Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Idiopathic inflammatory myopathies (IIM) are a heterogenous group of diseases characterized by muscle weakness (which is often more pronounced proximally) and inflammatory changes in skeletal muscle. IIM comprise different subtypes: dermatomyositis, polymyositis, inclusion body myositis, and amyopathic dermatomyositis. Juvenile dermatomyositis (JDM) accounts for 80% of all children with IIM. It typically affects skin and muscle; however, disease manifestations are variable and can involve other organ systems.
JDM occurs in all regions in the world. The estimated incidence rates of JDM in the United States from 1995 to 1998 ranged from 2.5 to 4.1 cases per million children per year. The retrospective surveys conducted in Sweden and the United Kingdom and Ireland demonstrated 2.4 and 1.9 cases per million children per year, respectively. , In the United States the annual incidence rates are similar in white and African American non-Hispanics, whereas Hispanics appear to have a lower incidence rate.
Non-Hispanic whites were most commonly represented in a US register (65.1%), followed by Hispanics (14.2%), African Americans (11.4%), and Asian or Pacific Islanders (1.3%). These findings are similar to the National Registry study in the United Kingdom and Ireland, which revealed a predominance in white (83%), followed by black (8%) and Asian (7%) patients.
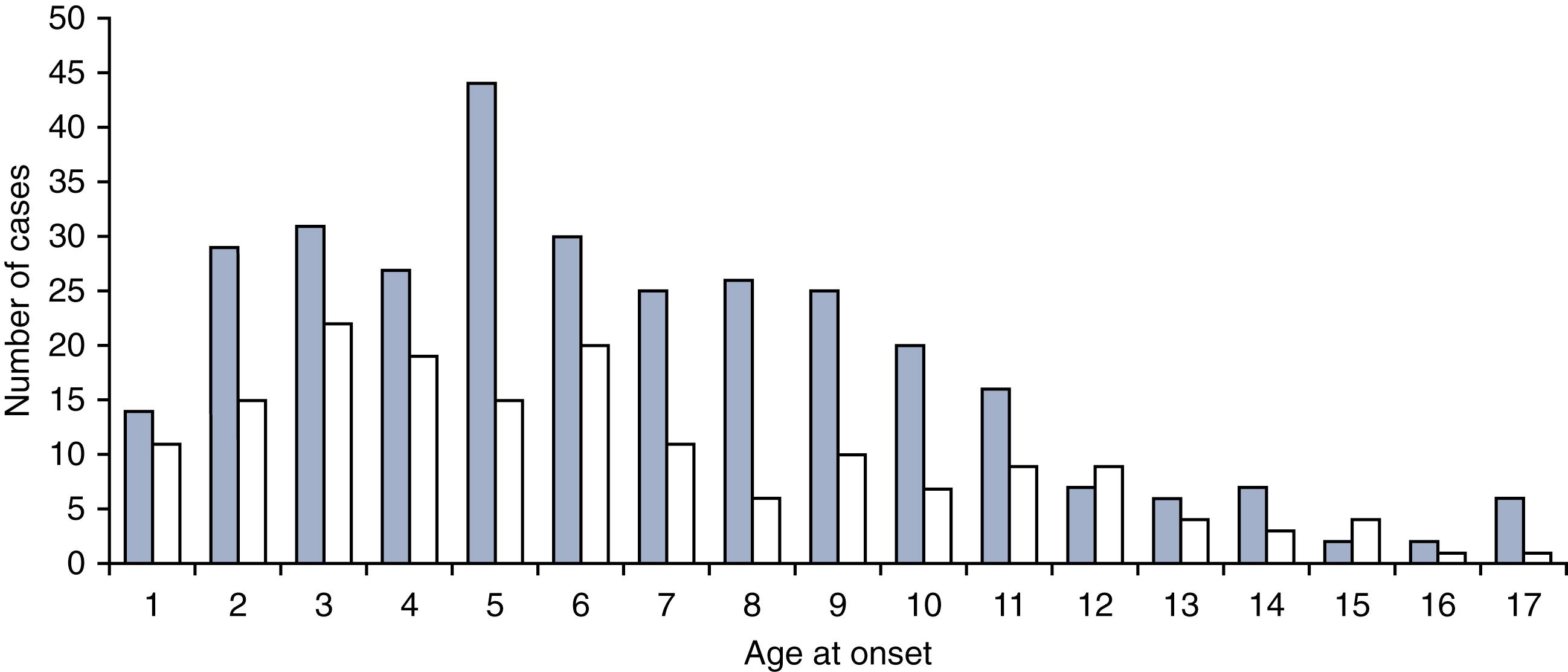
The peak age at onset of JDM is approximately 7 years ( Fig. 26.1 ). JDM is more common in girls than boys, with a ratio of 1.6:1 to 2.5:1. , ,
The etiology of JDM remains unclear. However, it appears that JDM is the result of an autoimmune reaction in genetically susceptible individuals triggered by environmental factors.
There are reports that certain human leukocyte antigen (HLA) alleles are associated with JDM and contribute to risk of the disease. HLA DRB1∗0301 allele has been found to potentially be a major immunogenetic risk factor, whereas DQA1∗0301 and DQA1∗0501 alleles are additional risk factors for JDM in Caucasian patients. These alleles are similarly found in adult dermatomyositis patients with myositis-specific or associated autoantibodies, as part of the 8.1 ancestral haplotype. , HLA DQA1∗0202, DQA1∗0101, and DQA1∗0102 alleles are identified as protective factors, found significantly less frequently in Caucasian patients with JDM compared with controls. ,
In addition to HLA alleles, non–HLA-associated genes, including genetic polymorphisms in tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1 receptor antagonist, have been implicated in pathogenesis of JDM. The TNF-α-308A and TNF-α-238GG alleles have been identified more frequently in patients with JDM compared with controls, , and TNF-α-308A has been associated with a prolonged disease course (≥36 months) and calcification, although how much this effect is driven by linkage to major histocompatibility complex (MHC) alleles within the HLA region remains controversial. Patients with TNF-α-308A synthesize more TNF-α, which may be associated with persistent immune activation contributing to a longer disease course. The TNF-α-308A allele is associated with small-vessel occlusion and increased concentration of circulating thrombospondin-1 (TSP-1), a mediator enhancing proliferation and migration of vascular smooth muscle cells. , The IL-1 receptor antagonist gene, A1 allele, is associated with increased proinflammatory activity and is a risk factor for the development of juvenile IIM in Caucasians, but not in African Americans, in whom the A3 allele is a possible risk factor.
Several environmental factors have been reported to be related to disease flare in patients with dermatomyositis. An online survey of adults and children with dermatomyositis from the United States and Canada examined environmental factors contributing to disease exacerbation. This survey demonstrated that patients with flares more often report unusual sun exposure and nonsteroidal antiinflammatory drug (NSAID) use within the preceding 6 months compared with those without flare. Patients who flared were more likely to have used antihypertensive medicines and psychiatric medications and to have received the human papillomavirus vaccine within 6 months of the flare.
It has been proposed that infection is a trigger for initiation of JDM. A study, in which parents of children with newly diagnosed JDM were interviewed, demonstrated that more than half of patients had a history of respiratory symptoms, and 30% of patients had gastrointestinal complaints, in the 3 months preceding disease onset. This study did not determine specific infectious agents, however infections with parvovirus B19, coxsackievirus B, enterovirus, hepatitis, influenza, group A streptococcus, Borrelia burgdorferi, and toxoplasma have all been documented in association with JDM.
Both adaptive and innate immune systems have been hypothesized as contributing to the pathogenesis of IIM. In many ways, immunologically, JDM looks like the body’s ineffective attempt to clear a chronic viral infection.
Plasmacytoid dendritic cells appear to play a key role in the pathogenesis. Plasmacytoid dendritic cells circulate in peripheral blood, and upon activation by viral infection, which causes upregulation of CCR7, they migrate to lymphoid organs. These cells recognize viruses through Toll-like receptor (TLR)9 and, subsequently, initiate an immune response. These processes induce the maturation of dendritic cells, causing activation of T cells by delivering signals through T-cell receptors and costimulatory molecules. Plasmacytoid dendritic cells are considered to be the main type 1 interferon (IFN)-producing cells.
Type 1 IFN pathway dysregulation has been implicated in the pathogenesis of JDM. Normally, type 1 IFN upregulates MHC class I and has an immunomodulatory function by activating the cytotoxic effects of natural killer cells, promoting activated T-cell survival, and supporting dendritic cell maturation. , Overexpression of MHC class I might cause activation of the endoplasmic reticulum stress response leading to muscle fiber injury. Type 1 IFN also induces proinflammatory cytokines and chemokines, including CXCL9/MIG, CXCL10, and CXCL11/I-TAC. In addition, type 1 IFN enhances autoantibody production and B-cell proliferation. Endothelial cells in the affected muscle produce IL-1 and other cytokines and promote inflammation through upregulation of vascular cell adhesion molecule (VCAM)-1 and endothelial intracellular adhesion molecule (ICAM)-1. Affected endothelial cells express various cytokine binding sites, leading to attraction of inflammatory cells and angiogenesis.
The presence of a gene signature characteristic of type 1 IFN pathway activation was identified in affected muscle tissue and later found in peripheral blood cells from patients with dermatomyositis. Serum IFN-α activity in patients with JDM is higher than healthy controls. Serum IFN-α activity is highest in newly diagnosed patients with a short duration of untreated disease, suggesting that IFN-α may be important in the early phase of disease. A type 1 IFN signature was found to be associated with disease activity and may be a useful marker for disease activity monitoring. , ,
Type 2 IFN (IFN-γ) may also be involved in the pathogenesis of JDM. It is predominantly produced by natural killer cells and activated T cells. Upregulated type 2 IFN and expression levels of type 2 IFN-inducible genes were found to be higher in muscle biopsies of JDM patients compared with muscular dystrophy patients and healthy controls.
Maternal chimerism may contribute to JDM. Maternal cells transfer to the fetus through the placenta and persist for decades after birth. These maternal cells can be benign or may cause autoimmunity in the child. There appears to be increased chimerism in peripheral blood mononuclear cells and muscle tissues in JDM patients compared with either population controls or healthy siblings. ,
The clinical presentation of JDM is variable. Almost all patients with JDM have at least some degree of muscle weakness. JDM can present with an acute or insidious onset; the median duration from symptom onset to diagnosis is about 3 to 7 months. , , , , , Table 26.1 shows the frequency of common clinical features at disease presentation and over time.
| Clinical Features | At Presentation | Over Time |
|---|---|---|
| Constitutional Symptoms and Signs | ||
| Fatigue | 82%–100% | 86%–100% |
| Fever | 21%–53% | Not applicable |
| Weight loss | 20%–61% | 36%–65% |
| Lymphadenopathy | 23%–25% | 21%–26% |
| Musculoskeletal Disease | ||
| Muscle weakness | 85%–96% | 98%–100% |
| Muscle pain or tenderness | 48%–75% | 52%–61% |
| Arthritis | 26%–45% | 28%–43% |
| Mucocutaneous Disease | ||
| Heliotrope rash | 62%–84% | 69%–80% |
| Gottron papules | 59%–91% | 76%–93% |
| Photosensitive rash | 51% | 43% |
| Malar rash or facial erythema | 34%–79% | 66%–71% |
| Periungual nailfold capillary changes | 21%–68% | 41%–100% |
| V sign or shawl sign | 5% | 5%–25% |
| Raynaud phenomenon | 5%–28% | 13%–24% |
| Cutaneous ulceration | 6%–23% | 24%–44% |
| Calcinosis | 0%–11% | 15%–44% |
| Lipodystrophy | 4% | 3%–10% |
| Cardiopulmonary Involvement | ||
| Cardiac involvement | 2.5%–25% | 2%–22% |
| Lung involvement | 8%–12% | 12%–17% |
| Gastrointestinal Disease | ||
| Dysphagia or dysphonia | 11%–25% | 8%–25% |
| Gastrointestinal symptoms | 8%–25% | 3%–20% |
Fever, fatigue, anorexia, and weight loss may be seen in patients with JDM (but are not universal) and may depend on the severity of the presentation. Some patients have lymphadenopathy, which is nonspecific.
Typically, the muscle weakness is symmetrical and more severely affects the limb-girdle musculature, anterior neck flexors, and trunk muscles. Patients may have difficulty climbing stairs, getting out of bed, raising their arms above their head, or combing their hair. Some patients complain of pain in their muscles. Physical examination demonstrates symmetrical muscle weakness, predominantly in the proximal muscle groups. Affected children may be unable to sit up from the supine position, rise from a seated position without the use of arms, or perform a deep knee bend. Patients may have a positive Gower sign, reflecting proximal lower extremity weakness. The Trendelenburg sign indicates gluteal muscle weakness. It is not uncommon to have muscle tenderness and edema of the overlying subcutaneous tissues of the affected muscles.
In severe presentations, patients have generalized weakness, affecting both proximal and distal muscles. Palatal and pharyngeal muscles become involved, causing dysphagia, dysphonia, aspiration, and reflux of food through the nose. Respiratory muscle weakness may occur and lead to respiratory failure. Patients who present with muscle weakness, accompanied by difficulty swallowing, nasal voice, tachypnea, or shortness of breath should be monitored closely for respiratory failure and for (potentially fatal) aspiration. Later in the disease, muscle atrophy may develop.
As a result of muscle weakness, patients with JDM frequently have impairments in their maximal aerobic and anaerobic exercise capacity. , Anaerobic exercise capacity is reduced almost 30% on average, compared with healthy children.
Patients with JDM may have arthralgia or arthritis; arthritis—commonly associated with early disease—may present long before the onset of rash or weakness or late in the course of disease. Oligo- or polyarthritis may be seen. The arthritis is nonerosive and nondeforming. Tenosynovitis can be found in JDM.
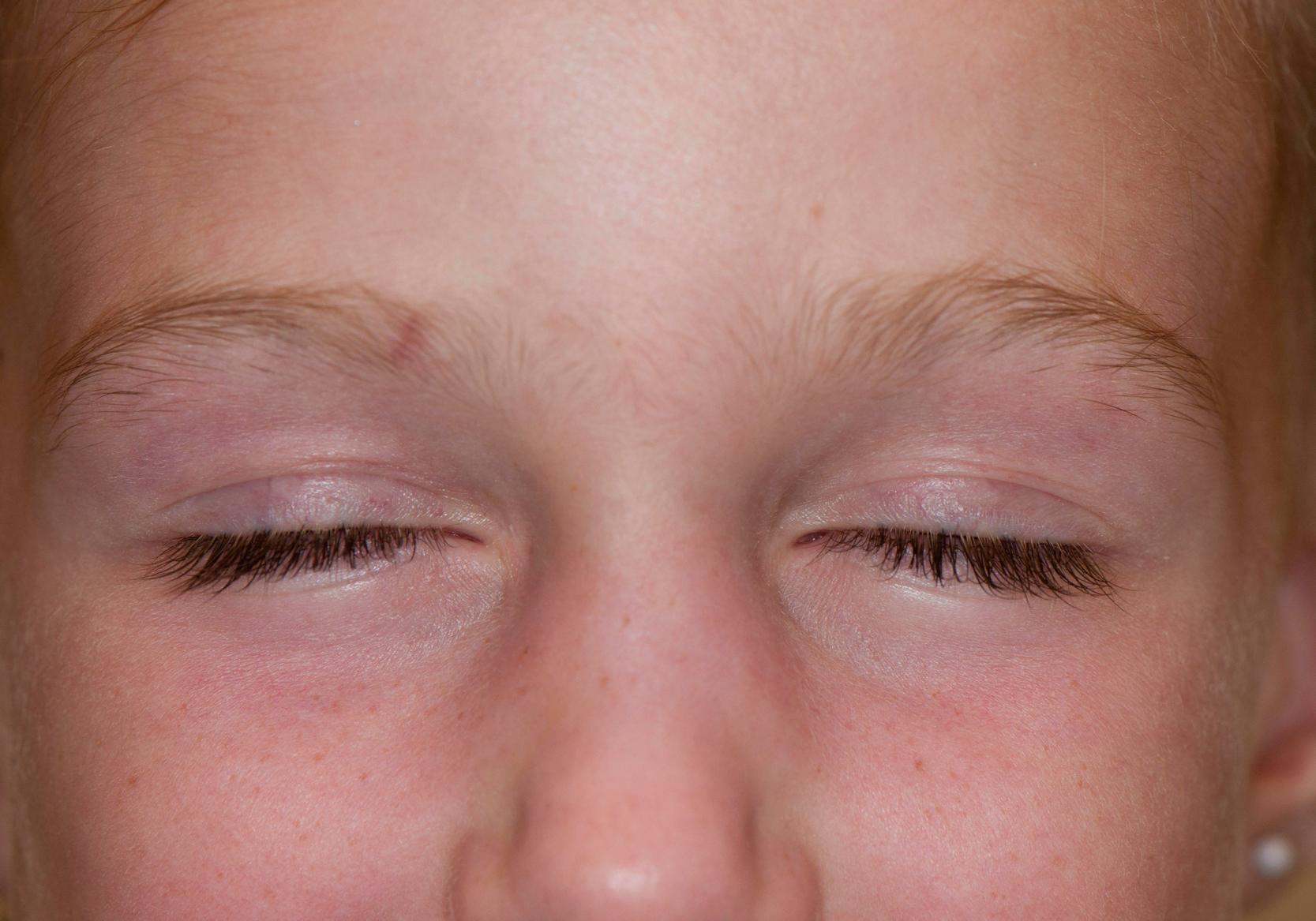
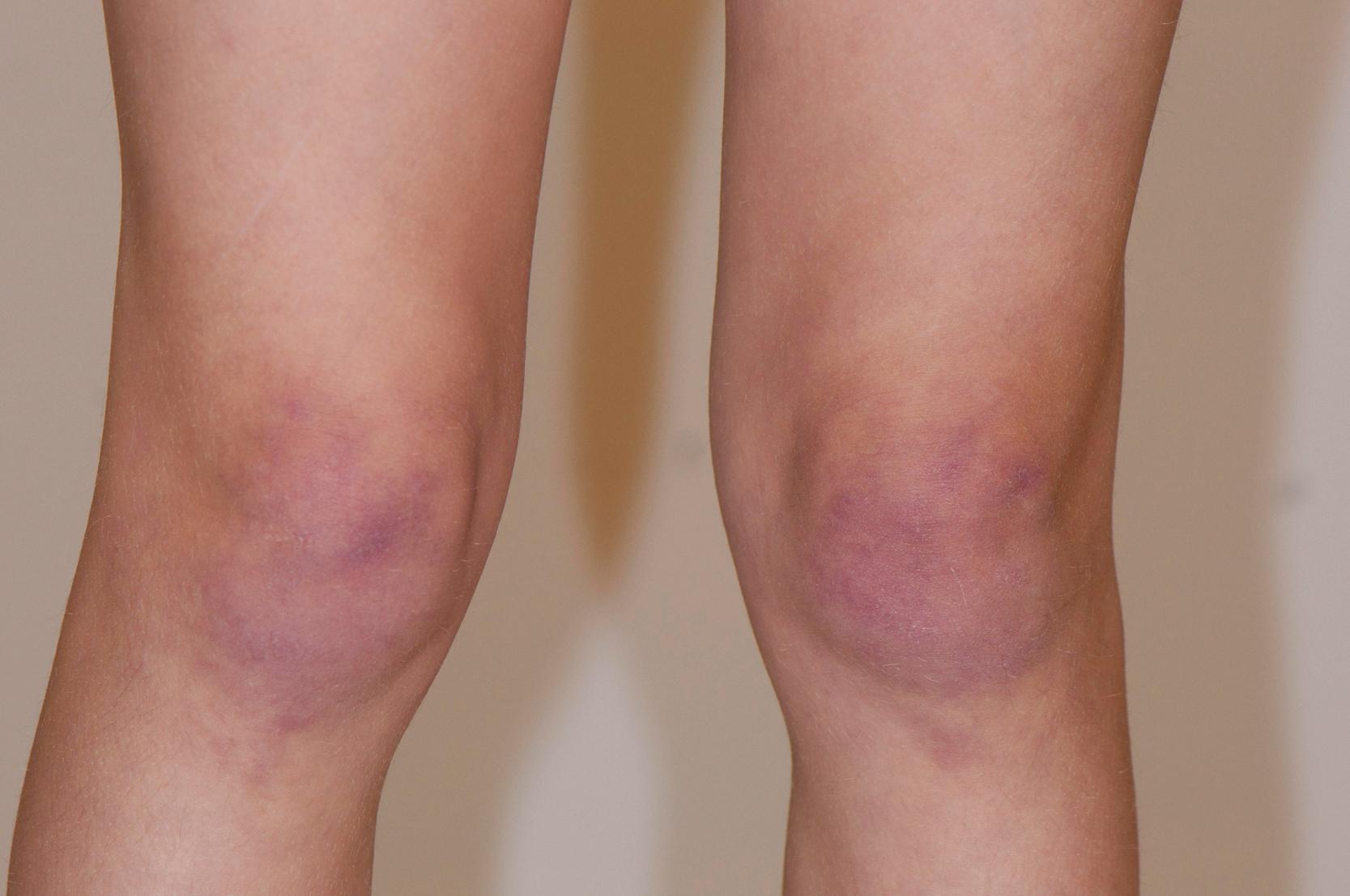
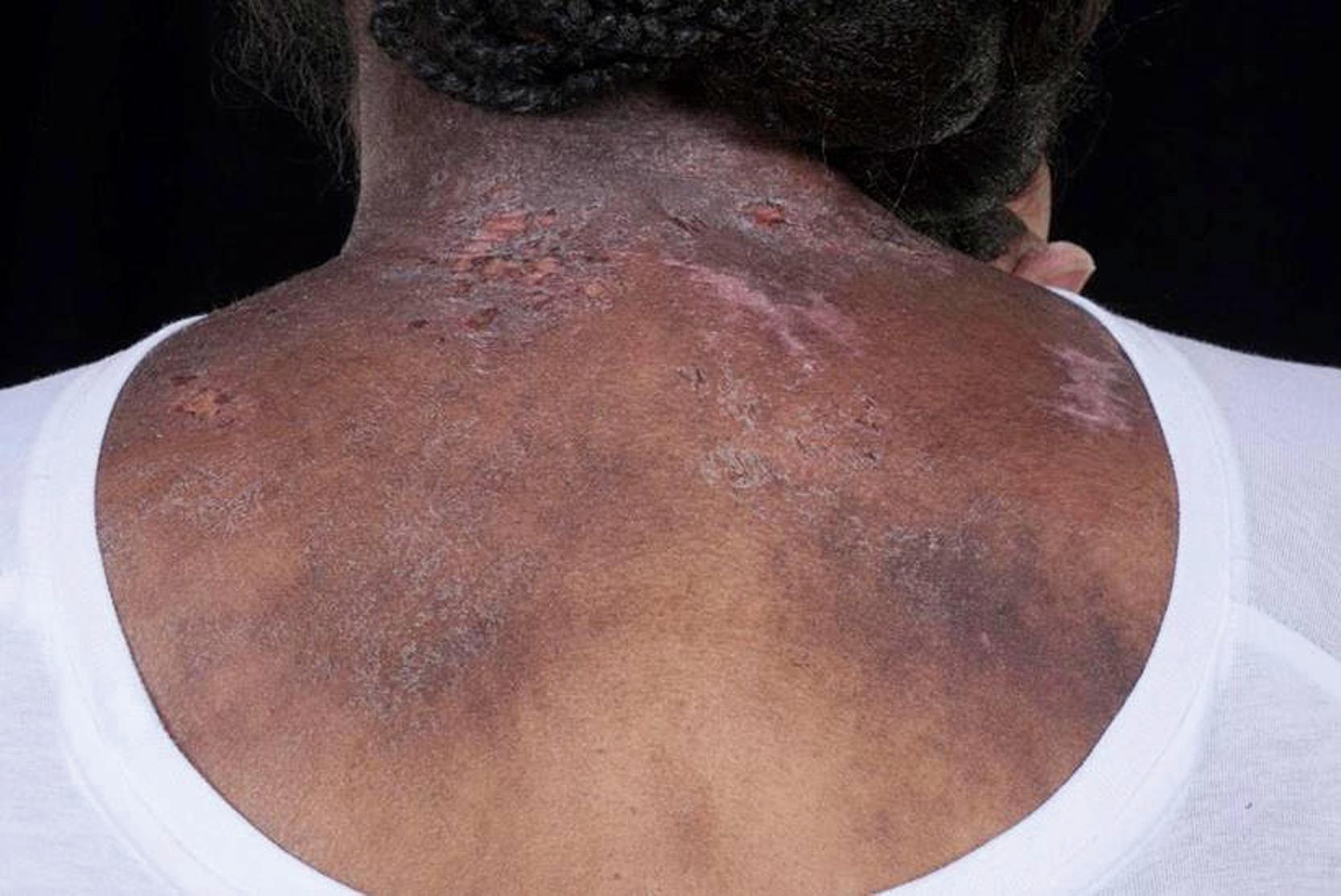
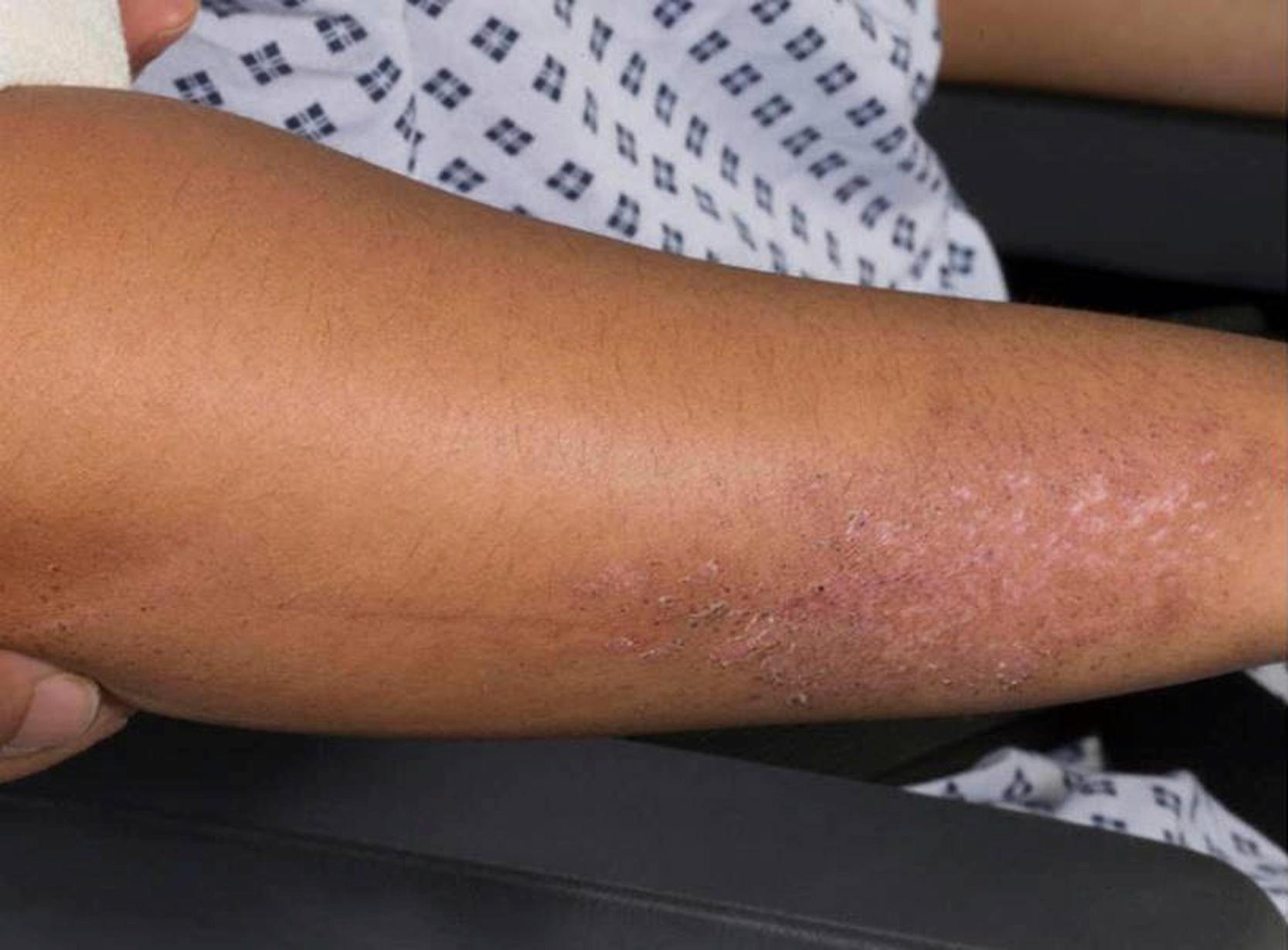
Common cutaneous manifestations include heliotrope rash, Gottron papules, shawl sign, V sign, skin ulceration, nailfold capillary change, and calcinosis. Heliotrope rash refers to a symmetrical erythematous to violaceous rash of the upper eyelids, with or without periorbital edema, and occasionally scaling on the eyelids ( Fig. 26.2 ). Malar or facial erythema may be present. Gottron papules are flat-topped, erythematous, papular eruptions—sometimes scaly (i.e., papulosquamous)—over the metacarpophalangeal and interphalangeal joints ( Fig. 26.3 ). Some patients may have similar lesions over the extensor aspect of the elbows, knees, and medial malleoli ( Fig. 26.4 ). The shawl sign is characterized by erythematous patches on the posterior neck, shoulders, and upper back ( Fig. 26.5 ). The V sign refers to an erythematous patch on the anterior neck and the upper part of chest. The holster sign is erythema over the outer surface of hips or thighs. Skin ulceration can occur over flexor surfaces, trunk, or medial canthus of the eyelid; ulcerative disease is often a sign of more extensive vasculopathy and portends more serious disease ( Fig. 26.6 ). Some patients may develop “mechanic’s hands,” which is a scaly rash over the tips and lateral aspects of the fingers, but it is rarely seen in children.
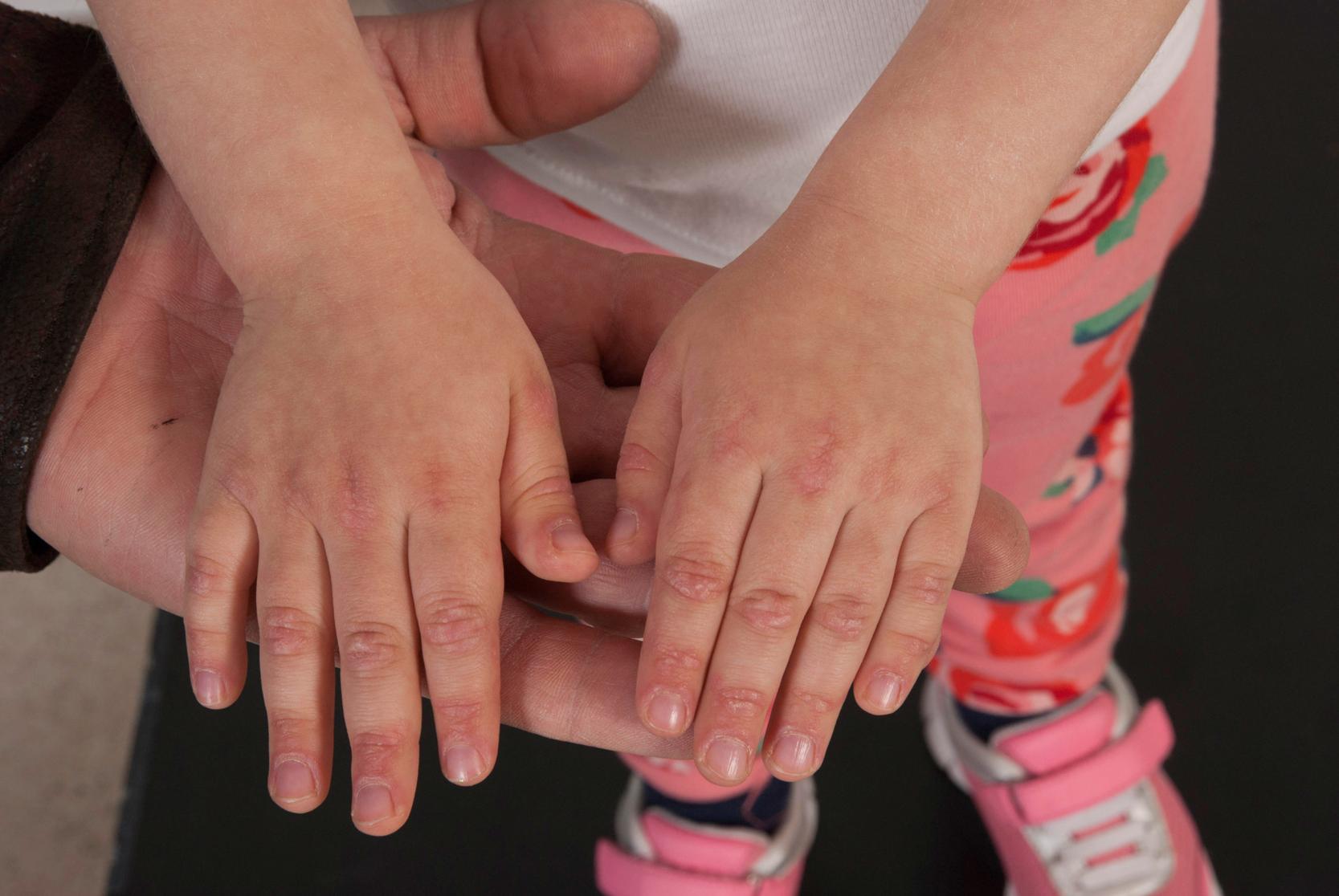
Nailfold capillary changes reflect small-vessel vasculopathy in JDM. Nailfold capillaroscopy is a noninvasive and nonexpensive technique to visualize the capillary network directly and assess abnormalities of microcirculation. In the area just proximal to the nail, capillaries from the subpapillary venous plexus run parallel to the skin surface. The normal capillary pattern is characterized by a regular disposition of capillary loops along the nail bed. Normal capillary density values are usually between 7 and 11 capillaries per millimeter. , In JDM, the changes seen include capillary dropout, enlarged and elongated capillaries (sometimes becoming “giant capillaries”), bushy capillaries, and areas of hemorrhage—all of which result in a reduced nailfold capillary density ( Fig. 26.7 ). Changes may be difficult to see in patients with darker skin.
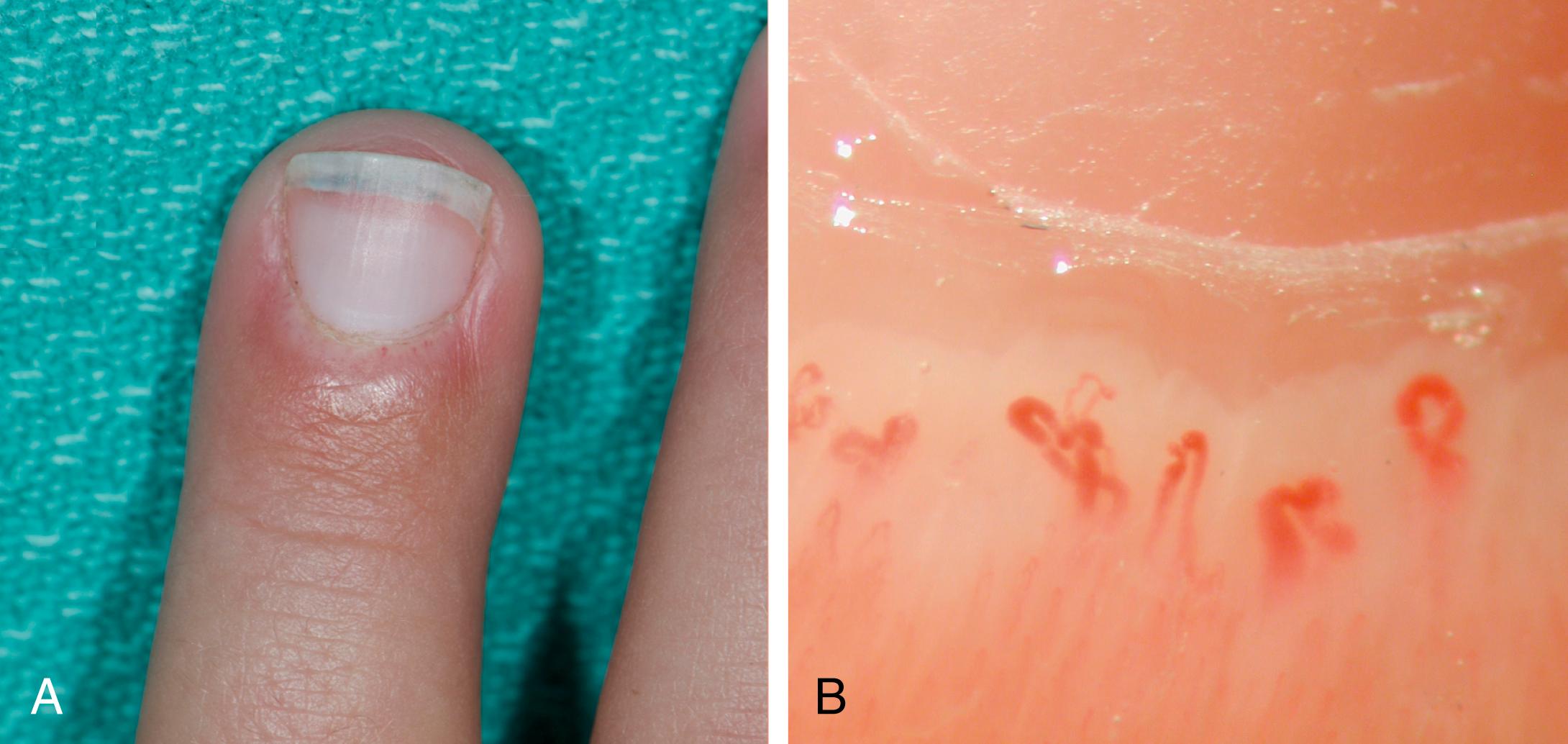
Several studies have demonstrated the diagnostic and prognostic roles of nailfold capillaroscopy in JDM. Patients with active disease have a greater degree of nailfold capillary changes and decreased nailfold capillary density compared with those with inactive disease. , The presence of more normal nailfold capillary density in children who fulfill the diagnostic criteria for JDM is associated with a shorter duration of untreated disease and lower skin disease activity. Nailfold capillary density is associated with skin and muscle disease activity and tracks clinical improvement over time after treatment.
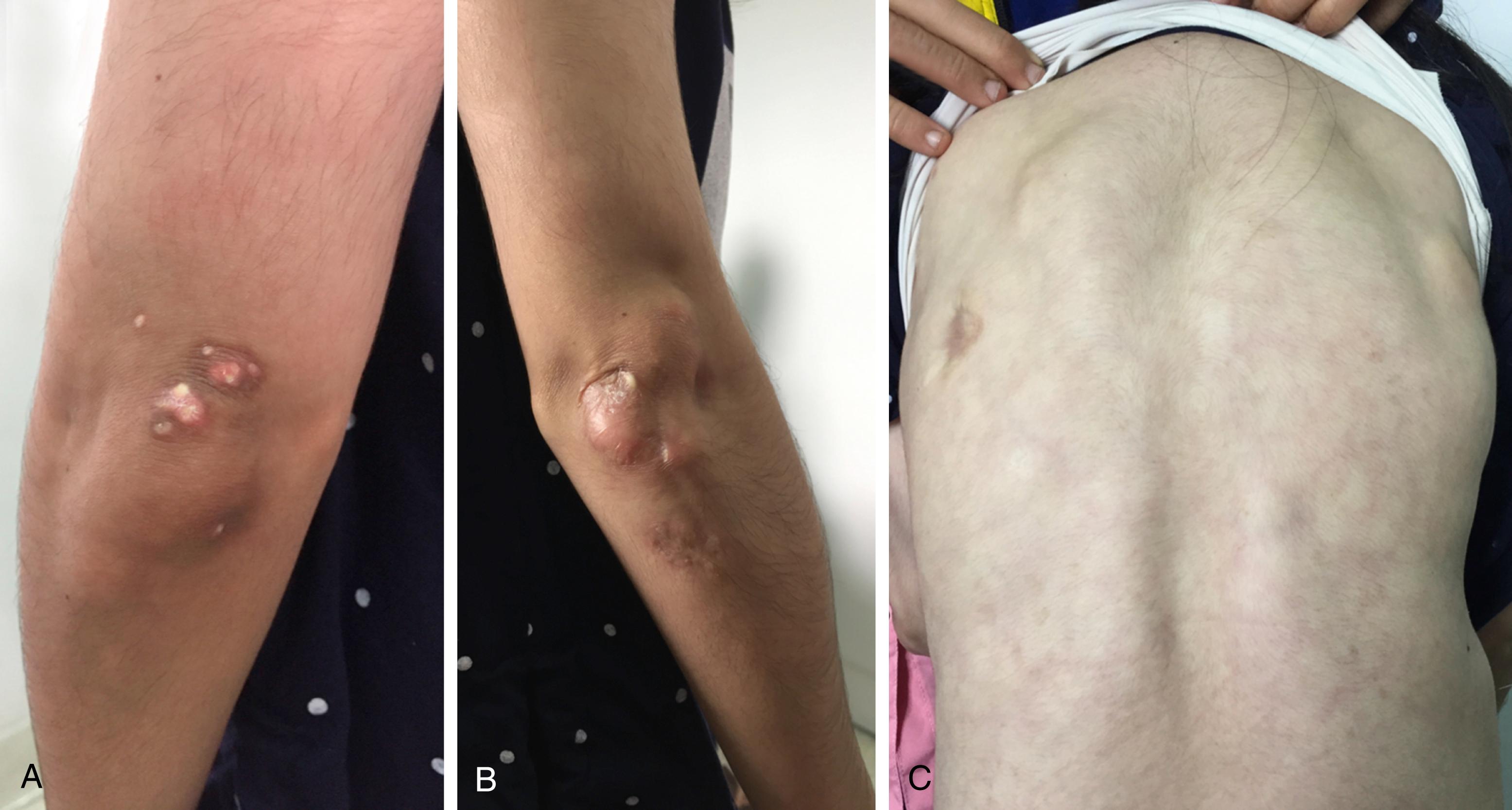
Calcinosis reflects a dystrophic deposition of the mineral calcium hydroxyapatite in the skin, soft tissue, or muscle. Calcinosis tends to be at pressure points, such as elbows, knees, and buttocks, but may occur anywhere over the body ( Fig. 26.8 ). It is rare as a presenting feature of JDM; it usually develops during the disease course and is thought to be a scarring response. Calcinosis may present in different patterns, including superficial plaques or nodules (“calcinosis superficialis”), deep tumoral calcinosis (“calcinosis circumscripta”), diffuse collections along fascial planes of tendon or muscles (“calcinosis universalis”), or extensive exoskeleton-like calcium deposits. Calcinosis may result in overlying skin ulceration, pain, or functional disability from joint contractures.
Lipodystrophy is reported in 3% to 10% of patients. , , , Lipodystrophy is characterized by a progressive partial or generalized loss of subcutaneous fat. It appears to be associated with metabolic abnormalities such as insulin resistance, hypertriglyceridemia, acanthosis nigricans, hirsutism, fat redistribution, polycystic ovary disease, and nonalcoholic steatohepatitis. , Most likely, muscle inflammation and damage leads to a loss of insulin receptors and resulting insulin resistance, with lipoatrophy as a secondary consequence.
Cardiac involvement is rare in JDM. In a retrospective study of North American patients with JDM, 9% of patients had abnormal electrocardiographic findings such as prolonged corrected QT interval, whereas 48% of patients had abnormal echocardiographic findings at disease presentation. Many of these findings were either mild or unlikely to be a result of JDM; for example, mild tricuspid regurgitation and/or pulmonary insufficiency. None of the patients presented with serious cardiac dysfunction. Another study demonstrated left ventricular diastolic dysfunction among patients with JDM when followed up years after; however, the patients did not have symptoms of cardiac diseases. The Single Hub and Access point for pediatric Rheumatology in Europe (SHARE) initiative recommends echocardiography and electrocardiography in all patients with JDM at diagnosis.
Interstitial lung disease (ILD) in children with JDM is rare, but one of the most serious complications. Respiratory symptoms are initially mild or absent in most patients at the time of ILD diagnosis. Pulmonary function tests, including carbon monoxide diffusion are recommended in all patients at the time of diagnosis. Restrictive ventilatory defects, as seen on a pulmonary function test, and decreased diffusing capacity for carbon monoxide are indicators of ILD. High-resolution computed tomography (HRCT) of the chest is suggested for confirmation, as a restrictive pattern may occur from respiratory muscle weakness alone. The usual HRCT features of ILD include consolidation, reticulonodular infiltration, ground-glass opacities, and peribronchovascular thickening. The lesions are often bilateral and predominantly in the lower lobe.
Early detection of ILD is important, as early aggressive treatment likely leads to better outcomes. High-serum Krebs von den Lungen-6 (KL-6) is found to be associated with the presence of ILD and can be detected in the early phase of ILD, regardless of respiratory symptoms. ILD is particularly associated with antimelanoma differentiation–associated gene 5 (MDA5) antibodies, with rapidly progressive ILD associated with high fatality, especially in Japan. ,
Other respiratory involvement in JDM patients includes aspiration pneumonia secondary to pharyngeal and esophageal dysmotility, and alveolar hypoventilation secondary to respiratory muscle weakness.
Gastrointestinal involvement includes abdominal pain, ulceration, and hemorrhage. Clinically important gut vasculopathy is rare, but it is one of the important causes of death in JDM. There is a case report of two JDM patients with severe gastrointestinal ulceration or perforation who required surgery. These gastrointestinal findings occurred when the JDM was only mildly active. Abdominal pain or a change in stooling pattern may be important clues to early gastrointestinal involvement.
There are a few described cases of neurological/neuropsychiatric involvement in patients with JDM including seizure, pseudoseizure, sensory neuropathy, depression, and fatal brainstem infarction. ,
Renal involvement occurs in up to one-fifth of adults with IIM, but it is rare in juvenile-onset myositis, including glomerulonephritis, nephrotic syndrome, acute kidney injury, and immunoglobulin (Ig)A nephropathy. In fact, in any JDM patient with renal disease a diagnosis of systemic lupus erythematosus is probably more likely. Most cases that are described as juvenile IIM with renal involvement have responded well to the conventional treatment of IIM and do not require additional therapy for renal disease.
About half of patients with JDM have eye and eyelid involvement. Besides heliotrope rash, scarring and blepharitis of the lids are found. Posterior subcapsular cataracts, when seen, are iatrogenic as a consequence of corticosteroid therapy. Retinopathy is rare.
The diagnosis of JDM is commonly confirmed using the Bohan and Peter criteria published in 1975. The diagnosis requires the presence of pathognomonic rash (heliotrope rash, Gottron papules) and three of the four following features for a “definite” diagnosis: proximal muscle weakness, elevated serum levels of muscle enzymes, electromyographic changes of chronic inflammatory myositis, and histopathological changes of inflammatory myositis. The presence of typical rash with two of these criteria leads to a diagnosis of “probable” JDM.
Muscle biopsy and even electromyography (EMG) are invasive investigations. Several studies have shown that only about half of patients undergo a muscle biopsy and between 31% and 60% of patients have an EMG. , , In clinical practice, magnetic resonance imaging (MRI) is currently widely used to assess muscle inflammation and extension of disease as a noninvasive technique, although it is not part of the Bohan and Peter criteria.
The European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) developed and validated classification criteria for adult and juvenile IIM in 2017 ( Table 26.2 ). The criteria provide a score that is calculated from age of onset, variables of muscle weakness, skin manifestations, other clinical manifestations, laboratory measurements, and muscle biopsy findings. Because fewer pediatric patients undergo muscle biopsies, there is an additional scoring algorithm that does not include biopsy variables. Definite IIM corresponds to a probability of 90% or greater (total score of ≥7.5 without muscle biopsy or ≥8.7 with muscle biopsy). A total score of 5.5 or greater to less than 7.5 without biopsy or 6.7 or more to less than 8.7 with biopsy is classified as probable IIM, with a probability of 55% or more to less than 90%. A total score of less than 5.3 without biopsy or less than 6.5 with biopsy is classified as possible IIM with a probability of 50% or more to less than 55%.
| When no better explanation for the symptoms and signs exist, these classification criteria can be used | ||||
|---|---|---|---|---|
|
|
|
||
| Classification Criteria | Present in This Patient? | Score Without Muscle Biopsy | Score With Muscle Biopsy | Points assigned |
|
|
|
|
|
|
|
|
|
|
| Muscle Weakness | Present in This Patient? | Score Without Muscle Biopsy | Score With Muscle Biopsy | Points Assigned |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Skin Manifestations | Present in This Patient? | Score Without Muscle Biopsy | Score With Muscle Biopsy | Points Assigned |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other Clinical Manifestations | Present in This Patient? | Score Without Muscle Biopsy | Score With Muscle Biopsy | Points Assigned |
|
|
|
|
|
| Laboratory Measurements | Present in This Patient? | Score Without Muscle Biopsy | Score With Muscle Biopsy | Points Assigned |
|
|
|
|
|
|
|
|
|
|
| Muscle biopsy features or presence of: | Present in This Patient? | Score Without Muscle Biopsy | Score With Muscle Biopsy | Points Assigned |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Score | ||||
Age at symptom onset younger than 18 years of age distinguishes juvenile from adult IIM. Patients who meet the criteria and have characteristic skin rash are classified as JDM. Those who do not have the required rash criteria are classified as juvenile myositis other than JDM. A muscle biopsy is not required for a definite diagnosis if the pathognomonic skin rash is present. Future research on muscle biopsies may yield additional insights into prognosis.
Laboratory tests reveal elevated serum muscle enzymes such as creatine kinase (CK), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and aldolase. Serum levels of muscle enzymes are raised in 80% to 96% of JDM patients. , , , , However, there are a number of patients with normal levels of muscle enzymes at presentation despite active disease. Furthermore, the levels of elevated muscle enzymes at disease onset do not correlate with disease outcome.
A complete blood count is often normal, but in some cases, leukocytosis and anemia can be found. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) may be elevated during active disease, but active disease can be seen with normal ESR and CRP. Serum levels of von Willebrand factor may increase but are neither sensitive nor specific for active disease. Galectin-9 and CXCL10 are biomarkers, which have been tested in several cohorts and have been proposed to distinguish between JDM patients with active disease and those in remission. Persistent high or rising levels suggest an imminent flare, up to several months before clinical symptoms, even in the absence of elevated CK levels.
Antinuclear antibody (ANA) is found in up to 76% of patients with JDM, although by itself it is neither specific nor diagnostic. , , , , ,
Myositis antibodies include both myositis-specific autoantibodies (MSA) and myositis-associated autoantibodies (MAA). MSA are present specifically in patients with IIM, whereas MAA occur in patients with other autoimmune conditions that are associated with myositis ( Table 26.3 ). MSA are identified in approximately 40% to 60% of children with IIM. , The detection of MSA suggests a diagnosis of myositis, but more research is needed to corroborate this statement. Because of the associations with distinct clinical features, these antibodies may predict clinical phenotypes and outcome in IIM, at least for groups of patients; individual patients may or may not follow representative courses.
| Autoantibodies | Target Autoantigen | Frequency (% JDM) | Clinical Complications |
|---|---|---|---|
| Myositis-Associated Autoantibodies (MAA) | |||
| Anti-PM-Scl | Exosome-associated PM-Scl-75 | 3–5 | ILD, cardiac involvement, arthritis, mechanics hands, Raynaud phenomenon and CK level; overlap syndromes |
| Anti-U1-snRNP | U1-snRNPs | 3–8 | Considered good prognosis in myositis; overlap syndromes |
| Anti-Ku | 70- and 80-kDa Ku hetero-dimers | <1 | ILD, Raynaud disease, arthralgia, and reflux |
| Anti-Ro52 | Ro52 | 6 | Commonly occur in myositis patients; associated with other MSAs (especially anti-Jo-1 and anti-MDA5 autoantibodies); overlap syndromes |
| Myositis-Specific Autoantibodies (MSA) | |||
| Anti-p155/140 | TIF-1γ | 22–29 | Severe cutaneous disease (lipodystrophy, skin ulceration, and edema) |
| Anti-MJ | NXP2 | 18 | Predicts poor prognosis; frequent muscle cramps, atrophy, joint contractures, and dysphonia; gastrointestinal ulcerations and bleeding |
| Anti-MDA5 (CADM-140) | MDA5 | 7–33 | Rapidly progressive ILD; higher IL-18, IL-6, and ferritin; fever and milder muscle disease (low CK levels) |
| Anti-Mi-2 | NuRD complex | 4–10 | Cutaneous features; better prognosis (milder muscle, decreased risk of ILD, malignancy, responds well to standard therapies) |
| Anti-SAE | SAE | <1 | Initially amyopathic disease in adult DM |
| Anti-ARS (anti-Jo-1, anti-OJ, anti-EJ, anti-KS, anti-PL-7, anti-Zo, anti-Ha, anti-PL-12) | ARS | 6–12 1–3 |
Antisynthetase syndrome; fever, ILD, Raynaud phenomenon, mechanics hands, nonerosive arthritis, mortality rate; better prognosis compared with anti-Jo-1 negative |
| Immune-Mediated Necrotizing Myopathy | |||
| Anti-SRP | SRP | Extremely rare | Severe symmetrical muscle weakness, Raynaud phenomenon, very high CK levels, cardiac disease, dysphagia, ILD |
| Anti-HMGCR | HMGCR | <3 in JM | Necrotizing autoimmune myositis, muscle weakness; worse disease course |
Antitranscription intermediary factor 1 gamma (anti–TIF-1γ) antibodies were originally described as anti-p155 and anti-155/140 antibodies. , These antibodies are among the most frequently found in children with IIM, 35% of juvenile IIM patients in a large US registry and 18% of juvenile-onset myositis patients in the UK registry. , In adult dermatomyositis, anti-TIF-1γ antibodies have a strong positive association with malignancy. In juvenile-onset patients, these antibodies are not associated with malignancy, but rather are associated with greater muscle weakness. Patients with anti-TIF-1γ antibodies tend to have cutaneous ulceration , and show typical skin features such as Gottron papules, shawl sign, and poikiloderma. , , Patients with these antibodies are more likely to receive more aggressive treatment (e.g., intravenous cyclophosphamide and/or biological treatments).
Antinuclear matrix protein 2 (anti-NXP2) antibodies, formerly known as anti-MJ antibodies , target a 140-kDa autoantigen protein. These antibodies are detected in 15% to 25% of patients with juvenile IIM. , , , Anti-NXP2 antibody–positive patients tend to have a younger age at disease onset, compared with patients with other MSAs. Clinical characteristics of the anti-NXP2 antibody–positive patients include muscle cramps, dysphonia, a greater degree of muscle weakness, and calcinosis. , , ,
Antibodies directed against MDA5 (anti-MDA5) are present in 6% to 7.4% of juvenile IIM patients. , The presence of anti-MDA5 autoantibodies may predict the development of ILD, as has been reported in adult dermatomyositis. , , In a predominantly Caucasian cohort of JDM patients in the United Kingdom, 19% of patients with positive anti-MDA5 autoantibodies had radiological evidence of ILD, but there were no cases with rapidly progressive ILD. These findings are different from Japanese reports, in which a higher level of these antibodies was associated with rapidly progressive ILD. , Patients in the UK registry with anti-MDA5 antibodies were found to have less severe muscle disease and developed more skin ulceration. An association with arthritis was identified in both UK and Japanese studies. Serial testing may help in monitoring the response to treatment as the titer of this antibody sometimes declines to normal values, or to undetectable levels, at remission.
Anti-Mi-2 antibodies target the Mi-2 nuclear antigen with a frequency of less than 5% of patients with juvenile IIM. , The majority of patients with these antibodies have “classical JDM” with typical skin and muscle signs. Anti-Mi-2 antibodies were strongly associated with edema and a greater degree of weakness in the UK registry, and with malar rash, Gottron papules, and heliotrope rash in the US registry. Patients with these antibodies usually have high ANA titers.
Antisignal recognition peptide (anti-SRP) autoantibodies are found in 1.6% to 2% of juvenile IIM patients, and more frequently in African Americans. , Anti-SRP autoantibody positivity is described in polymyositis and immune-mediated necrotizing myopathy. Patients with these antibodies have a higher frequency of severe muscle weakness and cardiac abnormalities on electrocardiogram or echocardiogram. Associations with Raynaud phenomenon and skin ulceration have been reported. These children often have a chronic continuous disease course, which can be difficult to control.
Anti-tRNA synthetase autoantibodies target the cytoplasmic aminoacyl-tRNA synthetase enzymes. The antisynthetase autoantibodies include Jo-1, PL-12, OJ, EJ, PL-7, KS, Zo, and Ha antigens and have been described in 2% to 5% of juvenile IIM. , Antisynthetase autoantibody–positive patients usually have an older age at onset and are more likely to have an insidious onset compared with other MSA groups. An association of these antibodies and the development of ILD has been identified. In the UK registry, patients with antisynthetase autoantibodies had less muscle weakness and more lipoatrophy, whereas in the US registry, children had more arthralgia, arthritis, and mechanic’s hands.
Antibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR ) have been reported in 1% of juvenile IIM patients. , About 60% of adults with these antibodies have a history of statin exposure, but no statin exposure, has been observed in anti-HMGCR–positive children. , Like adults, patients with anti-HMGCR antibodies frequently present with profound weakness and have only a partial response to immunosuppressive medications, requiring aggressive treatment. Intravenous immunoglobulin (IVIG) seems to be effective for these children. Children with anti-HMGCR antibody myositis may mimic a limb girdle muscular dystrophy. Muscle biopsies show distinctive features with muscle fiber necrosis, degeneration, and regeneration. These children may not have skin involvement.
Anti–small ubiquitin-like modifier activating enzyme (anti-SAE) antibodies are detected in adult patients with dermatomyositis. In children, the frequency of anti-SAE is about 1% of patients with juvenile IIM. No specific phenotype associations were identified in a large UK cohort.
MAA are present in 5% to 16% of patients with childhood-onset myositis. , MAA may be helpful to clarify the diagnosis in patients with overlap features such as scleroderma. Antibodies to antigens in the MAA group include anti-Ro (6.3%), anti-ribonucleoprotein (RNP) (4% to 5.6%), and anti-polymyositis scleroderma (PM-Scl) (3.7% to 5%). , Patients with anti-RNP antibodies have older age at onset and the disease phenotype is often polymyositis, or polymyositis overlap with systemic lupus erythematosus. Almost half of patients with anti-PM-Scl antibodies are classified as overlap with scleroderma, complicated by the development of calcinosis and lipoatrophy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here