Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
Intracranial pressure monitoring is recommended for conditions that may lead to an increase in intracranial pressure, such as traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), intracranial tumor, intracranial hemorrhage, stroke, hydrocephalus, CNS infection, and fulminant hepatic failure.
The American College of Surgeons recommends intracranial pressure monitoring in TBI patients with a Glasgow Coma Scale score ≤8 and structural brain injury on CT.
There are a variety of modalities available to monitor intracranial pressure, although use of the extraventricular drain and intraparenchymal bolt is preferred in current practice.
Several noninvasive methods of intracranial pressure monitoring are currently under investigation.
The importance of intracranial pressure (ICP) was first recognized by Alexander Monro more than 200 years ago and is now referred to as the Monro-Kellie doctrine or the Monro-Kellie hypothesis. The Monro-Kellie doctrine states that (1) the brain is housed in the nonexpandable skull, (2) brain parenchyma is fairly noncompressible, and (3) the volume of blood is relatively constant, and outflow of venous blood is necessary for the inflow of arterial blood. Later, CSF was recognized as a component of brain volume in addition to brain parenchyma and blood, and it was incorporated into the doctrine. If there is a new intracranial mass lesion such as a tumor or hematoma or an abnormal increase in the volume of any of the components, such as CSF (during hydrocephalus) or parenchyma (during brain edema), the volume of venous blood or CSF or both will decrease to accommodate. However, this compensatory reserve is limited, and any further increase in the volume of the pathologic lesion will lead to an increase in ICP because of the rigid, nonexpandable skull. An increase in ICP will then result in a decrease in perfusion pressure and cerebral blood flow and eventually cerebral herniation and death.
For more than a century, there has been clinical interest in measuring ICP. Early efforts to measure ICP were based on the observation that because the cranial and spinal CSF compartments communicate with each other, their pressure should be equal. Measurement of spinal CSF pressure through lumbar puncture should therefore reflect cranial CSF pressure, or ICP. , However, it was soon recognized that measurement of opening pressure via lumbar puncture is associated with a risk for cerebral herniation in the presence of an intracranial mass lesion and that it may not reflect ICP if there is any obstruction to CSF flow between the cranial and spinal CSF compartments.
During the first half of the 20th century, several investigators measured ventricular fluid pressure in a small number of patients. Its clinical use, however, was limited until the 1960s, at which time the pioneering neurosurgeon Nils Lundberg started to measure ICP with a ventricular catheter connected to an external strain-gauge pressure transducer and a standard ink-writing potentiometer recorder. CSF drainage was also used to reduce ICP. This method of ICP monitoring and CSF drainage was used in more than 400 patients, many of whom had traumatic brain injury (TBI), and this marked the beginning of the modern era in ICP monitoring.
Today, ICP monitoring is an integral part of neurocritical care. ICP monitoring has been used in the management of patients with TBI, subarachnoid hemorrhage (SAH), intracranial tumor, intracranial hemorrhage, stroke, hydrocephalus, CNS infection, and fulminant hepatic failure. However, despite the adoption of ICP monitoring in the modern critical care unit and the establishment of an association of intracranial hypertension with increased mortality, the benefits of ICP-directed management strategies have been equivocal. Results from the first randomized controlled trial to evaluate ICP-directed therapy in severe TBI patients, the Benchmark Evidence from South American Trials: Treatment of Intracranial Pressure (BEST TRIP), have generated further controversy. This multicenter trial conducted in Bolivia and Ecuador compared ICP-directed management versus a novel CT imaging and clinical examination–guided management protocol and found no differences in morbidity or mortality measured at 6 months after injury. Analyses of the trial have scrutinized its design and challenged its generalizability given the study’s locale. Ultimately, the BEST TRIP study was not a trial of whether or not one should monitor ICP, but rather a comparison of two different management strategies for severe TBI. It highlights the need for a deeper understanding of the pathophysiology of TBI and the interpretation of ICP in context of other clinical, radiographic, and monitoring information to individualize care. While a consensus-based interpretation of the BEST TRIP data advises against changing practice if ICP is already routinely monitored, , results of the BEST TRIP study can also assist clinicians in developing an algorithm for treating suspected intracranial hypertension in the absence of ICP monitoring.
The application of ICP monitoring is recommended in guidelines from several national and international societies. The Brain Trauma Foundation (BTF) published the first evidence-based guidelines for managing severe TBI in 1996. ICP monitoring and ICP-lowering therapy recommendations composed the mainstay of treatment options for severe TBI in the early versions of the guidelines. , The latest edition of the BTF guidelines continues to recommend using information from ICP monitoring to reduce postinjury mortality in severe TBI patients. However, as a result of changes in methodologies for grading quality of supporting evidence, the recommendation for placement of an ICP monitor in all patients with GCS ≤8 and an abnormal CT scan was removed. Meanwhile, the American College of Surgeons Trauma Quality Improvement Project (ACS TQIP) best practices guidelines, developed using a combination of best available evidence and expert census, still advocate for the use of ICP monitoring in patients with a Glasgow Coma Scale (GCS) score ≤8 and structural brain injury on CT. Furthermore, they suggest ICP monitoring for patients with a higher GCS score who have structural injury with a high risk for progression, such as those with large contusions or coagulopathy and for patients who require urgent surgery for extracranial injuries. Consensus statements published by the Neurocritical Care Society and the European Society of Intensive Care Medicine also strongly recommend the use of ICP monitoring for other acute brain injuries such as SAH and encephalitis as part of structured management protocols.
Since the 1960s, there has been a continuous effort to develop new technology for ICP monitoring. Nils Lundberg outlined the basic requirements for an ICP monitor, which still apply today: minimal trauma during placement, negligible risk for infection, no CSF leakage, easy to handle, reliable, and able to continue to function during various diagnostic and therapeutic procedures. The Association for the Advancement of Medical Instrumentation developed the American National Standard for Intracranial Pressure Monitoring Devices, which specifies that an ICP monitoring device should have a pressure range of 0 to 100 mm Hg, accuracy of 2 mm Hg in the range of 0 to 20 mm Hg, and a maximal error of 10% in the range of 20 to 100 mm Hg. Throughout the years, many different ICP monitors have been developed, but only very few are in active clinical use today.
An external ventricular drain (EVD), or ventriculostomy drain, connected to an external strain gauge is currently the “gold standard” for measuring ICP. It remains the preferred method for monitoring ICP among neurosurgeons in the United States and is recommended by the ACS TQIP guidelines as the first monitor of choice. An EVD can be placed at the bedside in the emergency department, ICU, or operating room, depending on local practice tradition. Most practitioners use anatomic landmarks (freehand technique) to insert the ventricular drain into the lateral ventricle with the tip in the foramen of Monro ( Fig. 37.2 ). , The catheter can then be tunneled subcutaneously to minimize CSF leakage and infection. Ventricular fluid pressure, which represents ICP, is transmitted to an external strain-gauge transducer via the fluid-filled EVD. The strain-gauge transducer can be recalibrated without manipulation of the EVD. It can be connected to many standard ICU monitoring systems and allows ICP measurements to be displayed along with other physiologic data such as pulse, blood pressure, or central venous pressure.
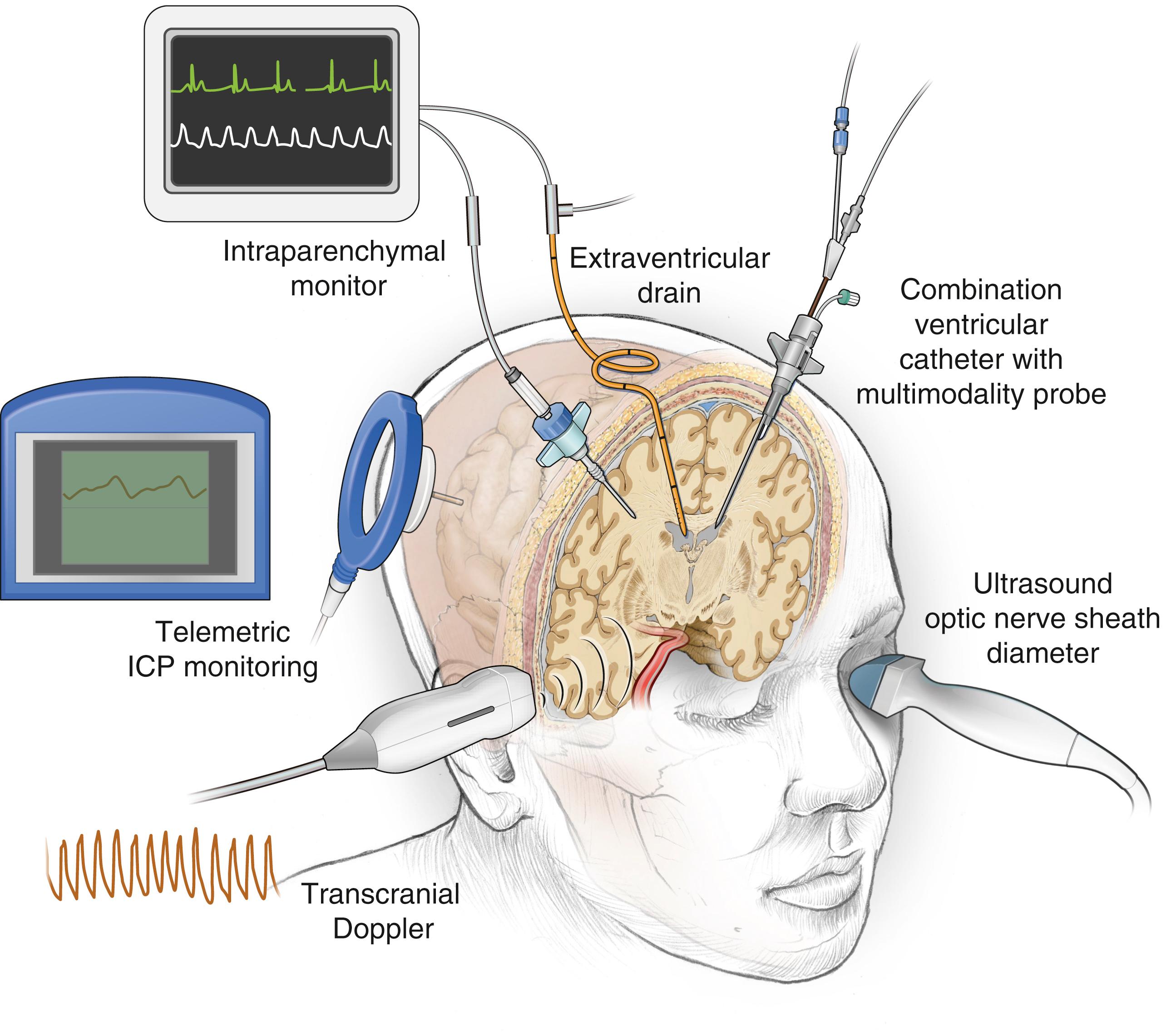
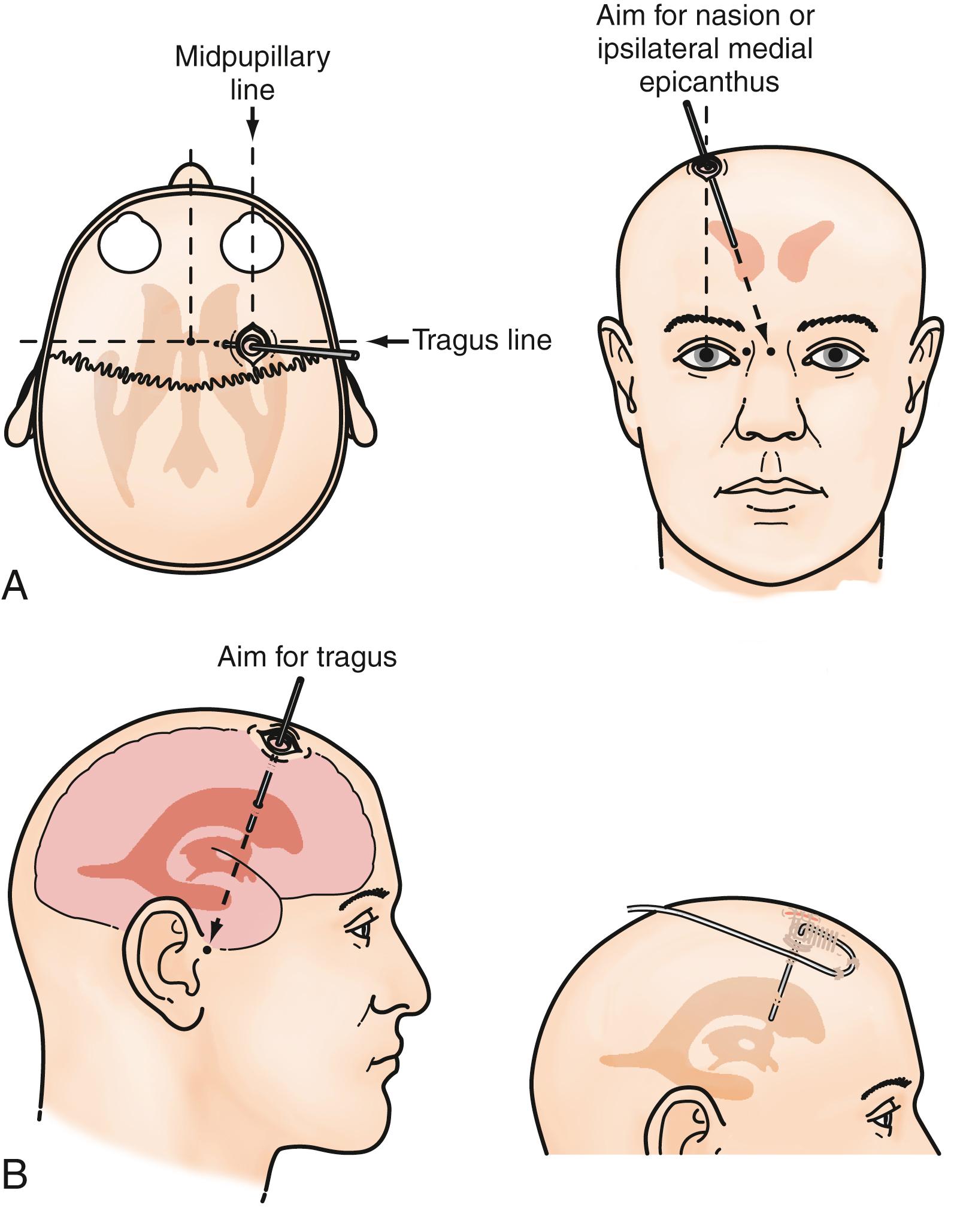
Advantages of the EVD as an ICP monitoring device include its extensive history, low cost, and reliability. , Most important, an EVD can also serve as a therapeutic device to remove CSF and lower ICP. , In patients with SAH or intraventricular hemorrhage, in which the elevated ICP is frequently a result of hydrocephalus, an EVD is the most appropriate ICP monitoring device, given its monitoring and therapeutic capabilities. However, an EVD has several weaknesses. Accurate placement of an EVD may be difficult with the freehand technique. In a recent survey of practicing neurosurgeons and residents, the success rate of cannulation of the ventricle was just 82%, even in the hands of practicing neurosurgeons. Currently, there is an EVD placement guide available that may increase the accuracy of placement of EVDs, although it is not widely used in the neurosurgical community. In some patients, it is simply not possible to place an EVD because of the small size of some ventricles or ventricular shift as a result of a mass lesion or severe edema.
Complications from EVD placement for ICP monitoring and CSF diversion include malposition, occlusion, hemorrhage, and infection. The malposition rate of EVDs ranges from 4% to 20%. Most of the misplaced EVDs did not cause any significant clinical sequelae, but about 4% of these EVDs did require replacement. , , Occlusion by brain matter or blood clot occurs frequently, especially in patients with intraventricular hemorrhage or SAH. Most of the occlusions can be resolved by flushing the EVD catheter. Hemorrhage secondary to EVD placement occurs infrequently. Hemorrhage rates ranging from 0% to 15% have been reported in the literature, with an average rate of 1.1%. , , Fortunately, most patients are asymptomatic from EVD-associated hemorrhage. , Clinically significant hemorrhage requiring surgical evacuation occurs about 0.5% of the time and results in intracerebral, subdural, and epidural hematoma. , , Coagulopathy is thought to be associated with an increase in hemorrhage rate, and therapeutic anticoagulants and antiplatelet agents are also known to be associated with an increased risk for hemorrhage. , In addition, laceration of a cortical artery can lead to traumatic pseudoaneurysm formation, and this complication has been reported with placement of ICP monitors.
The most significant risk associated with an EVD is infection. Biofilms have been isolated on 73% of EVD catheters after a median length of use of just 4 days. Lozier and coworkers performed an extensive review of all literature on infection associated with EVDs. The range of infection in all of the series was 0% to 22%, with a cumulative incidence of 8.8%. More recent studies have found a similar rate of infection. Clinical characteristics that have been identified to be related to increased EVD-associated infection include intraventricular hemorrhage, SAH, craniotomy, CSF leakage, systemic infection, increased CSF output, a history of diabetes mellitus, and depressed skull fracture. , , , Technical factors that may contribute to CSF infection include the duration of catheterization and irrigation of the catheter. In 17 studies reviewed by Lozier and colleagues, 10 studies found an association between the duration of catheterization and infection, whereas 7 did not find such an association. Careful inspection of the raw data of the latter group showed that there was an increased risk for infection after day 10 in one study. More recent studies also showed an increased risk for infection with longer duration of catheterization. , , Most studies have reported that there are few infections during the first 5 days of drainage and monitoring with an EVD but that the infection rate increases significantly after 5 to 10 days of catheterization. , ,
Because of the relatively high rate of CSF infection in patients with EVDs, multiple interventions have been used in an attempt to minimize the infection rate. However, most studies are retrospective in nature and often do not have enough statistical power to detect small absolute differences in the incidence of infection. Such interventions are discussed in the following sections.
Lozier and coworkers analyzed five studies that looked at whether there is a difference in infection rates in EVDs placed in the operating room, ICU, or emergency department All but one of the studies revealed no significant difference in infection rate whether the EVD was placed in the operating room, ICU, or emergency department. Two more recent studies also did not find statistically significant differences in infection rate related to the venue of ventriculostomy drain insertion. ,
Subcutaneous tunneling of the EVD catheter was reported by Friedman and Vries in 1980 as a way to reduce the infection rate. Other investigators then extended the distance of tunneling to the upper part of the chest or abdomen and had an infection rate of 4%. Two studies reported conflicting results. Sandalcioglu and Stolke reported that there was a significant difference in infection rate (83% vs. 17%) for catheters that were tunneled less than 5 cm subcutaneously versus catheters that were tunneled more than 5 cm, respectively. However, patient details were not available, and the infection rate of 83% in this study is significantly higher than most reported infection rates. Leung and coauthors, in contrast, did not find a significant difference in infection rate with long-tunneled EVDs. Most EVD insertion kits today contain a trocar for subcutaneous tunneling in excess of 5 cm.
The observation that the infection rate rises with increased duration of drainage from an EVD prompted several investigators to advocate prophylactic catheter exchange. , Several retrospective studies, however, showed no benefit of prophylactic catheter exchange, and there was, in fact, a higher incidence of infection in the group in which catheters were routinely exchanged. This was also observed in one prospective, randomized trial comparing the infection rate in a group that underwent prophylactic catheter exchange versus a control group that did not undergo prophylactic catheter exchange.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here