Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fillers are used to soften the appearance of superficial wrinkles and to recontour and smooth the deep folds of the face
Other indications include reducing the appearance of atrophic, traumatic and acne scars; lip augmentation; and volumizing the face in areas of lipoatrophy or senescence-associated fat loss
Agents include hyaluronic acid derivatives, synthetic materials, and autologous fat
Selection of an agent depends on the size, depth and location of the volume deficiency as well as the cost and longevity of the product
Agents are injected into the dermis or subcutaneous fat or periosteally by various techniques, such as serial puncture, linear threading, fanning, cross-hatching or depot injection
Fillers can be used as monotherapy or as an adjunct to other rejuvenating injectable techniques or surgical procedures in order to maximize longevity and aesthetic correction
Complications are technique-dependent or material-related, and include hypersensitivity reactions, granuloma formation, bacterial biofilms, contour irregularities and vascular occlusion, in addition to common injection-related phenomena such as pain, edema and purpura
Interventions for the aging face can be broadly categorized into the four “R's” of facial rejuvenation: R esurfacing (chemical peels, dermabrasion, and ablative and non-ablative lasers); R edraping (the various pulling and lifting facial surgical procedures); R elaxing (chemodenervation with paralytic agents); and R eplacement/ R econtouring (the use of filling agents for superficial and deep soft tissue augmentation). This fourth “R” has recently witnessed remarkable growth as patient demand for safe and effective minimally invasive aesthetic techniques has increased. In concert, the number of available dermal fillers and agents for soft tissue augmentation has expanded exponentially. More recently, there has been a shift from filling wrinkles and lines to replacing volume loss associated with atrophied fat and bone .
Patients often do not adequately appreciate the degree and significance of their volume deficit, and must first be educated about the underlying anatomy of what is producing their “wrinkle”. In addition to explaining the significance of volume deficits adjacent to the areas of primary concern, a distinction must be made between dynamic versus static lines as well as cutaneous changes due to photodamage versus those due to chronologic aging (senescence) ( Table 158.1 ). Although these defects often appear in combination, they must be assessed individually. Patients should be made aware of newer, commercially available injectable products so that the physician is able to personalize their treatment and cater to subtle nuances of their faces. Many of the newer products have unique characteristics and indications that make them more suitable for certain patients ( Table 158.2 ).
| CAUSES OF CUTANEOUS DEFECTS AND CONTOUR CHANGES AMENABLE TO FILLERS FOR CORRECTION |
| Chronologic aging |
|
| Photodamage |
|
| Other |
| SOFT TISSUE AUGMENTATION INDICATIONS |
FDA-approved (see Table 158.4 for indications for specific products)
|
Off-label
|
While the type of defect is clearly important, so are its size, depth and location, in addition to the appearance and integrity of the adjacent tissue. A discussion regarding therapeutic options should include alternatives such as surgery (e.g. rhytidectomy, excision, punch grafting), resurfacing (see Ch. 137 ), and chemodenervation agents (see Ch. 159 ), as well as no intervention at all. Patients should understand the indications, technique, advantages, disadvantages and inherent risks of each procedure, and they should be given an estimate of the financial cost and “downtime”. As with any treatment, the degree of overall success is largely proportional to the creation (and achievement) of realistic and appropriate goals and expectations by both the physician and the patient. Patients undergoing elective rhytide effacement with injectable fillers must realize that replacing lost or atrophic subcutaneous tissue is often temporary. Monotherapy with one agent may not be adequate, and multiple agents may be required. All wrinkles are not created equal; they come in many shapes and sizes and there is no one filler agent that is suitable for all defects. The choice of an appropriate dermal or subcutaneous implant requires knowledge of the available options and their characteristics.
Dating back to 1893, Neuber used blocks of autologous fat harvested from the arms for tissue augmentation of depressed facial defects. In 1899, Gersvny injected paraffin into the scrotum as a testicular prosthesis for a patient with advanced tuberculosis. Twelve years later, Brunings first described using the syringe technique to transfer free fat. Then, in 1953, Baronders published a review of permanent soft tissue augmentation with liquid silicone. Finally, in 1981, bovine collagen became the first US Food and Drug Administration (FDA)-approved xenogeneic agent for soft tissue augmentation, and it remained the standard filler for over 20 years.
In 1981, Zyderm® I was the first bovine collagen filler approved by the FDA, followed by Zyderm® II and Zyplast®. As a group, they have a long safety track record having been used in more than a million patients . All three products were derived from a closed US cattle herd carefully monitored to prevent transmission of bovine spongiform encephalitis, a prion-mediated disease. Cutaneous hypersensitivity reactions to these bovine collagen fillers occurred in 3% of patients; therefore, pre-treatment skin testing became mandatory . After the introduction of other fillers, especially the newer hyaluronic acid derivatives, the popularity of bovine collagen fillers waned because of their limited duration of results (2–3 months), manufacturing cost, and risk for hypersensitivity reactions. While no longer available in the US ( Table 158.3 ), the bovine collagen fillers are important because they set the standard to which other temporary fillers are often compared.
| TEMPORARY SOFT TISSUE FILLERS THAT ARE NO LONGER AVAILABLE IN THE US |
Bovine-derived collagen
Porcine-derived collagen
Non-cadaveric human collagen
Autologous human collagen
Cadaveric human collagen
Cadaveric fascia lata
|
Porcine-derived gelatin
|
Avian-derived hyaluronic acid
Non-animal stabilized hyaluronic acid
|
Autologous fibroblast therapy
|
In an attempt to reduce the risk of hypersensitivity reactions, a dermal filler consisting of bioengineered human collagen derived from a single human fibroblast cell line was developed. The CosmoDerm®/CosmoPlast® family of dermal fillers closely resembled the bovine-derived products except that no skin tests were required. However, they are no longer commercially available due to high manufacturing costs and low demand.
In 2008, Evolence®, a porcine form of type I collagen, was introduced for the correction of moderate to deep facial wrinkles and folds. Benefits included the rarity of hypersensitivity reactions, thus precluding skin testing, as well as a possibly longer duration of correction (up to 1 year) . It was recommended that Evolence® not be injected into the lips for augmentation because of the risk of subsequent nodule formation . As of 2009, manufacturing was discontinued due to low market demand.
In the late 1980s, research began into the use of preserved particulate allograft material derived from human cadaveric connective tissue, including dermis and fascia lata. The indications for Dermalogen®, Cymetra®, and Fascian® were similar to those for bovine collagen: facial rhytides, atrophic scars, and lip enhancement. Their use also declined with the introduction of newer soft tissue fillers.
In 2003, the FDA approved Restylane®, which ushered in a new era of agents made from hyaluronic acid (HA). Many of the HA formulations proved to be superior in longevity compared to their collagen predecessors. Globally, HA quickly became the most popular soft tissue filler. Currently available FDA-approved formulations of HA are outlined in Table 158.4 . Historic formulations of HA include Hylaform®, which was derived from rooster combs, and Captique®, a similar but bacterially derived formulation. Additional HA formulations no longer available in the US include Elevess® and Prevelle Silk® (see Table 158.3 ).
| FDA-APPROVED INDICATIONS FOR SOFT TISSUE FILLERS CURRENTLY EMPLOYED IN THE US | |
|---|---|
| Trade name ( ®, ™) | FDA-approved indication |
| Hyaluronic acid | |
| Restylane, Restylane-L * , Restylane Refyne * , Restylane Defyne * |
|
| Restylane Silk * |
|
| Restylane Lyft * |
|
| Perlane, Perlane-L * |
|
| Belotero Balance |
|
| Juvéderm Ultra, Juvéderm Ultra XC * |
|
| Juvéderm Ultra Plus, Juvéderm Ultra Plus XC * |
|
| Juvéderm Voluma XC * |
|
| Juvéderm Vobella XC * |
|
| Juvéderm Vollure XC * |
|
| Synthetic ** | |
| Polymethylmethacrylate microspheres plus bovine collagen (Bellafill) * |
|
| Poly-L-lactic acid ^ (Sculptra) |
|
| Calcium hydroxylapatite ^ (Radiesse) |
|
| Calcium hydroxylapatite plus lidocaine (Radiesse +) * |
|
| Silicone |
|
| Autologous | |
| Autologous fat |
|
| Azficel-T (LAVIV) |
|
** Bellafill® contains bovine collagen.
Longer-lasting injectable products are also commercially available including calcium hydroxylapatite (Radiesse®), poly-L-lactic acid (Sculptra®), and the permanent polymethylmethacrylate microspheres (Bellafill®) .
There is an ever-expanding array of materials and devices for soft tissue augmentation (see Table 158.4 ), and Table 158.5 lists the characteristics of the ideal filler. While soft tissue fillers can be subdivided based upon derivation, depth of cutaneous placement or duration of effect, most physicians will develop preferences based on location of desired augmentation.
| PARTIAL LIST OF THE CHARACTERISTICS OF THE IDEAL FILLER SUBSTANCE | |
|---|---|
|
|
During the initial consultation, baseline asymmetry, subjective defects, and realistic expectations should be discussed with the patient ( Table 158.6 ). In order to fully appreciate the depth and scope of the defect, patients should be placed in a gravity-dependent or seated position with adequate lighting. The latter should be at an acute angle in order to accentuate surface irregularities and deficits. A careful patient selection process will assist in determining if the patient is an ideal candidate for soft tissue augmentation. Contraindications include prior allergy to the filler material or its constituents (e.g. lidocaine). Pre-existing conditions in the planned treatment area such as dermatitis, herpetic lesions, or impetigo may require postponement of the procedure. The expected level of improvement and longevity also need to be discussed with the patient during the initial consultation.
| SOFT TISSUE AUGMENTATION: PREINJECTION CONSIDERATIONS |
| Medical background |
|
| Defect parameters |
|
| Patient goals |
|
Prior to the procedure, patients should be instructed to abstain from all nonessential medications that can inhibit coagulation and platelet aggregation. For example, patients may be asked to avoid aspirin for 10–14 days and NSAIDs for 5–7 days in order to decrease the risk of bleeding and bruising. In addition, omega-3 fatty acids, fish oil, and a number of over-the-counter supplements should be discontinued (see Table 133.3 ). Prior to the procedure, written consent is obtained and both make-up and cutaneous debris are removed . Pre- and post-treatment photographs are strongly recommended.
Clean technique is critical in preventing contamination during the initial handling, mixing, and injecting of the filler. Cleansing the skin with alcohol or chlorhexidine and changing gloves after intraoral manipulation is recommended. Percutaneous injections of fillers can be painful, necessitating the consideration of adding anesthesia to the treatment protocol. Although the physician can combine fillers with lidocaine in order to reduce the pain at the time of injection, a number of commercial products are now premixed with lidocaine (see Table 158.4 ). Some injectable products may require nerve blocks and/or regional anesthesia (see Ch. 143 ). More commonly, however, ice or topical anesthetic preparations that contain lidocaine, prilocaine, tetracaine, and/or benzocaine are placed on the areas to be injected.
Proper placement of the filler material is crucial. Injection techniques that are commonly used include serial puncture, linear threading, fanning, cross-hatching, and depot injection . In the serial punctur e technique , small amounts of filler are sequentially deposited along the wrinkle or fold. The injections are placed close to one another so that the filler can blend in a continuous fashion; post-injection massage can also help to blend the filler so that it is evenly distributed. Using the linear threading technique , the full length of the needle is inserted into the proper dermal or subcutaneous plane, and the filler is injected in a retrograde manner as the needle is withdrawn. Conversely, the material can be injected as the needle is advanced (anterograde), creating blunt dissection within the tissue space. The fanning technique involves multiple passes in different, but evenly spaced, directions without withdrawing the needle from its original site of insertion. The cross-hatching or radial injection technique involves evenly spaced, linear injections in a grid-like pattern; this technique is used for filling large areas and for the oral commissures . Finally, depot injections are useful when placing volumizing fillers within the subcutaneous fat or along the periosteum near the malar eminence. Occasionally, small depot aliquots are also placed along the infraorbital ridge. Depot placement of filler is immediately followed by massage by the injector in order to blend the product into the natural contour of the region.
Hyaluronic acid derivatives are currently the most commonly employed soft tissue fillers ( Table 158.7 ). The term hyaluronic acid is derived from the Greek word for glass, hyalos , and is so named because of its clear, glassy appearance. Hyaluronic acid (HA) is a key glycosaminoglycan (GAG; previously referred to as mucopolysaccharides) within the extrafibrillar matrix of the dermis (see Ch. 95 ). It is a ubiquitous component of connective tissue and is conserved amongst mammals, making it species-nonspecific. Within connective tissue, HA forms a gelatinous matrix that surrounds collagen and elastin fibers and it creates volume via its water-binding capability. HA also plays a role in cellular migration and therefore is important in the development and remodeling of tissues. A direct correlation exists between the amount of HA in the dermis and its water content as well as between HA content and the viscoelastic properties of the extracellular matrix. The concentration of naturally occurring HA within the skin decreases with age, resulting in a decreased ability to retain water, rendering the dermis less voluminous and increasing its propensity to wrinkle.
| BENEFITS OF HYALURONIC ACID DERIVATIVES |
|
The HA molecule consists of a polysaccharide chain comprised of numerous repeating disaccharides of N-acetylglucosamine and D-glucuronic acid. The longer the chain, the higher the molecular weight. All currently available, FDA-approved HA fillers are derived from bacterial ( Streptococcus ) fermentation. “Raw” or non-crosslinked HA is supplied as a powder that forms a viscous liquid when exposed to water. If injected in this unmodified state, exogenous HA imparts a duration of correction of only 12 to 24 hours. In order to increase tissue residence time, HA molecules must be stabilized via chemical crosslinking of the polysaccharide chains; this modification slows enzymatic breakdown and provides an acceptable duration of correction. Commercially available, FDA-approved HAs are crosslinked by BDDE (1,4- b uane d iol d iglycidyl e ther), with each filler differing in its manufacturing process.
Following the initial crosslinking, HA exists as a gel similar in consistency to Jell-O®. Subsequent post-crosslinking modifications, which vary depending upon the specific product, impart different attributes. For example, with the Restylane®/Perlane® family, the gelatin is passed through a sieve that creates microscopic particles of identical shape and size. Restylane® (approved in 2003) is then suspended in phosphate-buffered saline at a concentration of 20 mg/ml with 100 000 gel particles/ml. Compared to Restylane®, Perlane® has a larger mean gel particle size with 10 000 particles/ml. The Juvéderm® family relies on a proprietary technology that creates particles of random shapes and sizes. Juvéderm® products (first approved in 2006) have an HA concentration of 24 mg/ml, with Juvéderm® Ultra Plus being more heavily crosslinked than Juvéderm® Ultra ( Fig. 158.1 ). Belotero Balance® is a monophasic, non-particulate gel crosslinked in two consecutive reactions, resulting in a cohesive HA gel with varying zones of crosslinking density. The HA concentration in Belotero Balance® (approved in 2011) is 22.5 mg/ml.
The pivotal, phase III trials leading to FDA approval of Restylane®, Juvéderm®, and Belotero Balance® were six-month, randomized, blinded, multicenter, split-face, controlled trials that compared Zyplast® collagen to the respective HA for the treatment of moderate to severe nasolabial folds . At six months, all three HA fillers demonstrated superiority over Zyplast® collagen, with inter-study design and results having more similarities than differences ( Fig. 158.2A ). The HA products have been shown to be safe in all skin types. Further studies, focusing on repeat treatment after six months with the three families of HA, suggested that less volume is required to regain optimal correction . Often, an extended duration of 1–2 years or more was observed ( Fig. 158.2B ).

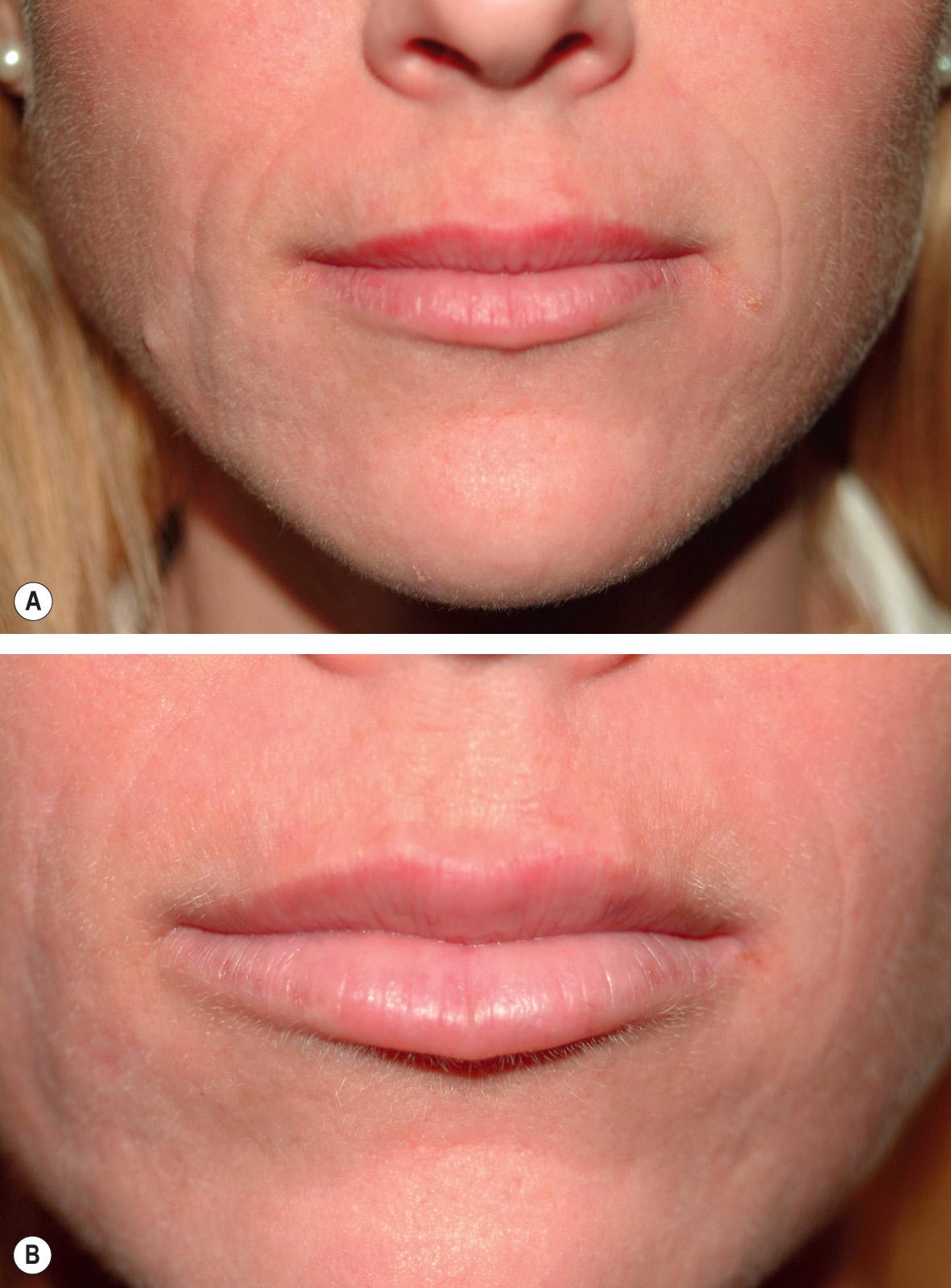
For the treatment of moderate to severe wrinkles and folds, the Restylane® and Juvéderm® families are usually injected into the immediate subdermal plane. If injected too superficially into the dermis, contour irregularity as well as a bluish discoloration due to the Tyndall effect can occur. The latter results from the optical scatter of different wavelengths of light encountering a clear substance. Recent studies suggest that Belotero Balance®, if injected more superficially within the dermis, integrates more smoothly into the dermis as compared to the Restylane® and Juvéderm® families. The former is also less likely to create a Tyndall effect . This makes Belotero Balance® particularly well suited for treatment of superficial, etched-in lines such as vertical lip lines ( Figs 158.3 & 158.4 ). Both Restylane® and Juvéderm® Ultra are FDA-approved for lip augmentation ( Fig. 158.5 ), although Belotero Balance® is often used off-label for this indication .


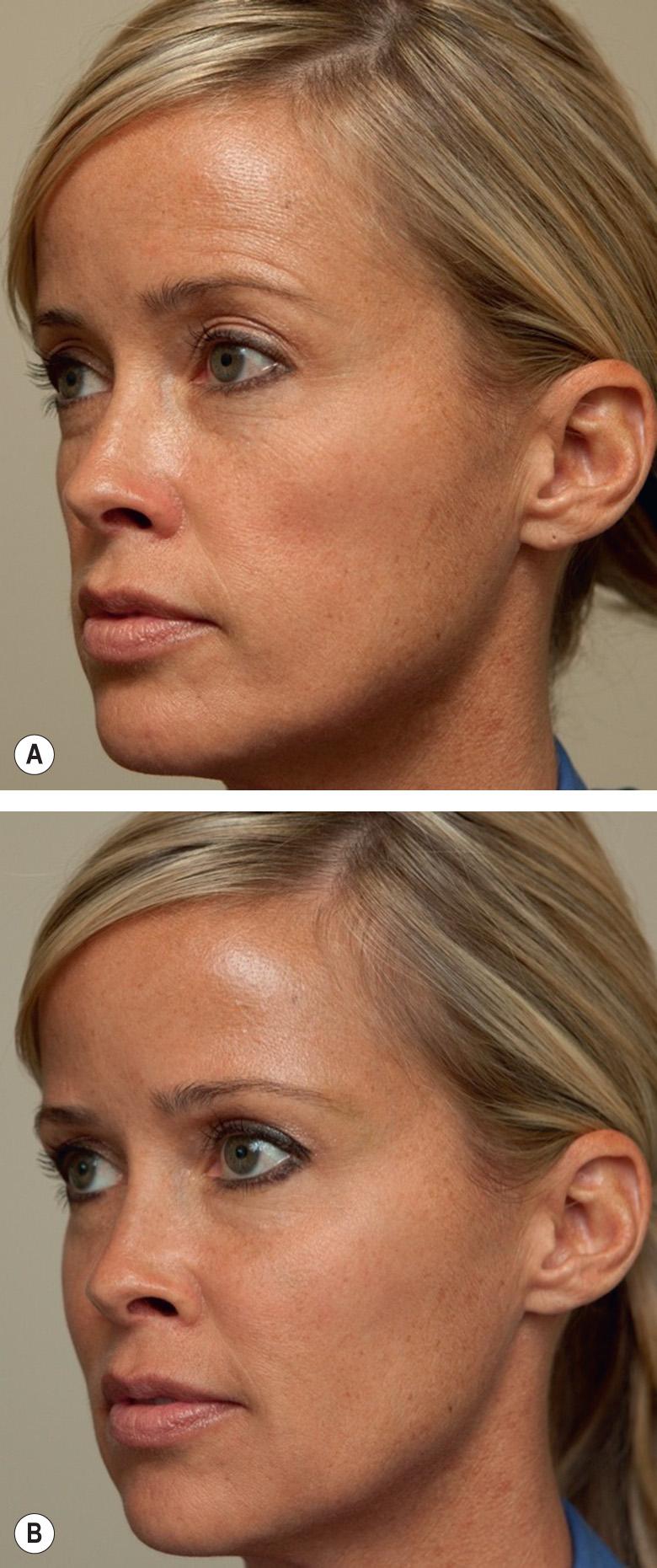
In 2013, Juvéderm Voluma® XC (“Voluma”) was the first HA filler to be FDA-approved for deep injection for cheek augmentation as a means of restoring age-related volume loss in the mid face ( ![]() ). This filler consists of a 20 mg/ml mixture of low- and high-molecular-weight HA. Voluma® differs from other HA fillers in that its lower-molecular-weight component, composed of shorter polysaccharide chains, allows for more efficient crosslinking. This in turn produces a highly cohesive gel with a greater lift capability. Results from the pivotal phase III multicenter, single-blinded, randomized, controlled study showed significant improvement in moderate to severe, age-related midfacial volume loss at six months (compared to the no-treatment control group; Fig. 158.6 ). Data suggest that nearly half of the patients maintained correction up to 24 months, emphasizing its long-term effectiveness. Adverse events were similar to other HA fillers and included swelling, tenderness and purpura, which resolved within a week in the majority of individuals . Juvéderm Voluma® XC is intended for injection into deeper subcutaneous and periosteal planes and should not be injected intradermally; nor should it be injected into the lip or glabellar areas.
). This filler consists of a 20 mg/ml mixture of low- and high-molecular-weight HA. Voluma® differs from other HA fillers in that its lower-molecular-weight component, composed of shorter polysaccharide chains, allows for more efficient crosslinking. This in turn produces a highly cohesive gel with a greater lift capability. Results from the pivotal phase III multicenter, single-blinded, randomized, controlled study showed significant improvement in moderate to severe, age-related midfacial volume loss at six months (compared to the no-treatment control group; Fig. 158.6 ). Data suggest that nearly half of the patients maintained correction up to 24 months, emphasizing its long-term effectiveness. Adverse events were similar to other HA fillers and included swelling, tenderness and purpura, which resolved within a week in the majority of individuals . Juvéderm Voluma® XC is intended for injection into deeper subcutaneous and periosteal planes and should not be injected intradermally; nor should it be injected into the lip or glabellar areas.

More recently, in 2016 and 2017, two additional fillers based upon Vycross® technology received FDA approval. Vobella XC® is indicated for lip augmentation and correction of perioral rhytides in adults over the age of 21 years whereas Vollure XC® corrects moderate to severe wrinkles and folds such as the nasolabial fold (see Table 158.4 ). Unlike previously FDA-approved products with these indications, Vollure® is the first HA filler reported to last up to 18 months in the pivotal trials.
A unique feature of HA is its reversibility, and complications from HA fillers can often be easily corrected. Injections of hyaluronidase (Vitrase®, Hylenex®) can dissolve previously injected HA; this is very helpful if the filler has been misplaced, if there is a complication post-injection (e.g. vascular occlusion, delayed granulomatous reaction), or if there is impending vascular necrosis. A recent in vitro , dose-response study suggested that Juvéderm® was more resistant to hyaluronidase when compared to Restylane®. In the authors' experience, 10 units (U) of hyaluronidase (Vitrase®) per 0.1 cc of Juvéderm or 5 U per 0.1 cc of Restylane® may be the most appropriate dose to dissolve the HA . The need for the greater amount of hyaluronidase for Juvéderm® correction likely reflects the more highly crosslinked nature of the product.
With the exception of Belotero Balance®, all currently FDA-approved HA families have a product that is pre-packaged admixed with lidocaine, which significantly reduces pain on injection (see Table 158.4 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here