Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
External beam radiotherapy has been accepted as a nonsurgical means of curative treatment for localized prostate cancer since the 1950s and 1960s, coinciding with the advent of cobalt and megavoltage X-ray units that allowed for sufficient energy deposition in the prostate without unacceptable skin toxicity. Since, there have been advances in radiation delivery and treatment planning that has significantly improved the control of cancer as well as reducing the toxicity of treatment. The experience of patients undergoing external beam radiotherapy in the modern era of image-guided, intensity-modulated radiation therapy (IMRT) is one of minimal toxicity with a high likelihood of cure. Nonradiation oncology physicians or those who have trained in the era prior to modern prostate radiotherapy may not be aware of the substantial improvements that have been made to patient quality of life and cancer control. This chapter outlines the historical development and practical aspects of modern prostate radiotherapy.
The general principle of radiotherapy is to provide adequate dose to a target of interest (tumor) to attain adequate tumor control, while minimizing dose to adjacent normal tissues in order to limit acute and late toxicity to adjacent normal tissue and preserving quality of life. Advances in technology have allowed for the development of conformal radiation delivery whereby radiation beams can be “shaped” to mimic the contour of the targeted tissue. The mechanism by which conformal radiation fields have been designed and delivered has evolved as improved technologies have become available. The most notable advances have been possible due to the advent of computed tomography (CT), computer-based radiation treatment planning systems, and in the more modern era, development of multileaf collimators (MLCs) that have allowed for intensity modulation of radiation beams. The exponential increase in computer processing capabilities have also allowed for the development of inverse planning software that have the capacity to predict radiation dose distributions based on complex mathematical modeling of radiation interaction with matter in three dimensions. The evolution of external beam radiation techniques for prostate cancer is summarized here.
At its introduction in prostate cancer therapy, radiation was planned based in two dimensions (2D), using plain X-ray films and fluoroscopy-based planning techniques. Four coplanar beams (anterior–posterior (AP), posterior–anterior (PA), right lateral (RL), and left lateral (LL)) were designed to encompass a large pelvic field, using bony anatomic landmarks to predict prostate location, with radiation dose being calculated by hand with the aid of depth-dose tables and approximations of patient contours and anatomy. For AP and PA radiation treatment fields, the superior border was placed at the midsacroiliac joints, the inferior border at the lower edge of the ischial tuberosities, and lateral borders 1.5–2.0 cm lateral to the pelvic brim. On the lateral fields, the anterior border was often the pubic symphysis and posteriorly the S2/S3 interspace with the superior and inferior borders designed to match the AP and PA fields. After initial treatment to this larger pelvic field, a set of smaller radiation fields were then designed by shrinking the field by lowering the anterior border to the top of the acetabulum and laterally including two-thirds of the obturator foramen. This second set of radiation fields (known in radiation oncology parlance as a “cone down”) were typically delivered once the course of treatment to the larger fields were completed. Dose was defined and prescribed at a single point at the geometric center of the beams and calculated by hand based on single plane dose distributions for each beam. Though 2D treatment planning required significant skill as well as mathematical and physical knowledge, the technology of the time still required large volumes of normal tissue to be treated; the source of significant treatment-related toxicity. Furthermore, modern virtual CT-based reconstruction of these standard 2D fields has shown that such fields are more likely than modern treatment to have incomplete inclusion of bulky tumors, tumors that invade seminal vesicles, or locally advanced disease.
While 2D planning is no longer used routinely, the outcomes served as historical controls as 3D planning was introduced and investigated. For T1/T2 disease, 2D treatment planning led to 5 and 10 year PSA relapse-free rates that ranged from 60% to 80% and approximately 40%, respectively, as opposed to 25–32% and approximately 10%, respectively, for T3/T4 disease. When stratified by pretreatment PSA, PSA relapse-free rates ranged from 44% to 65% for PSA 4–10 ng/mL, 27% to 72% for PSA 10–20 ng/mL, and 11% to 28% for PSA >20 ng/mL. Additionally, the results of RTOG studies 7506 and 7706 helped establish 70 Gy to the prostate delivered by 2D planning as the maximum tolerated dose owing to significant GI toxicity. Despite this, there was evidence that higher prostate doses improved biochemical control, thus much interest was directed at how to deliver conformal therapy to allow for dose escalation while minimizing rectal dose.
With the advent of CT imaging and its increased availability in the 1980s and 1990s, 3D conformal radiation therapy (3D-CRT) techniques were developed and rapidly adopted. Though 3D-CRT treatment planning and patient positioning can vary between institutions, patients are typically placed in a reproducible supine position using an immobilization device (typically customized to the individual patient), after which a CT scan is acquired and target tissues such as the prostate, and normal tissues such as rectum, bladder, and femoral heads (organs at risk – OARs) are contoured on the axial images. Radiation target volumes are defined such that gross tumor volume (GTV) is considered the area of all known disease, radiographic or otherwise, whereas clinical target volume (CTV) is the GTV plus any areas considered to potentially harbor microscopic disease. In early prostate 3D-CRT, classically the CTV was the prostate plus any at risk volume such as seminal vesicles and/or lymph nodes if advanced disease was suspected. A final expansion of these volumes to a planning target volume (PTV) is then applied to account for systematic errors, which include daily patient set-up error and internal organ motion. This CTV to PTV expansion varied by institution, but was typically between 1.0 cm and 1.5 cm. Computer-based planning then allows generation of virtual 3D volumes representing the CTV and OARs in axial image sets and in the beams eye view of each of the radiation treatment beams. Furthermore, the planning software permits virtual arrangement of a desired number of beams (often four to six) around the patient, after which blocks (either by MLC or cerrobend cutouts) can be designed for each beam using the beam’s eye view function in order to “shape” the radiation field to the contour of the PTV while blocking exposure to OARs. Dose volume histograms (DVHs) are generated and show a graphical representation of the radiation dose delivered to any defined volume. Plans can be optimized by adjusting number and angles, as well as shape of blocking, with the best plan providing highest prostate coverage and simultaneously minimizing dose to OARs.
3D-CRT was quickly adopted for prostate cancer therapy over 2D treatment as it allowed higher prostate dose delivery while limiting dose to surrounding normal tissue. Although early dose calculation studies showed that reduction of dose to surrounding tissues, such as the bladder or rectum, was on the order of 14%, this translated to a clinically significant reduction in patient toxicity. This was similar to the results of a randomized study of 266 patients comparing toxicity with 3D-CRT or conventional treatment to 66 Gy, where grade 2 GI toxicity was reduced to 19% from 32% using 3D-CRT. Furthermore, studies showed that 3D-CRT not only led to lower rates of normal tissue toxicity, but it also allowed for safe delivery of higher doses of radiotherapy with at least equal, and in most cases improved, PSA relapse-free rates compared to conventional 2D therapy historical controls. The data regarding dose escalation therapy are reviewed later in this chapter.
Although 3D-CRT is a significant improvement over conventional 2D planning, it has limitations. 3D-CRT beams have uniform radiation intensity across the field, thus cannot conform to concave structures as may be encountered at the prostate/rectum or prostate/bladder interfaces. Concave conformality requires the ability to modulate radiation beam intensity across a field, which was not readily possible or practical with earlier technology. In the 1990s, rapid improvements in computing power combined with improvements in the design and capabilities of linear accelerators and multileaf collimation systems, resulted in the emergence of IMRT.
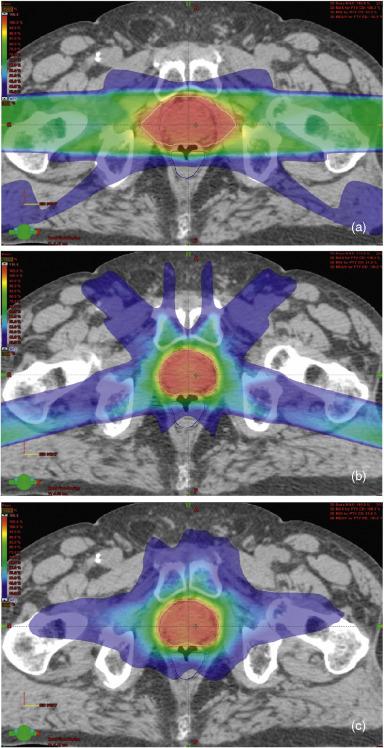
IMRT improved upon 3D-CRT delivery by allowing nonuniform intensity across a radiation field. Originally, this was done by breaking up each beam into multiple segments with MLCs at different positions, treating each segment separately, with the beam turned off between each segment treatment (step and shoot). While step and shoot methods are still used, newer technology has emerged such that MLCs can be rapidly moved across the field while the radiation beam is on (dynamic multileaf collimation), similarly allowing for modulation of the beam intensity across the field, but resulting in faster delivery of an IMRT plan. Further advances include the ability to rotate the gantry of the linear accelerator (arc-based therapy) simultaneously while using dynamic MLCs to modulate beam intensity, thus effectively delivering radiation from an infinite number of gantry angles further increasing target conformality. While all modalities if planned properly provide comparable and sufficient target coverage, arc-based therapies have higher treatment efficiency than the other methods described. Figure 45.1 demonstrates a comparison of the dose distribution of 3D-CRT versus IMRT versus arc-based therapy in a localized prostate cancer case.
IMRT planning required further technical advances in computer-based planning. Instead of forward planning as occurs with 3D-CRT whereby the physician and dosimetrist choose beam angles, beam energies, and beam weighting, and designs field blocks upfront, followed by calculation of the dose distribution to the target and OARs, IMRT makes use of inverse planning. Through inverse planning, the physician and dosimetrist still chooses the beam angles (or arcs, if using arc-based therapy), and beam energies up front. They must then define the criteria that a plan must achieve, such as percentage of the PTV that must reach a specified dose, maximum and/or minimum point doses, and dose-volume limits to OARs and assign the priority of each of these input variables. The planning algorithm then uses these criteria to run through various iterations of an IMRT plan adjusting MLC positions and beam intensity until a plan is generated that meets the input criteria. Due to the complexity of the calculations and modeling assumptions used to generate IMRT plans, IMRT also requires an extra level of quality assurance (QA). QA measures require that each plan undergo a check with a medical physicist, and be tested on a phantom where the dose delivered is measured and verified before it can ever be used for the treatment of a patient.
The use of IMRT was quickly introduced in the treatment of prostate cancer in the 1990s at a number of centers. Multiple groups assessed and confirmed the improved conformality of IMRT compared to 3D-CRT methods. Furthermore, reduced toxicity has been shown to be achieved when using IMRT over 3D-CRT. In one of the largest series evaluating toxicity with IMRT, Zelefsky et al showed rates of grade 2 rectal toxicity lowered to 2% with IMRT when delivering a dose of 81 Gy to the prostate compared to 14% for 3D-CRT. This reduction in toxicity was not at the cost of unacceptable tumor control. With longer follow up, actuarial PSA relapse-free survival rates for favorable, intermediate, and unfavorable risk groups were 85%, 76%, and 72%, respectively. Similarly, Vora et al. reported on long-term experience using image-guided IMRT in 302 patients treated to a median dose of 75.6 Gy (range 70.2–77.4 Gy), and noted that with a median follow up of 91 months, IMRT resulted in durable biochemical control rates of 77.4%, 69.6%, and 53.3% for low-, intermediate-, and high-risk patients, respectively. Reported chronic grade 3 GU and GI toxicity was 0% and 0.7%, respectively.
As much as 3D-CRT became standard over conventional therapy, even in the absence of randomized control trials, IMRT became accepted as a treatment standard for localized prostate cancer, due in large part to the preponderance of prospective and retrospective data showing its efficacy and safety. In 2001, the Radiation Therapy Oncology Group (RTOG), in a randomized control trial, RTOG 0126, was initiated and is using a 2 × 2 design to compare clinical outcomes and toxicity of 3D-CRT versus IMRT to 70.2 Gy or 79.2 Gy. While hormonal therapy was not allowed in this study, thus likely confounding the applicability of the results in modern era treatment, the biochemical outcomes are eagerly awaited. Recently, the preliminary toxicity analysis was released, showing a reduction in the volumes of bladder and rectum receiving 65, 70, and 75 Gy with IMRT, which correlated with reduced grade 2 acute GI/GU toxicity compared to 3D-CRT ( Table 45.1 ).
| Reported toxicity | 3D-CRT (%) | IMRT (%) | p |
|---|---|---|---|
| Acute effects | |||
| Combined Grade 2+ GI/GU | 15.1 | 9.7 | 0.042 |
| Grade 2+ erectile impotence | 5.4 | 4.8 | – |
| Grade 2+ urinary frequency/urgency | 10.0 | 7.6 | – |
| Late effects | |||
| Grade 2+ GI | 22.0 | 15.1 | 0.039 |
| Grade 3+ GI | 5.1 | 2.6 | 0.09 |
| Grade 2+ erectile impotence | 67.6 | 58.6 | – |
| Grade 2+ urinary frequency/urgency | 24.8 | 24.2 | – |
| Grade 2+ urinary incontinence | 5.8 | 6.6 | – |
IMRT is also promising for locally advanced disease where risk of lymph node disease necessitates pelvic radiotherapy. Ashman et al. compared dosimetric and toxicity outcomes of whole pelvic treatment using 3D-CRT or IMRT. IMRT resulted in a 60% reduction in the volume of bowel receiving 45 Gy compared to 3D-CRT and a decrease in incidence of grade 2 GI toxicity from 58% for 3D-CRT to 8% with IMRT. Similar outcomes have been reported by others showing decreased dose to OARs with associated reductions in acute toxicity. To date, phase I and II data suggest pelvic IMRT is feasible and tolerated, but results regarding the effect on biochemical disease-free survival are still waiting to mature.
IMRT has also been introduced into the adjuvant and salvage settings following radical prostatectomy. Dosimetric studies have demonstrated IMRT can significantly reduce dose delivered to the bladder and rectum compared to whole pelvic and 3D-CRT techniques. Extrapolating from the IMRT experience in the intact prostate, this is expected to translate into reduction of severity and overall rates of acute toxicity. Goenka et al. demonstrated that in a group of 285 patients treated with 3D-CRT or IMRT (72% receiving 70.2 Gy), IMRT resulted in a reduction of grade 2 GI toxicity from 10.2% to 1.9%. Given the relatively new adoption of IMRT for prostate bed radiotherapy, biochemical outcomes still need time to mature. At present, while there is no evidence to indicate definitively that IMRT should be used over 3D-CRT, there is likewise no evidence to refute its use.
Given the phase I and II evidence presented earlier that conformal therapy can provide lower morbidity and equal or improved biochemical control in part due to the ability to deliver higher doses to the prostate, a series of dose escalation trials were initiated with the advent of conformal therapy. The results of a select set of randomized and prospective trials are compiled in Table 45.2 . Each of these studies showed improved biochemical control with dose escalation, although in some studies, this was a significant improvement in only intermediate and high risk disease. Confounding some of the dose escalation data is the widespread recognition that short- or long-term androgen deprivation therapy may be synergistic with radiotherapy, such that the contribution of dose escalation or addition of hormones is less certain. In all, however, these series of studies were paramount for establishing the safety and efficacy of dose-escalated treatment, and improvements in IMRT techniques combined with improved image guidance holds promise for more precise delivery with fewer side effects while not sacrificing disease control.
| Institution/study group | Stage | n | Dose (Gy/fractions) | Biochemical PFS | Follow-up | Late toxicity |
|---|---|---|---|---|---|---|
| Randomized phase III trials | ||||||
| MRC RT01 (1998–2001) | T1b–T3a | 843 | 64/32 versus 74/37 (3D-CRT) | 43% versus 55% ( p = 0.003) | 10 years | Grade 3 GI 6% versus 10%; Grade 3 GU 2% versus 4% × 5 years |
| GETUG (1999–2002) | T2–T3, or T1 + G7+ or PSA 10–50 | 306 | 70/35 versus 80/40 (3D-CRT) | 61% versus 72% ( p = 0.036) | 5 years | Grade 3 GI 2% versus 6%; Grade 2+ GU 10% versus 18% |
| Dutch CKVO96-10 (1997–2003) | T1b–4 | 664 | 68/34 versus 78/39 (3D-CRT) | 47% versus 54% ( p = 0.04) | 70 months | Grade 3+ GI 4% versus 6%; Grade 3+ GU 12% versus 13% |
| PROG 95-09 (1996–1999) | T1b–T2b, PSA < 15 | 393 | 70.2/39 versus 79.2/44 (proton/photon, conformal) | 68% versus 83% ( p <0.001) | 10 years | Grade 3+ GI 2% versus 2%; Grade 3+ GU 1% versus 1% |
| MDACC (1993–1998) | T1b–T3 | 301 | 70/35 versus 78/39 (3D-CRT) | 59% versus 78% ( p = 0.004) | 8 years | Grade 3+ GI 1% versus 7%; Grade 2+ GU 8% versus 13% |
| Prospective trials | ||||||
| MSKCC (1996–1998) | T1c–T4, clinically localized | 170 | 81/45 (5 field IMRT) | 81% (low-risk), 78% (intermediate-risk), 62% (high-risk) | 10 years | Grade 2+ GI 2%; Grade 2+ GU 17% |
| MSKCC (1997–2008) | Localized prostate cancer | 1002 | 86.4/48 (5–7 field IMRT) | 99% (low-risk), 86% (intermediate-risk), 68% (high-risk) | 7 years | Grade 2+ GI 4.4%; Grade 2+ GU 21.1% |
| RTOG 9406 (1994–2000) | Localized prostate cancer | 1051 | (I) 68.4/38, (II) 73.8/41, (III) 79.2/44, (IV) 74/37, (V) 78/39 (3D-CRT) | (I) 21–26% (II) 23–37% (III) 16–48% (IV) 13–45% (IV) 45–61% | 9.2–11.7 years | Grade 3+ GI/GU (I) 3–6% (II) 2–4% (III) 6% (IV) 7–9% (IV) 9–12% |
Presently, dose-escalated external beam radiotherapy, defined as ≥75.6 Gy, has been widely adopted. In 2006, 70.7% of patients in the United States receiving definitive radiotherapy for prostate cancer received dose-escalated therapy; this increased to 90% of cases in 2011. The most recent National Comprehensive Cancer Network (NCCN) clinical guidelines for prostate cancer recommend doses of 75.6–79.2 Gy for low-risk prostate cancer, whereas doses up to 81 Gy are appropriate for improved biochemical control in intermediate- and high-risk disease. Given that dose escalation is now considered standard, several groups have proposed dose constraints based on clinical and radiobiologic data as a guideline for minimizing toxicity. Tables 45.3 and 45.4 summarize a select set of commonly referenced parameters.
| Organ | Dose constraint | Toxicity rate |
|---|---|---|
| Rectum (whole organ) | V50 < 50%; V60 < 35%; V65 < 25%; V70 < 20%; V75 < 15% | <15% (≥Grade 2 late toxicity); <10% (≥Grade 3 late toxicity) |
| Bladder , * (whole organ) | V65 ≤ 50%; V70 ≤ 35%; V75 ≤ 25%; V80 ≤ 15% | |
| Penile bulb (whole organ) | Mean dose to 95% of organ <50 Gy | <35% rate of severe erectile dysfunction |
| Minimum dose to hottest 90% of organ <50 Gy | ||
| Dose to 60–70% penile bulb <70 Gy | <55% rate of severe erectile dysfunction | |
| Small bowel | V15 <120 cm 3 (bowel loops contoured) | <10% (≥Grade 3 acute toxicity) |
| V45 <195 cm 3 (whole potential space-peritoneal bowel “bag”) |
* Based on RTOG 0415 recommendations, based on ≥ Grade 3 late toxicity.
| Volume | RTOG 0126 – 79.2 Gy | MSKCC – 81 Gy | MSKCC – 86.4 Gy |
|---|---|---|---|
| GTV (prostate) | D min – 79.2 Gy | D min – 81 Gy | D min – 86.4 Gy |
| PTV * | No more than 2% PTV can receive <79.2 Gy; D max – 84.7 Gy | No more than 10% of PTV can receive ≤77 Gy ** ; D max – 89 Gy | D max – 95.5 Gy |
| Rectum † | V75 Gy <15%; V70 Gy <25%; V65 Gy <35%; V60 Gy <50% | V47 Gy <53%; V75.6 Gy <30%; D max – 82 Gy | V47 Gy <53%; V75.6 Gy <30%; D max – 85.5 Gy |
| Bladder | V80 Gy <15%; V75 Gy <25%; V70 Gy <35%; V65 Gy <50% | V47 Gy <53% | V47 Gy <53%; V75.6 Gy <30% |
| Bowel | None given | D max (large bowel) – 60 Gy D max (small bowel) – 53 Gy |
|
| Penile bulb | Mean dose less ≤52.5 Gy | None given |
* For RTOG 0126, an initial PTV included at least 0.5 cm expansion from a CTV containing the prostate and proximal 1.0 cm seminal vesicles, the PTV for MSKCC 81–86.4 Gy included prostate and seminal vesicles with a 1.0 cm expansion, except 0.6 cm posteriorly at rectum.
** The <81 Gy dose restricted to PTV/rectal volume interface, otherwise, D min – 81 Gy.
† Rectum contoured from anus (level of ischial tuberosities) for 15 cm length or rectosigmoid flexure for RTOG 0126, but only rectum 0.5 cm superior and inferior of the PTV was contoured for the MSKCC 81–86.4 Gy studies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here