotent precursors that give rise to all of the nephron epithelial cell types. Nephron segmentation into glomerular and tubular domains is initiated by the sequential formation of two clefts in the renal vesicle. The lower ( vascular ) cleft gives way to the comma-shaped body and subsequent generation of an upper cleft leads to formation of an S-shaped body, which is characterized by three segments or limbs (see Fig. 2.1 D). The middle limb gives rise to the proximal convoluted tubule and the upper limb to the descending and ascending limbs of the loops of Henle and the distal convoluted tubule.
Formation of the glomerulus begins as the vascular cleft broadens while the lower limb of the S-shaped body forms a cup-shaped structure (see Fig. 2.1 D and F). Epithelial cells lining the inner wall of this cup will comprise the visceral glomerular epithelium, or podocyte layer. Cells lining the outer wall of the cup will form the parietal glomerular epithelium that lines the Bowman capsule (see Fig. 2.1 F). The glomerular capillary tuft is formed via recruitment and proliferation of endothelial and mesangial cell precursors. Recruitment of angioblasts and mesangial precursors into the vascular cleft form a primitive vascular plexus (see Fig. 2.1 E). Podocytes of these capillary loop stage glomeruli lose mitotic capacity and begin to form actin-based cytoplasmic extensions, or foot processes, and specialized intercellular junctions, termed slit diaphragms. Subsequent development of the glomerular capillary tuft involves a poorly defined sequence of capillary branching and formation of endothelial fenestrae. Mesangial cells, in turn, populate the core of the tuft and provide structural support to capillary loops via deposition of extracellular matrix. The full complement of glomeruli in the fetal human kidney is attained by 32–36 weeks. It should be kept in mind that the process of nephrogenesis at each UB tip occurs approximately 1 million times in each kidney. Moreover, the requirement for reciprocal induction implies that signaling defects in either compartment can have pleiotropic effects across the entire urinary tract.
Urinary tract malformations are classified under the overall term C ongenital A nomalies of the K idney and U rinary T ract (CAKUT). These malformations are the most frequently detected abnormalities during intrauterine life (0.1 to 0.7 pregnancies) and are the major cause of childhood kidney failure. In 30% of affected patients, CAKUT occur in combination with nonkidney malformations as part of a genetic syndrome. Over 200 distinct syndromes feature some type of kidney and urinary tract malformation ( Table 2.1 ).
| Syndromes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Chromosomal Abnormalities |
|
|
|
|
|
|
|
| Metabolic Disorders |
|
|
|
|
|
CAKUT represent a spectrum of developmental malformations including:
Aplasia (agenesis): congenital absence of kidney tissue
Simple hypoplasia: kidney length > 2 standard deviations below the mean for age with reduced nephron number but normal kidney architecture
Dysplasia ± cysts: malformation of tissue elements
Isolated dilatation of the kidney pelvis ± ureters (collecting system)
Anomalies of position including ectopic and fused (horseshoe) kidney
These malformations may be unilateral or bilateral and are associated with structural abnormalities of the lower urinary tract in 50% of affected patients. These ureteral abnormalities include vesicoureteral reflux (VUR) (25% of cases), ureteropelvic junction obstruction (11%), and ureterovesical junction obstruction (11%). Kidney dysplasia is a polymorphic disorder characterized at the microscopic level by abnormal differentiation of mesenchymal and epithelial elements, decreased nephron number, loss of the demarcating zone between the cortex and the medulla, and metaplastic transformation of mesenchyme to cartilage and bone. Dysplastic kidneys range in size from large, distended kidneys with multiple large cysts to small kidneys, with or without cysts. A small dysplastic kidney without macroscopic cysts, imaged by ultrasound, is classified as hypoplastic/dysplastic in the absence of a pathologic examination, which distinguishes between simple hypoplasia and dysplasia. The multicystic dysplastic kidney (MCDK) is an extreme form of kidney dysplasia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here