Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Angiography alone is often insufficient to assess intermediate and, at times, severe stenoses.
Although adjunctive stress testing has limitations, fractional flow reserve (FFR) provides an accurate detector of lesion specific myocardial ischemia.
Deferring revascularization of a coronary stenosis with a non-“ischemic” FFR has a favorable long-term outcome.
Percutaneous coronary intervention (PCI) guided by FFR is clinically and economically superior to angiography-guided PCI in patients with multivessel coronary artery disease.
Instantaneous wave-free pressure ratio (iFR) demonstrated noninferiority to FFR in low-risk patient subgroups.
The goal of coronary revascularization is the relief of ischemia and restoration of coronary blood flow. The treatment of coronary stenosis with stenting improves exercise tolerance, reduces antiischemic medications, and improves survival in patients with ST elevation myocardial infarction (STEMI). However, in patients with stable coronary artery disease (CAD), stent placement is of no benefit if the angiographic stenoses are not responsible for ischemia.
The rationale for physiologic lesion assessment is simple: for lesions of intermediate severity, the angiogram cannot be relied on exclusively to direct coronary revascularization. Coronary angiography produces two-dimensional (2D) luminograms, a silhouette image of the three-dimensional (3D) vascular lumen in a given projection. Angiography does not truly identify atherosclerosis, a disease within the vessel wall, but instead provides a “shadow” without intraluminal details sufficient to characterize a plaque. Furthermore, the eccentric shapes of plaques do not permit the observer to determine whether such an opening is limiting coronary blood flow. The accurate identification of both “normal” and “diseased” vessel segments by angiography further complicates the determination of a lesion’s significance in the setting of diffuse CAD, which cannot easily be seen on an angiogram. Angiographic artifacts that include contrast streaming, branch overlap, vessel foreshortening, calcifications, and ostial origins further make the interpretation of some luminal narrowings unreliable. Despite numerous attempts to improve the angiographic imaging of complex anatomy, the angiographer is still confronted by a visual dilemma in which no single view or multiple views can provide an answer. Moreover, even sophisticated imaging modalities such as densitometry, rotational angiography, and computed tomography angiography (CTA) with 3D reconstruction (without the computational fluid-dynamic addition of fractional flow reserve computed tomography [FFRCT]) do not reliably reflect the physiologic significance of a given lesion.
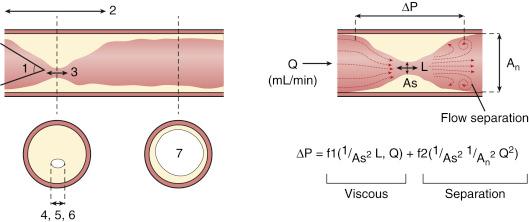
Coronary blood flow can increase from a resting level to a maximum (i.e., coronary reserve), depending on increases in myocardial oxygen demand or in response to neurogenic or pharmacologic hyperemic stimuli. Normally, large epicardial vessel resistance to blood flow is negligible. Most of the regulation of coronary flow occurs in the myocardial precapillary arteriolar resistance vessels. In a normal adult artery that supplies normal myocardium, coronary blood flow can increase more than threefold. However, several conditions—including left ventricular hypertrophy, myocardial ischemia, and diabetes—can affect the microcirculation, blunting the maximal absolute increase in coronary flow or increasing resting flow above the expected level for myocardial oxygen demand at rest. The regulation of coronary vasomotor tone and the influence of several mechanisms such as α-adrenoreceptor–mediated vasoconstriction have been extensively reviewed elsewhere and are beyond the scope of this chapter. A significant atherosclerotic stenosis produces epicardial conduit resistance and, depending on flow, loss of distal pressure. In response to the loss of perfusion pressure and flow to the distal (poststenotic) vascular bed, the small resistance vessels dilate to maintain satisfactory basal flow appropriate for myocardial oxygen demand. Viscous friction, flow separation forces, and turbulence at the site of the stenosis produce energy loss at the stenosis ( Fig. 5.1 ). Energy (heat) is extracted, which reduces pressure distal to the stenosis and thereby produces a pressure gradient between the proximal and distal arterial regions. The pressure loss or pressure gradient increases with increasing coronary flow in a curvilinear manner. An absolute poststenotic myocardial perfusion pressure threshold exists, below which myocardial ischemia may be easily induced.
As the severity of an epicardial stenosis increases, maximal coronary flow becomes attenuated, and coronary flow reserve (CFR) decreases. CFR, the ratio of maximal to basal arterial flow, is a combined measure of the capacity of the major resistance components—the epicardial coronary artery and the supplied vascular bed—to achieve maximal blood flow in response to hyperemic stimulation. Although there is no true “normal CFR,” a higher CFR implies that both epicardial and microvascular bed resistances are low (i.e., normally low resistance; Fig. 5.2 ). However, a lower CFR (<2.0) does not indicate which component is affected, a fact that limits the clinical applicability of this measurement for lesion assessment. Although early studies in animals and humans indicated an absolute “normal” range for CFR of 3.5 to 5, the CFR in adult patients undergoing cardiac catheterization with chest pain syndromes and CAD risk factors with “angiographically normal” vessels was 2.7 ± 0.6, suggesting a degree of patient variability and microvascular disease.
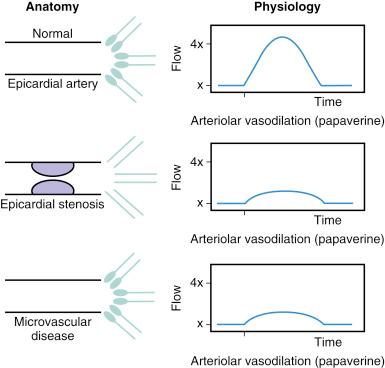
CFR may be reduced in patients with normal coronary arteries and either essential hypertension or aortic stenosis (AS), in part related to myocardial hypertrophy and an abnormal microvasculature. Furthermore, CFR can be altered by changes in basal and hyperemic flows, both of which are influenced by hemodynamics, loading conditions, and contractility. For example, tachycardia increases basal flow and decreases hyperemic flow, thus reducing CFR by 10% for each 15-beat increase in heart rate. In clinical terms, CFR is best used to assess the microcirculation in the absence of epicardial artery narrowings.
As the limitations of invasive CFR were recognized, the development of pressure-sensor guidewires yielded a new concept of lesion assessment. As is the case from the earlier discussion regarding coronary flow, resistance to blood flow in a normal epicardial coronary artery is negligible, with virtually no energy loss as blood traverses the vessel. The aortic pressure thus remains constant throughout the conduit—including all branch vessels, regardless of their size—in the absence of epicardial disease. In the case of epicardial coronary narrowing, the resistance to flow and associated energy loss results in a pressure drop and is proportional to flow. To maintain resting myocardial perfusion at a constant level, a decrease in myocardial resistance compensates for any resistance of flow caused by the epicardial narrowing. The pressure ratio between normal and poststenotic pressure at constant maximal flow can represent an index of the physiologic consequences of a given coronary narrowing on the myocardium. Pressure measurements, made across a lesion during maximal hyperemia, are termed the myocardial fractional flow reserve , a concept introduced in 1993 by Pijls and colleagues. FFR is the ratio of the maximal myocardial blood flow in the presence of a stenosis relative to expected normal flow in the absence of a stenosis, and it can be expressed as a fraction of its normal expected value if there were no lesion. Derived from pressure data alone for the myocardium, FFR measurement is based on several assumptions regarding translesional pressure measured during maximal hyperemia. The proposed equations have been derived from a theoretic model of the coronary circulation and have been validated experimentally in instrumented dogs, and later in humans by comparison with myocardial flow measured by positron emission tomography (PET). During maximal hyperemia (induced pharmacologically), coronary resistance is at the lowest level and remains constant, so that flow is directly related to the measured pressure. The total myocardial blood flow (Qn) in an area served by a coronary artery with a stenosis is the sum of the flow through the stenosis (Qs) and the collateral flow (Qc). Fractional flow reserve is then simply defined as the ratio of the measured flow (Qs) to the maximal flow that should be present without any stenosis (Qn):
and
where Pd is distal coronary pressure, Pa is aortic pressure, Pv is venous pressure (or right atrial pressure), and R is resistance of the myocardial vascular bed. In this model, Pv is assumed to be negligible, hence:
Given maximal hyperemia, resistance becomes constant and “near zero” in both the numerator and denominator, thus:
FFR can thus be estimated as the ratio of the mean distal coronary blood pressure to the mean aortic blood pressure. Because each myocardial territory serves as its own control, FFR is a lesion-specific index. Furthermore, because FFR is measured only at maximal hyperemia, it is independent of microcirculation, heart rate, blood pressure, and other hemodynamic variables.
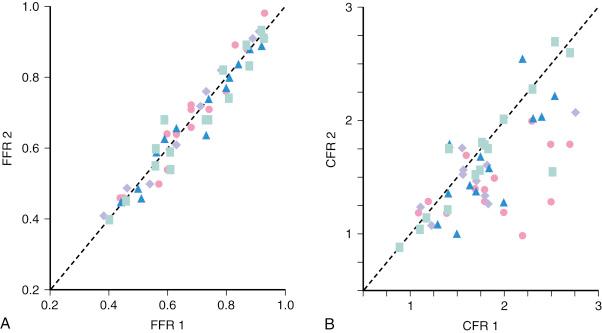
Unlike most other physiologic indexes, FFR has a normal value of 1.0 for every patient and every coronary artery. FFR has high reproducibility and low intraindividual variability ( Fig. 5.3 ). Moreover, unlike CFR, FFR is independent of sex or CAD risk factors, such as hypertension and diabetes, and has less variability with common doses of adenosine. De Bruyne and associates demonstrated that in humans, FFR is independent of hemodynamic conditions. Changes in heart rate affected by pacing, changes in contractility affected by dobutamine infusion, and changes in blood pressure affected by nitroprusside infusion did not alter FFR. The coefficient of variation between two consecutive measurements was 4.2%, which is lower than the 17.7% for CFR measured with a Doppler wire.
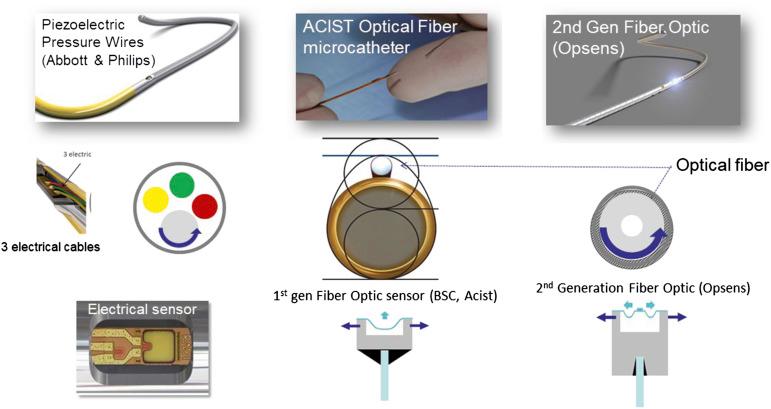
The measurement of coronary pressure is similar to performing an angioplasty in that a sensor guidewire is passed through an angioplasty Y-connector attached to a guiding catheter, with anticoagulation (intravenous [IV] heparin) given beforehand. To minimize measurement variability caused by vessel spasm, intracoronary (IC) nitroglycerin (100 to 200 μg) is given before the guidewire is advanced into the artery. For coronary pressure measurements, a guidewire with a pressure sensor can be used to measure distal coronary pressure. The angioplasty sensor guidewires ( Fig. 5.4 ) have mechanical properties close to standard “workhorse” guidewires and have a pressure sensor located 3 cm from the tip, at the junction of the radiopaque and radiolucent portions of the wire. The wire tip can be shaped to facilitate delivery to the distal vessel. Recently, a novel 0.022-inch pressure-sensing monorail microcatheter has been made available to measure distal coronary pressure over any 0.014-inch standard guidewire of the operator’s choice. The microcatheter has a pressure sensor associated with a marker that sends its signal via a fiberoptic pathway to the table-mounted analyzer (see Fig. 5.4 ).
All currently available systems have an “auto zero” feature that activates when the guidewire/catheter is connected to the analyzer. This signal should be zeroed against the “zero” from the guiding catheter transducer. The sensor is then introduced and positioned at the tip of the guiding catheter, where the guiding catheter and wire pressures are equalized (or normalized)—a step that electronically assimilates both pressure signals so that they are identical. Next, the sensor is advanced down the vessel and across the stenosis (or to the most distal part of the coronary artery for assessment of serial lesions or diffuse disease). A pharmacologic hyperemic stimulus (e.g., adenosine, discussed later) is then administered through the guiding catheter, or it can be given by IV infusion. The mean and phasic pressure signals are continuously recorded, and at peak hyperemia—represented by the steady-state nadir, or lowest, distal pressure once stable hyperemia is reached—the FFR is calculated as noted earlier ( Fig. 5.5 ).
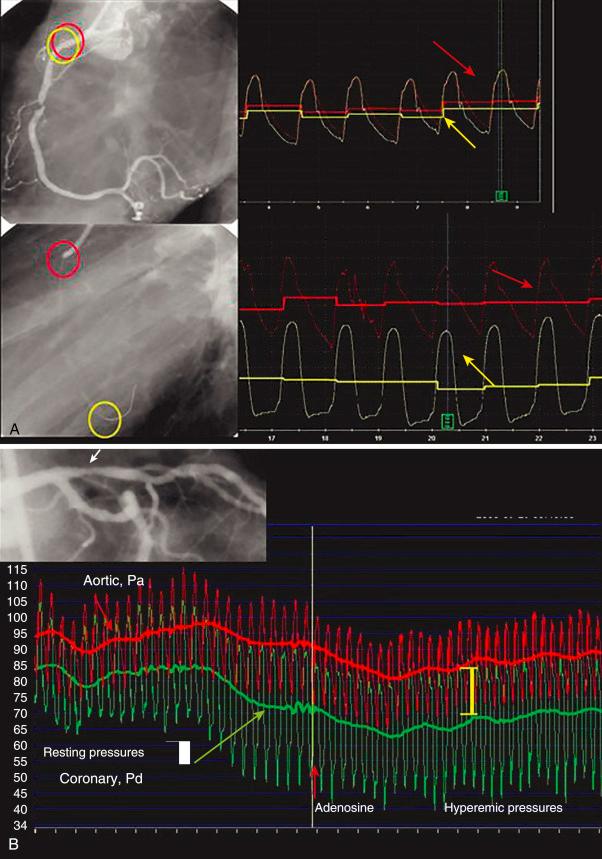
To study the distribution of abnormalities along a diseased coronary artery (with serial lesions or diffuse disease), the pressure sensor can be pulled back slowly during intravenously induced hyperemia. Simultaneously observing the location of the wire by fluoroscopy and the pressure tracings can pinpoint the location of hemodynamically significant atherosclerotic abnormalities. On pulling back the pressure sensor in a vessel with diffuse but not focal obstructions, gradual pressure recovery along the course of the vessel can be observed. In contrast, a vessel with a focal stenosis will demonstrate an abrupt increase in pressure proximal to the lesion. By moving the sensor back and forth, the exact location of a pressure drop, representing a focal obstruction to flow, can be determined. FFR is often measured using 6-Fr guiding catheters, but 5-Fr guides and diagnostic catheters as small as 4 Fr have been successfully used. In general, the smaller the lumen of the catheter, the greater care the operator must take at flushing the catheter to optimally achieve an accurate aortic pressure signal (Pa). As with any technique in the catheterization lab, attention to detail is required to reduce inaccuracies in the measurement of FFR. A more complete description of the application and nuances of coronary pressure measurements can be found elsewhere.
Qian and coworkers examined the safety of IC Doppler wire measurements in 906 patients. Of these, 15 patients (1.7%) had severe transient bradycardia after administration of IC adenosine, 14 in the right coronary artery (RCA) and 1 in the left coronary artery (LCA). Nine patients (1%) had coronary spasm during passage of the Doppler guidewire, five in the RCA and four in the left anterior descending (LAD) coronary artery. Two patients (0.2%) had ventricular fibrillation (VF) during the procedure. Hypotension with bradycardia and ventricular asystole occurred in one patient. Transplant recipients had more of these complications than did patients undergoing either diagnostic or interventional procedures. All complications were easily managed, and no long-term adverse consequences were observed. These data support the safe clinical practice of sensor-wire measurements with IC adenosine.
Maximal coronary hyperemia is required for in-lab coronary physiologic lesion assessment ( Table 5.1 ). The most widely used maximal vasodilator agent at this time is adenosine. Hyperosmolar ionic and low-osmolar nonionic contrast media do not produce maximal vasodilation. Nitrates increase volumetric flow, but because these agents also dilate the epicardial conductance vessels, the increase in coronary flow velocity is less than with adenosine or papaverine. IC nitroprusside has similar hyperemic effects compared with IC adenosine. Papaverine is no longer used for IC hyperemic stimulation because of the occasional QT interval prolongation and associated ventricular tachycardia (VT) or VF.
| Drug | Dose | Plateau (s) | Half-Life (min) | Side Effects | Comments |
|---|---|---|---|---|---|
| Papaverine IC | 15 mg LCA 10 mg RCA |
30–60 | 2 | Transient QT interval prolongation, torsades de pointes | Rarely used |
| Adenosine IV | 140 μg/kg/min | 60–120 | 1–2 | Decreased blood pressure (10%–15%), chest burning | Avoid in patients with history of bronchospasm |
| Adenosine IC | 100–200 μg LCA 50–100 μg RCA |
5–10 | 0.5–1 | Transient AV block when injected into the dominant artery | Must repeat with escalating doses to ensure that maximal hyperemia is reached |
| Dobutamine IV | 20–40 μg/kg/min | 60–120 | 3–5 | Tachycardia, increase in blood pressure | May induce ischemia |
| Nitroprusside IC | 0.3–0.9 μg/kg | 20 | 1 | Decreased blood pressure (20%) | |
| Regadenoson IV | 0.4 mg | 30 | 2–4 a | Tachycardia | Exact length of hyperemia and ability to repeat bolus not studied in the cardiac catheterization lab for FFR |
a Half-life is triphasic, with second phase lasting approximately 30 minutes and third phase lasting approximately 2 hours.
A principal advantage of adenosine is that it has a short half-life, with a return to basal flow within 30 to 60 seconds after cessation of infusion. Adenosine is benign in a wide range of dosages (IC and IV). Typically, IV adenosine will result in approximately a 10% drop in mean arterial pressure (MAP) and may be accompanied by symptoms of chest burning. IC adenosine down the dominant coronary artery will result in atrioventricular (AV) block at high enough doses that it will result in a significant, although transient, decline in MAP.
IV adenosine has the advantages of simplicity and weight-adjusted dosing (140 μg/kg/min) and is required for the evaluation of ostial lesions or for the assessment of diffuse disease during pullback recordings. IV administration, however, tends to have a higher incidence of side effects such as flushing, chest tightness, bronchospasm, nausea, and transient AV block or bradycardia compared with IC dosing. It should also be noted that all the validation studies utilizing IV adenosine administered this infusion through a central vein. Administration through a peripheral vein may be accompanied by a long latent period before the onset of hyperemia without a clear-cut stability of the hyperemic phase.
IC adenosine appears to be equivalent to IV infusion for determination of FFR in most patients. Jeremias and colleagues examined differences in FFR between IC adenosine (15 to 20 μg in the RCA or 18 to 24 μg in the LCA) and IV adenosine (140 μg/kg/min) in 52 patients with 60 lesions and found a strong linear relationship between IC and IV adenosine (regression coefficient [ r ] = 0.978, P < .001). The mean measurement difference for FFR was 0.004 (standard deviation [SD] ± 0.03). A small random scatter in both directions of FFR was noted in 8.3% of stenoses, where the IC adenosine FFR value was 0.05 greater than the IV adenosine FFR value, suggesting a suboptimal IC hyperemic response for which a repeated, higher IC adenosine dose may be helpful. Changes in heart rate and blood pressure were significantly greater with IV adenosine. Two patients with IV adenosine, but none with IC adenosine, had side effects such as bronchospasm and nausea. Despite these doses being correlated with hyperemic effects of IV adenosine, current dose recommendations are 50 to 100 μg in the RCA and 100 to 200 μg in the LAD to ensure maximal hyperemia is achieved.
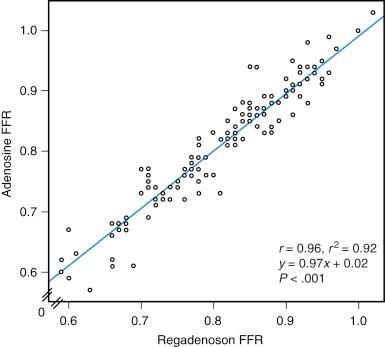
Regadenoson is a low-affinity α-2A adenosine receptor agonist that causes coronary vasodilation and increased myocardial blood flow and has been reported to be equivalent to adenosine. It selectively targets the α-2A receptor in coronary arteries and therefore has fewer adverse effects compared with adenosine. Regadenoson has a longer half-life of 2 to 3 minutes in the initial phase, 30 minutes in the intermediate phase, and 2 hours in the terminal phase, and it may prove to be easier to use. With a single infusion bolus of regadenoson (0.4 mg), maximum coronary hyperemia is achieved and maintained equivalent to that achieved with a constant infusion of adenosine. However, questions regarding the amount of time that hyperemia persists after this bolus and whether a similar hyperemic effect can be achieved with a second bolus demand further investigation. At this time, regadenoson is a useful agent for simple measurement of FFR ( Fig. 5.6 ).
Bartunek and associates examined FFR in response to IC adenosine and IV dobutamine (10 to 40 μg/kg/min) in 22 patients with single-vessel CAD. Peak dobutamine infusion produced similar distal coronary pressures and pressure ratios (Pd/Pa, 60 ± 18 and 59 ± 18 mm Hg; FFR, 0.68 ± 0.18 and 0.68 ± 0.17, respectively; all P = not significant [NS]). An additional bolus of IC adenosine given at peak dobutamine in nine patients failed to change the FFR. As shown by angiography, high-dose IV dobutamine did not modify the area of the epicardial stenosis, and, much like adenosine, it fully exhausted myocardial resistance regardless of inducible left ventricular dysfunction.
IC nitroprusside may be an alternative to IC adenosine. Parham and associates examined coronary blood flow velocity, heart rate, and blood pressure in unobstructed LAD arteries in 21 patients at rest, after IC adenosine (boluses of 30 to 50 μg), and after three serial doses of IC nitroprusside (boluses of 0.3, 0.6, and 0.9 μg/kg). IC nitroprusside produced equivalent coronary hyperemia with a longer duration (∼25%) compared with IC adenosine. IC nitroprusside (0.9 μg/kg) decreased systolic blood pressure by 20% with minimal change in heart rate, whereas IC adenosine had no effect on these parameters. FFR measurements with IC nitroprusside were identical to those obtained with IC adenosine ( r = 0.97). In doses commonly used for the treatment of the no-reflow phenomenon, IC nitroprusside can produce sustained coronary hyperemia without detrimental systemic hemodynamics. Sodium nitroprusside also appears to be a suitable hyperemic stimulus for coronary physiologic measurements.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here