Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The inherited macular dystrophies are characterized by bilateral central visual loss and symmetrical macular abnormalities. Most present in the first two decades of life with a wide range of clinical, electrophysiological, psychophysical, and histological findings. Inheritance may be autosomal dominant, autosomal recessive, X-linked recessive or associated with disease-causing variants in mitochondrial DNA. There is great genetic heterogeneity even amongst these subtypes. The molecular basis of inherited macular disease is now well understood, providing insights into the pathogenesis ( Table 47.1 ). The recent advances in next generation sequencing have greatly improved the ability to make a precise molecular diagnosis using either multigene panels or whole exome sequencing (see Chapter 10 ).
| Macular dystrophy; OMIM number | Mode of inheritance | Chromosome locus | Mutated gene |
|---|---|---|---|
| Stargardt disease; 248200 | Autosomal recessive | 1p21−p22 (STGD1) | ABCA4 |
| Macular dystrophy with central cone involvement; 616170 | Autosomal recessive | 4q28.2 | MFSD8 |
| Autosomal recessive bestrophinopathy; 611809 | Autosomal recessive | 11q13 | BEST1 |
| Autosomal recessive (atypical) vitelliform dystrophy; 616151 | Autosomal recessive | 6q14.1 | IMPG1 |
| Stargardt-like macular dystrophy; 600110 | Autosomal dominant | 6q14 (STGD3) | ELOVL4 |
| Stargardt-like macular dystrophy; 603786 | Autosomal dominant | 4p (STGD4) | PROM1 |
| Autosomal dominant bull's eye macular dystrophy; 608051 | Autosomal dominant | 4p (MCDR2) | PROM1 |
| Autosomal dominant (atypical) vitelliform dystrophy; 616151 | Autosomal dominant | 6q14.1 | IMPG1 |
| Best macular dystrophy; 153700 | Autosomal dominant | 11q13 | BEST1 |
| Pattern dystrophy; 169150 | Autosomal dominant | 6p21.2-cen | PRPH2 |
| Doyne honeycomb retinal dystrophy; 126600 | Autosomal dominant | 2p16 | EFEMP1 |
| North Carolina macular dystrophy; 136550 | Autosomal dominant | 6q14−q16.2 (MCDR1) | Non-coding variants at the MCDR1 locus, each thought to modify expression of the retinal transcription factor PRMD13 |
| Autosomal dominant macular dystrophy resembling MCDR1; 608850 | Autosomal dominant | 5p15.33−p13.1 (MCDR3) | Non-coding variants at the MCDR3 locus thought to modify expression of genes affecting macular development, including IRX . |
| North Carolina-like macular dystrophy associated with deafness | Autosomal dominant | 14q (MCDR4) | Not identified |
| Progressive bifocal chorioretinal atrophy; 600790 | Autosomal dominant | 6q14−q16.2 | Non-coding variant upstream of PRMD13 |
| Sorsby fundus dystrophy; 136900 | Autosomal dominant | 22q12.1−q13.2 | TIMP3 |
| Central areolar choroidal dystrophy; 215500 | Autosomal dominant |
|
|
| Adult-onset macular dystrophy; 602225 | Autosomal dominant | 19q13.33 | CRX |
| Juvenile retinoschisis; 312700 | X-linked | Xp22.2 | RS1 |
| X-linked recessive atrophic macular degeneration | X-linked | Xp11.4 | RPGR |
We review pediatric macular dystrophies but not those that present later, such as Sorsby fundus dystrophy, dominant drusen, and adult vitelliform macular dystrophy. Systemic disorders with macular dystrophy will be discussed in Chapters 10 and 65 .
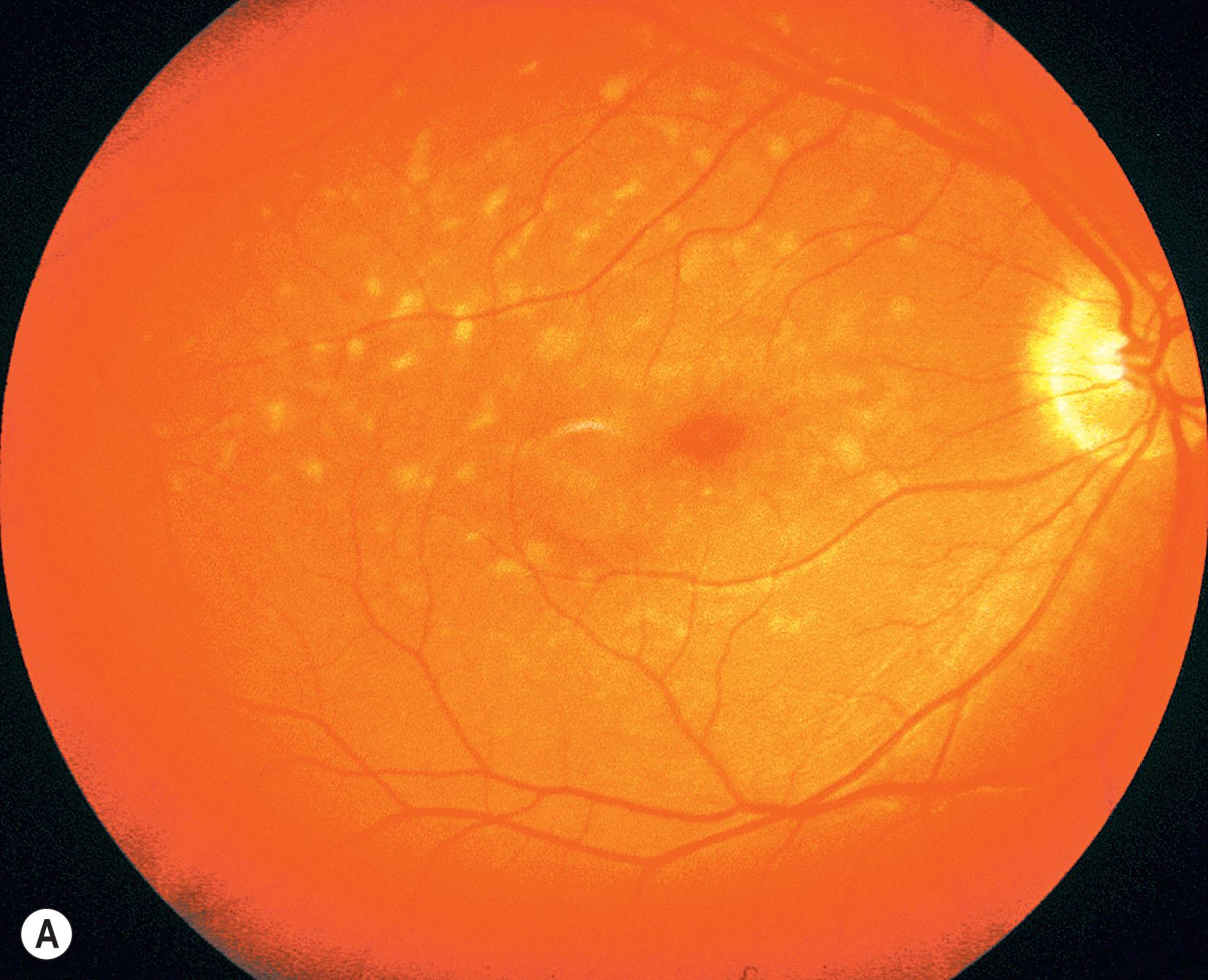
Stargardt macular dystrophy (Stargardt disease, STGD) is the most common inherited macular dystrophy, with a prevalence of 1 in 10,000; it is inherited as an autosomal recessive (AR) trait. Most cases present with central visual loss in the early teens. There is typically macular atrophy with yellow-white flecks at the level of the retinal pigment epithelium (RPE) at the posterior pole. The flecks may be “fish-shaped” (pisciform), round, oval, or semilunar. The oval area of macular atrophy may, in early stages, have a “beaten bronze” appearance ( Fig. 47.1 ). However, there may be no evidence of flecks at presentation (up to a third of children), the only abnormality being macular atrophy, but in these patients, flecks usually develop over time. The now infrequently used term “fundus flavimaculatus” (FFM) was originally coined to describe the phenotype of retinal flecks occurring without macular atrophy. STGD and FFM are caused by disease-causing sequence variants in the same gene ( ABCA4 ); both patterns may be seen in the same family. Patients who present with FFM develop macular atrophy over time.
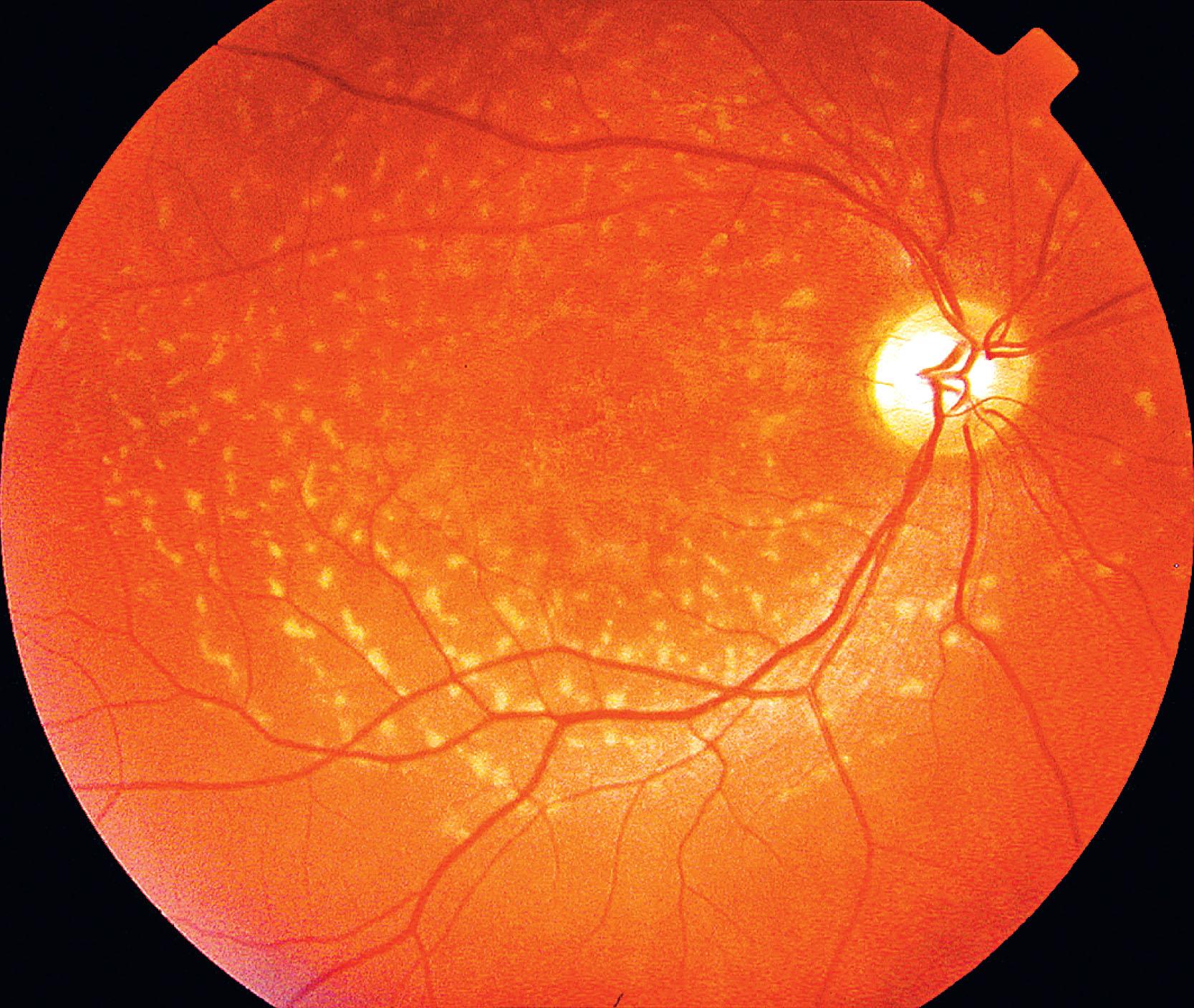
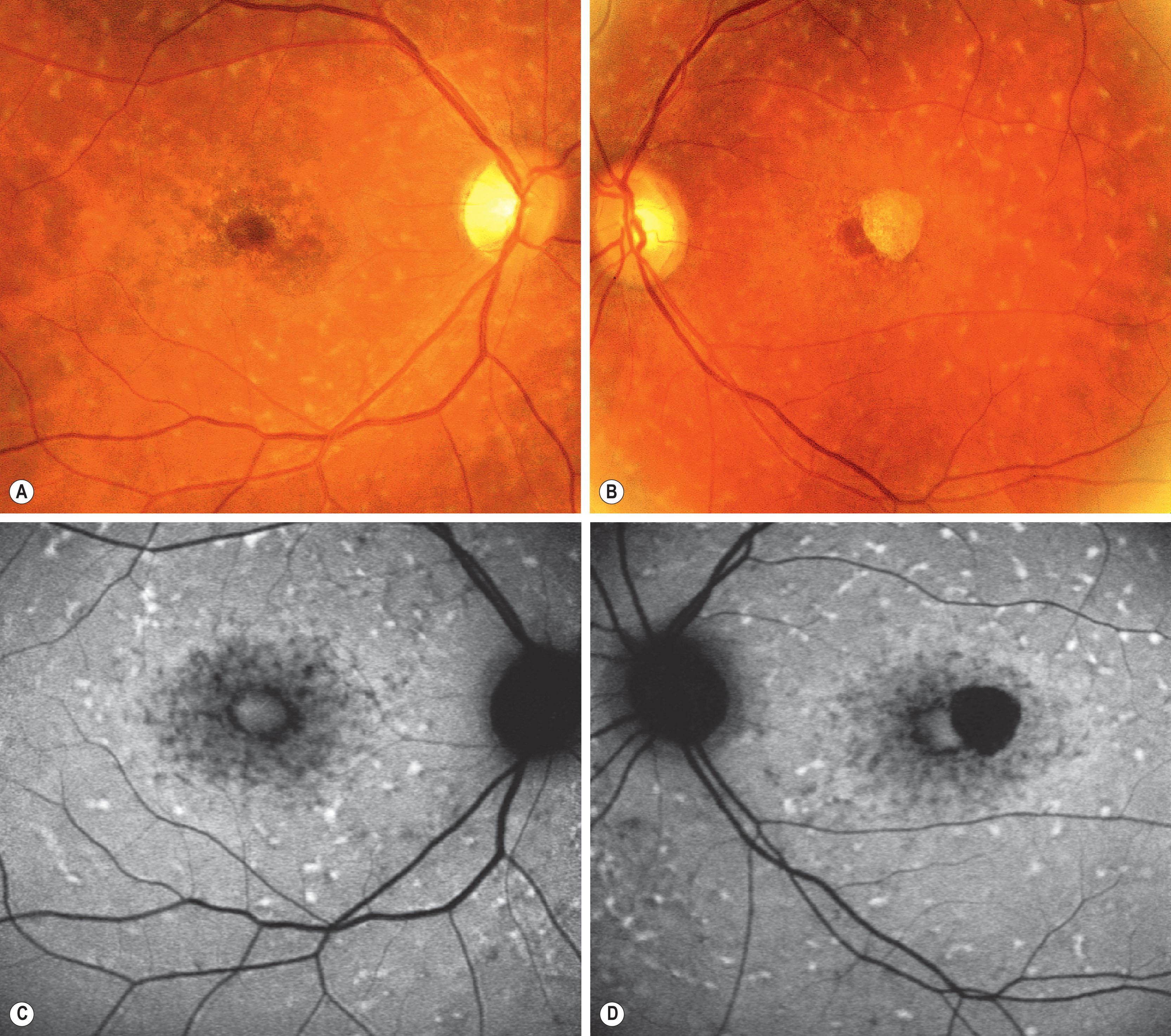
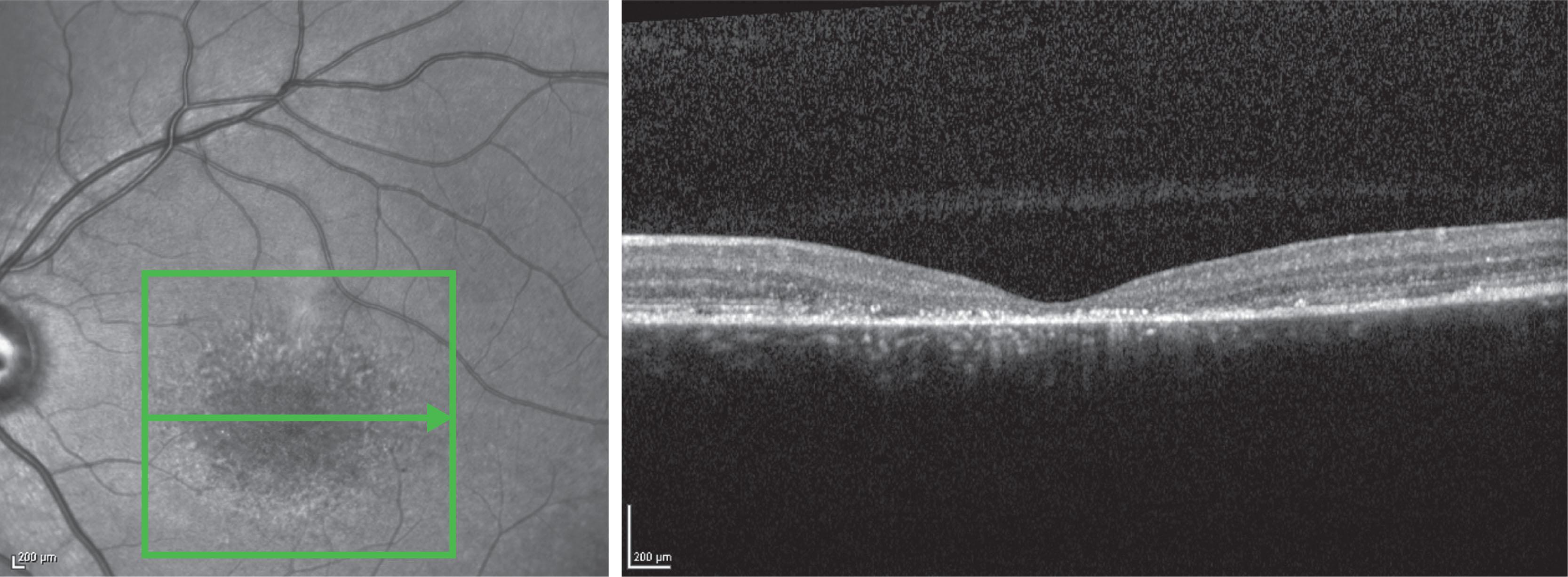
In both STGD and FFM, fundus fluorescein angiography (FFA) classically reveals a dark or masked choroid ( Fig. 47.2 ) in the early phase. This is due to excess lipofuscin accumulation in the RPE, which obscures fluorescence from choroidal capillaries. The retinal flecks appear hypofluorescent on FFA early in their evolution, but later they appear hyperfluorescent due to RPE atrophy. Fundus autofluorescence (FAF) imaging, using the intrinsic fluorescence from lipofuscin in the RPE, has superseded FFA in confirming the diagnosis. The abnormal accumulation of lipofuscin, the presence of active and resorbed flecks, and RPE atrophy with peripapillary sparing are characteristic on FAF imaging ( Fig. 47.3 ). These FAF features are also metrics that can be reliably used to determine change over time and be employed in clinical trials. In children with normal fundoscopy and visual loss from macular dysfunction, FFA may be helpful; a subtle window defect in the central macula or a dark choroid helps confirm the diagnosis. However, this has now been superseded by high-resolution optical coherence tomography (OCT), which shows abnormal foveal structure at very early stages of disease. In more established disease, OCT reveals loss, or marked disruption, of central outer retinal macular architecture with relative preservation of peripheral macular structure ( Fig. 47.4 ).
Electrophysiological abnormalities in STGD are variable. An abnormal electro-oculogram (EOG), suggestive of generalized RPE dysfunction, is common. The pattern electroretinogram (PERG) and focal electroretinogram (ERG) are usually abolished or markedly reduced, suggesting macular dysfunction. The full-field ERG may be normal at diagnosis (group 1) or suggest widespread retinal dysfunction (group 2 or 3):
Group 1: severe pattern ERG abnormality with normal full-field ERG
Group 2: additional generalized cone dysfunction
Group 3: generalized cone and rod dysfunction.
These groups are not explained by differences in age of onset or duration of disease and electrophysiological classification at onset of disease may be thereby helpful in informing prognosis. Patients in group 1 have the best visual prognosis, with those in group 3 the worst.
Disease-causing sequence variants in the gene ABCA4 underlie STGD, and have also been implicated in cone and cone–rod dystrophy. ABCA4 encodes a transmembrane rim protein in the outer segment discs of rod and cone photoreceptors involved in transport of retinoids from photoreceptor to RPE. Failure of this transport results in deposition of a lipofuscin fluorophore, A2E ( N -retinylidene- N -retinylethanolamine), in the RPE, which is deleterious to the RPE and leads to secondary photoreceptor degeneration.
More than 1200 sequence variations in ABCA4 have been reported, demonstrating the high allelic heterogeneity and highlighting the difficulties in assigning disease-causing status to sequence variants detected when screening such a large (50 exons) and polymorphic gene. A further complication is that in some patients with classical Stargardt disease only one disease allele is identified on sequencing the coding region of ABCA4; it is now evident that the missing alleles include variants affecting splicing and also deep intronic variants that can regulate gene expression. Null variants with a major effect on the encoded protein can be confidently predicted to be disease-causing, but for other variants assigning pathogenicity is more problematic (see reference for detailed review). Direct evidence of pathogenicity can only be established by functional analysis of the encoded mutant protein and there are a limited number of such studies.
Correlation between the type and combination of ABCA4 variants with the severity of the phenotype is sometimes possible. For example, biallelic null variants usually lead to an early-onset severe cone–rod dystrophy phenotype rather than STGD. Variable retinal phenotypes within families may be explained by different combinations of ABCA4 variants segregating within a single family, but it is very likely that other modifier genes or environmental factors may also influence intrafamilial variability.
ABCA4 is an ATP-binding transporter that is involved in clearing all trans retinal from the outer segments following phototransduction so that it can be recycled to form 11- cis retinal, the light-sensitive chromophore. The delayed clearance of all trans retinal associated with ABCA4 dysfunction is toxic to the retina through the formation of the bisretinoid A2E which is a major component of lipofuscin. Stargardt disease is associated with the accumulation of high levels of lipofuscin-related products in the RPE causing dysfunction and ultimately leading to photoreceptor cell death. The development of an ABCA4 knockout mouse ( abca4 −/− ) has, despite the limitation of lacking a macula, greatly improved our understanding of disease mechanism and possible therapies. The abca4 −/− show elevated levels of A2E and other lipofuscin-related fluorophores in the RPE. A2E synthesis can be reduced by raising abca4 −/− mice in total darkness, by inhibiting the visual cycle pharmacologically and is increased by feeding the mice supplemental vitamin A. It seems reasonable to therefore advise patients with STGD to avoid vitamin A supplementation and to wear ultraviolet light-blocking sunglasses. We also recommend an antioxidant-rich diet, which slows photoreceptor cell death in animal models of retinal dystrophies. Affected children may be helped by low vision aids and educational support.
Families should also be offered genetic counseling. STGD is inherited as an AR trait and thus younger siblings of an affected child have a 1 in 4 risk of developing the disorder. Older siblings are at a lower risk, as the age of onset is usually similar between affected siblings. The carrier frequency of STGD is 1 in 50; thus, there is a 1 in 50 chance that an asymptomatic partner of an affected individual carries a disease-causing sequence change in ABCA4 . Therefore, there is a 1% risk of an affected individual having an affected child (higher if the partner is a close relative).
New pharmacological interventions for STGD include drugs that target the adenosine triphosphate (ATP)-dependent transport mechanism, thereby augmenting ABCA4-related retinoid transport, or slow the visual cycle, reducing the production of A2E. Directly inhibiting the toxic effects of A2E or facilitating removal of lipofuscin/A2E accumulation are other approaches. Pharmacological agents aimed at these targets have been developed and are in ongoing human clinical trial or anticipated to start in the near future. Such agents may also be helpful in other macular degenerations associated with lipofuscin accumulation, such as Best disease. Other approaches to therapy include gene replacement therapy and for advanced disease stem cell-based RPE cell transplantation. There are more than 15 different registered clinical trials for Stargardt disease ( http://www.clinicaltrials.gov ).
Few macular dystrophies show X-linked inheritance. The commonest is juvenile X-linked retinoschisis which is covered in detail in Chapter 50 and will not be considered further here. Some disease-causing variants in RPGR are associated with cone or cone–rod dystrophy rather than RP, and may in the initial stages have funduscopic abnormalities confined to the macula. However, the full field ERG abnormalities help distinguish this from a purely macular dystrophy. Most affected patients are myopic and have an atrophic appearance of the central macula. FAF imaging generally shows hypoautofluorescence in the central macula, often with a hyperautofluorescent ring more peripherally.
Compared to the recessive disorder, individuals with autosomal dominant (AD) STGD-like dystrophy have a milder phenotype with better vision and minimal color vision defects. Individuals usually present in the first or second decades with visual loss, which may precede retinal changes. Typically, there is progressive macular atrophy with or without flecks. Fundus autofluorescence imaging shows patchy hyperautofluorescence at the macula and spectral domain OCT shows early loss of the outer nuclear layer with RPE atrophy occurring at a later stage. The “dark choroid” sign on FFA is uncommon in the dominant form.
Individuals with AD STGD-like dystrophy often have no significant EOG or full-field ERG abnormalities; ERG changes when present are mild.
Inheritance patterns may distinguish the two forms of STGD, but pseudodominance is a confounding factor − the carrier frequency of AR STGD is sufficiently common (1 in 50) for an affected individual to have an asymptomatic partner carrying a disease-associated sequence change in ABCA4 . Pseudodominance is also common in consanguineous families.
Two chromosomal loci have been identified: 6q14 (STGD3) and 4p (STGD4). Heterozygous variants in the gene ELOVL4 which encodes the Elongation of Very Long chain fatty acids-4 protein are associated with STGD3 and other macular dystrophies, including pattern dystrophy. There is no associated systemic phenotype. The retinal phenotype is associated with four different variants in exon 6 of the gene, each of which result in a truncated protein. More recently two additional STGD3 causative variants in the promoter of the gene have been identified; these are thought to reduce gene expression. ELOVL4 is also implicated in other disorders; heterozygous missense variants in ELOVL4 are associated with a late onset form of spinocerebellar ataxia (SCA34) and homozygous variants result in a severe infantile onset neurological disorder with seizures, developmental delay, and ichthyosis.
A heterozygous missense variant, p.Arg373Cys, in PROM1 co-segregates with disease in STGD4. PROM1 encodes human prominin 1, which has a role in outer segment disc formation and maintenance. The same PROM1 missense mutation, p.Arg373Cys, occurs in patients with an early-onset autosomal dominant “bull's eye” macular dystrophy (MCDR2). Biallelic variants in PROM1 result in a more severe generalized retinal dystrophy.
Onset is from the end of the first to the sixth decade, with most affected individuals presenting with reading difficulties. RPE mottling in younger subjects progresses to a bull's eye maculopathy (BEM) and, later, macular atrophy. Some patients have typical features of RP (see Chapter 45 ). Generally affected individuals have a mild to moderate reduction in visual acuity, except when associated with RP, where markedly reduced central vision is common.
Electrophysiological findings range from isolated macular dysfunction without generalized photoreceptor dysfunction, to a very severe rod–cone dysfunction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here