Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Colorectal cancer (CRC) is a complex heterogeneous disease with a variety of factors influencing genetic and epigenetic changes that drive tumor initiation and progression. Alterations in the intricate system of biological checks and balances can lead to a malignant change of the normal colorectal mucosa. The underlying changes, whether inherited or sporadic, influence the genotype and phenotype of that particular cancer and patient. Understanding CRC in this context, and grouping patients based on the underlying cancer pathway, helps to study the disease and provide more precise clinical management. This chapter provides an overview of the underlying changes in colorectal carcinogenesis and the hereditary CRC syndromes.
Cancer is fundamentally a genetic disease as tumors develop through genetic or epigenetic somatic alterations in cells. A relatively small proportion of gastrointestinal (GI) cancer, probably less than 5%, develops as a consequence of a highly penetrant germline mutation in one gene, resulting in a familial GI polyposis or cancer syndrome. The tumors that develop in these syndromes then follow the same evolutionary paths as sporadic tumors, but the risk for cancer is greatly elevated in the mutation carrier, the tumors have a substantially earlier median age of onset, and the patients are at risk for multiple metachronous tumors. An understanding of the biological basis of sporadic CRCs has provided insight into the special instances of the familial syndromes, all of which are quite distinct genetically and clinically.
There are multiple signaling pathways that regulate cell growth. Consequently, there are multiple possible pathways for tumorigenesis, which explains why CRCs—and the cancer syndromes—are heterogeneous genetically and clinically. However, there is a discrete number of parallel regulatory pathways, which crosstalk with one another, and the function of any pathway can be altered by a defect in any one of the serial links.
There are three classes of genes involved in the evolution of cancer: proto-oncogenes, which become activated through mutation, amplification, or chromosomal rearrangement; tumor suppressor genes, which become inactivated through mutation, chromosomal deletion, or promoter methylation; and genes involved in maintaining genomic stability, which become dysregulated—usually by inactivation—leading to genomic instability and the rapid accumulation of more mutations. Moreover, without some form of genomic instability, it would take a very long time to accumulate mutations in these specific genes that drive cancer development.
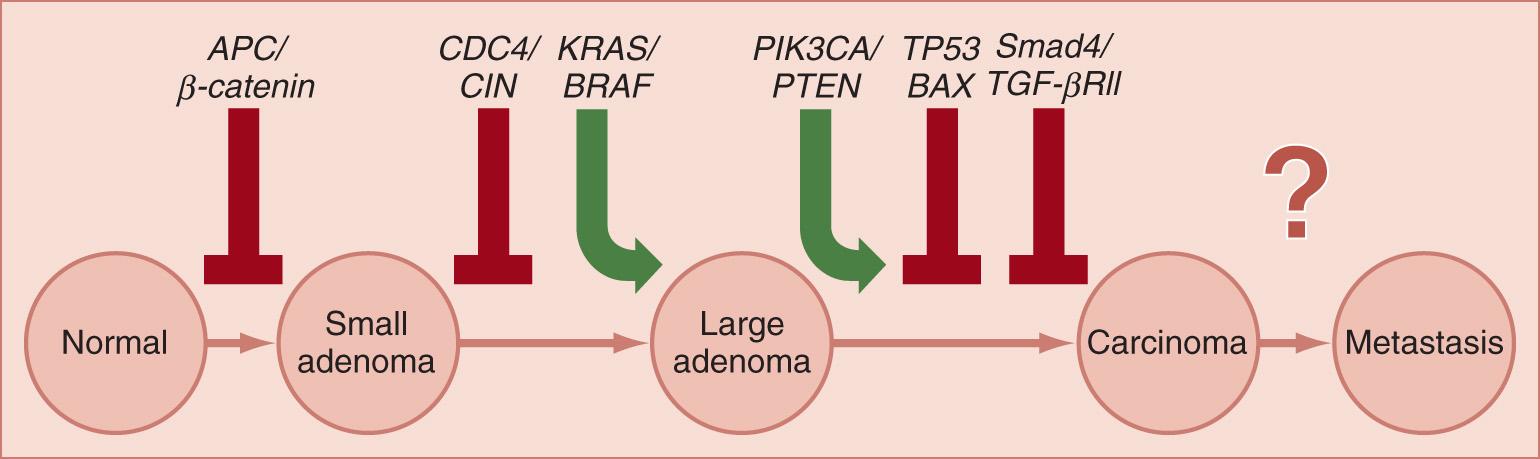
We can classify CRCs into three broad classes of genetic or epigenetic instability, but there is some overlap among them, which can be initially confusing. The first is manifested by chromosomal instability (CIN), and was first proposed in 1990 by Vogelstein in the context of multistep carcinogenesis. The first step in the CIN pathway is inactivation of the WNT signaling pathway, followed, in sequence, by activating mutations in key growth-stimulating genes, such as KRAS , CDC4 , PIK3CA, and others, followed by disruption of the negative growth regulatory network of transforming growth factor (TGF)-β signaling (typically through inactivation of one or more of the SMAD genes), and the cataclysmic conversion of benign to malignant behavior by mutations and allelic losses of the p53 (TP53) gene, which abrogates the G1/S cell cycle checkpoint and permits the accumulation of chromosomal rearrangement and aneuploidy. This pathway is illustrated in Fig. 165.1 . More than half of all CRCs develop through this pathway. There is considerable heterogeneity in the evolution of this pathway, and only three of these, APC , KRAS and p53 , are mutated in greater than 11% of all CRCs.
The initial genetic alteration in the classic pathway is inactivation of the WNT signaling pathway, which is a key concept for understanding familial adenomatous polyposis (FAP). This usually occurs by biallelic inactivation of the APC gene, removing the protein that regulates the intracellular concentration of β-catenin, which is a transcription factor that helps turn on the growth program, and it is also involved in the intercellular adhesion complex that holds colonic epithelial cells together. However, the same effect can be achieved through a stabilizing mutation in the β-catenin gene that renders the protein incapable of being degraded by the adenomatous polyposis coli (APC) protein. Indeed, additional downstream events involving T-cell factor/lymphoid enhancer-binding factor (TCF or LEF) proteins can do this as well. The WNT signaling pathway is depicted in Fig. 165.2 .
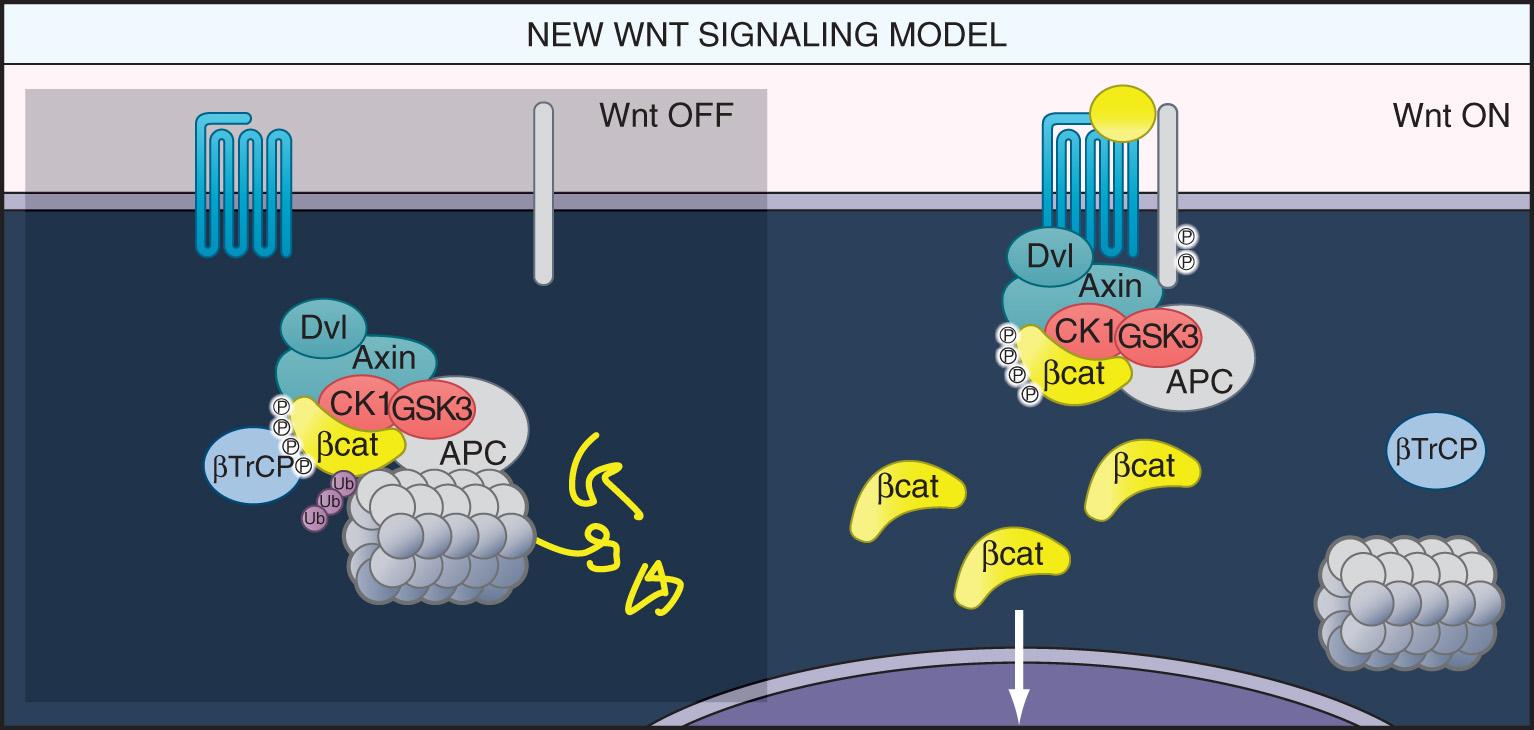
Once WNT signaling is dysregulated, the colonic epithelial cell can undergo uncontrolled divisions and fails to differentiate. The colorectal adenoma is a collection of epithelial cells that continue to grow outside of the colonic crypt, do not fully differentiate, and form a mass lesion. The actual “drivers” of the process are the β-catenin gene (now upregulated), and eventually, other activated oncogenes. CIN begins in benign adenomas, which can remain stable for decades, or grow slowly. But once the p53 gene is lost (typically by one inactivating mutation and loss of the other allele), there is an increase in the accumulation of abnormal chromosomal rearrangements, which results in large numbers of deleted, duplicated, and rearranged genes. These changes synergize to drive the neoplastic process forward. Because of the very large number of specific genetic alterations that can contribute to the process, CRCs that evolve through the classic pathway can be quite heterogeneous in appearance and clinical behavior.
Most families with FAP have a germline mutation in the APC gene. Occasional families with FAP have no detectable mutation in APC ; however, there are no convincing examples of germline mutations in any other gene that cause typical FAP. Even a mutation that results in the deletion of a long-range promoter sequence in APC (literally over 46,000 bases upstream of the start codon) can cause full-blown FAP.
Since biallelic mutations in APC are required for neoplastic colonic epithelial cell growth to occur, the phenotype in a person with a germline mutation in APC is normal at birth, but over time, losses of the other “wild type” allele occurs in individual epithelial cells, which results in adenomas. Over time, unregulated growth predisposes the adenoma to the accumulation of more mutations in the multistep pathway. Illustrating the long time frame in which this occurs, the median age for the first adenoma in FAP is 16, but the median age for cancer is 39. There are no instances of inheriting biallelic APC germline mutations as that phenotype is embryonically lethal.
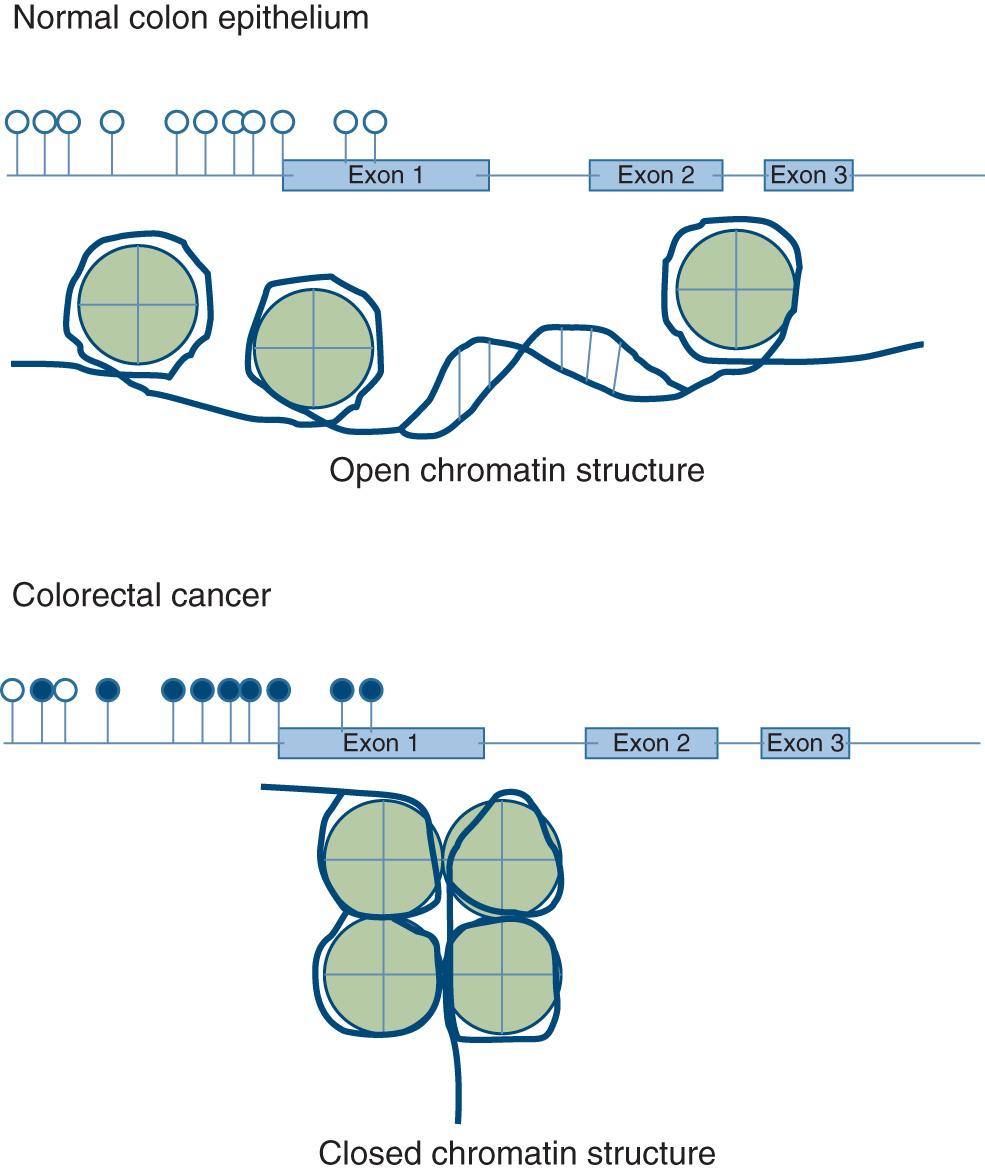
The second CRC pathway involves the inactivation of tumor suppressor genes through hypermethylation of gene promoters, a normal physiologic process for silencing genes that becomes accelerated through a process that is not completely understood. The sequences in the promoters that are hypermethylated are C-G dinucleotide pairs (called CpG sequences, in which the p stands for the phosphodiester bond between the C and G). These sequences are infrequent in the genome except in gene promoters (the on–off switch or rheostat for gene expression), and they occur in clusters called CpG islands. When there is an excess of promoter methylation in the cancer genome, this is the CpG island methylator phenotype, or CIMP . This occurs as a predominant epigenetic “lesion” in about 32% of CRCs, but increased CpG methylation is also seen in many other CRCs, so the methylation must be quantitated at selected promoters for the CRC to be categorized as CIMP. CIMP often (but not always) occurs in association with a mutation in the BRAF gene (V600E), and this is associated with more aggressive, lethal CRCs compared with tumors arising from the other pathways.
Tumors in the CIMP pathway are thought to begin as sessile serrated adenomas (SSAs) rather than as typical adenomatous polyps; consequently, this has been called the serrated carcinoma pathway. Both SSAs and CIMP CRCs occur predominantly in the proximal colon. The genetic or epigenetic events that drive the SSA from a benign, nonneoplastic-appearing lesion to CRC are not specifically known, although the genes that commonly undergo promoter methylation in CIMP include p16 , CDKN2A , THBS1 , HPP1, and MLH1 . The underlying “instability” involves excessive promoter methylation, whereas it is the silencing of tumor suppressor genes that acts as the “driver” of carcinogenesis in CIMP-related tumors. Additionally, a number of microRNAs, which are key regulators of gene expression, are silenced by methylation in cancer, and are an increasingly important part of the driving force in CIMP-mediated carcinogenesis. Fig. 165.3 illustrates the CIMP and methylation-mediated gene silencing.
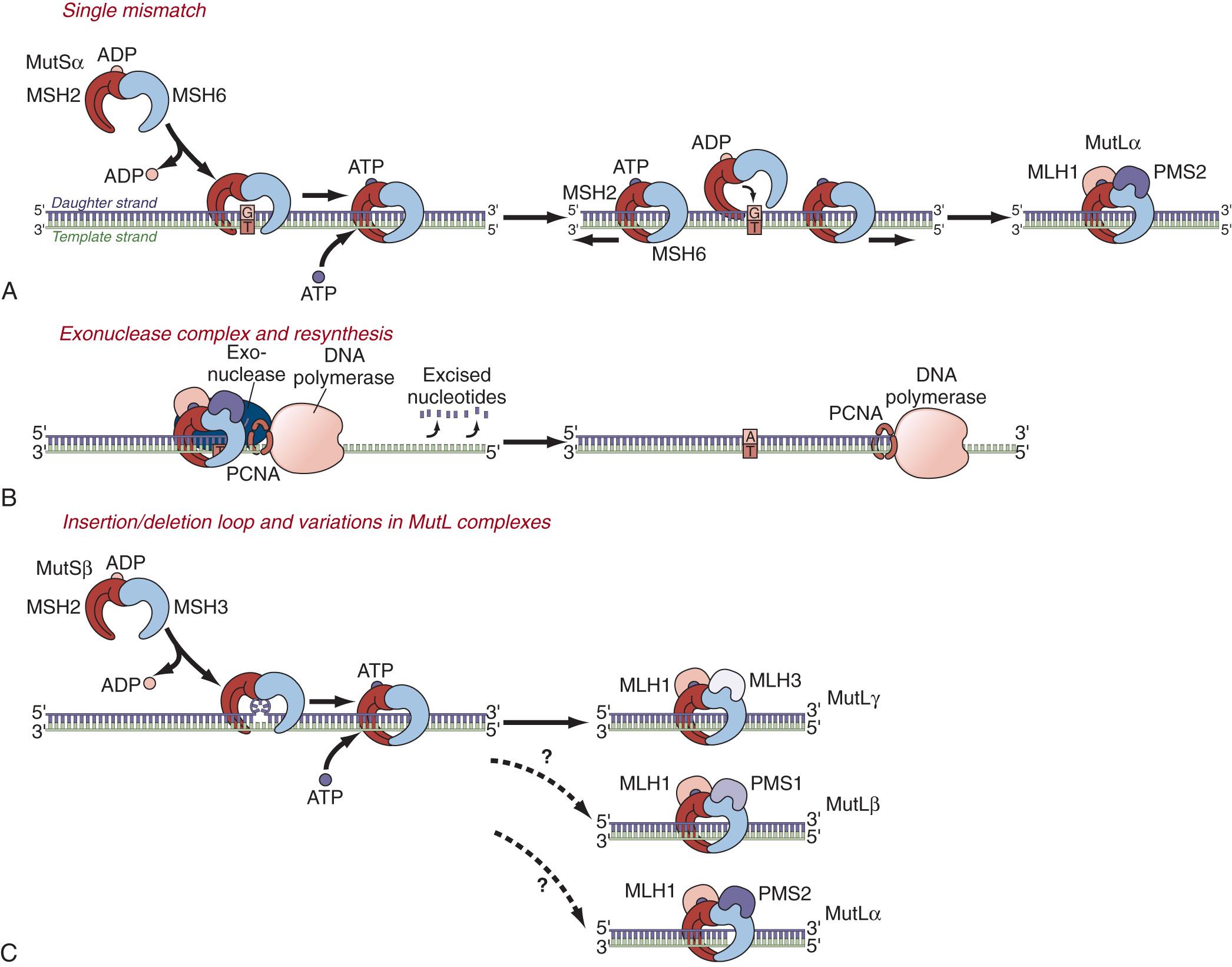
The third pathway for colorectal carcinogenesis is a secondary consequence of either CIN (in the setting of Lynch syndrome) or CIMP, and is called “ microsatellite instability ” or MSI. The nucleus has multiple enzyme systems that monitor DNA for damage, and either repair the mutation or prevent the cell from replicating. One of these is mainly an S-phase monitor for errors introduced by DNA polymerase (also playing a role outside of DNA synthesis), called the DNA mismatch repair (MMR) system. During DNA replication, DNA polymerases are prone to errors while copying the correct number of nucleotides at simple repetitive sequences (mono-, di-, tri-, and tetra-nucleotide repeats) called microsatellites. The DNA MMR system identifies these small DNA copying errors and removes the faulty sequences, which permits the cell to resynthesize the sequence correctly. In the absence of DNA MMR activity, there is greater than 100-fold increase in synthetic errors in microsatellite sequences, which is where the term MSI was derived. The DNA MMR system is shown in Fig. 165.4 .
CRCs with MSI can occur in three circumstances. First, approximately 12% of all CRCs begin as CIMP tumors, but when methylation occurs at both alleles of one of the DNA MMR genes ( MLH1 ), MSI ensues and overwhelms the CIMP background, as the accumulation of mutations occurs rapidly. These tumors have a background of CIMP, but are recognized as MSI tumors because of this overwhelming phenotype, and often have an activating mutation in BRAF . If they do not have a mutation in BRAF , the prognosis is better than for CIN or pure CIMP CRCs.
Second, about 3% of CRCs occur in the setting of Lynch syndrome (previously referred to as hereditary nonpolyposis CRC [HNPCC]), of which most have MSI. There is evidence that neoplasms in Lynch syndrome arise initially as otherwise ordinary adenomatous polyps, and then develop MSI when there is loss of the wild-type allele of the DNA MMR gene responsible for the Lynch syndrome. However, it is possible that some neoplasms may arise directly from a nonneoplastic colonic crypt with defective DNA MMR activity, which is present in large numbers in the nonneoplastic epithelium of the colon. Once there is a DNA MMR-deficient cell, the progeny accumulate mutations at DNA sequences that encode mononucleotide repeat nucleotides (i.e., C n , T n , G n , or A n where n = 6 to 10). There are about 30 genes in the human genome that have microsatellites in coding sequences, and these include a number of critical tumor suppressor genes (such as TGF-β receptor 2, BAX, Caspase-5, and others) that are frequently mutated at the microsatellite by deletion or insertion of one base pair in the sequence, which inactivates the gene. As such, these microsatellite-encoding genes are the actual “drivers” of carcinogenesis once mutated.
Third, a very small proportion of CRCs begin in the CIN pathway, and develop biallelic mutations at one of the DNA MMR genes, after which MSI ensues, which rapidly overtakes the prior phenotype. This is called “Lynch-like syndrome.” Curiously, it can be familial, which is not understood.
MSI can occur in the context of CIMP, in which approximately 90% of the tumors are in the proximal colon, and the patients are on average about 5 years older than those with sporadic CRCs. It can also occur in the context of Lynch syndrome, in which case about two-thirds of the tumors are in the proximal colon and the patients are 15 to 20 years younger than others with CRC. It can also occur due to biallelic somatic mutations in DNA MMR genes, and will masquerade as Lynch syndrome.
Because the “driver” mutations are different for each of these pathways, the clinical behavior of the tumors is different for each group. Importantly, however, the CIN or classic pathway is the sequence seen in the disease FAP, which is always associated with a germline mutation in the APC gene. The MSI pathway is present in essentially all CRCs that occur in Lynch syndrome, which is caused by germline mutations in one of the four DNA MMR genes ( MLH1 , MSH2 , MSH6, and PMS2 ). However, most CRCs with MSI are not Lynch syndrome, but are a consequence of methylation-induced silencing of MLH1 , or less commonly, Lynch-like syndrome. There has been no identifiable form of a familial CIMP syndrome.
The hamartomatous polyposis syndromes are quite distinct from either FAP or Lynch syndrome. In these instances, the germline mutations cause benign lesions to develop in the small and/or large intestine, usually in the context of a unique, identifiable phenotype. However, all of the hamartoma syndromes are associated with a variety of cancers in the gut and elsewhere because of the dysregulation of growth they cause. These are discussed later in this chapter.
There are several hereditary syndromes of CRC predisposition that are associated with a strong tendency toward multiple colorectal polyps. Polyposis simply means “lots of polyps,” but in practical terms it is defined as more than 100 polyps at one examination. Fewer numbers of polyps, from 10 to 100, are often referred to as attenuated polyposis or “oligopolyposis.” Polyposis syndromes also can be classified according to polyp histology (the phenotype) and according to the gene that is mutated in the patients' germline (genotype). The various syndromes, along with their clinical and genetic criteria, are listed in Table 165.1 . Management of the polyposis syndromes involves diagnosis, evaluation, and management with the goals to (1) prevent death from cancer and (2) maintain adequate quality of life.
| Syndrome | Polyp Histology | Gene(s) | Number of Polyps for Diagnosis | Inheritance Pattern |
|---|---|---|---|---|
| Familial adenomatous polyposis | Adenoma | APC | Severe/profuse: >1000 Mild: 100–1000 Attenuated: <100 |
Dominant |
| MutYH-associated polyposis | Adenoma | MutYH | Any | Recessive |
| NTHL1-associated polyposis | Adenoma | NTHL1 | Any | Recessive |
| Polymerase proofreading-associated polyposis | Adenoma | POLD, POLE | Any | Dominant |
| Lynch syndrome | Adenoma | MLH1, MSH2, MSH6, PMS2 EPCAM |
>10 | Dominant |
| Serrated polyposis | Serrated polyp | ? | >20 of any size and location >5 proximal to the sigmoid, 2 >10-mm diameter |
? |
| Hereditary mixed polyposis syndrome | Adenoma, serrated polyp, hamartoma | GREM1 | >5, at least 1 juvenile polyp | Dominant (Ashkenazi Jews) |
| PTEN hamartoma tumor syndrome | Juvenile polyp, hamartoma, lipoma, fibroma, neurofibroma, serrated polyp | PTEN | — | Dominant |
| Juvenile polyposis | Juvenile polyp | SMAD4 BMPR1A |
>5 >1 plus a family history of juvenile polyposis |
Dominant |
| Peutz-Jeghers polyposis | Peutz-Jeghers polyp | STK11 | >1 plus a family history of Peutz-Jegher polyposis | Dominant |
FAP is rare, with an incidence of approximately 1 in 10,000 live births. Prevalence is estimated at 1 in 24,000 to 1 in 60,000 and depends on the average size of families in a country or region, and the completeness of registration of the disease. FAP affects all races and both genders equally. The expression of the disease may vary according to genotype and vary even within patients who share the same mutation, due to modifying factors, such as gender. FAP is caused by a germline APC mutation, which results in a generalized growth disorder expressed as benign and malignant tumors in a variety of tissues. Manifestations associated with the mutation are summarized in Table 165.2 . The severity of the growth disorder and the risks and age at onset of cancer vary between and within families.
| Organ | Benign | Malignant |
|---|---|---|
| Colon and rectum | Adenoma | Carcinoma |
| Stomach | Adenoma Fundic gland polyp |
Carcinoma |
| Small intestine | Adenoma | Carcinoma |
| Thyroid | — | Papillary carcinoma |
| Adrenal gland | Adenoma | Carcinoma |
| Bone | Osteoma | — |
| Skin | Epidermoid cyst | — |
| Teeth | Extra teeth | — |
| Fibroblasts | Desmoid disease | — |
| Brain | — | Medulloblastoma |
| Liver | — | Hepatoblastoma |
Most patients with FAP are diagnosed based on family history. For a dominantly inherited syndrome with close to 100% penetrance, the family history of CRC and other manifestations of the APC mutation is compelling. Children of an affected parent usually undergo genetic testing at puberty and, if affected, start colonoscopy at that time. Testing in infancy is generally discouraged because of the possible effect of a positive result on the parent's attitude to their affected children, but this fear may not be borne out in practice. However, the possibility of hepatoblastoma, a rare manifestation of the germline APC mutation, is a reason to perform genetic testing of newborns and then hepatoblastoma screening (6-monthly liver ultrasound and alpha fetoprotein) until the age of 7.
Approximately 25% of patients with FAP have no knowledge of a family history of the disease. Reasons for this include adoption, deliberate withholding of information by affected family members, nonpaternity, germline mosaicism, and a de novo mutation at conception. These patients are unaware of the risk posed by the unsuspected germline mutation and usually present with symptoms of their polyposis or cancer, such as rectal bleeding, abdominal pain, and diarrhea. They have a high chance of having CRC at diagnosis.
Clinical diagnosis of FAP is usually made on colonoscopy followed by efforts to establish a genetic diagnosis so that the family can be triaged. The odds of identifying an APC mutation are greater than 80% in patients with greater than 100 adenomas. If a germline APC mutation is found, then all at-risk relatives can be offered screening. Other genetic mechanisms may cause FAP and include deletion of the APC promoter 1B, chromosomal loss (often associated with reduced mental capacity), and APC haploinsufficiency. If genetic testing is negative, sometimes extracolonic manifestations can be used to predict an affected relative.
Care of patients with FAP and their families is best given by centers of experience and excellence. Such centers are scattered around the world, and are often linked by organizations such as the Collaborative Group of the Americas for Inherited CRC ( cgaicc.com ) and the International Society for Gastrointestinal Hereditary Tumors (InSiGHT). Patients and their families who enroll in a registry benefit at least from enhanced survival due to organized surveillance.
CRC is the main risk in patients with FAP and is the most common cause of death. In classic FAP (>100 synchronous adenomas), the average age at cancer diagnosis is 39 years, with much younger onset in patients with profuse polyposis. The most effective way of preventing cancer is to remove the colon, and thus thought should be given regarding the timing of prophylactic surgery once a diagnosis is established. Symptomatic patients should undergo colectomy using an oncologic technique as occult cancer risk is elevated in these patients. For patients who do not require a colectomy around the time of diagnosis, annual colonoscopy should be performed with removal of larger polyps and continued thoughtfulness of the timing of surgery.
Almost all patients with FAP develop fundic gland polyps in the stomach, and adenomas in the duodenum. Duodenal cancers are the third most common cause of death in FAP (after CRC and desmoid disease) and upper GI surveillance begins at 20 to 25 years old. An esophagoduodenoscopy should be done with a side-viewing duodenoscope so that the duodenal ampulla can be examined.
CRC is rare in FAP patients younger than 20 years old. For patients diagnosed in their teenage years, the timing of surgery depends primarily on the severity of the polyposis. Factors governing the timing of colectomy are shown in Table 165.3 . While the top priority is to avoid cancer or to remove it as early as possible, lifestyle concerns are real and need to be considered. Many FAP patients are in their teens or twenties when faced with the prospect of surgery, and are often asymptomatic. They have academic, financial, social, and developmental concerns, and the idea of a stoma or the chance of a complication is scary. This is especially the case where other members of the family have had unfortunate outcomes. Therefore, appropriate surgery selection is key to decrease the cancer risk, maintain the quality of life, and minimize the complications. Note that increased risk of desmoid disease is listed as an indication to defer surgery as long as possible in Table 165.3 . This is because approximately 80% of desmoid disease in FAP develops after abdominal surgery, presumably secondary to the intervention.
| Urgency | Timing | Indication |
|---|---|---|
| Immediate | Next available list | Cancer Symptoms Complications of colonoscopy |
| Soon | Within 3 months | Profuse polyposis (>1000 adenomas) Multiple large (>1 cm) adenomas High-grade dysplasia in an adenoma |
| Sometime | On a year-by-year basis | Mild polyposis (100–1000 adenomas) Asymptomatic Social, intellectual, academic, financial, family factors |
| Defer | Put off as long as possible | High risk of desmoid disease High comorbidity |
There are three main options for prophylactic surgery in patients with FAP: colectomy with ileorectal anastomosis (IRA), total proctocolectomy with ileal pouch–anal anastomosis (IPAA), and total proctocolectomy with end ileostomy (TPC-EI). These surgeries can be done by open or minimally invasive techniques, and the IPAA can be done with a stapled anastomosis or with a mucosectomy and handsewn anastomosis. Tables 165.4 and 165.5 show the factors that favor or disfavor the different surgeries. An oncologic technique with high ligation of the feeding vessels and removal of the mesentery and the omentum should always be used because unsuspected cancer may be found in the specimen.
| Advantages | Disadvantages | Indications | Contraindications | |
|---|---|---|---|---|
| Ileorectal anastomosis | Quick, safe, uncomplicated Good quality of life Bowel function acceptable Controls colonic polyposis Avoids any ileostomy Surveillance easy |
Risk of rectal cancer Surveillance required |
<20 rectal adenomas <1000 colonic adenomas |
Rectal cancer |
| Ileal pouch–anal anastomosis | Controls most colorectal polyposis Avoids permanent ileostomy Reasonable quality of life |
High complications Bowel function unpredictable Ileostomy needed Risk of pouch polyposis Risk of ATZ cancer Quality of life unpredictable Surveillance can be difficult |
>20 rectal adenomas Rectal cancer |
High desmoid risk Lack of surgical expertise <20 rectal adenomas |
| Proctocolectomy and ileostomy | Least risk of complications Complete removal of cancer risk in lower GI tract |
Permanent ileostomy | Low rectal cancer IPAA impossible Poor anal sphincter function |
Good sphincters No rectal cancer |
| Minimally invasive | Less trauma, less pain, reduced length of stay, quicker recovery, minimal scars | Expertise required, slower, may cause issues with IPAA | Slim, young patients Expertise available |
Severe desmoid risk IPAA |
| Open | Less operative time | More pain, longer recovery, scar | Best for IPAA patients with high desmoid risk | Patients where cosmesis is an issue, high morbidity |
| Stage | Size | Symptoms | Growth |
|---|---|---|---|
| I | <10 cm | None | None |
| II | <10 cm | Mild | <25% in 6 months |
| III | 11–20 cm | Moderate | 25% to 50% in 6 months |
| IV | >20 cm | Severe | >50% in 6 months |
In practical terms, the decision for IRA or IPAA is driven by the severity of the polyposis: the more severe the polyposis the higher the risk of metachronous rectal polyposis and/or rectal cancer. Church et al. showed that when there were 5 or fewer rectal adenomas and 1000 or fewer colonic polyps, no patient needed a subsequent proctectomy. When there were 6 to 20 rectal adenomas, 15% of patients needed later proctectomy; however, with 20 or more rectal adenomas, the incidence of later proctectomy was more than 50%. Recently this “rule” has been stretched in favor of a conservative approach. Teenagers who might otherwise need proctectomy and a pouch have been treated with a laparoscopic IRA, for the benefits of a quicker, safe surgery, better bowel function, lower risk of desmoid disease, no stoma, and quicker recovery. These are considerable benefits for young, active patients; however, annually, careful proctoscopy is needed to keep the rectal polyposis in check.
The need for postoperative surveillance should be considered when planning the surgery. The ideal length of the rectum is 15 cm, which provides reasonable bowel function while still allowing easy endoscopic evaluation. With an IRA at 15 cm from the anal verge, patients on average have between 4 and 5 semiformed bowel movements without urgency or incontinence. A diverting stoma is not routine and recovery is quick, particularly after a laparoscopic approach. Preoperatively, all large rectal polyps must be removed to assure there is not cancer. Small (<5 mm) polyps may be left for future surveillance and removal as needed. Postoperatively, the rectal polyp burden may spontaneously reduce, likely because of the different types of stool the mucosa encounters. The IRA itself can be tricky to construct because of the disparity in luminal diameter between the ileum and the rectum. A technical point to limit this disparity is to resect the terminal ileum flush with the ileocecal valve to preserve the “trumpet-like” flare of the bowel just before it reaches the cecum. The ileal mesentery can also be a source of trouble as the mesenteric defect can allow an internal hernia, which could cause small bowel obstruction. Small mesenteric defects should be closed.
The main differences between IRA and IPAA are (1) the loss of the rectum; (2) the extent of surgery that requires deep pelvic dissection; and (3) the different physiology of an ileal pouch. If the low rectum is relatively free of adenomas, a stapled IPAA can be performed with an ileal J pouch. This gives decent function with the expectation of an average of 5 to 6 daily bowel movements and good control with the ability to defer the movement for 1 to 2 hours. If the anal transition zone (ATZ) and the low rectum are carpeted by adenoma, then mucosectomy and handsewn IPAA should be performed. An S pouch is the preferred configuration under these circumstances. A handsewn IPAA is prone to stricture and leak, and has a higher rate of seepage and a need for the patients to wear pads. Surgeons performing IPAA should be familiar with either anastomotic technique, and be able to perform the operation open as well as laparoscopically. Taking the pouch down to the anus without twists is critical to good function, as even a twist of 90 degrees can cause shelves in the pouch that hinder defecation. A diverting loop ileostomy is routine. However, if the IPAA procedure is stapled, the operation goes well, the stapler donuts are intact, and the leak test on the anastomosis is negative, then omitting the temporary loop ileostomy may be considered.
For patients who prefer an operation that completely removes the chance of large bowel cancer, and who do not mind an EI, this is the best choice. It is critical that the ileostomy be well positioned and well constructed, with a 2-cm spout, so that pouching is easy and the skin-protective appliance lasts 3 to 4 days.
Patients with an IRA and an IPAA need surveillance of their rectum or pouch, as adenomas and cancers can occur. Adenomas and cancers can also occur on an EI, although cancer takes at least 15 years to develop. Surveillance of the rectum and ileal pouch is performed yearly, usually as an office procedure without sedation, after two fleet enemas. The terminal ileum above the IRA or pouch is examined for at least 15 cm. The anastomosis itself is checked and the body of the pouch or rectum is examined for adenomas. Polyps may be hyperplastic or lymphoid follicles, and pouch adenomas can be quite subtle. Because the ileal mucosa has villi, the adenomas tend to blend into the surrounding epithelium. Ulcers may be seen at the IRA and at the entry of the ileum into the pouch. They should be biopsied, but they are common and do not have any clinical significance in asymptomatic patients. The ATZ is a “hot spot” for adenomas. Whereas pouch adenomas usually take several years to form (42% of pouches have adenomas by 7 years follow-up), ATZ adenomas may be there at the time of surgery and can sprout quite quickly. They are twice as common after a stapled IPAA as after a handsewn IPAA and, if not dealt with, can turn to cancer. They can often be simply snared, but carpeting with villous adenoma requires stripping of the ATZ and a handsewn reanastomosis if possible. Sometimes this needs a formal redo of the IPAA with transabdominal pouch mobilization; the preferred technique is to do this transanally, with half the circumference done initially and the other half after the ATZ has healed from the first procedure. Pouch cancer is rare. EIs should be checked once a year and the patient educated in the appearance of adenomas on the stoma.
Chemoprevention in FAP means treating colorectal, ileal, and duodenal adenoma risk with medications to suppress the neoplasia, as an alternative to surgery and polypectomy, or at least to postpone it. Many studies have tested a variety of agents in patients with FAP, mostly because the accelerated adenoma to carcinoma sequence in FAP allows the effects of putative chemotherapeutic agents to become obvious relatively quickly. The most tested drug is sulindac (Clinoril), a nonsteroidal antiinflammatory agent with cyclooxygenase (COX)-1 and COX-2 inhibiting activity. However, sulindac can cause GI distress, bleeding, intestinal ulceration, and renal effects, and is not tolerated in 20% of patients. That being said, a dose of 150 to 200 mg twice daily suppresses colorectal adenomas and desmoid disease, but its effectiveness depends on strict compliance with taking the drug. There is a concern that cancers can still develop in patients whose adenomas have been suppressed for years. For this reason, and because no chemotherapeutic agent has been shown to be completely effective in FAP, we do not favor chemoprevention as a routine option for colorectal adenomas in FAP. However, chemoprevention with sulindac is reasonable in FAP patients with pouch polyposis as the surgical alternative is pouch removal and likely an EI. Other agents that have shown some effect include difluorometylornithine (DFMO) and celecoxib (Celebrex), although neither is used in standard practice. For duodenal adenomas in FAP, the epidermal growth factor receptor inhibitor erlotinib (Tarceva), in combination with sulindac, has recently shown great promise in the treatment of duodenal adenomas in FAP. Combination chemotherapy, whereby multiple pathways to cancer are targeted at once, holds great promise for the future.
Desmoid disease is a manifestation of the APC mutation that is found in 30% of FAP patients, mostly in the abdominal wall, in the small bowel mesentery, and in the retroperitoneum. It is an abnormal proliferation of fibroblasts that can produce tumors, or hard white sheets. Desmoid tumors can grow rapidly and be fatal, while even desmoid sheets can pucker adjacent organs, causing bowel or ureteric obstruction, and enterocutaneous fistulas. The clinical behavior of desmoid disease varies from patient to patient and even within a patient, becoming less aggressive over time. Overall, about 12% regress, 7% are lethal, and the remainder never disappear, but rather grow and shrink to a small extent while remaining relatively asymptomatic. There is no predictably effective treatment for desmoid disease and thus, for patients at high risk (see later), deferring its onset by avoiding abdominal surgery needs consideration. Desmoid disease can interfere with plans for an IPAA by restricting the length of the small intestinal mesentery so that the pouch will not reach the anus. A recent Cleveland Clinic study of patients undergoing proctectomy after an initial IRA showed that desmoid disease was present in 26 of 67 patients. Although proctectomy can still be completed in nearly all patients, desmoid disease prevented a planned IPAA in 8 of 62 patients. This possibility should be discussed with patients preoperatively.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here