Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
THE USE OF CATHETERIZATION in the care of children with congenital heart disease (CHD) was first described by Dexter and colleagues in 1947 with the first interventional procedure, balloon atrial septostomy, subsequently described by Rashkind and Miller in 1966. Over the intervening decades the discipline of pediatric interventional cardiology has vastly expanded within the field of pediatric cardiovascular medicine.
Technologic advances have increased the scope of pediatric cardiac catheterization procedures. As echocardiography, computed tomography (CT), and magnetic resonance imaging (MRI) diagnostic capabilities have increased, the need for purely diagnostic cardiac catheterization has waned. There has been a transition from diagnostics toward more therapeutic interventions, with interventional procedures now accounting for more than two-thirds of all pediatric cardiac catheterizations. Interventional cardiology has afforded patients a wider range of nonsurgical options for the management of CHD, postponing or replacing the requirement for surgery. Hybrid procedures, combining interventional cardiology with cardiac surgery, have enabled the management of more complex cardiac lesions. As more children with CHD survive into adulthood, there has been a shift in patient demographics. Children presenting for interventional cardiology represent a diverse group from premature neonates to older adolescents, with simple to complex pathophysiology, requiring procedures of varying complexity. Catheterization laboratories are often remote sites and can be challenging environments. Anesthesiologists providing care to children undergoing cardiac catheterizations must have a good understanding of the patient's pathophysiology and tailor anesthesia to the patient- and procedure-specific requirements. Adverse events are common during catheterization procedures. The anesthesiologist must be able to anticipate, prevent, and treat complications that may arise in this high-risk population.
In this chapter, we outline the main procedures performed in interventional cardiology, describe the challenges and complications faced by anesthesiologists, and address the principles and details of anesthetic techniques.
Diagnostic catheterization allows accurate documentation of pressure and oxygen content from different regions of the circulation. Interpretation of these hemodynamic data allows quantification of the degree of intracardiac shunting and calculation of vascular resistances. This information is necessary to guide critical decisions, such as the suitability of a child to undergo palliative or reparative surgery for congenital heart lesions, and to direct medical therapy. Consistent and reproducible physiologic conditions are required during the procedure to allow correct interpretation and comparison of results. Expected values for hemodynamic variables are listed in Table 22.1 . There are no absolute values for these variables, which vary with the age of the child. The use of angiocardiography during catheterization to define complex cardiac anatomy is waning because of the increased use and capabilities of noninvasive imaging modalities such as echocardiography, CT, and MRI.
| Structure | Value (mm Hg) |
|---|---|
| Right atrium | 3–5 (mean) |
| Right ventricle | 20–25/3–5 (systolic/end-diastolic) |
| Pulmonary artery | 12–15 (mean) |
| Left atrium | 7–10 (mean) |
| Left ventricle | 65–110/3–5 (systolic/end-diastolic) |
| Aorta | 65–110/35–65 (systolic/diastolic) |
Interventional procedures now account for approximately two-thirds of all cardiac catheterization cases in children of all ages.
Atrial septostomy involves passing a balloon-tipped catheter across the atrial septum, typically through the membranous foramen ovale. The balloon is inflated and pulled back through the septum creating a larger intraatrial communication that improves mixing of oxygenated and deoxygenated blood at the atrial level. The procedure is most commonly performed in neonates with d -transposition of the great vessels ( d -TGA) as an emergency procedure. Children with d -TGA have systemic and pulmonary circulations in parallel rather than in series; the right ventricle pumps deoxygenated blood to the aorta, and the left ventricle pumps oxygenated blood to the main pulmonary artery. Atrial septostomy allows critical mixing of oxygenated and deoxygenated blood, thereby increasing systemic oxygen saturation. The procedure can be performed in the catheterization laboratory under fluoroscopic guidance or undertaken at the bedside in the intensive care unit using echocardiographic guidance. Other indications for atrial septostomy include tricuspid atresia, mitral atresia, and pulmonary atresia with intact ventricular septum. The major risks associated with the procedure are vessel injury, paradoxical embolism, arrhythmia, and cardiac perforation. More recent concerns of an association with increased brain injury do not seem to have been evidence-founded.
With the development of specifically designed closure devices, atrial septal defect (ASD) closure has become one of the most commonly performed endovascular procedures. The technique is intended for closing secundum ASDs, which are defects located in the region of the fossa ovalis. Defects falling outside this area, such as sinus venosus and primum ASDs, are generally not suitable for percutaneous closure.
To warrant closure, children need to demonstrate clear evidence of volume loading of the right heart structures and a defect that is unlikely to close spontaneously in the short to medium term. Devices have evolved from the early bulky meshes and a wide variety of sizes and designs are now available. The choice of closure device depends on the size and margins of the defect, but brand selection depends more on the clinician's preference than objective performance ( Fig. 22.1 ). Daily aspirin in a dose of 3 to 5 mg/kg is recommended for a minimum of 6 months after the procedure. The main perioperative complications associated with ASD device closure include vessel injury, cardiac arrhythmia, cardiac perforation, and device embolization. In the long term, devices are well tolerated with atrial arrhythmias the most common problem, although life-threatening complications such as cardiac erosion have been reported. ASD closure devices can also be used to close surgically created fenestrations when they are no longer required, such as between the atrium and venous conduits after a Fontan operation.
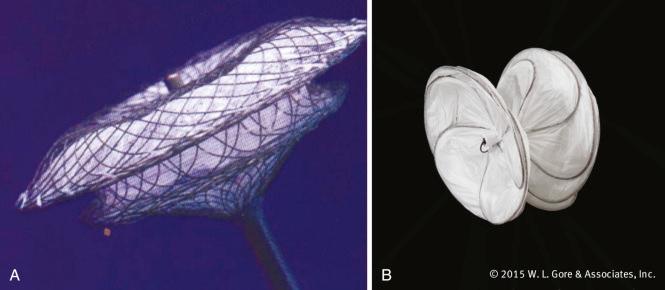
Ventricular septal defect (VSD) closure presents a technically greater challenge than ASD closure and is associated with a greater incidence of complications. Defects of the midmuscular septum or apex are most suitable for device closure, although perimembranous VSDs may also be closed percutaneously. A combined surgical and interventional approach may be appropriate for certain cases, such as the small infant with an anterior apical VSD that is difficult to reach surgically.
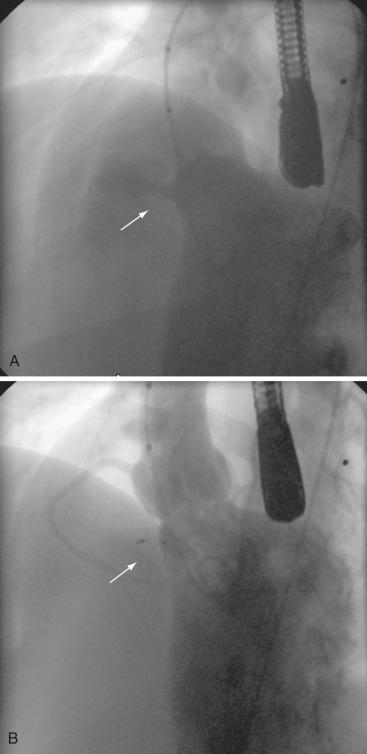
When using a device to close a VSD, a snare is placed in the right side of the heart to capture a guidewire that has been passed across the VSD from the left ventricle. The guidewire is brought outside the body via the venous access to form an arteriovenous rail. The delivery sheath for the VSD device is then advanced over the wire to approach the VSD from the right side of the heart. For anterior and high muscular defects, the wire is best snared and exteriorized through a femoral vein approach, whereas for defects in the middle to low muscular septum, the wire is best snared and exteriorized through a jugular venous approach ( Fig. 22.2 ). Complications include dysrhythmias, blood loss, valve dysfunction, low cardiac output state, and device embolization. Device closure of perimembranous VSDs in particular has been associated with an increased incidence of complete heart block and aortic valve disruption owing to the close proximity of conduction pathways and the aortic valve apparatus. This has led to some controversy in their use, with many centers considering the risk unacceptable, given the good outcomes following surgical VSD closure.
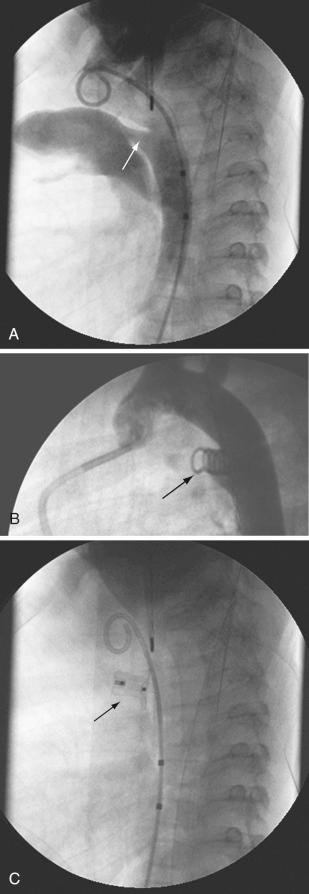
Transcatheter closure of patent ductus arteriosus (PDA) was the second specific intervention developed for children with CHD, and the procedure continues to be performed using techniques similar to the original methods pioneered by Rashkind and associates. The procedure has a high occlusion rate with the smallest incidence of adverse events of all interventional procedures and is indicated in any child with evidence of left ventricular overload. PDA morphology varies from short and broad to narrow and tortuous, so the customary approach is to perform an aortogram to define the size and geometry. For closure of small and moderate PDAs, stainless steel coils (e.g., Gianturco, Cook Medical, Bloomington, IN) are most commonly used, whereas for larger PDAs, occluder devices (e.g., Amplatzer, St. Jude Medical, St. Paul, MN) are more appropriate. Devices can be deployed with a retrograde or anterograde approach. Embolization of the device at the point of deployment is a risk but there are several techniques that can help control device release. Embolization may also be delayed, so proper follow-up is required; the other major risk associated with this procedure is vessel injury. Despite impressive technical evolution over the years, PDA device closure in premature or low body weight infants remains prohibitively challenging and further catheter design is required in this area. These techniques can also be used to close other unwarranted vascular connections such as major aortopulmonary collateral arteries (MAPCAs) and Blalock-Taussig (BT) shunts that are no longer required ( Figs. 22.3 and 22.4 ).
Balloon angioplasty techniques are used to dilate stenotic valves, most commonly pulmonary and aortic, as well as stenotic segments of the aorta or pulmonary arteries and surgically placed shunts or pulmonary artery bands. Neonatal membranous pulmonary atresia can be treated using this technique, crossing the pulmonary valve membrane with the stiff end of a guidewire or with radiofrequency catheters before subsequent dilatation of the valve annulus. Balloon valvuloplasty of isolated pulmonary stenosis in older infants and children is often a curative procedure, whereas neonates with critical pulmonary stenosis or atresia often require further interventions to supplement pulmonary blood flow such as ductal stenting or a BT shunt. Inflation of the balloon temporarily arrests right ventricular output and is often accompanied by transient hypotension but is generally well tolerated. Paradoxically, those with tighter stenosis and little antegrade flow may maintain hemodynamic stability better, particularly if the duct is still open, as cardiac output is less disrupted.
The role of balloon valvuloplasty for critical aortic stenosis remains controversial; in some centers, it remains the treatment of choice, whereas others prefer a primary surgical approach. Concerns relate to longer-term outcomes and the rate of surgical intervention required in those treated initially with valvuloplasty. When performed, balloon dilation of aortic stenosis in the neonate is a particularly high-risk procedure. These infants often present in a low–cardiac output state requiring ventilation, inotropic support, and prostaglandin E 1 (PGE 1 ) infusion to maintain ductal patency. Catheterization can be complicated by arrhythmias (including asystole), the development of significant aortic regurgitation (which may require surgical intervention), and sudden death resulting from acute coronary ischemia. The complication rate in older children is less than in younger children, yet transient hypotension, bradycardia, and left bundle branch block are commonly reported.
Stents are sometimes implanted across focal areas of persistent stenosis in the systemic and pulmonary circulations or within surgically placed shunts. The technique of implantation requires great precision for correct positioning of the stent, and the cardiac interventionalist needs to take into account the inevitable shortening that occurs with stent implantation when selecting a device for a particular lesion. There is also a risk of stent malposition with the potential for dislodgment. Although rare, late aneurysm formation has been reported after stenting the aorta for coarctation.
Bonhoeffer and colleagues first described the technique of replacing a dysfunctional valve in a right ventricle to pulmonary artery (RV-PA) conduit with a catheter-implanted valve in 2000. The Melody valve (Medtronic Inc., Minneapolis, MN) is one such device that has been approved for use in surgically placed RV-PA conduits meeting specific requirements. Studies have confirmed a high procedural success rate and satisfactory short-term and medium-term valve function after implantation of the Melody valve. The need for careful patient selection and for adequate relief of right ventricular outflow tract (RVOT) obstruction at the time of valve implantation are paramount in achieving results comparable to those of surgical replacement of dysfunctional conduits. Off-label use has additionally demonstrated promising results with implantation of Melody valves in children with native RVOTs. Unfortunately, most children who are managed with transannular patch repair of tetralogy of Fallot are currently unsuitable for transcatheter valve replacement because of an aneurysmal RVOT. New hybrid procedures are, however, emerging to circumvent this, allowing more options for patients with tetralogy of Fallot, many of whom are likely to require recurrent interventions over their lifetime. Alternative devices are also being developed to reduce the size of the delivery systems to enable use of these technologies in younger and smaller children. As with surgical valve replacements, thrombus formation and infective endocarditis are concerns after transcatheter valve implantation.
Endocardial catheters that record an electrocardiogram first became available in the early 1960s. Intracardiac electrograms, supplemented by external electrocardiogram leads, record electrical activity in the heart, and programmable stimulators aim to induce and terminate tachyarrhythmias. This allows arrhythmia mechanisms to be defined and arrhythmogenic foci to be mapped. Ablation catheters can then be guided to deliver local energy to the endocardium to destroy the source of the arrhythmia or interrupt aberrant conduction pathways. Ablation can be delivered as radiofrequency, cryoablation, laser therapy, or direct currents. Ablative procedures are indicated for the treatment of cardiac arrhythmias, particularly atrial tachyarrhythmias.
The procedures are complex, requiring specialized equipment, specially trained staff, and use of multiple catheters to measure electrical signals. These procedures are often of greater duration than other catheterization procedures. Ablation of ectopic foci has a good success rate and small complication rate. The main risks with this procedure are heart block, cardiac perforation, vessel injury, and stroke.
The most common approach for cardiac catheterization is the femoral route. Femoral venous catheterization avoids the risk of pneumothorax and the vessel is often easier to access than the internal jugular vein in the unanesthetized child. In children who are likely to have a cavopulmonary shunt placed surgically for palliation, avoiding routine cannulation of the internal jugular vein may decrease the risk of compromising the superior vena cava. Internal jugular vein access, however, is sometimes required, such as during some VSD closures, investigation of cavopulmonary connections in children, and when the cardiac interventionalist is unable to obtain femoral access. In neonates, the umbilical vein may be used, although difficulty can be encountered attempting to cross the ductus venosus to access the inferior vena cava. Patency of the ductus venosus can be assessed by ultrasound before the procedure to avoid unnecessary manipulation of the umbilical vein. An alternative route is transhepatic puncture. This route has been used for temporary access during catheterization and for long-term vascular access.
The pediatric cardiac catheterization laboratory is a high-risk environment associated with significant morbidity and mortality. Of the 373 anesthesia-related cardiac arrests reported in the Pediatric Perioperative Cardiac Registry, 34% occurred in children with cardiac disease and 17% of these occurred in the catheterization laboratory. Additionally, there are complications directly attributable to the procedures. Successful practice in this setting requires a thorough understanding of pathology, vigilance, excellent communication between team members, and appropriate backup from surgeons and other specialties. Standard procedures for predictable emergencies should be in place. An intensive care bed should be available for high risk procedures. The facility for cardiopulmonary bypass or extracorporeal membrane oxygenation (ECMO) for high-risk patient groups or procedures may be beneficial, although this type of therapy may not be available everywhere. Local policies on appropriate case mix should be based on experience and infrastructure and clear referral pathways for high-risk cases should be established.
Recent studies of pediatric catheterization have reported mortality rates in the immediate perioperative period (in theatre or recovery) of between 0.05 and 0.28%. However, the IM proving P ediatric and A dult C ongenital T reatment (IMPACT) database of more than 16,000 pediatric catheterizations reports all-cause mortality for children undergoing catheterization during their admission as 2.1%, with the majority of deaths occurring within 14 days of the procedure. Despite the increasing complexity of both patients and procedures being undertaken, the mortality rate is decreasing.
Adverse events occur in 8.8% to 10.6% of catheterization procedures overall. Morbidity may be classified as either major or minor. Major complications are life-threatening events requiring immediate medical or surgical intervention (e.g., cardiac arrest, removal of an embolized device) or which result in a significant permanent lesion (e.g., embolic stroke, vessel aneurysm). These types of adverse events occur during 1% to 2% of procedures. Minor complications are transient and resolve with specific treatment (e.g., tolerated dysrhythmias, transient arterial thrombosis). Complication rates have remained largely constant despite technologic advances, reflecting the changing nature of the patient population and interventions.
Certain patient and procedural characteristics confer increased risk of complications. Neonates are particularly vulnerable, with a reported rate of major complications of 4.7% for interventional catheterization, which may be explained by reduced physiologic reserve, presence of uncorrected or partially palliated congenital heart defects, and an increased risk of obstruction to great vessels and cardiac chambers by wires and devices. Age younger than 1 year and low body weight have been identified as independent risk factors for complications. Specific cardiac lesions are also associated with greater risks of both morbidity and mortality, notably single ventricle physiology, whether unrepaired or palliated, and significant left ventricular outflow tract obstruction (e.g., Williams-Beuren syndrome and hypertrophic cardiomyopathy). The presence of pulmonary hypertension, particularly with an idiopathic etiology and suprasystemic pulmonary artery pressures, increases perioperative risk substantially. With respect to procedural risk, interventional procedures have greater complication rates than diagnostic procedures for all age groups except neonates, in whom the complication rates are similar. Specifically, high-risk procedures include VSD device closure; atrial septostomy where the septum is restrictive; balloon interventions for the mitral valve, pulmonary vein, pulmonary artery, and neonatal aortic valve; and stent placement in surgical shunts. At the other end of the spectrum, PDA and ASD device closures have the least risks of complications.
Vascular complications are the most common complication of pediatric catheterizations, accounting for almost a third of the total. They may be acute, leading to unexpected hemodynamic instability, or delayed, leading to longer-term morbidity. Many factors may contribute to unexpected hemodynamic instability, including blood loss, balloon- or catheter-induced interruptions in blood flow or coronary perfusion, arrhythmias, tamponade, vessel rupture, acute valve dysfunction, and device malposition.
Arterial thrombosis is common after pediatric cardiac catheterization, with a reported incidence in two large studies of 4.3% and 11.4%. However, diagnosis based on clinical assessment of pulses is likely to lead to underestimation; when measured by Doppler ultrasound, 32% of infants had compromised arterial blood flow to the leg. Young age, small size, larger sheath size, and repeated arterial catheter exchanges are independent risk factors for thrombosis; polycythemia and dehydration may increase risk further.
Prophylactic heparin reduces but does not remove the risk of arterial thrombosis ; and despite widespread use, there is no consensus on the appropriate dose. Common schedules prescribe 50 to 100 IU/kg; larger doses do not appear to add additional benefit. Most cases of reduced or absent pulse will resolve either spontaneously or with additional heparin anticoagulation. Thrombolysis is a viable next step, but surgical intervention will be needed for thrombosis resistant to medical therapy, arterial tears or avulsion, and pseudoaneurysms. Percutaneous thrombectomy may be suitable in older children. Occasionally, a pulse may be persistently reduced in a clinically well-perfused limb despite intervention. The long-term concern is delayed limb growth; cases have been reported but one small study failed to demonstrate limb-length discrepancy after median follow up of 3½ years. Currently there is no means to identify those at risk.
Isolated cases of femoral or iliofemoral venous occlusions with limb edema have been published as part of large series, but two small prospective observational studies found an incidence of 0% and 1.6%, respectively. All affected children responded to heparin therapy without the need for further intervention. Using the smallest catheter necessary and heparin prophylaxis should assist in limiting the incidence.
Vessel rupture can occur at the site of vessel entry or at the site of intervention. It is a rare but potentially catastrophic event. One death caused by intraabdominal hemorrhage after rupture of a femoral vein in a neonate was reported in a series of 4454 catheterizations. Arterial or venous perforations were responsible for four major complications and six minor complications in a series of 4952 procedures, and significant groin hematoma occurred in 25 cases.
Vessel rupture appears most common during balloon dilation of branch pulmonary arteries and during RVOT valve implantation procedures, but it has also been reported along the ascending aorta and arch after dilation on the aortic valve. Occasionally, arterial dissection, aneurysm, and pseudoaneurysm formation may occur. Rupture may cause hemopericardium or hemothorax, or both; pulmonary artery disruption after balloon dilation may manifest as hemoptysis. If rupture and hemorrhage occur, hypertension should be avoided, the trachea should be intubated (if the airway is not already secured), and any circulating heparin should be reversed. In extremis, blood from pericardial or pleural drains may be returned directly to the patient via femoral cannulae until surgical intervention can be achieved.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here