Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Juvenile myasthenia gravis is a major category of neuromuscular junction disorders that shares many pathophysiologic and clinical features with adult autoimmune myasthenia gravis, yet displays distinct demographic patterns, clinical features, and therapeutic challenges. These considerations underscore the need to discuss this disease separately from its adult counterpart.
Juvenile myasthenia gravis affects a broad range of ages within the pediatric population and has a worldwide geographic distribution. In North American and European populations, juvenile myasthenia gravis tends to be rare compared to adult myasthenia gravis, having been estimated to comprise 10% to 20% of myasthenia cases overall. The incidence of juvenile myasthenia gravis is estimated to be 0.9 to 2.0 new cases per million per year in Canada, and 1.5 new cases per million children per year in the United Kingdom. Although incidence and prevalence studies are rare, the disease appears to be more common in some parts of Asia. One series from China found that 50% of myasthenia gravis patients were less than 15 years old, while another series from Hong Kong found that 39.3% of myasthenia gravis cases were of juvenile onset. Gender ratios in juvenile myasthenia gravis are either balanced or show a slight female predominance prior to puberty, whereas affected females are much more common than males in adolescence, mimicking the gender ratio in adults.
Epidemiological data regarding neonatal myasthenia gravis are hard to come by and mild cases may not be recognized. Overall, it appears to be relatively rare and the incidence may be declining as maternal myasthenia gravis is better controlled. One study found a 30% incidence of neonatal myasthenia gravis among infants born to mothers with known cases of myasthenia gravis. Even rarer are the more severe variants: the fetal akinesia sequence and the fetal acetylcholine inactivation syndrome (see Chapter 7 ).
As in the adult form of the disease, juvenile myasthenia gravis is caused by antibodies attacking the postsynaptic membrane at the neuromuscular junction. Neonatal myasthenia gravis is somewhat different in etiology compared to juvenile myasthenia gravis, as the former is caused by transplacental transmission of maternal antibodies. Presynaptic autoimmune injuries typically cause a slightly different disease, Lambert-Eaton myasthenic syndrome, which has been reported in childhood but is exceedingly rare in this population. Genetic mutations that impair the function of the neuromuscular junction lead to congenital myasthenic syndrome (see Chapter 26 ).
The most common pathogenic antibody binds to the acetylcholine receptor (AChR), which is a key component of the synaptic signaling mechanism. Acetylcholine, the primary neurotransmitter at the neuromuscular junction, is released into the synapse in response to depolarization of the presynaptic motor nerve membrane with its accompanying influx of calcium ions. The acetylcholine diffuses rapidly across the synapse and binds to the acetylcholine receptors, triggering a depolarization of the postsynaptic membrane and a cascade of depolarizations that culminate in contraction of the muscle fiber ( Figure 27.1A ). All of this occurs in a blink of an eye.
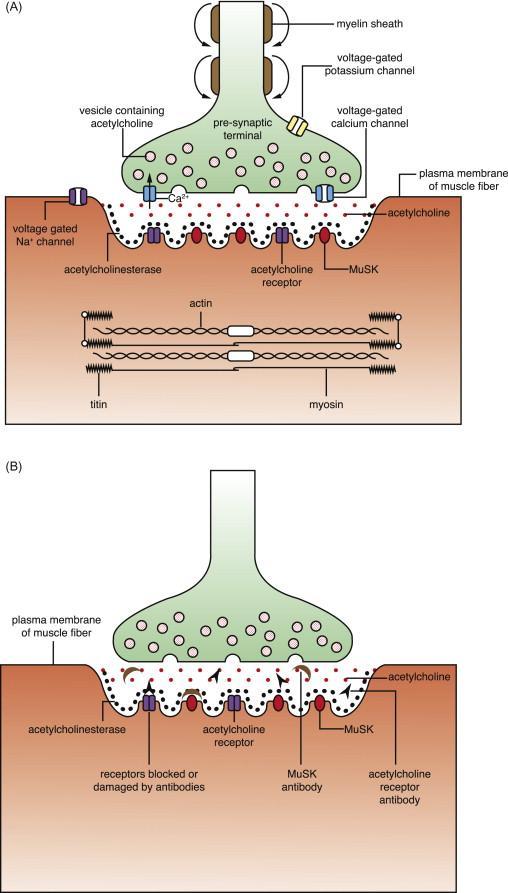
The acetylcholine receptor is a pentameric complex that exists in two forms: α 2 βδγ in fetal and denervated muscle, and α 2 βδε in adult muscle. Most antibodies bind to the acetylcholine receptor and are detected by a radioimmunoprecipitation assay that is widely available. These antibodies bind mainly to the α and γ subunits, but since the γ subunit is not present in mature muscle, the less common antibodies to ε subunits can be important in some patients. These antibodies activate the complement cascade and can lead to destruction of the postsynaptic membrane and loss of the high density of acetylcholine receptors. The antibodies can also cause internalization of the acetylcholine receptors, without damage, because being divalent they can cross-link adjacent acetylcholine receptors via binding to the two acetylcholine α subunits; however, this process may not be very important in myasthenia gravis as it is accompanied, in experimental animals, by an increased synthesis of acetylcholine receptors. More rarely, blocking antibodies prevent the normal ion channel function of the acetylcholine receptors. The end result of any of these three mechanisms is impairment of neuromuscular transmission ( Figure 27.1B ), leading to myasthenia gravis (the original Greek term translates as “muscle weakness severe”). It should be noted that most antibody tests are designed to help diagnosis and not to make accurate and standardized measurements for evaluation of long-term treatment efficacy.
In the 15% of adult myasthenia gravis patients who do not have elevated acetylcholine receptor antibody titers, other antibody-mediated attacks on the neuromuscular junction are likely to cause the disease. The main known target is muscle specific kinase (MuSK). Antibodies to MuSK tend to be elevated in the absence of elevations of acetylcholine receptor antibodies. Myasthenia gravis associated with MuSK antibodies tends to be less common in northern Europe or Canada. MuSK antibody positive juvenile myasthenia gravis is rare, but has been reported on multiple occasions, in children as early as 14 months. Patients with MuSK antibody-associated myasthenia gravis often do not respond to pyridostigmine, so it is important to identify the immunological basis of individual cases whenever possible as a guide to potential treatments.
Titin and ryanodine receptors are intracellular muscle proteins and the antibodies that bind to them serve as biomarkers for the presence of thymoma in adults younger than 60 years. They are often, though not always, accompanied by elevations in acetylcholine receptor antibodies in adults. Children and adolescents with juvenile myasthenia gravis occasionally have isolated elevations of this antibody, but the association with thymoma appears to be reduced or absent in this age group based on a small number of cases. Antibodies to LRP4, another postsynaptic membrane protein, have also been reported recently in myasthenia gravis, but their frequency and clinical associations with the disease have not been characterized in detail to date, especially in children and adolescents.
Thymomas are found in some cases of juvenile myasthenia gravis, just as they are in the adult form of the disease, but are much less common in children and adolescents than in adults. This association with thymoma places myasthenia gravis within the broad category of paraneoplastic diseases. Histologic examinations of thymus tissue obtained from children with juvenile myasthenia gravis who have undergone thymectomy have found a high incidence of lymphoid follicular hyperplasia.
Some evidence suggests a link between Epstein Barr virus (EBV) and some autoimmune diseases. An early hint that this may be the case for myasthenia gravis was the discovery of the presence of Epstein Barr virus genome in thymus tissue derived from a few affected patients. A more recent study found evidence for active Epstein Barr virus infection in all 17 thymuses obtained from myasthenia gravis patients, compared to none of 6 control thymuses. However, 2 subsequent studies found no evidence for such an association, casting doubt on this theory. This proposed association remains controversial.
Nearly all patients with juvenile myasthenia gravis demonstrate ocular involvement, principally the levator palpebrae and/or extraocular muscles, leading to ptosis, diplopia, and sometimes frank ophthalmoparesis or ophthalmoplegia. The ocular symptoms are often the first or primary manifestation of the disease at all ages. In rare cases, ocular symptoms are absent at presentation, but in those cases, bulbar or vocal symptoms are typically present, and ocular symptoms may develop later. Dysphagia, dysarthria, nasal speech, extremity weakness, and respiratory distress may occur in conjunction with the ocular symptoms. It is rare for myasthenia gravis to present with extremity fatigue in the absence of cranial nerve-innervated muscle involvement.
The bimodal anatomical distribution of involvement in most cases of myasthenia gravis has yielded its subcategorization into pure ocular and generalized variants. Both the ocular and generalized forms of myasthenia gravis may occur at any age. Ocular myasthenia gravis is more common in children than in adults and tends to occur more often in prepubertal children, whereas generalized myasthenia gravis is more common in postpubertal adolescents. Most patients who develop generalized myasthenia gravis display symptoms and signs of generalization soon after onset; thus, it is usually possible to assign individual patients to one of these anatomical subcategories early in the course of the disease. However, in rare cases, generalization may occur unexpectedly after years of pure ocular symptoms and/or remission from pure ocular involvement.
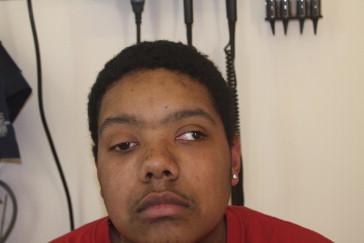
The physical examination of a child or adolescent with suspected myasthenia gravis is critical. Ptosis may be unilateral, bilateral, and asymmetric ( Figure 27.2 ), or bilateral and symmetric. When bilateral and symmetric, the ptosis may be difficult to discern on physical examination, as some children may appear to have mild ptosis at baseline. In such situations, it is helpful to examine any older photos of the child that are available for comparison. When unilateral or asymmetric, the ptosis is usually more obvious. One finding that can help verify the presence of abnormal ptosis is the curtain sign. To elicit the curtain sign, the examiner should lift each eyelid in turn. The affected side(s) will drop when the contralateral eyelid is raised. Ophthalmoparesis may be detected by lack of conjugate gaze in one or more directions, and is supported by the patient’s report of subjective diplopia in those directions ( Figure 27.2 ). Quantification of misalignments is most accurately performed by a neuro-ophthalmologist. Ptosis and/or ophthalmoparesis may become more obvious in response to a maneuver to fatigue the eyes, such as sustained upgaze for 1 minute. The ice pack test is used by some practitioners in the clinic or at the bedside; clinical signs of myasthenia gravis should improve in response to cold temperatures. Neck flexion strength is especially useful to assess while examining a patient with suspected myasthenia gravis; however, it is important to remember that children under the age of approximately 10 years tend to have apparent neck flexion weakness at baseline. While examining the extremities, some practitioners attempt to elicit fatigability in the deltoid muscle by asking the patient to abduct and adduct the arms repeatedly. When basic pulmonary function testing is available, measurement of vital capacity and/or negative inspiratory force is useful to assess respiratory function. When such equipment is not readily available, a good bedside assessment of vital capacity can be performed by asking the patient to take a deep breath and count numbers out loud as high as possible on one breath. Adolescents and adults should be able to count beyond 30 on one breath.
In all suspected cases of myasthenia gravis, serum thyroid studies are indicated, as symptoms of thyroid disorders may mimic those of myasthenia gravis, and thyroid disorders are sometimes present concurrently with myasthenia gravis.
A differential diagnosis should be considered in the appropriate settings. For pure ocular presentations, alternative diagnoses may include ptosis due to hypoplasia of the levator palpebrae muscles. The Marcus Gunn jaw winking phenomenon also occurs at times in children, and should be suspected when the ptosis occurs during chewing movements. When generalized symptoms are present, the possibility of Lambert-Eaton myasthenic syndrome should be considered, though this diagnosis is exceedingly rare in children compared to adults. Botulism may sometimes be a diagnostic consideration. A typical history for infant botulism includes living in an endemic geographic area (Pennsylvania, Utah, and California have high rates of infant botulism in the United States), a family member who works in the construction industry (spores are often stirred during construction projects), and constipation in addition to weakness. In older children and adolescents, rare cases of botulism may occur primarily as a result of ingesting botulinum toxin in improperly canned foods. Clues on physical examination that may indicate the presence of botulism include internal as well as external ophthalmoplegia (sluggish pupillary reactions in addition to eye movement abnormalities) and flaccid paralysis; tendon reflexes may be preserved. When generalized weakness appears to be chronic, the diagnosis of congenital myasthenic syndrome is a consideration (see Chapter 26 ), as well as other inherited disorders associated with ptosis including myotonic dystrophy (see Chapter 37 ) and congenital myopathies (see Chapter 28 ). Serum creatine kinase levels and electromyography may help sort out this differential diagnosis.
Juvenile myasthenia gravis, like adult myasthenia gravis, is ultimately a clinical diagnosis. A strongly suggestive history and physical examination may be sufficient to make the diagnosis; however, in a number of cases the clinical presentation is not clear-cut, and it is usually helpful to have positive results on one or more standard diagnostic tests. It is important to remember that some of these diagnostic tests do not always have high sensitivities, especially in cases of ocular myasthenia gravis.
Antibody testing is usually highly specific for myasthenia gravis. In the appropriate clinical setting, positive antibody titers confirm the diagnosis, and further testing may only be indicated if the clinical presentation is atypical. Antibody testing only involves serum testing and, especially in the case of acetylcholine receptor antibodies, is relatively inexpensive and straightforward. Thus, this test is easy to obtain in children. Some laboratories distinguish between binding, blocking, and modulating antibodies, but the binding antibody titer is usually sufficient and the other two are not universally available.
As in adults, children with generalized juvenile myasthenia gravis are more likely to have elevated acetylcholine receptor antibodies than children with pure ocular juvenile myasthenia gravis. Because the acetylcholine receptor antibody was the first and most common antibody to be associated with myasthenia gravis, patients who have elevated acetylcholine receptor antibody titers have traditionally been termed “seropositive,” with the remainder categorized as “seronegative,” even if they are found to have elevated titers of other antibodies. Seropositive adult patients tend to have more severe clinical courses, though the antibody titers do not correlate with clinical status or history of thymectomy. In pediatrics, seronegative myasthenia gravis is more common before puberty. In adults, a significant proportion of seronegative myasthenia patients have been shown to have elevated antibodies to muscle-specific kinase (MuSK), ranging from a majority in the first report to a lower proportion in other reports from the Netherlands, Thailand, and Sri Lanka. In the United States, MuSK antibody testing is expensive and elevated MuSK antibody titers are rare in seronegative juvenile myasthenia gravis, but can be identified in some generalized cases. A prepubertal child with MuSK antibody positive juvenile myasthenia gravis has been reported to present with ocular symptoms, only developing a brief period of generalized symptoms 2 years after onset. There are rare reports of patients, including an adolescent, found to have elevated titers of both acetylcholine receptor antibodies and MuSK antibodies. It is important to identify the occasional patients with elevations in both types of antibodies, as the acetylcholine receptor antibody elevation is typically low and less relevant than the elevation in the MuSK antibody.
Among the rare instances when striated muscle antibody titers are elevated in juvenile myasthenia gravis, they are sometimes associated with acetylcholine receptor antibody elevations and sometimes are found in isolation. When any antibody titer is borderline or mildly elevated, or if the clinical presentation is discordant with the antibody titers, repeat testing may help clarify the situation, though any intervening immunotherapies may reduce the sensitivity of further rounds of testing. Antibody titers do not generally correlate with the clinical status of affected individuals, though some reports suggest that a correlation may exist for MuSK antibody positive cases.
The ice test is a traditional bedside test in which ice is placed on the eyes of a patient with ptosis who is suspected of having myasthenia gravis. A positive result is determined when cooling for approximately 2 minutes results in a 2 mm or greater improvement in the ptosis. The ice test has been determined in numerous adult studies to have a reasonably high sensitivity and specificity, but should not be relied on alone for diagnostic confirmation. The ice test has not been studied extensively in juvenile myasthenia gravis, but its use has been documented in children, and one study that included adults and children over the age of 9 years found that the ice test had reasonably good sensitivity but low specificity.
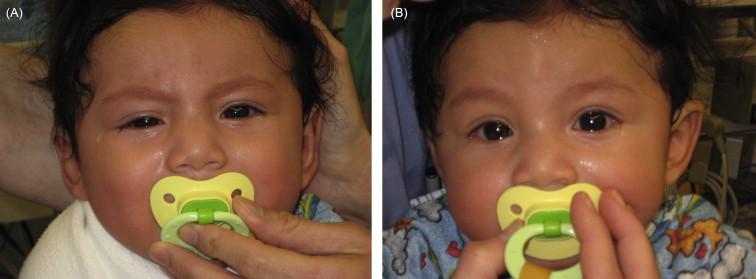
Edrophonium is a short-acting acetylcholinesterase inhibitor that is used only for diagnostic purposes; the half-life is so short that this medication has no therapeutic value. Tensilon is an old brand name for edrophonium, and the diagnostic test that makes use of this medication is often still called the Tensilon test, but given the multiple brands currently in use, it would be more accurate to call it the edrophonium test. The test is most useful when there is a clear physical sign such as unilateral ptosis that can be objectively observed for a response to this medication. It is best if this testing is performed in a monitored setting (i.e. with cardiac telemetry) where advanced cardiorespiratory support is available in case of bradycardia or asystole, which in rare instances occurs during this testing, especially when higher doses are used. An intravenous line should be placed, with a 3-way stopcock attached close to the catheter. One port should be attached to a syringe of normal saline flush, and the other to a syringe of edrophonium. At its typical 10 mg/mL concentration, edrophonium should either be drawn up in a 1 mL tuberculin syringe or diluted for use in a larger syringe. There is some conflict in the literature regarding the optimal dose of edrophonium in infants, with one report recommending 0.1 mg only, and another documenting 0.1 mg/kg. A pharmacokinetic study of edrophonium that included infant data suggests that 0.1 mg/kg is needed to counteract neuromuscular blockade. It may be reasonable to start with a dose of 0.1 mg as a test dose, and then administer 0.1 mg/kg (with a maximum of 0.5 mg) once or twice. For children older than a year who are below 35 kg in weight, the test dose should be 0.5 mg, followed by subsequent 1 mg doses several minutes apart to a maximum total dose of 5 mg. For children and adolescents who are above 35 kg, adult dosing ranges may be used, i.e. a test dose of 1 mg and subsequent doses of 1 to 2 mg each administered several minutes apart to a maximum total dose of 10 mg. The physical sign in question (e.g. ptosis, dramatic ophthalmoparesis, nasal speech) should be monitored before and after each dose to determine if there is a transient improvement. The heart rate should be tracked throughout, and dosing paused if there is a substantial dip. If bradycardia ensues, atropine is an effective antidote, and should be administered intravenously immediately. When the parents consent, photos taken before and after the test may be useful to document any possible response ( Figure 27.3 ). There are many instances when the results of this testing are equivocal, so the parents should be cautioned ahead of time that the findings may not yield a clear diagnosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here