Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The inherited retinal disorders are clinically and genetically heterogeneous and many become symptomatic in childhood. They are usually an isolated abnormality in an otherwise healthy child but, in syndromic form, they may be associated with systemic abnormalities (see Chapter 46 ). Recent advances in genetic sequencing technologies, so called next-generation sequencing (NGS), have greatly improved molecular diagnosis (see Chapter 10 ) and many genes causing childhood-onset retinal disorders have now been identified (see https://sph.uth.edu/retnet/ ). Previously genetic testing depended on Sanger sequencing individual genes and, given the great heterogeneity of many retinal dystrophies, this was an expensive and time-consuming process; many patients did not receive a molecular diagnosis. NGS techniques using either gene panels or whole exome sequencing allow the parallel sequencing of all known retinal genes and these powerful techniques are now readily available. Most patients can now get a specific molecular diagnosis; this greatly improves genetic counselling and also allows the identification of suitable patients for clinical trials of novel therapeutic interventions as they become available.
There is considerable genetic heterogeneity for individual clinical disorders, and disease-causing variants in a single gene may give rise to several different phenotypes. The situation is further complicated by the fact that some genes that are usually associated with syndromic retinal disease may also be implicated in isolated retinal disease, depending on the causative variant. This may make discussions about long-term prognosis more challenging.
From the clinical standpoint, the disorders can be usefully divided according to whether they are stationary or progressive; have generalized retinal or localized macular dysfunction; and if the former, exhibit predominantly rod or cone involvement. Disorders with predominantly macular dysfunction are covered in Chapter 47 ; in this chapter we consider generalised retinal disorders that are not associated with a systemic phenotype.
Stationary disorders usually present at birth or in the early months of life, and as most do not show significant progression they are best referred to as dysfunction syndromes. Progressive conditions, which typically present later, are termed dystrophies.
These include the forms of stationary night blindness and the cone dysfunction syndromes.
There are three main forms of stationary night blindness: (i) congenital stationary night blindness (CSNB), in which the fundus is normal or shows myopic changes; (ii) fundus albipunctatus; and (iii) Oguchi disease. Both (ii) and (iii) have a distinctive fundus appearance.
CSNB is characterized by night blindness, variable visual loss, and a largely normal fundus. It may be inherited as an autosomal dominant (AD), autosomal recessive (AR), or X-linked (XL) disorder.
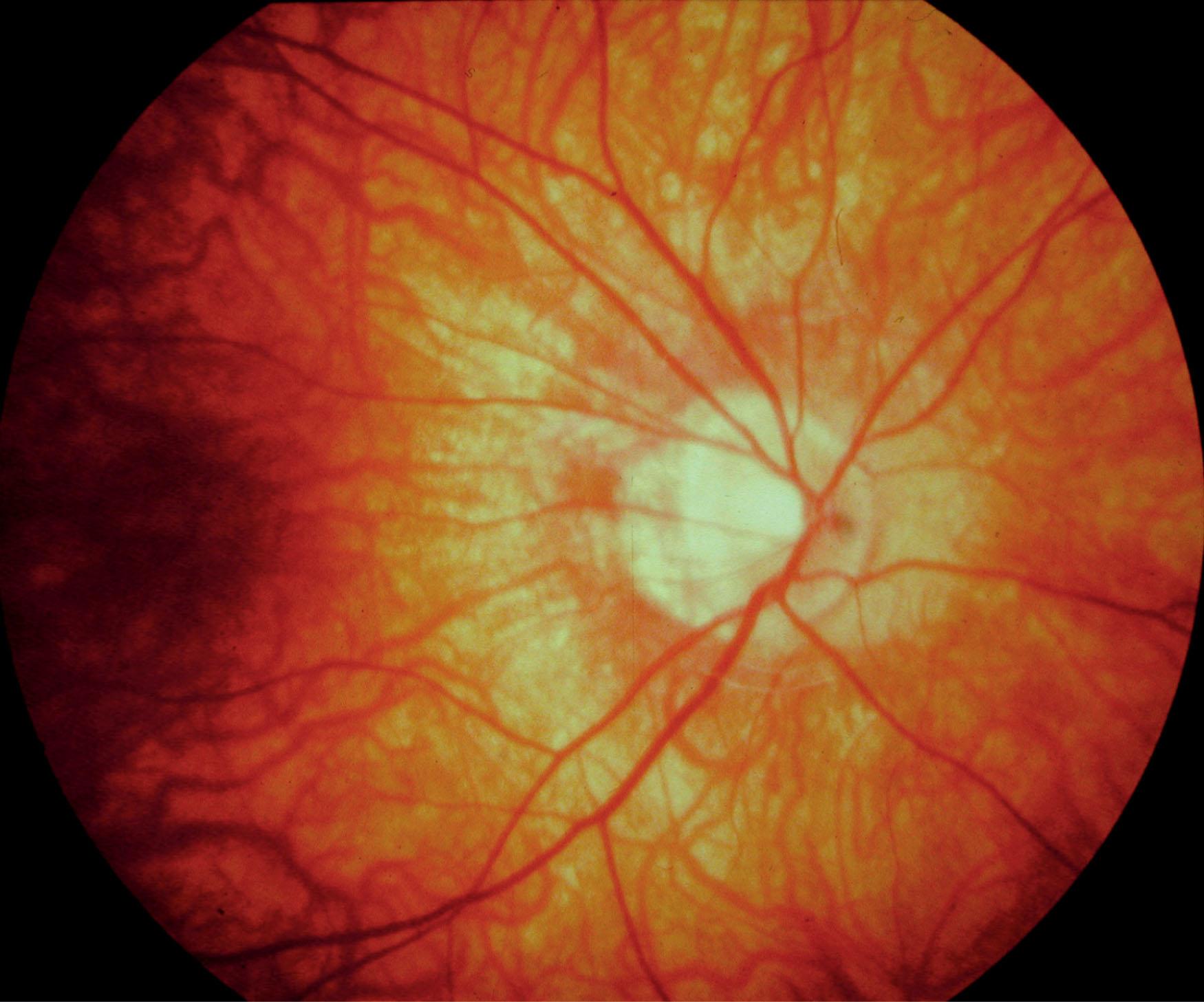
The visual acuity is usually normal or mildly reduced in the AD form, whereas mild-to-moderate central visual loss is common in the AR and XL subtypes. Other features of XL and AR CSNB include moderate-to-high myopia, nystagmus, strabismus, and paradoxical pupil responses. Fundus examination is usually normal but some patients have myopic fundi and pale or tilted optic discs ( Fig. 45.1 ). Patients with AD CSNB usually present with symptomatic night blindness, but patients with XL or AR CSNB may present in infancy with nystagmus, strabismus, and reduced vision. Nystagmus is not invariable and some patients are not diagnosed until late childhood or adulthood. The diagnosis is easily missed without electroretinography (ERG) or comprehensive molecular genetic testing. XL and AR CSNB may be further subdivided into complete and incomplete forms. This differentiation, originally proposed in XL disease using electrophysiological and psychophysical criteria, was subsequently shown to reflect genetically distinct disorders.
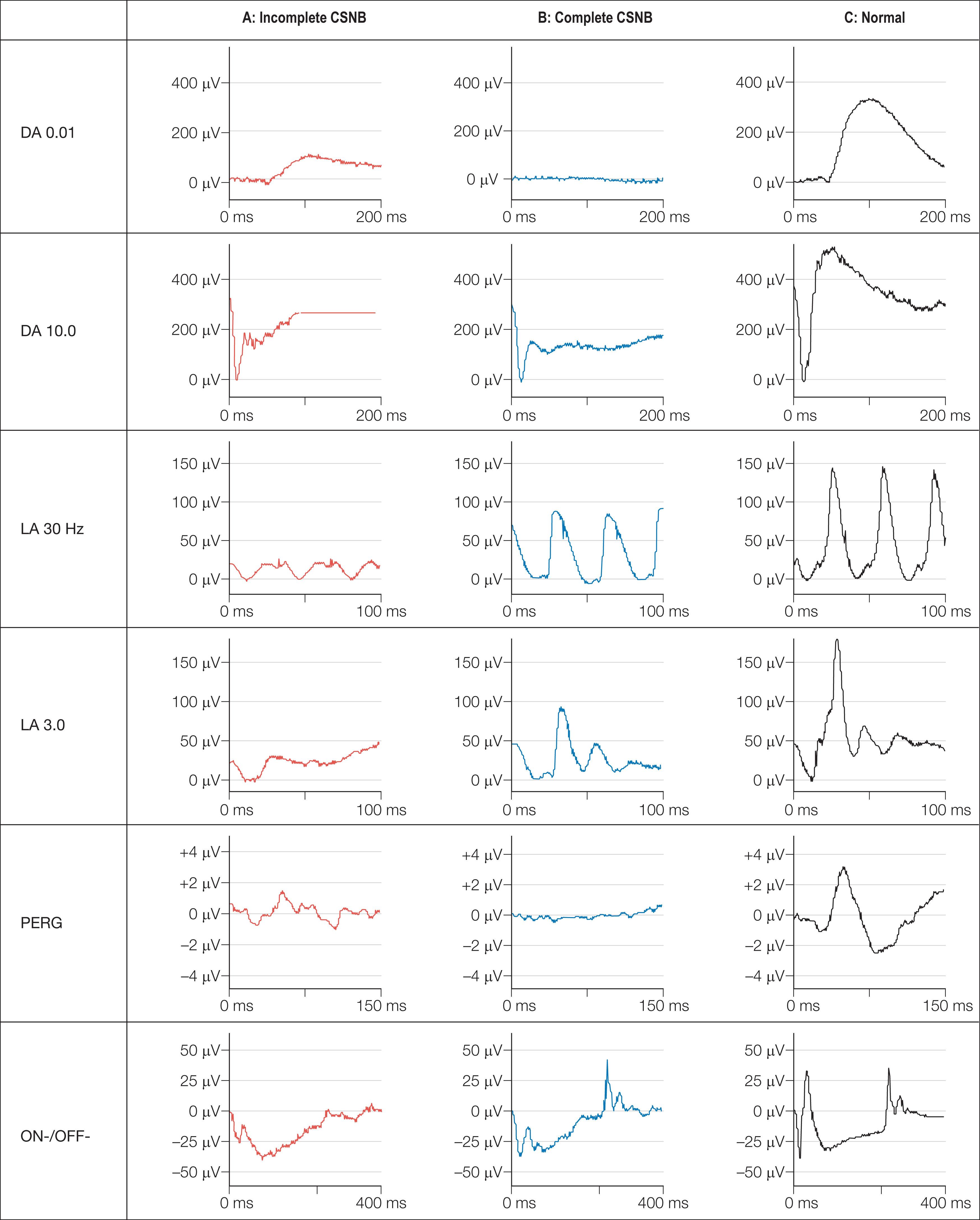
International Society for Clinical Electrophysiology of Vision (ISCEV) standard ERGs should be performed, but this may not be possible in infants, for whom a simplified protocol is more appropriate (see Chapter 7). Four main responses are defined by the adaptive state of the eye (DA: dark adapted; LA: light adapted) and the flash strength in cd.s.m 2 : a rod-specific ERG (DA 0.01) and a bright flash response (DA 10.0) performed under scotopic conditions, and two measures of cone function, a 30 Hz flicker ERG (LA 30 Hz) and a single flash photopic ERG (LA 3.0). Both complete and incomplete CSNB show a “negative ERG”: the largely photoreceptor-derived a-wave in the bright flash response is normal, but there is selective reduction in the inner nuclear-derived b-wave, so that it is smaller than the a-wave, indicating predominantly inner retinal dysfunction. There is no detectable DA 0.01 ERG in complete CSNB and a profoundly negative DA 10.0 response. Cone ERGs show subtle abnormalities reflecting ON bipolar cell dysfunction ( Fig. 45.2 ). There is a detectable rod-specific ERG in incomplete CSNB, and a profoundly negative bright flash response. Cone ERGs are much more abnormal than in complete CSNB, reflecting involvement of both ON and OFF bipolar pathways. They show a characteristic triphasic appearance in the 30 Hz flicker response (see Fig. 45.2 ).
ERG evidence of inner retinal rod system dysfunction may also occur in AD CSNB but in association with normal ISCEV cone ERGs. In other cases of AD CSNB, ERG rod responses are attenuated with normal cone responses, but the standard bright flash response does not have a negative waveform.
Disease-causing variants in the genes encoding three components of rod-specific phototransduction have been reported in AD CSNB: rhodopsin ( RHO ), the α-subunit of rod transducin ( GNAT1 ), and the rod cyclic guanosine monophosphate (cGMP) phosphodiesterase β-subunit ( PDE6β ).
Two causative genes ( CACNA1F and NYX ) have been identified, accounting for most families with XL CSNB. Incomplete CSNB is associated with variants in CACNA1F , which encodes the retina-specific α1F-subunit of the voltage-gated L-type calcium channel. The expression of CACNA1F appears limited to photoreceptors and is prominent in the synaptic terminals; it is essential for the formation and function of the photoreceptor ribbon synapse. Most disease-causing variants are inactivating truncations. The loss of functional channels impairs the calcium flux into rod and cone photoreceptors required to sustain tonic neurotransmitter release from presynaptic terminals. This results in the inability to maintain the normal transmembrane potential of bipolar cells, so the retina remains in a partially light-stimulated state, unable to respond to changes in light levels.
Complete CSNB is associated with variants in NYX , the gene encoding the leucine-rich proteoglycan nyctalopin. Leucine-rich repeats are believed to be important for protein interactions, with many of the sequence variants identified within these repeats. Nyctalopin is expressed in photoreceptor inner segments, outer and inner nuclear layers, and ganglion cells. Nyctalopin may guide and promote the formation and function of the retinal ON pathway.
Several genotype–phenotype studies have been performed in individuals with either CACNA1F or NYX variants. There is considerable inter- and intra-familial phenotypic variability associated with CACNA1F variants, even with an identical sequence variant suggesting that other genetic or environmental factors modify the phenotype. Although most patients with XL CSNB have non-progressive disease, Nakamura et al. reported two brothers with a CACNA1F mutation and progressive decline in vision, with eventually undetectable rod and cone ERGs. Since then there have been a number of other reports of patients with a cone–rod dystrophy phenotype associated with disease-causing variants in CACNA1F . Patients with complete XL CSNB ( NYX mutations) are invariably myopic and have more pronounced night blindness.
Biallelic disease-causing variants in GRM6 , TRPM1 , GPR179 or LRIT3 lead to complete CSNB. GRM6 encodes a metabotropic glutamate receptor (mGluR6) located on the dendrites of rod and cone ON bipolar cells, mediating the sign inversion that occurs at the first synapse, such that glutamate release in the dark by photoreceptors causes hyperpolarization of the ON bipolar cell membrane. TRPM1, a transient receptor potential cation channel, subfamily M, member 1, is probably involved in effecting the membrane voltage change in ON bipolar cells in response to glutamate. GPR179 encodes a G protein-coupled receptor expressed in bipolar cells and LRIT3 is a cell adhesion protein which plays a role in cone ON bipolar cell synaptic development. Each of the genes involved in AR complete CSNB is involved in photoreceptor-bipolar cell synaptic development or function (reviewed in reference ).
Variants in CABP4 have been associated with AR incomplete CSNB. CABP4, a member of the calcium-binding protein (CABP) family, is specifically located in photoreceptor synaptic terminals, where it is directly associated with the C -terminal domain of CACNA1F .
Sequence variants have been identified in SLC24A1 , in patients with AR CSNB without a negative ERG; in the standard scotopic bright flash response, both the a- and b-waves are equally reduced. SLC24A1 is a member of the solute carrier protein superfamily located in inner segments, outer and inner nuclear layers, and ganglion cells.
Åland Island eye disease (AIED) is an X-linked recessive disorder similar to incomplete CSNB, characterized by reduced visual acuity, nystagmus, nyctalopia, mild red–green dyschromatopsia and myopia. Affected males may show iris translucency, foveal hypoplasia and decreased fundus pigmentation. The clinical appearance may resemble X-linked ocular albinism (XLOA) but in XLOA color vision is usually normal and patients with AIED do not show the typical intracranial visual pathway misrouting associated with albinism.
The symptoms of night blindness and the psychophysical and ERG changes seen in AIED are similar to those in the incomplete form of the XL CSNB. The disorders are allelic, with disease-causing variants now identified in CACNA1F in AIED.
Patients with a contiguous gene syndrome (which includes glycerol kinase deficiency, congenital adrenal hypoplasia, Duchenne muscular dystrophy [DMD] and ocular abnormalities known as Oregon eye disease, with a deletion of Xp21) have ocular features in common with affected males with AIED and have similar predominantly inner retinal ERG abnormalities. Furthermore, some males with Duchenne muscular dystrophy, associated with disease-causing variants in the dystrophin gene at Xp21, have ERG abnormalities similar to those of CSNB. The ERG phenotype is determined by the site of the variant within the dystrophin gene. However, in contrast to Oregon eye disease, affected patients have no ocular symptoms and there is no associated visual dysfunction. These multisystem disorders have a non-progressive form of predominantly rod system dysfunction.
Oguchi disease is a rare autosomal recessive stationary night blindness with a grayish or green–yellow discoloration of the fundus at the posterior pole or extending beyond the arcades, which reverts to normal on prolonged dark adaptation (Mizuo phenomenon) ( Fig. 45.3 ). Exposure to light then usually leads to the gradual reappearance of the abnormal discoloration in 10–20 minutes. The results of optical coherence tomography (OCT) and adaptive optics scanning laser ophthalmoscopic imaging (AOSLO) studies suggest that the change in fundus appearance is related to changes in rod photoreptor reflectivity.
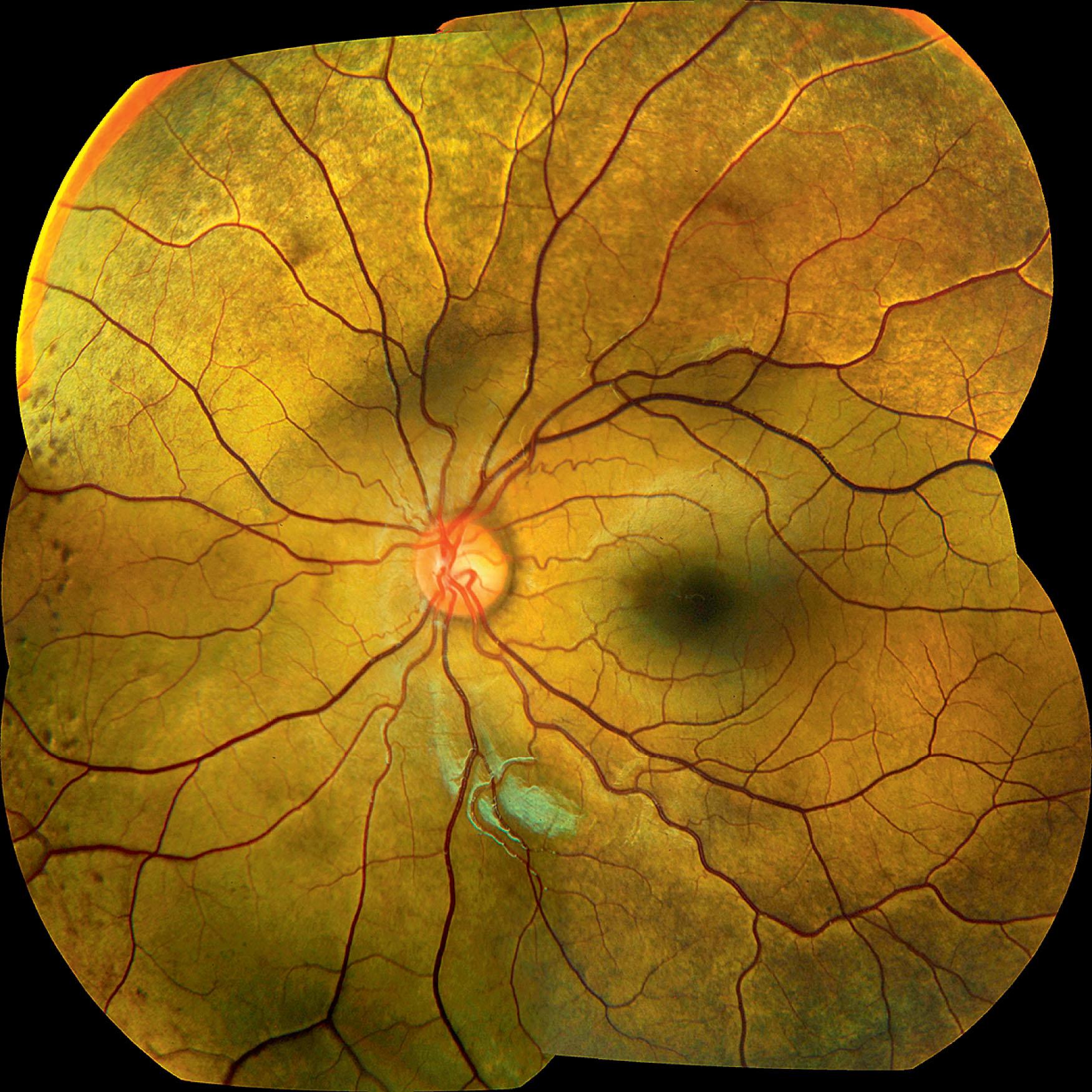
Most patients present with poor night vision. Visual acuity is normal or mildly reduced and photopic visual fields and color vision are normal. Most reported cases are from Japan, but it can occur in other races.
There are two types of Oguchi disease depending on the dark adaptation findings:
Type 1: rod adaptation is markedly slowed; full recovery of sensitivity takes several hours and the absolute threshold is normal or only minimally elevated.
Type 2: there is no recognizable rod adaptation; the abnormal retinal appearance is less marked and the Mizuo phenomenon may be absent.
Most patients with Oguchi disease have a “negative” bright flash ERG, confirming the site of dysfunction to be post-phototransduction, as in AR and XL CSNB. In contrast to fundus albipunctatus (see below), the ERG remains abnormal even after prolonged dark adaptation.
Oguchi disease in some patients is a consequence of a truncating deletion in SAG , encoding arrestin. Reduced activity of arrestin is likely to result in prolonged activation of transducin and rod phosphodiesterase following light exposure. cGMP levels would thereby be maintained at a low level in even dim light exposure and the outer segment cation channels would remain closed resulting in prolonged rod hyperpolarization. The rods would behave as if they were light adapted and would be unresponsive to light at low levels of illumination, explaining the psychophysical abnormalities.
Null alleles in GRK1 , encoding a second component of the rod phototransduction pathway, rhodopsin kinase (RK), have also been identified in Oguchi disease. The key function, of both RK and arrestin, in the normal deactivation and recovery of the photoreceptor after exposure to light, explains the delayed recovery in Oguchi disease.
Evidence from knockout mice models suggests that patients with SAG or GRK1 variants may be more susceptible to light-induced retinal damage; it may therefore be advisable for affected patients to wear tinted spectacles.
This autosomal recessive form of stationary night blindness, most commonly related to disease-causing variants in RDH5 , usually has a characteristic fundus appearance with multiple white dots throughout the retina; however, rarely fundus examination is normal. Patients either present with night blindness or an abnormal retinal appearance on routine fundoscopy. The visual acuity is usually normal and the condition non-progressive.
The deposits appear as discrete dull-white lesions at the level of the retinal pigment epithelium (RPE). They are most numerous in the mid-periphery and are usually absent at the macula; the optic discs and retinal vessels are normal. Fluorescein angiography shows multiple areas of hyperfluorescence, which may not directly correlate to the deposits seen clinically. In some patients there is good correlation between the white dots and increased autofluorescence on fundus autofluorescence (FAF) imaging, but not in others. The differential diagnosis is from other causes of flecked retina (see Chapter 49 ).
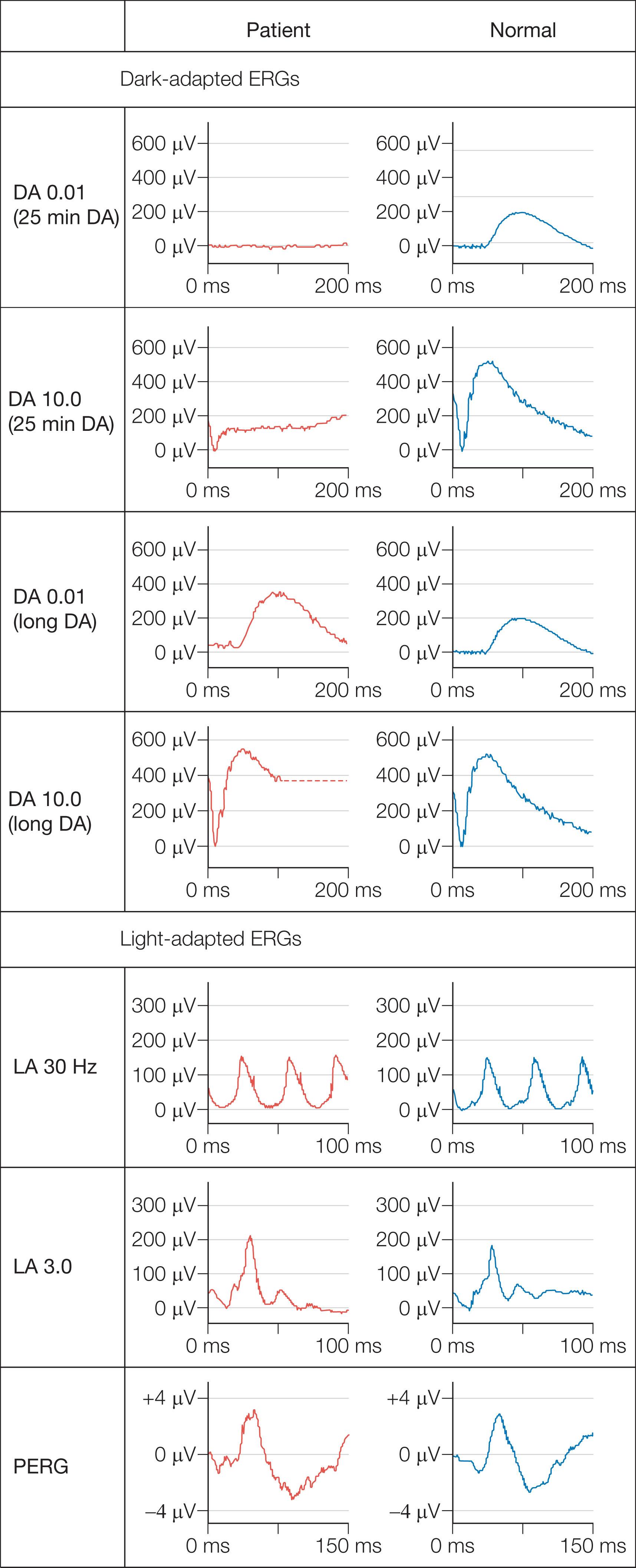
Dark adaptation is severely delayed in fundus albipunctatus (FA), reflecting abnormal regeneration of rhodopsin. The rod–cone break is delayed and full rod adaptation may take many hours. Rod ERGs are markedly abnormal, with the rod-specific ERG (DA 0.01) usually being undetectable under standard conditions, but normalizing following prolonged dark adaptation ( Fig. 45.4 ). The dark-adapted bright flash ERG (DA 10.0), which after a standard period of dark adaptation arises in dark-adapted cones, can have a low b:a ratio; a red flash stimulus under dark adaptation shows a normal cone component but an undetectable rod component and prevents confusion with CSNB associated with a negative ERG. It is necessary to considerably exceed the ISCEV ERG standard recommendations for dark adaptation to confirm the diagnosis of FA. Most but not all patients with RDH5 mutations show full recovery of rod function with extended dark adaptation. This contrasts with the findings in retinitis punctata albescens (see below), related to mutation in RLBP1 , and usually helps to distinguish between the two disorders.
There are two forms of FA, one in which cone ERGs are normal, and a rarer form described as fundus albipunctatus with cone dystrophy and negative ERG.
Disease-causing variants in three genes ( RDH5 , RLBP1 , and RPE65 ), encoding components of the visual cycle, have been identified to date in patients with a white dot fundus phenotype similar to FA. The gene RDH5 encoding 11- cis -retinol dehydrogenase accounts for most cases. Recombinant mutant 11- cis -retinol dehydrogenases have reduced activity compared with recombinant enzyme with wild-type sequence. The function of the protein product of RDH5 is consistent with the delay in the regeneration of photopigments characteristic of the disorder.
Sequence variants have also rarely been reported in RLBP1 , encoding the cytosolic cellular retinaldehyde binding protein (CRALBP). RLBP1 variants usually cause retinitis punctata albescens (see below) and are generally associated with progressive retinal disease. CRALBP has been localized to the RPE, Müller cells, and ciliary epithelium. It can select 11- cis -retinaldehyde from a mixture of retinoids and protect it from photoisomerization, suggesting a possible role in the generation of 11- cis -retinoids in the visual cycle and in keeping with the phenotypic features of FA.
Bilallelic sequence variants in RPE65 have also been identified in patients with a retinal appearance similar to FA.
The cone dysfunction syndromes include congenital color vision disorders where there is normal visual acuity but defective color vision, and the various forms of cone dysfunction associated with reduced central vision and often nystagmus and photophobia ( Table 45.1 ).
| Cone dysfunction syndrome | Alternative names | Mode of inheritance | Visual acuity | Refractive error | Nystagmus | Color vision | Fundi | Mutated gene(s) or chromosome locus |
|---|---|---|---|---|---|---|---|---|
| Complete achromatopsia | Rod monochromatism Typical achromatopsia | Autosomal recessive | 6/36–6/60 | Often hypermetropia | Present | Absent | Usually normal | CNGA3CNGB3GNAT2PDE6CPDE6HATF6 |
| Incomplete achromatopsia | Atypical achromatopsia | Autosomal recessive | 6/24–6/60 | Often hypermetropia | Present | Residual | Usually normal | CNGA3PDE6C |
| Oligocone trichromacy | Oligocone syndrome | Autosomal recessive | 6/12–6/24 | Equal incidence of myopia and hypermetropia | Often absent | Normal | Normal | – |
| RGS9 / R9AP retinopathy | Bradyopsia | Autosomal recessive | 6/12–6/24 | Equal incidence of myopia and hypermetropia | Often absent | Normal | Normal | RGS9R9AP |
| Cone monochromatism | – | Uncertain | 6/6 | – | Absent | Absent or markedly reduced | Normal | – |
| Blue cone monochromatism | X-linked atypical achromatopsia X-linked incomplete achromatopsia | X-linked | 6/24–6/60 | Often myopia | Present | Residual tritan discrimination | Usually normal or myopic | (i) Deletion of the locus control region. (ii) Single inactivated L/M hybrid gene |
| Bornholm eye disease | – | X-linked | 6/9–6/18 | Moderate-to-high myopia with astigmatism | Absent | Deuteranopia Protanopia | Myopic | L/M opsin array |
Color vision in humans is trichromatic; there are three classes of cone photoreceptor that contain visual pigments maximally sensitive at 560 nm (L-cones [red]), 535 nm (M-cones [green]), and 440 nm (S-cones [blue]). The genes for the protein component (opsin) of the L- and M-cone pigments are on the long arm of the X chromosome, and the S-cone opsin gene on chromosome 7. About 8% of men and 0.5% of women have a red–green color vision defect, which is associated with abnormalities of the red and green cone opsin genes. Tritanopia is an uncommon autosomal dominant disorder in which there is a specific deficiency of S-cone sensitivity associated with mutations of the S-cone opsin gene.
This group of disorders, their clinical characteristics, and the molecular pathology are reviewed elsewhere.
Achromatopsia is genetically heterogeneous and characterized by a lack of functioning cones in the retina. It affects about 1 in 30,000 live births. It may occur in complete and incomplete forms depending on whether there is an absence of, or some residual, cone function.
The usual presentation is with reduced vision, nystagmus, and marked photophobia at birth or early infancy. Parents often comment that vision is much better in dim illumination. Pupil reactions are sluggish or may show paradoxical constriction in the dark. High hyperopic refractive errors are common and the fundus appearance is normal. The nystagmus and photophobia, marked in infancy, may improve with age. The visual acuity is usually in the region of 6/60 in complete achromatopsia, with complete color blindness. Patients with incomplete achromatopsia have better visual acuity and may show some residual color vision. Peripheral visual fields are normal but a central scotoma can often be detected. Achromatopsia is generally a stationary disorder with little change in visual acuity over time but slow deterioration and macular atrophy may occur.
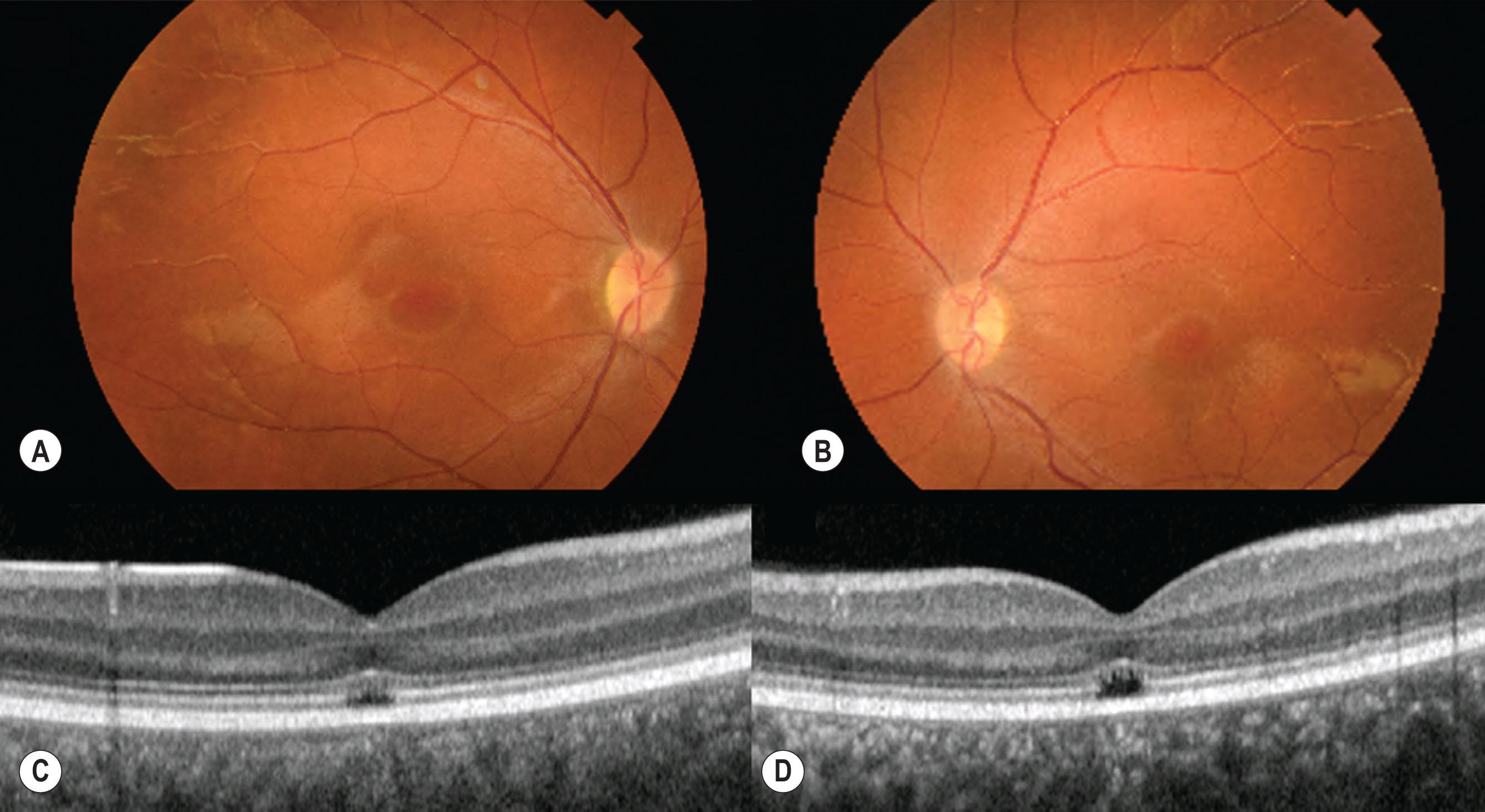
Although in most cases the fundus appearance is normal, detailed spectral domain OCT imaging shows a variety of abnormalities of the central macula, including foveal hypoplasia ( Fig. 45.5 ). Sundaram et al. have described five distinct appearances on spectral domain OCT: (1) continuous ellipsoid zone (EZ); (2) EZ disruption; (3) EZ absence; (4) presence of hyporeflective zone; (5) outer retinal atrophy and RPE loss. That study showed no association of the imaging findings with age or genotype. Histopathologic studies of donor eyes have demonstrated cone-like structures in the retina and recent advances in AOSLO imaging have supported the view that cones are present in the achromat retina in reduced numbers but are non-functioning. This has led to optimism that gene-based therapies may be able to restore some cone function in patients with achromatopsia.
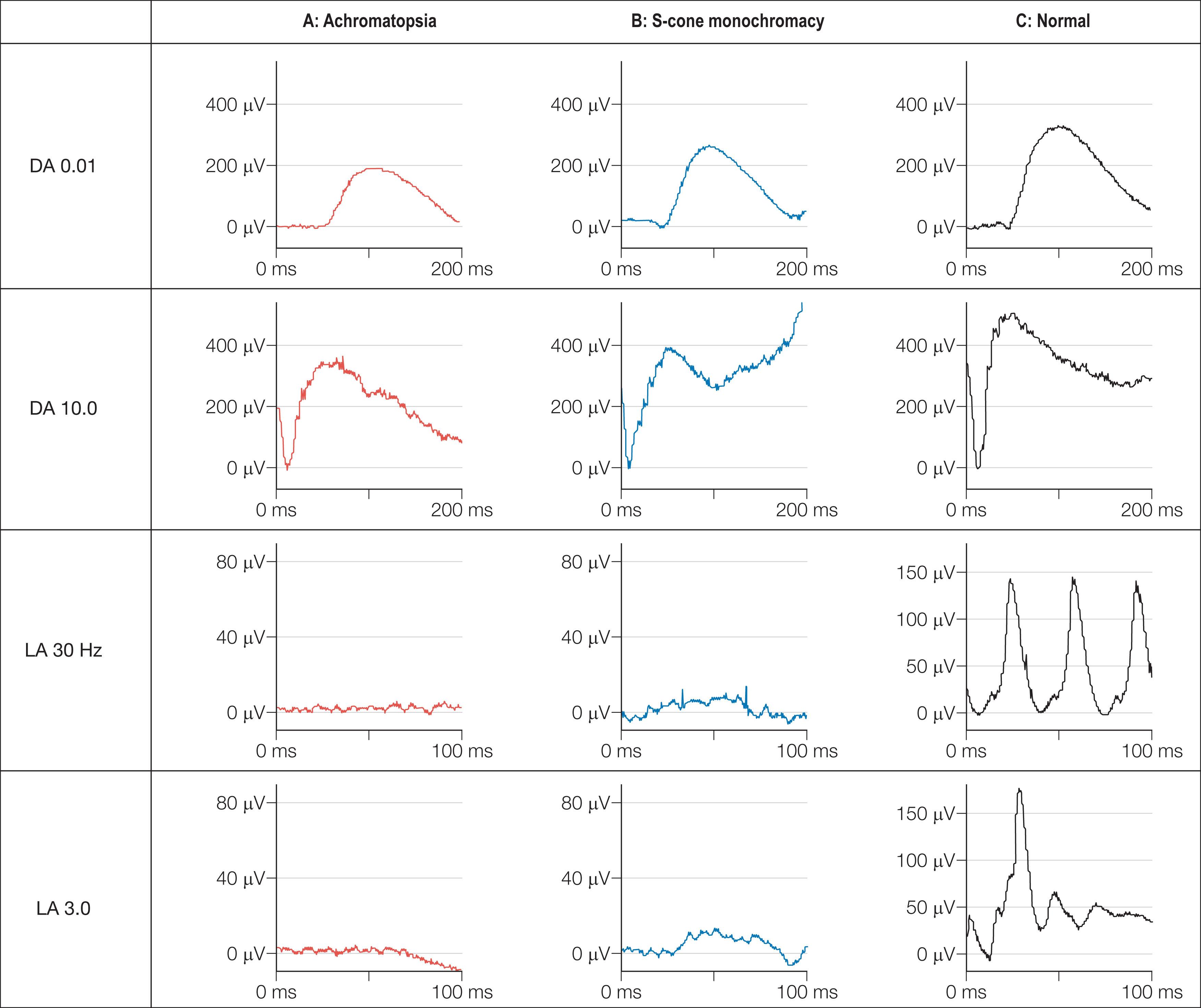
In complete achromatopsia the dark-adaptation curve is monophasic with no evidence of a cone contribution. There is no detectable color vision. Spectral sensitivity studies show that rods mediate threshold under both photopic and scotopic conditions; there is no evidence of a Purkinje shift. Electroretinography ( Fig. 45.6 ) generally shows normal rod-derived ERGs but no detectable cone-derived responses. Patients with incomplete achromatopsia may have some residual color vision but there are usually no detectable cone responses on ERG.
Achromatopsia is recessively inherited and genetically heterogeneous. Six causative genes have been identified, CNGA3 , CNGB3 , GNAT2 , PDE6C , PDE6H , and ATF6 . About 70%–80% of achromatopsia results from disease-causing variants in CNGA3 and CNGB3 , with GNAT2 , PDE6C , PDE6H , and ATF6 each accounting for less than 1%–2%; further causative genes remain to be discovered. Most patients with incomplete achromatopsia have sequence variants in CNGA3 , but this phenotype has also been reported in association with variants in CNGB3 , PDE6C , and GNAT2 . With the exception of ATF6 , all causative genes encode components of the cone phototransduction pathway. Such genes are likely to affect cone function rather than photoreceptor development (see reference for summary of gene function). The disease mechanism in ATF6 -related disease is unclear. ATF6 is a transcription factor involved in the cellular response to endoplasmic reticulum stress. It is ubiquitously expressed and it is not clear why the phenotype is restricted to cone photoreceptors.
Blue cone monochromatism (BCM; also known as S-cone monochromacy) is an X-linked recessive disorder, affecting approximately 1 in 100, 000 individuals, in which affected males have normal rod and S-cone function but lack L- and M-cone function. The clinical features are similar to complete achromatopsia but less severe. Affected infants have photophobia and nystagmus from early infancy. They are usually myopic, in contrast to achromatopsia where most patienst are hyperopic. The nystagmus often reduces with time. Fundus examination is usually normal or shows myopic changes in childhood, but macular atrophy may occur over time. Spectral domain OCT shows variable macular thinning even at a young age. AOSLO imaging shows residual cones in greater density than that expected for S-cones, suggesting that there is survival of some L- or M-cones that may be responsive to gene replacement therapy.
Achromatopsia and BCM may be differentiated by the mode of inheritance, findings on psychophysical testing, and electrophysiology. There is some preservation of the single flash photopic ERG (LA 3.0) in BCM (see Fig. 45.6 ), and specialized spectral ERG techniques to measure S-cone ERGs can also be used (see Fig. 45.6 ). Female carriers of X-linked BCM may have abnormal cone ERGs and mild anomalies of color vision.
Color vision tests that probe the tritan color axis, such as the Hardy, Rand, and Rittler (HRR) plates, are necessary in order to distinguish between achromatopsia and BCM, with relative preservation of tritan discrimination in BCM. Blue cone monochromats display fewer errors along the vertical axis in the Farnsworth 100-hue test (fewer tritan errors), and may display protan-like patterns on the Farnsworth D-15.
BCM is associated with disease-causing variants in the L- and M-opsin gene array ; there are two main molecular mechanisms of disease:
A normal L- ( OPN1LW ) and M- ( OPN1MW ) pigment gene array is inactivated by a deletion in the locus control region (LCR), located upstream of the L-pigment gene. A deletion here abolishes transcription of all genes in the pigment gene array and therefore inactivates both L- and M-cones.
The LCR is preserved but changes within the L- and M-pigment gene array lead to loss of functional pigment production. The most common genotype in this class consists of a single inactivated L/M hybrid gene. The first step in this second mechanism is unequal crossing over, reducing the number of genes in the array to one, followed in the second step by a mutation that inactivates the remaining gene. The most frequent inactivating mutation that has been described is a thymine-to-cytosine transition at nucleotide 648, which results in a cysteine-to-arginine substitution at codon 203 (Cys203Arg), a mutation known to disrupt the folding of cone opsin molecules.
The data suggest that 40% of BCM results from the one-step mutational pathway (deletions in the LCR). The remaining 60% of BCM is predominantly secondary to the aforementioned two-step pathway. Other less common mutational mechanisms include the deletion of an entire exon in a single opsin array gene, rare haplotypes (“L/M interchange haplotypes”), or gene conversion transferring a disease-causing variant between OPN1LW and OPN1MW (see reference for summary).
Oligocone trichromacy is characterized by reduced visual acuity (6/12 to 6/24), mild photophobia, normal fundi, reduced cone ERGs with normal rod responses, and normal color vision. Patients have been described both with and without nystagmus. These patients appear to have a reduced number of normal functioning cones (oligocone) with preservation of the three cone types in the normal proportions, thereby permitting trichromacy; this hypothesis is supported by high-resolution quantitative retinal imaging.
Color vision testing either reveals normal or slightly elevated color discrimination thresholds. Slightly elevated discrimination thresholds are compatible with a reduction in cone numbers.
Oligocone trichromacy is inherited as an autosomal recessive trait; the condition is likely to be genetically heterogeneous and some patients have been reported with variants in CNGB3 and CNGA3 . Patients with either RGS9 / R9AP mutations (“bradyopsia”, see below) or oligocone trichromacy have similar clinical phenotypes, characterized by stationary cone dysfunction with normal color vision but the electrophysiologic features associated with RGS9 and R9AP are distinctive.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here