Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Inherited bone marrow (BM) failure is defined herein as decreased production of one or more of the major hematopoietic lineages; i.e., red blood cells (RBCs), neutrophils, and platelets, due to germline mutations that were derived from the parents or occurred de novo ( Table 30.1 ). Although outdated, the term “constitutional” has been used interchangeably with “inherited” and similarly implies that a genetic abnormality causes the BM dysfunction. The designation “congenital” has a looser connotation and refers to conditions that manifest early in life, often at birth, but does not imply a particular causation. Therefore, “congenital BM failure” is not necessarily inherited and may be caused by acquired factors such as viruses, drugs, or environmental toxins. Nevertheless, some inherited BM failure syndromes (IBMFSs) have been historically termed congenital (e.g., severe congenital neutropenia [SCN]) and remain so.
| Disorder | Inheritance | Gene |
|---|---|---|
| IBMFSs With Multilineage Cytopenia and Familial MDS/AML | ||
| Fanconi anemia | AR | FANCA, FANCC, FANCD1/BRCA2, FANCD2, FANCE, FANCF, FANCG/XRCC9, FANCI, FANCJ/BRIP1, FANCL/PHF9, FANCM, FANCN/PALB2, FANCO/RAD51C, FANCP/SLX4, FANCQ/ERCC4, FANCR/RAD51, FANCS/BRCA1, FANCT/UBE2T, FANCU/XRCC2, FANCV / REV7 , FANCW/RFWD3 |
| XLR | FANCB | |
| Mixed Fanconi anemia/xeroderma pigmentosa/Cockayne syndrome) | AR | ERCC1/XPF |
| Shwachman-Diamond syndrome | AR | SBDS, DNAJC21, EFL1 |
| Dyskeratosis congenita | XLR | DKC1 |
| AD | TINF2, TERC, TERT, RTEL1, PARN, ACD(TPP1) | |
| AR | TERT, RTEL1, PARN, ACD(TPP1), NOP10, NHP2, WRAP53(TCAB1), CTC1, POT1, RPA1 | |
| Congenital amegakaryocytic thrombocytopenia | AR | MPL |
| SRP72 -associated hereditary aplastic anemia/MDS | AD | SRP72 |
| ERCC6L2 -associated hereditary aplastic anemia/MDS | AR | ERCC6L2 |
| THPO -associated hereditary aplastic anemia/MDS | AR/AD | THPO |
| Reticular dysgenesis | AR | AK2 |
| Cartilage-Hair hypoplasia | AR | RMRP, POP1, NEPRO |
| Pearson syndrome | Maternal | mDNA |
| Familial thrombocytopenia with predisposition to AML | AD | RUNX1/CBFA2 |
| AD | ETV6 | |
| GATA2-associated disorders (MonoMac syndrome, Emberger syndrome, familial MDS syndrome) | AD | GATA2 |
| Bone marrow failure and diabetes | DUT | |
| Familial MDS/AML (Others) | CEBPA | |
| Familial MDS/AML (Others) | DDX41 | |
| Seckel syndrome | AR | CEP152, CENPJ, CEP63, NIN, PLK4, CDK5RAP2, ATR, RBBP8, ATRIP, DNA2 |
| Schimke immunoosseous dysplasia | AR | SMARCAL1 |
| Dubowitz syndrome | AR | NSUN2, LIG4 , |
| AD | -14q32, -17q24, -19q13 | |
| Rothmund-Thomson syndrome | AR | RECQL4 |
| Nijmegen breakage syndrome | AR | NBN |
| IBMFSs With Predominantly Anemia | ||
| Diamond-Blackfan anemia | AD | RPS7, RPS10, RPS15, RPS15a, RPS17, RPS19, RPS24, RPS26, RPS27, RPS27a, RPS28, RPS29, RPL5, RPL9, RPL11, RPL15, RPL18, RPL26, RPL27, RPL31, RPL35, RPL35a |
| XL |
|
|
| AR | EPO | |
| AR | ADA2/CECR1 | |
| Inherited sideroblastic anemia | XL | ALAS2 |
| XL | ABCB7 | |
| AR | SLC19A2, GLRX5, PUS1, SLC25A38, YARS2, TRNT1 | |
| Maternal | MT-ATP6 | |
| Congenital dyserythropoietic anemia type I | AR | CDAN1, CDIN1 |
| Congenital dyserythropoietic anemia type II | AR | SEC23B |
| Congenital dyserythropoietic anemia type III | AD | KIF23 |
| Congenital dyserythropoietic anemia - unclassified | AR | KLF1 |
| IBMFSs With Predominantly Neutropenia | ||
| Kostmann/Severe congenital neutropenia | AD | ELA2, GFI1, TCIRG1 |
| AR | HAX1, CSF3R, G6PC3, VPS45, JAGN1 | |
| XL | WAS | |
| Cyclic neutropenia | AD | ELA2 |
| WHIM syndrome | AD | CXCR4 |
| Glycogen storage diseases Ib | AR | G6PT1/SLC37A4 |
| Barth syndrome | XL | TAZ |
| Poikiloderma with neutropenia | AR | USB1/C16orf57 |
| Neutropenia, immune deficiency, skeletal dysplasia and glycosylation defect | AR | PGM3 |
| Cohen syndrome | AR | COH1/VPS13B |
| Dominant intermediate Charcot-Marie-Tooth | AD | DNM2 |
| Hermansky-Pudlak syndrome, type 2 with neutropenia | AR | AP3B1 |
| Hyper-IgM syndrome | XL | CD40LG |
| Autosomal dominant severe congenital neutropenia | AD | SEC61A1 |
| Mitochondrial DNA depletion syndrome 13 | AR | FBXL4 |
| IBMFSs With Predominantly Thrombocytopenia | ||
| Thrombocytopenia absent radii syndrome | AR | RBM8A |
| Thrombocytopenia with radio-ulnar synostosis | AD | HOXA11 |
| Familial autosomal dominant non-syndromic thrombocytopenia | AD | MASTL, ANKRD26, ACBD5, CYCS |
| Familial platelet disorder with AML | AD | CDC25C |
| X-linked thrombocytopenia | XL | WASP |
| Mediterranean platelet disorder | AD | GP1BA |
| Familial thrombocytopenia | AD | GFI1B |
| Familial thrombocytopenia | AR | FYB, SBF2 |
| Gray platelet syndrome | AR | NBEAL2 |
| Epstein/Fechtner/Sebastian/May-Hegglin/Alport syndrome | AD | MYH9 |
| Familial macro-thrombocytopenia | AR | FLNA, ABCG5, ABCG8, ACTN1, MYSM1, PRKACG |
| Familial macro-thrombocytopenia | AD | TUBB1, ITGA2, ITGA2B, ITGB3 |
| Stormorken syndrome (thrombocytopenia with anemia) | AD | STIM1 |
| Autosomal dominant non-syndromic sensorineural deafness type Dfna and thrombocytopenia | AD | CDCL1 (MCM2) |
Hematopoiesis is an orderly but complex interplay of stem and progenitor cells, growth factors, BM stromal elements, and positive and negative cellular and humoral regulators. Thus, BM failure can potentially occur at several critical points in the hematopoietic lineage pathways. With regard to IBMFSs, germline mutations interfere with orderly hematopoiesis and cause the BM failure. The discovery of specific, high-penetrance mutant alleles associated with discrete IBMFSs provides evidence for this. Many of these alleles are of genes that directly affect physiologic cell survival, differentiation, and function in pathways that are essential for normal hematopoiesis (e.g., DNA repair, telomere maintenance, ribosome biogenesis, microtubule stabilization, chemotaxis, signaling from hematopoietic growth factors, signal transduction related to hematopoietic cell differentiation, and granulocytic enzymes). Modifying genes, epigenetic processes, acquired factors, and chance effects may also be operative and interact with the mutant genes to produce overt disease with varying clinical expression. Hence, the disorders listed in Table 30.1 are transmitted in a Mendelian pattern determined primarily by mutant genes with inheritance patterns of autosomal dominant, autosomal recessive, or X-linked types. Newly discovered IBMFSs may follow similar inheritance patterns or be multifactorial in origin caused by an interaction of multiple genes and a variety of exogenous or environmental determinants.
Data from the Canadian Inherited Marrow Failure Registry (CIMFR) suggest an incidence of about 65 cases diagnosed per million live births per year. As of December 2020, the most prevalent IBMFSs in this registry according to decreasing order are Diamond-Blackfan anemia (DBA) (22.2%), Fanconi anemia (FA) (14.2%), Shwachman-Diamond syndrome (SDS) (13.9%), dyskeratosis congenita (DC) (11.0%), Kostmann/SCN (8.0%), thrombocytopenia absent radii (TAR) syndrome (1.9%), and congenital amegakaryocytic thrombocytopenia (CAMT) (1.3%). It is noteworthy that 30% of the patients in this registry are unclassified and cannot be assigned a syndromic or genetic diagnosis. In the Israeli registry the frequency of IBMFS in decreasing order were reported as FA (52%), SCN (17%), DBA (14%), CAMT (6%), DC (5%), SDS (2%), and TAR syndrome (2%). Importantly, none of these syndromes is restricted to the pediatric age group. Patients with IBMFSs may be detected for the first time in adulthood. Reported cases include patients with FA, SDS, DC, DBA, and SCN among others, who first became evident when reaching adulthood. The genetics and pathophysiology of IBMFSs are closely linked to several common pathological and physiological process such as cancer and aging.
Historically, the IBMFSs were classified as “benign” disorders in contrast sharply with hematologic cancers. Patients with IBMFSs often die early in life from complications of cytopenias. However, in the current era of advanced supportive care and availability of recombinant cytokines and other effective therapeutics, patients with these conditions usually survive the early years of life and beyond. With the extended lifespan of patients, the natural history of these disorders has dramatically changed. One of the most sobering observations is that the many IBMFSs confer an inordinately high predisposition to developing myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). These include conditions such as SCN, SDS, FA, DC, CAMT, DBA, and TAR syndrome, among others. Thus, the distinction between “benign” and “malignant” hematology in the context of the IBMFSs has become blurred, and a new clinical and hematologic continuum is evident. Clearly, these disorders are leukemia-predisposition syndromes and several of them (e.g., FA, DC, and DBA) are broader cancer-predisposition syndromes. There is reason to believe that the first genetic “hit” or leukemia-initiating step may be the syndrome-specific inherited genetic abnormality itself, which initially manifests as the single- or multiple-lineage marrow failure state. The “predisposed” progenitor, already initiated, could conceptually develop decreased responsiveness to the signals that regulate homeostatic growth, terminal cell differentiation, or programmed cell death. Leukemic promotion and progression with clonal expansion leading to MDS or AML could then ensue readily.
FA is inherited in an autosomal recessive manner in 98% of cases. In rare cases, it is transmitted in an X-linked recessive or autosomal dominant mode.
Although the original report of FA in 1927 by Dr. Guido Fanconi described pancytopenia combined with physical anomalies in three brothers, many publications thereafter have underscored the clinical variability of the condition. FA is a genomic instability disorder characterized by chromosomal fragility and breakage, a defect in DNA repair, progressive BM cell underproduction, peripheral blood cytopenias, developmental anomalies, and a strong propensity for hematologic and solid tumor cancers.
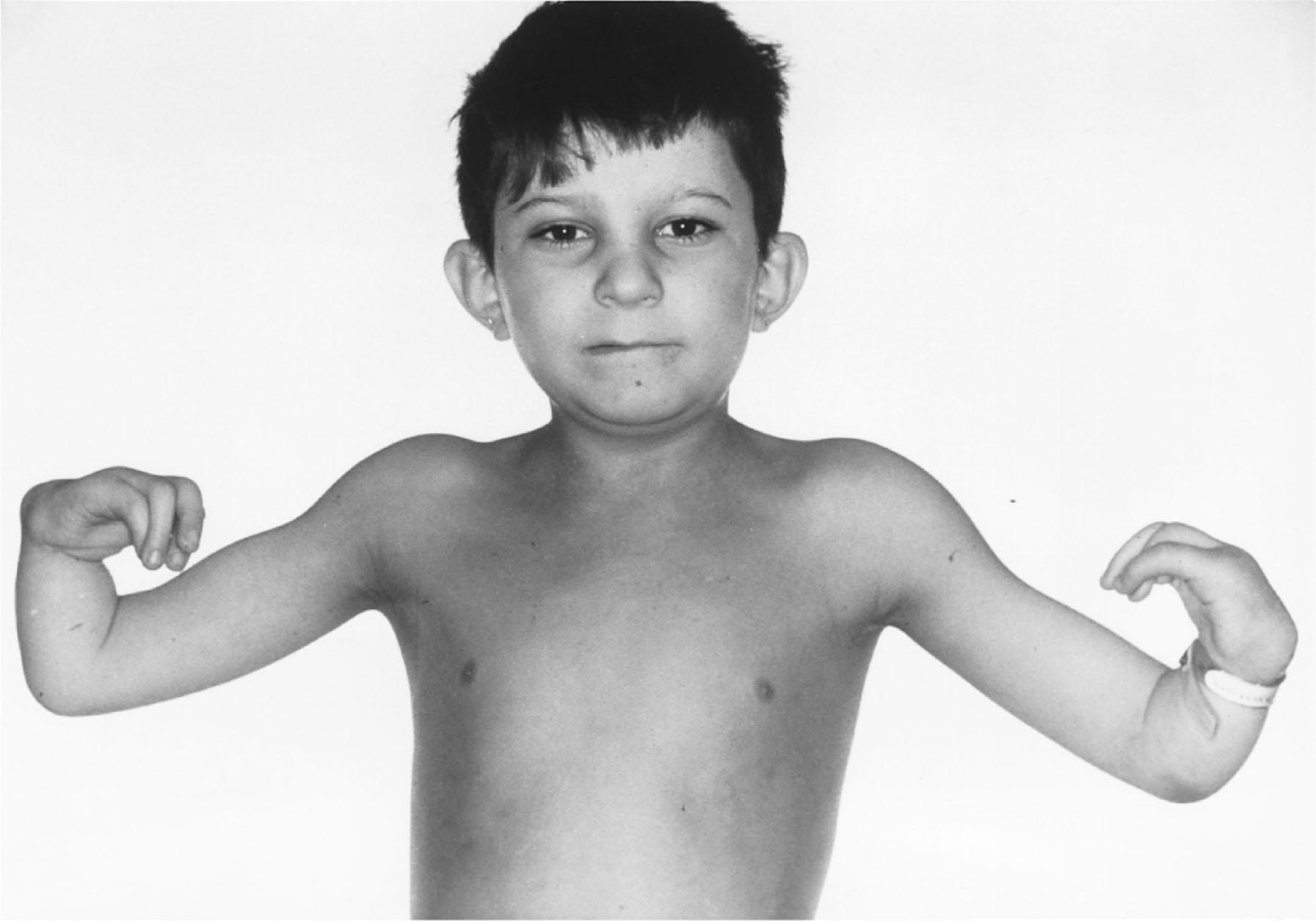
FA patients may present with either physical anomalies but normal hematology, or normal physical features but abnormal hematology, normal physical features and normal hematology, or physical anomalies and abnormal hematology ( Fig. 30.1 ). There can also be sibling heterogeneity in presentation with discordance in clinical and hematologic findings, even in affected monozygotic twins. Using published information, the median age at diagnosis of FA is about 6.5 years with a reported range from birth to 49 years. However, advances in genetic diagnostic tools may result in early diagnosis of young siblings or adults who do not have apparent clinical manifestations.>
The overall prevalence of FA is 1 to 5 cases per million with a carrier frequency of 1 in 200 to 300 in most populations. Data from the CIMFR showed a prevalence of 11.4 cases per million live births per year. It occurs in all racial and ethnic groups. Spanish Gypsies have the world’s highest prevalence of FA with a carrier frequency of 1 in 64 to 1 in 70 for a common founder mutation. A founder effect has also been demonstrated in Afrikaners in South Africa in whom one specific mutation is common (frequency, 1 in 83), as well as in Ashkenazi Jews (1 in 89), Moroccan Jews, Tunisians, sub-Saharan African Blacks, Indian, Israeli Arabs, Brazilians, and Japanese.
FA genes are all involved in DNA repair. The first clue for this defect was the abnormal chromosome fragility that is readily seen in metaphase preparations of peripheral blood lymphocytes or skin fibroblasts cultured with phytohemagglutinin (PHA) and is enhanced by adding a DNA interstrand cross-linking agent, such as mitomycin C (MMC) or diepoxybutane (DEB) (see Abnormal Chromosome Fragility section later). This feature was utilized to discover the first FA genes by complementation analysis. Complementation is considered when fusion of FA patient cells with cells from an individual who does not have mutations in the same gene (i.e., cell hybridization with cells from a healthy subject or a FA patient with mutations in another FA gene) result in correction of MMC or DEB hypersensitivity in growth inhibition or chromosomal fragility assays. Complementation has also been determined by transducing known FA gene cDNA into ungenotyped patient cells. The mutated gene was determined by a failure of fused cells with known mutation to correct (complement) the abnormal chromosome breakage in the patient’s T cells in culture on exposure to DEB/MMC. This is why FA genes are called FANC genes (FA complementation). Recently, next generation gene panel assays replaced complementation testing for identifying the mutant FA gene, and whole exome/genome sequencing replaced the complementation testing for FA gene discovery. So far, 22 genetic groups (termed types A, B, C, D1, D2, E, F, G, I, J, L, M, N, O, P, Q, R, S, T, U, V, W) have been proposed (see Table 30.1 ). The first FA gene, FANCC , was discovered in 1992 in Toronto, and then the other genes, corresponding to each of the other complementation groups, were subsequently cloned: FANCA, FANCB, FANCC, FANCD1/BRCA2, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ/BACH1/BRIP1, FANCL, FANCM, FANCN/PALB2, FANCO/RAD51C, FANCP/SLX4, FANCQ/ECCR4, FANCR/EXCC2 , and FANCS/BRCA1, FANCT/UBE2T, FANCU/XRCC2, FANCV/REV7, FANCW .
The most commonly mutated genes based on data from the International Fanconi Anemia Registry (IFAR) are FANCA (∼61%), FANCC (∼16%), and FANCG (∼10). As of 2020, the most commonly used tool for genetic investigation of FA patients is by either next generation sequencing gene panel assays or single gene analysis. Partial or complete gene deletions are common in FA; hence, analysis of deletions should always be considered when nucleotide sequencing does not reveal pathogenic variants. Deletion analysis can be done by either subjecting next generation sequencing gene panel data to special copy number variation analysis software programs, or by utilizing multiplex ligation-dependent probe amplification) or microarray copy number variation (CNV).
Cells and cell lines from FA patients are phenotypically similar regardless of the complementation group that they represent. A hypothesis was therefore formulated and subsequently substantiated that the various wild-type FA proteins function in a common response pathway to repair DNA damage incurred during DNA replication. A major function of FA pathway genes is to repair interstrand DNA crosslinks. Many exogenous agents (e.g. cisplatin, nitrogen mustards, and MMC) and endogenous agents (e.g. aldehydes and free oxygen radicals) can induce formation of interstrand DNA crosslinks.
There are three general steps in the FA DNA damage response pathway: (1) Core c omplex . Nine wild-type FA proteins (FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL, FANCM, and FANCT) and additional proteins (e.g., FAAP20, FAAP24, and FAAP100) form a single large nuclear protein “core complex” as the first step. The core complex functions as a ubiquitin ligase of which FANCT, an E2 ubiquitin-conjugating enzyme and FANCL, an E3 ubiquitin ligase, are the catalytic subunits. The FA core complex is recruited to fork-like DNA structures via FAAP24 and FANCM. (2) ID2 complex . The activated core complex converts a downstream heterodimer composed of two proteins, FANCI and FANCD2 (called the “ID2 complex” ), from unubiquitinated isoforms to monoubiquitinated isoforms. Monoubiquitination does not occur if the core complex upstream of the ID2 complex is not intact, and therefore FA cells from patients with upstream mutations do not show the monoubiquitinated FANCI/FANCD2. Recently, it has been shown that monoubiquitination occurs after the ID2 complex binds to an arrested replication fork at an ICL. (3) Downstream effector complexes . In normal cells after monoubiquitination of the ID2 complex, it forms a binding interface for single and double stranded DNA and downstream effector complexes with additional FA and other proteins. The scaffold protein FANCP/SLX4 binds first, followed by the endonucleases FANCQ(ERCC4), MUS81, SLX1, and ERCC1 that cleave DNA interstrand crosslinks, resulting in DNA adducts and dsDNA breaks. DNA adducts are resolved by the exonucleases FANCV/REV7, REV1, REV3 after forming a translesion synthesis complex. dsDNA breaks are repaired by homologous recombination. The exonucleases CTIP, MRN, XO1 cut the dsDNA breaks and generate ssDNA with 3′ overhang. Replication protein A (RPA) is recruited to the ssDNA break. FANCR/RAD51 recombinase localizes and loaded to the ssDNA/RPA complex by FANCD1/BRACA2 and FANCS/BRACA1. FANCR/RAD51 removes RPA and prevents it from self-binding. A recombination filament is generated by FANCR/RAD51 and the additional proteins that bind to the site in parallel or subsequently: FANCD1/BRCA2, FANCS/BRCA1, FANCO/RAD51C, FANCJ/BRIP1, FANCN/PALB2, and FANCU/XRCC1. Consequently, the recombination filament searches for homologous bases to repair DNA crosslinks.
There are three main DNA repair processes that the FA genes cooperate with: (1) nucleotide excision repair that excises one DNA strand flanking the interstrand crosslink (via interaction between FANCP/SLX4 and MUS81, SLX1, and others) followed by ligation. This process is utilized in quiescent cells; (2) translesion synthesis that involves one strand incisions around the interstrand crosslink (ICL), unhooking of the ICL, and extension of the uncut strand (via recruitment of translesion polymerase); (3) homologous recombination is initiated after nucleolytic incisions by endonucleases (FANCQ/ERCC4), MUS81, SLX1, ERCC1) and generation of dsDNA break. The dsDNA break is cut by exonucleases to generate a ssDNA break, to which FANCR/RAD51, FANCD1, FANCS, FANCN, FANCJ, FANCO, FANCU, and other proteins are recruited to form a recombination filament that searches for homologous bases for further repair. Other DNA-repair proteins such as MRE11-RAD50-NBS1, PCNA, and BLM are also involved in the later stages of the DNA repair response.
The exact link between the impaired ability to repair interstrand crosslinks and FA phenotype is still to be defined, but may be related to accumulation of DNA adducts, a failure to arrest DNA synthesis in response to DNA damage, impaired homologous recombination, defective nonhomologous end joining, abnormal induction of p53, induction of P53/CHK1 dependent G2/M cell cycle arrest, and increased apoptosis. In addition, homologous recombination and several FA proteins play a role in replication of telomeric G4 structures and possibly prevention of replication induced telomere damage. Loss of these functions may thus lead to short telomeres.
There are important protein-protein interactions between FA proteins and non-FA “binding partners” for cell survival. FANCC and FANCD2 form complexes with members of the signal transducer and activator of transcription (STAT) family of transcription factors in cytokine-mediated biologic responses. Secondly, heat shock proteins provide several cell survival functions, and FANCC protein specifically facilitates the anti-apoptotic role of Hsp70. FANCC also interacts with cdc2, PKR, and p53, suggesting that FANCC has other roles that are independent of DNA damage recognition and repair. GSTP1 is an enzyme that detoxifies byproducts of redox stress and xenobiotics and FANCC protein enhances GSTP1 activity in cells exposed to apoptosis inducers.
Several studies suggested a role of oxidative stress in the evolution of BM failure and leukemia in FA. Reactive oxygen species (ROS) were shown to be elevated in FA cells and high oxidative stress causes increased DNA damage, increased hematopoietic stem cell (HSC) senescence and a decreased HSC pool, thereby leading to BM failure. Further, in vivo and in vitro studies have demonstrated the ability of the antioxidant N-acetylcysteine to reduce DNA damage, reduce HSC senescence, and improve HSC reconstitution ability. Therefore, it is possible that patients with FA are particularly sensitive to ROS-induced DNA damage due to impaired DNA repair mechanisms. This increased sensitivity may be caused, at least in part, by impaired detoxification of ROS and naturally produced aldehydes. A deficiency in superoxide dismutase and poor cell growth at ambient oxygen have also been demonstrated in FA cells.
In FA patients’ skin fibroblasts, N-acetylcysteine was able to reduce ROS levels and apoptosis as measured by activation of caspase-3 and poly(ADP-ribose)polymerase (PARP) cleavage. In fancc −/− mice, N-acetylcysteine rescues hematopoietic colony formation that is impaired by spontaneous secretion of TNFα. It also reduces TNFα-mediated hematopoietic colony formation and HSC senescence and HSC reconstitution potential. Using a fancd2 −/− mouse model, treatment with the antioxidant drug resveratrol has also been shown to preserve HSC quiescence, partially correct the abnormal cell cycle status, and significantly improve the spleen colony-forming capacity of BM cells. Importantly, treatment of FA mice with N-acetylcysteine has been shown to reduce the accumulation of cytogenetic abnormalities (that are commonly seen in FA patients who transform to MDS/AML). In one study, the antioxidant tempol delayed cancer in tumor-prone fancd2 −/− /Trp53 +/− mice. However, in another study neither N-acetylcysteine nor the antioxidant resveratrol had this property in this mouse model.
Cell lines from FA patients have also been shown to feature increased autophagy and mitophagy that was attributed to elevated levels of mitochondrial fission caused by high oxidative stress. In another study, interestingly, cells from FA patients showed impairment of mitochondrial functions as evidenced by a high frequency of mtDNA genetic variants, downregulation of mtDNA complex-I and complex-III encoding genes of OXPHOS, and reduced expression of certain mitophagy-related genes (ATG, Beclin-1, and MAP1-LC3) that may lead to reduced ability to clear damaged mitochondria.
The level at which oxidative stress is linked to FA phenotype independently of DNA damage is still to be defined. The high oxidative stress and oxygen sensitivity phenotype of FA cells shorten cell survival. A cardinal phenotype of FA cells is an abnormality in cell cycle distribution with an increased number of cells with 4 N DNA content arising from a delay in the G 2 /M or late S phase of the cell cycle. The strongest evidence supporting an oxygen metabolism deficiency in FA is a reduction of FA cells with 4 N DNA content when grown at low oxygen levels and the unexpected appearance of 4 N DNA content when normal cells are grown at high oxygen levels. Of note, some wild-type FA proteins play a role in redox-related functions. FANCC associates with NADPH (nicotinamide adenine dinucleotide phosphate), cytochrome P-450 reductase, and glutathione S-transferase, proteins with redox functions. FANCA and FANCG are redox-sensitive proteins that multimerize after H 2 O 2 treatment, prompting the notion that the FA pathway may function in oxidative stress management.
Hematologic abnormalities in FA are evident at the hematopoietic stem and progenitor cell (HSPC) level with marked reduction in both multipotent (HSC, and multipotent progenitors, MPP) and oligopotent cells (common myeloid progenitors, CMP, megakaryocyte-erythroid progenitors, MEP), but a modest reduction in granulocyte-monocyte progenitors (GMP). Cure of FA BM failure by HSC transplantation (HSCT) supports the hypothesis that the hematopoietic defect starts at the HSC level. Clonogenic assays show reduced frequencies of CFU-E (colony-forming unit-erythroid), BFU-E (burst-forming unit-erythroid), and CFU-GM (colony-forming unit-granulocyte macrophage) colony-forming cells in almost all patients after aplastic anemia ensues as well as in a few patients before the onset of aplastic anemia. Besides low progenitor numbers, decreased colony numbers in these studies can also be interpreted as faulty proliferative properties which lead to an inability to form colonies in vitro. Indeed, there is a defective proliferative response of CFU-GEMM (colony-forming unit granulocyte, erythrocyte, macrophage, megakaryocyte), BFU-E, and CFU-GM progenitors to GM-CSF plus stem cell factor (SCF) (c-kit ligand) or to IL-3 plus SCF.
Additional factors are operative in FA BM failure. Telomeres, the non-encoding DNA at each end of chromosomes, shorten with each round of cell division in normal human somatic cells. Their length is a reflection of the mitotic history of the cell. Telomerase, a ribonucleoprotein reverse transcriptase that can restore telomere length, is variably present in hematopoietic progenitors. Leukocyte telomere length is significantly shortened in FA patients despite increased telomerase activity. In parallel, increased BM cell apoptosis has been demonstrated in FA patients and in knock-out mouse models and is mediated by Fas, a membrane glycoprotein receptor containing an integral death domain. FA cells exposed to tumor necrosis factor-α (TNF-α), interferon-γ (INF-γ), MIP-1α, Fas ligand, and double-stranded RNA undergo exaggerated apoptotic responses.
Studies of cytokines in FA patients have shown varied abnormalities. FA fibroblasts showed no deficiencies in SCF or M-CSF (macrophage colony-stimulating factor) production. Importantly, IL-6 production was found to be reduced in FA patients and TNF-α generation was markedly increased.
Initial attempts to generate induced pluripotent stem cells (iPSCs) from FA patients have been difficult since reprogramming causes increased DNA double-strand breaks and the FA pathway needs to be activated. This barrier could be bypassed by either correcting the genetic defect before reprogramming or performing the reprogramming under hypoxic conditions. Successful reprogramming resulted in cells that recapitulate the hematopoietic defect and identify the early pathogenetic defect at the stage of hemoangiogenic progenitors.
Interestingly, transforming growth factor-β (TGF-β) signaling was found to suppress FA cells. Blocking this pathway improved the survival and proliferation of HSPCs derived from FA mice and from FA patients. Further, inhibition of TGF-β signaling in FA HSPCs resulted in elevated homologous recombination repair with a decrease in non-homologous end-joining.
Multiple FA mouse models have been generated in which targeted disruption of genes like Fanca , Fancc , Fancd1 , Fancd2 , Fancg, Fancn, among others has been achieved . Knock-out mouse models largely do not recapitulate the marrow hypocellularity and cytopenias that characterizes FA, with a few exceptions (e.g., Slx4 −/− or combined Fancc −/− / Fancg −/− ). Consistent findings in some or all of the mice include impaired proliferation of BM hematopoietic progenitors, hypogonadism, impaired fertility, growth retardation, microphthalmia, development of cancers, hypersensitivity of BM progenitor cells to MMC, as well as to INF-γ or TNF-α in vitro and in vivo. The phenotype of these mutant mice shows abnormal G 2 /M progression of the cell cycle similar to FA patients. Interestingly, double knockout of several FA genes together with genes that play a role in balancing oxidative stress and other genotoxic agents (e.g., Fancd2 −/− /Foxo3a, Fancc −/− /Sod1 −/− , Fancd2 −/− /Aldh2 −/− ) leads to a phenotype that more closely resembles human FA. For example, Fancc −/− / Sod1 −/− mice develop a hypocellular BM; Fancd2 −/− / Foxo3a −/− mice feature an initial expansion followed by a progressive decline of BM stem and progenitor cells, and Fancd2 −/− / Aldh2 −/− have reduced progenitor cell numbers and develop leukemia. FancD2 −/− p53 +/- mice have a significantly increased incidence of tumors relative to either single mutant strain.
The diagnosis of FA can readily be made based on signs and symptoms related to aplastic anemia and the presence of characteristic congenital physical anomalies. However, about 30% of the patients have no physical anomalies, and about 25% may be diagnosed with FA based on physical anomalies without yet developing cytopenias. Interestingly, family screening may detect affected family members who have neither physical malformations nor cytopenias at the time of diagnosis (about 7% of the patients).
Table 30.2 lists the characteristic physical abnormalities and their approximate frequency based on more than 2000 published case reports. The two most common anomalies are skin hyperpigmentation and short stature , each with a frequency of 40% of cases. Characteristically, the hyperpigmentation is a generalized brown melanin-like splattering that is most prominent on the trunk, neck, and intertriginous areas that becomes more obvious with age. Café-au-lait spots are also common. Hypopigmentation and vitiligo may also be seen. In the minority of cases with short stature, growth failure is associated with endocrine abnormalities. In one report, spontaneous overnight growth hormone secretion was abnormal in all patients tested, and 44% had a subnormal response to growth hormone stimulation. Approximately 40% of patients also have overt or compensated hypothyroidism, sometimes in combination with growth hormone deficiency.
| Anomalies | Approximate Frequency (%) |
|---|---|
| Skin pigment changes or café-au-lait spots | 40 |
| Short stature | 40 |
| Upper limb anomalies (thumbs, hands, radii, ulnae) | 35 |
| Hypogonadal and genitalia changes (mostly male) | 27 |
| Other skeletal findings (head or face, neck, spine) | 25 |
| Eye, eyelid, or epicanthal fold anomalies | 20 |
| Renal malformations | 20 |
| Gastrointestinal or cardiopulmonary malformations | 11 |
| Ear anomalies (external and internal), deafness | 10 |
| Hips, legs, feet, toe abnormalities | 5 |
| Central nervous system imaging anomalies | 3 |
Malformations involving the upper limbs are common, especially hypoplastic, supernumerary, bifid, or absent thumbs. Hypoplastic or absent radii are always associated with hypoplastic or absent thumbs in contrast to TAR syndrome in which thumbs are always present. Less often, anomalies of the feet are seen, including toe syndactyly, short toes, a supernumerary toe, clubfoot, and flat feet. Congenital hip dislocation and leg abnormalities are occasionally seen. Male patients often have gonadal and genital abnormalities, including undescended, atrophic, or absent testes, hypospadias, an underdeveloped penis or micropenis, phimosis, and an abnormal urethra. Female patients occasionally have malformations of the vagina, uterus, or ovary. Renal anomalies such as ectopic, pelvic, or horseshoe kidneys are detected often, as are duplicated, hypoplastic, dysplastic, or absent organs. Occasionally, hydronephrosis or a hydroureter is present.
Many patients have a “ Fanconi facies,” and unrelated patients can resemble each other almost as closely as siblings. The head and facial changes vary but commonly consist of microcephaly; small eyes; epicanthal folds; and abnormal shape, size, or positioning of the ears (see Fig. 30.1 ). Anomalies in the tympanic membrane and middle ear ossicles are seen in almost 70% of patients, resulting in hearing loss in most affected patients. Approximately 10% of FA patients have cognitive deficiencies.
A cardinal feature is the gradual onset of BM failure with declining values in one or more hematopoietic lineages in the first decade of life, usually between 4 and 8 years of age. Fewer than 5% of patients develop hematological changes during the first year of life. Of 754 FA patients followed prospectively by the IFAR, 80% had hematologic abnormalities other than acute leukemia or MDS. The cumulative incidence of BM failure by 40 years of age was 90%. Thrombocytopenia with RBC macrocytosis usually develops initially, with subsequent onset of granulocytopenia and then anemia. Severe BM aplasia eventually ensues in most cases, but the degree of pancytopenia is variable and evolves over a period of months to years. The development of aplastic anemia can be accelerated by intercurrent infections or by certain drugs. Within families, there is a tendency for the hematologic changes to occur at approximately the same age in affected siblings, but this is not consistent.
The RBCs are macrocytic with mean corpuscular volumes (MCVs) often above 100 fL even before the onset of significant anemia. Erythropoiesis is characterized by increased fetal hemoglobin (HbF) levels. The increased HbF production has a heterogeneous distribution in contrast to most cases of hereditary persistent fetal hemoglobin. Elevation of RBC MCV and HbF is substantially higher than that seen in acquired aplastic anemia. Ferrokinetic studies indicate that most patients have an element of ineffective erythropoiesis. The RBC lifespan may be slightly shortened, but this is a minor contributory factor to the anemia.
In the early stages of the disease, the BM may not be hypocellular and can even show erythroid hyperplasia, sometimes with dyserythropoiesis, dysplastic changes, and even megaloblastic-appearing cells. Dysplastic changes may be very prominent with nuclear–cytoplasmic dyssynchrony, hypolobulated megakaryocytes, and binucleated erythroid cells; the findings are difficult to distinguish from MDS. As the disease progresses, the BM becomes hypocellular and fatty, sometimes in a patchy manner, and shows a relative increase in lymphocytes, plasma cells, reticulum cells, and mast cells. When full-blown BM failure occurs, the morphology of the BM biopsy is identical to severe acquired aplastic anemia.
Abnormal chromosome fragility is the hallmark of FA and the recommended diagnostic test. A major finding in FA is abnormal chromosome breakage seen in metaphase preparations of peripheral blood lymphocytes cultured with PHA. The karyotype is characterized by chromatid breaks, rearrangements, gaps, endoreduplications, and chromatid exchanges. Cultured skin fibroblasts also show the abnormal karyotype, underscoring the systemic nature of the disorder. The abnormal lymphocyte chromosome patterns and the number of breaks per cell have no direct correlation with the hematologic or clinical course of individual patients.
Although the breakage is increased in these baseline lymphocyte cultures, it is strikingly enhanced by adding a DNA interstrand cross-linking agent. Many oncogenic and mutagenic inducers such as ionizing radiation, SV40 viral transformation, alkylating agents (e.g., cyclophosphamide and nitrogen mustard), and platinum compounds. Nevertheless, DEB and MMC have supplanted all the above for diagnostic testing.
For a definitive diagnosis of FA, the IFAR has determined increased numbers of chromosome breaks per cell occurring after exposure to DEB with a range of 1.06 to 23.9 compared with the normal control range of 0.00 to 0.10. Further supportive features are unusual chromosome abnormalities such as tri-radial and quadriradial figures. This pattern of abnormal chromosome breakage can also be used to make a prenatal diagnosis of FA (see below). Results of DEB testing of heterozygote carriers overlap with the results from healthy individuals.
A small proportion of patients with clinical FA do not show increased number of cells with chromosome breakage when treated with DEB or MMC. These patients usually have hematopoietic cell somatic mosaicism as a result of a genetic correction in a hematopoietic stem or progenitor cell, resulting in one normal allele. The mechanisms for this phenomenon include gene conversion events, back mutations, or compensatory deletions or insertions. The end result is mixed populations of somatic cells, some with two abnormal alleles and some with one. However, combined analysis of a number of cells with breaks, number of breaks per cell, and number of breaks per aberrant cell may help overcome most of these challenges. If FA is strongly suspected and the chromosome fragility test on peripheral blood cells is negative, a skin biopsy is performed to assess chromosomal breakage in cultured fibroblasts with DEB or MMC rather than in lymphocyte cultures.
Immunoblotting of the FANCD2 protein has been proposed as a diagnostic test for most cases of FA and/or as a tool to direct specific gene testing. In this assay, FANCD2 protein is analyzed in primary lymphocytes or fibroblasts after exposure to MMC or radiation by immunoblotting. The blot can distinguish the unubiquitinated and monoubiquitinated FANCD2. The results may suggest one of three situations: (1) in FA patients with a positive chromosomal breakage assay, no detection of FANCD2 suggests biallelic null mutations in this gene; (2) detection of FANCD2 without its monoubiquitinated form suggests mutations in one of the upstream core complex genes are predicted; and (3) detection of monoubiquitinated FANCD2 suggests mutation in one of the downstream FA genes such as FANCD1/BRCA2, FANCJ/BACH1/BRIP1, FANCN/PALB2, FANCO/RAD51C, FANCP/SLX4 etc. Monoubiquitination of FANCD2 is normal in other chromosomal breakage disorders. With the development of relatively rapid and affordable molecular diagnostic tests such as next generation sequencing this test is no longer used for diagnostic purposes.
FA cells (e.g., lymphocytes and skin fibroblasts) exposed to DNA interstrand crosslinking agents arrest in the G 2 /M phase of the cell cycle, leading to a resultant 4 N DNA cellular content. This alteration can be detected by flow cytometry and has been used to diagnose FA. Transfection of a wild-type FA gene reduces G 2 /M arrested cells as determined by cell cycle kinetics using flow cytometry, thereby pinpointing the mutant gene. Due to concerns about specificity and reproducibility and instrumentation cost, this method is not used clinically for the diagnosis of FA.
The majority of FA patients have stable, elevated levels of serum α-fetoprotein expressed constitutively that are independent of liver disease or androgen therapy. Levels are also unchanged after HSCT. The clinical utility of these findings is limited.
Ultrasonographic examination of the abdomen may reveal intraabdominal anomalies in various organs such as kidneys, urogenital system, and the gastrointestinal tract. Echocardiography may reveal cardiac anomalies. Radiography and computed tomography (CT) can be informative in revealing bone, intestinal, or other anomalies; however, imaging using radiation should be minimized as much as possible because of the carcinogenic risk, and should be replaced by magnetic resonance imaging (MRI).
A major feature of the FA phenotype is the propensity to develop cancer. The chromosome fragility, defects in DNA repair, genomic instability, oxidative stress, and the cellular damage that occur in FA patients translate into a significant predisposition to develop a malignancy. Because many genes are associated with FA and because alterations in the FA pathway are relevant to the pathogenesis of common types of cancers, the disorder is a critical human model of the genetic determinants of hematologic cancers and solid tumors.
FA can be considered as a member of two families of cancer predisposition syndromes. The first is composed of genetic disorders of DNA repair that include ataxia telangiectasia, xeroderma pigmentosum, and Bloom syndrome. The close relationship between FA and these syndromes is underscored by data showing convergence of signaling pathways in these conditions and the identification of ERCC4 mutations in patients who manifest a complex phenotype of FA, xeroderma pigmentosa and Cockayne syndrome. The second family of predisposition syndromes consists of other inherited BM failure disorders described herein, including SDS and DC that have a propensity for malignant myeloid transformation or development of solid tumors.
The magnitude of the risk of developing malignancy in FA has been evaluated in several studies including literature reviews and analyses of registry data. In the National Cancer Institute study the observed to expected ratio of any malignancy was 19 in FA patients who have not received HSCT and was 55 in patients who received HSCT. The median patient age for the development of all cancers in a literature review was 16 years of age, which is strikingly different from the median age of 68 years for the same types of cancer in the general population. The risk of developing solid tumors in FA increases with advancing age.
In a study of the Canadian cohort, the cumulative risk of clonal and malignant myeloid transformation (of MDS and AML) after censoring patients transplanted for severe BM failure was 75% by the age of 18 years. The IFAR data indicate that the risk of acquiring clonal cytogenetic abnormalities was 67% by 30 years of age; the actuarial risk of MDS or AML was 52% by 40 years of age; and the cumulative incidence of leukemia was 33% by 40 years of age. This steady tempo of leukemic evolution implies a stepwise acquisition over time of additional, critical genetic “hits” before overt MDS/AML develops. In a literature review of 320 published FA cases, 14 patients developed leukemia. The risk of MDS was estimated as 5% to 10% and of leukemia as 5% to 10%. Studies from the IFAR showed that the risk of developing MDS and AML was higher for patients in whom a prior clonal BM cytogenetic abnormality had been detected. Monosomy 7, rearrangement or partial loss of 7q, rearrangements of 1p36 and 1q24-34, and rearrangements of 11q22-25 are frequent recurring cytogenetic abnormalities. Additional data indicate a strong correlation in FA BM cells of chromosome 3q26q29 partial trisomies and tetrasomies and rapid progression to MDS or AML. When interpreting the significance of clonal cytogenetic abnormalities in FA patients, note that clonal variation is frequent, including appearances of new clones, inability to detect established clones on repeat examination, and clonal evolution. In a study from France the investigators used SNP arrays to analyze whole marrow cells of FA patients with MDS/leukemia. They identified a relatively high frequency of somatic RUNX1 gene disruption compared to that typically seen in patients with de novo MDS/AML. In a study of FA patients without apparent MDS/AML the GMP-cell population was reduced, but to a lesser degree than other multipotent and oligopotent progenitors. These cells harbored higher mutation rates than control subjects with a nucleotide variant signature that was similar to that seen in AML; increased G>A/C>T variants, decreased A>G/T>C variants, increased trinucleotide mutations at Xp(C>T)pT, and lower mutation rates at Xp(C>T)pG sites compared to other Xp(C>T)pX sites. The same pattern was seen in blast cells from a patient with FA and AML. These data suggest that the CFU-GM represent a cellular reservoir for clonal evolution.
The IFAR data indicates that by the age of 40 years, the cumulative incidence of nonhematologic cancers is 28%. In a literature review of 320 published FA cases, 25 non-transplanted cases had one to three separate types of solid tumors. Among the NCI cohort 21 of 163 FA patients developed solid tumors with an observed/expected ratio of 19. The most frequent solid tumor reported was squamous cell carcinoma involving the head and neck (mostly tongue) and upper and lower esophagus followed by the vulva or anus, cervix, brain, and skin. There were additional cases of tongue and oral squamous cell carcinoma as well as thyroid cancer and lymphoproliferative diseases that occurred after HSCT. Liver tumors, benign and malignant, were the second most frequent. Most of the patients with hepatomas and adenomas had received prior androgen therapy for aplastic anemia. Androgen administration has therefore been implicated in liver tumor pathogenesis. In descending order of frequency, cancers were also reported in the brain, kidney, breast, and adrenal gland.
Heterozygote carriers of FA gene mutations do not develop peripheral blood cytopenias or aplastic anemia, and cell lines from heterozygote carriers do not show excessive chromosome fragility in culture when exposed to DEB or MMC. The mean chromosomal breakage level of lymphocytes from FA carriers tested in cultures with a clastogenic agent may be higher than controls, but individual carrier testing may show overlap with normal values and severely limits its diagnostic utility. Literature from the early 1980s describes congenital anomalies of the hand and the genitourinary system in relatives of patients with FA, and parents of children with FA may have short stature. FA carriers may have increased levels of HbF, decreased natural killer (NK) cell counts, and diminished reactivity to mitogen stimulation. However, these results need to be reexamined using modern genetic diagnostic testing to exclude homozygosity and coexisting other conditions.
Monoallelic carriers for several FA genes are at increased risk of developing cancer, including for example, FANCD1, FANCS , FANCN, FANCJ, FANCM , FANCP , and FANCO . Female carriers of FANCD1/BRCA2 and FANCS/BRCA1 have an increased risk of breast cancer ranging from 40% at age 80 to a lifetime risk of about 80% and of ovarian cancer with a risk of up to 20% at age 70. Male carriers have a 7% risk of breast cancer and a 20% risk of prostate cancer before age 80. Other FA genes that are more prevalent in patients with breast cancer than in the general population, but to a much lower degree than FANCD1 and FANCS, are FANCN, FANCJ, FANCO, FANCP, FANCU, FANCD2.
Heterozygous mutations in FANCN are associated with pancreatic cancer, medulloblastoma, and high-grade glioma. Heterozygous mutations in FANCJ , FANCP , and FANCO are also associated with ovarian cancer.
The clinical severity of FA is partly determined by the type of mutation and by the specific FA gene involved. Correlation between genotype and phenotype have been described but they were not consistently observed in different cohorts, which may be due to phenotype modifying variants that differ between cohorts and are possibly related to different ethnic backgrounds.
Several studies have found that patients with null mutations (e.g., deletions or nonsense point mutations in FANCA , FANCB , and FANCG ) or missense mutations that lead to little residual protein function tend to have more severe physical malformations and an earlier onset of BM failure than patients with milder mutations. An earlier onset of leukemia was also found to be associated with null mutations in FANCG .
With regard to the impact of specific genes, several studies have shown association between specific genetic groups and certain phenotypes.
Severe physical anomaly phenotype is associated with FANCB , FANCC IVS4+4 A>T in Ashkenazi Jews, FANCD1/BRCA2 , FANCD2 , FANCI, FANCN(PALB2)
Early-onset BM failure is associated with FANCC (IVS4+4 A>T in Ashkenazi Jews and FANCI.
A significant predisposition to develop solid tumors, AML is associated with FANCD1(BRCA2), FANCN(PALB2), and FANCM. FANCM mutations were found in patients with cancer, but without physical malformations or BM failure. Patients with FANCA mutations developed cancer at a significantly older age as compared to patients with mutations in other Fanconi genes.
VACTERL-H association (combination of vertebral, anal, tracheoesophageal, renal, limb, and cardiac abnormalities) is often seen in patients with mutations in FANCD1/BRCA2 , FANCC (IVS4+4 A>T in Ashkenazi Jews), and FANCB .
Ethnic backgrounds may affect phenotype severity in patients with the same mutations. For example, non-hematological phenotype that may include VACTERL-H association is often seen in Ashkenazi Jewish patients and mutations in FANCC (IVS4+4 A>T in), but not in Japanese patients with the same mutation.
About 30% of FA patients do not have physical anomalies, and such individuals may not be recognized until they present with aplastic anemia, MDS, AML, unilineage cytopenias, or macrocytic RBCs. Thus, FA should be part of the differential diagnosis in children and adults with unexplained cytopenias; characteristic birth defects; a diagnosis of aplastic anemia, MDS, or AML in patients mainly up to the age of 40 years, but sometimes also higher; unusual sensitivity to chemo- or radiotherapy; cancers typical of FA but at an atypical age such as cancer of the cervix when younger than 30 years or squamous cell carcinoma of the head and neck when younger than 50 years of age. Any of these should prompt consideration of FA as the underlying problem. All patients with idiopathic aplastic anemia who are younger than 40 years should have chromosomal fragility testing. However, if the test was not performed at diagnosis, patients with “idiopathic” aplastic anemia who fail to respond to immunosuppressive therapy with ATG and cyclosporine should be tested.
Although neutropenia is a consistent feature of SDS , anemia or thrombocytopenia (or both) is seen in more than 50% of the patients and can be confused with FA. Because growth failure is also a manifestation of SDS, differentiating between the two disorders can initially be difficult. The major difference between them is that SDS is a disorder of exocrine pancreatic dysfunction that may or may not produce gut malabsorption. This can be confirmed by fecal fat analysis, by showing reduced levels of serum trypsinogen, serum isoamylase, or fecal elastase, and by reduced levels of fat-soluble vitamins such as A, D, and E. Nowadays pancreatic stimulation studies using intravenous secretin or cholecystokinin and measuring enzyme secretion is rarely done. Ultrasonography, MRI of the pancreas may also demonstrate fatty changes within the pancreas. Other skeletal features found in some patients with SDS include short flared ribs, thoracic dystrophy at birth, delayed bone maturation, and metaphyseal dysostosis of the long bones. Chromosomal analyses do not show spontaneous breaks in SDS, and increased breakage after clastogenic stress testing using DEB or MMC does not occur in SDS. Genetic testing for mutations in one of the SDS genes can help distinguish SDS from FA.
Dyskeratosis congenita (DC) shares some features with FA, including development of pancytopenia, a predisposition to develop solid tumors and leukemia, and skin pigmentary changes. However, the pigmentation pattern is somewhat different in DC and manifests with a lacy reticulated pattern affecting the face, neck, chest, and arms, often with a telangiectatic component. At some point, usually in the first decade of life, DC patients also develop dystrophic nails of the hands and feet and, somewhat later, leukoplakia involving the oral mucosa, especially the tongue. Other findings seen only in DC and not in FA are teeth abnormalities with dental decay and early tooth loss, hair loss, and hyperhidrosis of the palms and soles. Chromosomal fragility with DEB testing is typically normal in DC patients, which contrasts sharply with FA patients. Molecular analysis of DC genes is positive in about three-quarters of the patients (see Table 30.1 ).
Congenital amegakaryocytic thrombocytopenia (CAMT) and TAR syndrome both present in the neonatal period with thrombocytopenia. Patients with CAMT develop impairment in other blood cell lineages soon after presentation. A neonatal hematologic presentation is atypical for FA; fewer than 5% of patients are diagnosed during the first year of life. Neither CAMT nor the various thrombocytopenia syndromes above show chromosome fragility, which separates them from FA. Genetic testing is available for many of these disorders (see Table 30.1 ). In the TAR syndrome, thumbs are always preserved and intact despite the absence of radii, but in FA, the thumbs are hypoplastic or absent when the radii are absent.
Seckel syndrome , or “bird-headed dwarfism” manifests with short stature; microcephaly; cognitive delay; sinopulmonary infections; and a predisposition to developing lymphomas, pancytopenia, and AML. Some patients may show increased chromosomal breakage in lymphocyte cultures with DEB or MMC and mimic FA. There are several genes that have been linked to Seckel syndrome: mutant ATR has been associated with Seckel Type 1, RBBP8 with Type 2, CENPJ with Type 4, CEP152 with Type 5, CEP63 with Type 6, and ATRIP with Type 8. Genotyping will distinguish FA from Seckel syndrome.
Nijmegen breakage syndrome (NBS) is an autosomal recessive disorder caused by mutations in the NBS1 gene and is characterized by stunted growth, microcephaly, a distinctive facies, café-au-lait spots, immunodeficiency, and a predisposition to develop lymphoid malignancies. Some patients resemble those with FA, have BM failure, and may show increased chromosome breakage in lymphocyte cultures with MMC. The genetic defect is a mutant NBS1 gene whose wild-type protein product is involved in DNA repair. Because NBS can mimic and be confused with FA, genotyping is essential and diagnostic.
Cells from patients with Bloom syndrome show abnormal spontaneous breakage, but unlike FA cells, the breakage does not increase in vitro in response to DEB. Ataxia telangiectasia is characterized by sister chromatid exchange without hypersensitivity to DEB or BM failure.
The most serious early consequence in most FA patients is BM failure. The exceptions are patients with biallelic FANCD1 / BRCA2 mutations who have a cumulative probability of 97% of developing a malignancy by age 6 years, including AML, Wilms tumor, and medulloblastoma. By age of 40 years, patients with mutations in genes other than FANCD1 have a cumulative risk of marrow failure, AML, and solid tumors of about 60%, 10%, and 15%, respectively. The risk of marrow failure plateaus at 70% at about 60 years of age, but the risk of solid tumors continues to rise. Treatment for cancer imposes additional problems and probably increases the risk for additional cancers secondary to therapy. Thus, the major causes of death in FA patients are sepsis and bleeding from BM failure, complications of HSCT, and progressive cancer or consequences of its treatment.
Despite these serious issues, the prognosis for FA patients is improving. Based on a literature review of more than 2000 FA case reports, the median survival from 1927 to 1999 was 21 years. In contrast, the median survival age from 2000 to 2009 was 29 years and 39 years in 2018. More than 80% of patients reach age 18 years or more. Earlier diagnosis, especially of mild cases, diagnosis of FA in young adults with AML or a solid tumor, comprehensive clinical and laboratory surveillance programs, timely therapeutic interventions, and HSCT are attributed to the improved outlook.
Because of their clinical and psychosocial complexity, patients with FA should be supervised by a hematologist at a tertiary care center using a comprehensive and multidisciplinary approach. On the initial visit, the practitioner should take a detailed personal and family history, a careful physical examination with emphasis on physical anomalies, complete blood counts and chemistries, a BM biopsy for cellularity and morphology, and an aspirate for additional morphology, cytogenetics, and an iron stain looking for ringed sideroblasts. Chromosome fragility testing with and without DEB and/or MMC on peripheral blood lymphocytes on patients should be arranged. If FA is confirmed by DEB or MMC testing, genetic diagnosis should be offered to the family. Subsequently, imaging studies should be requested to search for internal anomalies. Because of the carcinogenic risk, imaging using radiation should be limited to those that may change management and may provide information that cannot be obtained by other methods or in a timely fashion. When all the results from the workup have been compiled, a discussion should be held with the patient and guardians about the diagnosis, management options, and prognosis. A referral to a genetic counselor should ensue. Blood counts, hemoglobin variant analysis, and chromosome fragility testing of siblings should be offered. High-resolution human leukocyte antigen (HLA) typing of the patient and immediate family members is recommended shortly after the diagnosis is established to determine potential matched-related donors in case HSCT becomes necessary.
If the patient is stable, has only minimal to moderate hematologic changes, and does not have transfusion requirements, a period of observation is indicated. During this time, subspecialty consultations (e.g., with orthopedic surgeons, urologists, gynecologists, and otolaryngologists) can be arranged. Blood counts should be frequently monitored to determine their stability. In a stable patient with mild cytopenias, blood counts can be monitored every 3 months, and BM evaluation should be performed annually. Falling counts, a clonal BM cytogenetic abnormality, or prominent multilineage dysplasia require more frequent clinic visits and blood and BM sampling to monitor for progression to severe aplastic anemia, MDS, or AML. Spectral karyotyping (SKY), fluorescent in situ hybridization (FISH), and comparative genomic hybridization of BM cells can enhance the diagnostic capability ( Chapter 57 ). Analysis of mutations in specific cancer-related genes may be introduced into clinical practice in the future, but more research is necessary to determine their ability to predict progression to MDS/AML.
A surveillance program for solid cancers should be initiated at least annually. After the age of 10 years or one year after HSCT, the oral cavity should be examined every 6 months for signs of malignant changes because the risk in untransplanted FA patients is 700-fold that of the general population. Dentists, oral surgeons, or head and neck surgeons should be periodically recruited after the age of 10 years or after HSCT to screen for head and neck squamous cell carcinomas by rhinopharyngoscopy using a flexible endoscope. Beginning at age 13 years, all women with FA should be offered an annual gynecologic screening because the relative risk of vulvar squamous cell carcinoma is 4000-fold higher and cervical cancer is 200-fold higher than that of the general population. Human papilloma virus (HPV) DNA can be detected in 84% of FA squamous cell carcinoma specimens from various anatomic sites. Although the role of HPV in FA carcinogenesis is controversial, quadrivalent HPV vaccine is still recommended for boys and girls with FA at 9 years of age as a possible preventive approach.
Growth should be serially documented, and if growth velocity or stature falls below expectations, endocrine evaluation is needed to identify growth hormone deficiency. Diabetes mellitus occurs more commonly in FA, and random glucose levels should be evaluated annually or biannually. Based on the degree of hyperglycemia found on initial testing, fasting glucose levels and glucose tolerance tests should be performed. Screening for hypothyroidism should also be performed annually.
Androgen therapy has been used to treat FA for decades. The overall response rate in the literature is about 50% heralded by reticulocytosis and a rise in hemoglobin within 1 to 2 months. If the other lineages respond to androgens, white blood cells increase next and then platelets, but it may take many months to achieve the maximum response. Accepted indications for treating with androgens are one or more of the following: hemoglobin level less than 8 g/dL or symptoms from anemia, platelet count less than 30,000/mm 3 , and neutrophil count less than 500/mm 3 . Oxymetholone, an oral 17-α alkylated androgen, is used most frequently at 1 to 5 mg/kg once a day. Depending on disease severity, oxymetholone can initially be administered either at 5 mg/kg/day and be decreased to the lowest effective dose or be started at a low dose of about 1 mg/kg/day and be increased monthly if there are no major side effects and an insufficient response. Although unproven, some clinicians add corticosteroids to offset androgen-induced growth acceleration and to prevent thrombocytopenic bleeding by promoting vascular stability. For this purpose, 5 to 10 mg of prednisone is given orally every second day. There are increasing data on the efficacy of the attenuated androgen, danazol, in FA; however, there are no comparative data with oxymetholone. Claims of reduced masculinizing side effects in female FA patients treated with danazol compared with those treated with oxymetholone have not yet been validated in clinical trials. A maintenance danazol dose of 2 to 5 mg/kg/day is probably sufficient to maintain good blood counts in those who respond. Another androgen, oxandrolone, has been evaluated for FA at Cincinnati Children’s Hospital. Nine subjects completed this study and were followed for a median of 99 weeks (46 to 136 weeks). A third of the subjects developed mild sub-clinical virilization and continued treatment with a dose reduction. None of the patients had adverse behavioral changes. Two patients developed elevated liver function tests at 42 and 105 weeks. Seven patients had a hematologic response. Based upon this limited study, these investigators concluded that oxandrolone was well-tolerated, had a favorable toxicity profile in patients with FA, and may serve as an alternative androgen for the treatment of BM failure in FA patients.
If an injectable androgen is preferred to decrease the risk of liver toxicity and growth of hepatic tumors, nandrolone decanoate, 1 to 2 mg/kg/week, is given intramuscularly followed by the application of local pressure and ice packs to prevent the development of hematomas. When the response is deemed maximal or sufficient, the androgens should be slowly tapered but not stopped entirely.
Almost all patients relapse when androgens are stopped. The few who successfully discontinue treatment are often undergoing puberty when temporary “spontaneous hematologic remissions” have been observed. Most patients on long-term androgens eventually become refractory to therapy as BM failure progresses. Potential side effects include masculinization, which is especially troublesome in female patients, and elevated hepatic enzymes, cholestasis, peliosis hepatis , and liver tumors. Five complications of androgen therapy require consideration.
Peliosis hepatis is a cystic dilation of hepatic sinusoids that fill with blood and can be life threatening if they rupture. They may be clinically silent or produce right upper quadrant pain. Liver function test results are normal. Ultrasonographic examination is a safe way to diagnose the abnormality. The lesions may regress after stopping the androgens.
Androgens also damage hepatocytes nonspecifically. This may be manifest as cholestatic jaundice or elevated liver enzymes. Stopping androgen therapy usually leads to complete resolution. Hepatic cirrhosis may develop in patients on continued androgen therapy. If resolution of enzyme elevation does not occur after androgen withdrawal, a liver biopsy is indicated.
Hepatocellular adenomas are associated with androgen therapy. These are benign, noninvasive tumors. They can, however, rupture, leading to life-threatening bleeding. FA patients may develop these tumors rapidly, but they can be readily detected by imaging. The tumor may regress after stopping the androgens. If persistent, surgical resection or radiofrequency ablation may be necessary.
Hepatocellular carcinoma (HCC; hepatoma) occurs with androgen use, and some studies have suggested that FA patients on treatment may be at increased risk for HCC. The HCC associated with androgens is characteristically not associated with serum, α-fetoprotein elevations distinguishing it from de novo HCC. Patients developing HCC should discontinue androgen therapy.
Androgen therapy for FA patients has been found to be an adverse prognostic factor for those receiving a HSCT in one European study, but not in others. Unfortunately, comparative studies between androgen administration and HSCT from related or unrelated donors are not available and are probably not feasible due to the rarity of the disease.
Those receiving androgens should be evaluated serially with liver enzyme profiles every 2 to 3 months and ultrasonography of the liver every 6 to 12 months. If liver enzymes increase to above normal or if abnormalities appear on imaging, the androgen dose should be decreased or stopped.
Both granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) can induce a neutrophil response in neutropenic FA patients. G-CSF is indicated for a patient with recurrent or serious bacterial infection, especially if the neutrophil counts are less than 500/mm 3 . In a published clinical trial of G-CSF in 12 FA patients, all 12 had an increase in absolute neutrophil numbers, 5 had a significant increment in hemoglobin levels, and 4 had an increase in platelet counts. Concurrent with the impressive improvements in blood counts, 8 of 10 patients who finished 40 weeks of G-CSF treatment showed elevations in the percentage of BM and peripheral blood CD34 + cells. The starting dose for subcutaneous G-CSF is 5 μg/kg/day, and after a neutrophil response occurs, the dose can be decreased to every second day or two to three times a week. Long-acting pegylated G-CSF has not been studied in FA.
In another published clinical trial, combination cytokine therapy consisting of subcutaneous G-CSF 5 μg/kg once daily with erythropoietin 50 units/kg administered subcutaneously or intravenously three times a week was given to FA patients. Androgen therapy was added if the response was inadequate. Of 20 patients treated, 19 had improved neutrophil numbers, 6 had an increase in hemoglobin levels, and 4 achieved a sustained rise in platelets.
Because genomic instability and a marked predisposition to leukemia and cancer are features of FA, the wisdom of using granulopoietic growth-promoting cytokines on a long-term basis for FA is an issue. There may be a heightened risk of inducing or promoting expansion of a leukemic clone, especially one with monosomy 7. Therefore, before starting cytokine therapy, a baseline BM aspirate and biopsy is recommended, which then should be repeated every 6 months to document changes in morphology and cytogenetics.
HSCT is the only clinically available curative therapy for the hematologic abnormalities of FA: aplastic anemia, MDS, and AML, though this may change if ongoing clinical trials of gene therapy are proven feasible, effective, and safe. The best donor source is an HLA-matched sibling in whom thorough history, physical examination, blood counts, HbF, chromosome breakage testing, and ideally genetic testing have excluded a diagnosis of FA.
Absolute indications for a matched sibling donor HSCT are (1) severe underproductive cytopenias (platelet <20 × 10 9 L, ANC <0.5 × 10 9 L, hemoglobin <70 g/L) and/or transfusion dependency; (2) high-risk MDS with chromosomal clonal abnormalities like monosomy 7, partial trisomies and tetrasomies of 3q26q29 and deteriorating counts, or a BM blast count of greater than 5%; or (3) overt AML. Decision to transplant for milder, relative indications should be made on a case-by-case basis.
Three caveats about transfusional supportive care before HSCT are (1) more than 20 exposures to blood products is a risk factor that adversely affects engraftment and survival posttransplant; (2) use of directed donations from family members may cause alloimmunization to an antigen that can increase the risk of graft rejection after a matched sibling donor or haploidentical related donor; and (3) single-donor apheresis platelets should be requested when required and available and the product should be leukodepleted and irradiated.
Initial efforts to transplant FA patients using standard myeloablative preparative regimens and graft-versus-host disease (GVHD) prophylaxis were plagued by two serious and often lethal problems, severe cytotoxicity from chemotherapy and irradiation and exaggerated GVHD. In vivo studies confirmed hypersensitivity of FA patients to standard doses of radiation. These observation resulted in developing reduced-intensity HSCT protocols that remain myeloablative for FA patients for both matched related donors (cyclophosphamide 20 mg/kg, single dose of TAI/TBI 400 to 500 cGy and ATG, resulting in 75% to 100% overall survival) and for alternative donors (cyclophosphamide 40 mg/kg, TAI/TBI 400 to 500 once, ATG, resulting in ∼35% overall survival).
Over time, conditioning protocols utilizing irradiation have been almost entirely abandoned due to higher toxicity, lower long-term overall survival, and a potential increase in post HSCT solid tumors compared to regimens without radiation. Fludarabine, a purine antimetabolite with potent immunosuppressive and myeloablative properties with minimal toxicity to other tissues, has gained favor as an effective adjunct to preparative regimens. In the late 1990s/early 2000s fludarabine was introduced almost universally to conditioning regimens for patients with FA.
Modern protocols for HSCT incorporating fludarabine (e.g., cyclophosphamide 20–40 mg, fludarabine 140–180 mg/m 2 and horse or rabbit ATG, T-cell depleted BM or umbilical cord blood, and GVHD prophylaxis (based on cyclosporine or sirolimus + MMF or methylprednisolone) have resulted in 5-year overall survival of greater than 90%.
Using HLA-mismatched related donors, matched or one-antigen mismatched unrelated donors (MUDs) or unrelated cord blood carry a higher risk of complications and a lower disease-free survival than matched sibling donor HSCT ( Chapter 105, Chapter 106 ). Provided that no family members are suitable, BM or peripheral blood stem cell donors or cord blood from unrelated donors are sought and identified by searching donor and umbilical cord blood registries. Ideally, an unrelated donor is fully matched by high-resolution typing for all HLA-A, B, C, and DRB1 antigens, a so-called 8/8 match . A second choice is a one-antigen mismatch unrelated donor. Matched or one-antigen mismatched banked unrelated umbilical cord blood is equally suitable to a one-antigen mismatched BM donor in FA and is an option. The last choice of an unrelated donor is a two-antigen mismatched cord blood. Using cord blood cells from unrelated donors, engraftment and survival are comparable to related or unrelated BM, although engraftment is slower with cord blood. The incidence of acute and chronic GVHD is reduced with cord blood grafts even in 1 or 2 HLA antigen mismatched transplants.
Recently, radiation-free HSCT in FA with T-cell depletion has been shown to be highly promising in FA patients transplanted with a MUD. In a multicenter prospective study conducted between 2009 and 2014, 45 patients age 4.3 to 44 years with BM failure (76%) or MDS (11% MDS) or other indications (13%) were transplanted. The donors were matched unrelated (56%), misMUD (31%), or misMRD (13%). The graft source was peripheral blood CD34 + cells (1.1 to 63 × 10 6 cells/kg, Aim >2.5), CD3 cell depleted (0 to 5 × 10 4 cells/kg, Aim <5). The conditioning was intravenous busulfan (2.4 to 4 mg/kg with adjustment to <350 ng/mL), cyclophosphamide (40 mg/kg), fludarabine (140 mg/m 2 ), and rabbit ATG. One-year probabilities of overall and disease-free survival for the entire cohort were 80% and 78%, respectively
The best outcome predictors are recipients younger than 10 years old, recipient seronegativity for cytomegalovirus (CMV), history of fewer than 20 exposures to blood products, and use of fludarabine in the cytoreductive regimen. FA phenotype with three or more congenital malformations and prior administration of androgen therapy have been shown to adversely affect HSCT outcomes in some studies but not in others. Indications for an alternate donor HSCT are identical to those for a matched sibling donor HSCT.
HSCT from a haploidentical related donor have gained interest due to the almost universal availability of such donors (parents). Initially CD34 + selected cells with or without ex vivo T-cell-depletion have been used in few FA patients. Recently a study from India and a study from a group of investigators from the USA and Brazil published results of haploidentical HSCT using in vivo T-cell depletion by post HSCT cyclophosphamide in 19 and 30 patients with FA, respectively. The group from India used fludarabine (180 mg/m 2 ), cyclophosphamide (10 mg/kg), TBI 200cG, post-HSCT cyclophosphamide 25 m/kg day +3+4, N-acetylcysteine during cyclophosphamide, and peripheral blood (81%) or BM (19%) (median CD34 dose: 5 × 10 6 /kg). The group from USA/Brazil used fludarabine 150 mg/m 2 , TBI 200 to 300 cGy, ± cyclophosphamide 10 mg/kg, ± rabbit ATG, post-HSCT cyclophosphamide 25 m/kg day +3+4, and unmanipulated BM graft. The 5-year overall survival was 68% in the study in India and 67% in the USA/Brazilian study.
Treatment of patients with FA with MDS/AML is challenging, at least in part due to the limited ability to high-intensity induction therapy. A collaborative French/Brazilian clinical trial used tandem FLAG (fludarabine/ARA-C/G-CSF) followed by HSCT regardless of response. Eighteen FA patients with advanced MDS or AML were enrolled. The trial showed relatively promising results. The transplant conditioning included cyclophosphamide 40 mg/kg, fludarabine 120 mg/m², TBI 2 Gy, ATG. In case of haploidentical transplants the patients also received post HSCT cyclophosphamide 30 mg/kg on day +4 and +5. The results were relatively promising; at 7 years, overall survival was 53% and GVHD-free/relapse free was 48%. In an EBMT retrospective study patients with FA and MDS/AML were transplanted with busulfan-based or TBI-based HSCT regimens with or without pre-HSCT chemotherapy. Overall survival and event-free survival were 42% and 39%, respectively. However, in this study there was no statistical difference in outcome if the patients received pre-HSCT chemotherapy or not.
Molecular technology has led to preimplantation genetic diagnosis (PGD) coupled with in vitro fertilization and selection of HLA-matched embryos for implantation. This is an option for parents who have a child with FA and a defined FA mutation but without a matched sibling donor. The mother’s fertile eggs are harvested and fertilized with the father’s sperm in vitro, resulting in a number of blastomeres. Using single-cell polymerase chain reaction (PCR) technology, isolated cells from several blastomeres can be tested for an HLA match and for absence of biallelic FA mutation. The selected HLA-compatible normal blastomeres can then be transferred and implanted in utero, resulting in a successful pregnancy and subsequent birth of a matched unaffected sibling. Cord blood from the PGD-selected healthy infant siblings can be banked for use as a graft for a HSCT for the affected sibling. The notion of “designer babies” is still debated in ethical circles.
Despite the successes of HSCT in correcting the BM failure of FA patients, there is a subset of survivors who develop cancers , particularly squamous cell carcinoma of the head and neck (see also the section Predisposition to Malignancy above). These malignancies reflect the ongoing genetic susceptibility of FA nonhematopoietic tissue to cancer despite successful transplantation for aplastic anemia, MDS, or AML. Published data comparing cancer risks in transplanted and nontransplanted FA patients show a 4.4-fold increase in age-specific hazard rate of squamous cell carcinoma in the transplanted cohort. The causes for the increased cancer risk above the baseline seen in FA patients who have not been transplanted are not proven, but GVHD, especially chronic, and the preparative regimens of chemotherapy and irradiation are highly suspect. T-cell depletion has been introduced in some protocols to reduce GVHD, and irradiation has been reduced or eliminated in others to address this issue.
Gene therapy . Spontaneous mutation correction is common in FA patient cells and such cells may outnumber biallelically mutated cells. This is evident by mosaicism in chromosome breakage assays. Therefore, corrected cells through gene therapy are expected to have a growth advantage and expand in the BM. However, thus far gene therapy trials have not shown long-term preservation of substantial number of corrected FA cells in the transplanted patients or hematological improvement.
There are several challenges to gene therapy in FA. Mobilization, collection, and cryopreservation of enough peripheral blood CD34 + cells from FA patients is feasible, particularly with usage G-CSF or plerixafor. However, this must efficiently be done before the onset of severe pancytopenia, since at this stage the HSC pool has been exhausted.
Several gene therapy studies have been conducted in FA and several others are currently being conducted in Europe and USA. Potential steps that may heighten the success with gene therapy are improving the vectors and/or correction methods, transduction of true HSCs, infusion of sufficient numbers of corrected HSCs, better preparation of patients and possibly giving conditioning before cell infusion, confusion of mesenchymal stem cells to improve stromal function, ensuring safety and eliminating off-target effects.
Genetic counseling should be offered to all patients and families with FA. The discussion should include the implications of the identified (or not identified) underlying genetic lesion, important genotype phenotype correlations (e.g., high risk of early cancer in patients with FANCD1/BRAC2 mutations), mode of transmission, risk of having the disease in family members, risk of recurrence in future pregnancies, available diagnostic tests during pregnancy, and PGD/IVF and selection of HLA-matched embryos who do not have FA as potential donors for HSCT. Screening of all first-degree relatives should be offered according to the methods available: medical history, physical examination, complete blood counts, hemoglobin F level, chromosome fragility, and mutation analysis.
During pregnancy, gene testing and/or the abnormal chromosome breakage patterns characteristic of FA can be used to make a prenatal diagnosis of FA. Diagnostic testing can be performed on fetal amniotic fluid cells obtained at week 16 of gestation or on chorionic villus biopsy specimens at 9 to 12 weeks of gestation.
A manual for the management of FA patients is available by the Fanconi Anemia Research Fund (see www.fanconi.org for Fanconi Anemia Guidelines for Diagnosis and Management , 4th edition, 2014).
The premise for gene therapy in FA is based on the assumption that corrected hematopoietic cells would have a growth advantage. Strengthening this supposition are FA patients with hematopoietic somatic mosaicism who show spontaneous disappearance of cells with the FA phenotype. These mosaic patients may show spontaneous hematologic improvement, suggesting that hematopoiesis was derived from stem cells with a normal phenotype.
Despite encouraging preclinical studies since the early 2000s using retroviral vectors showing that wild-type FANCC and FANCA can be integrated into normal and FA CD34 + cells, the ensuing clinical trials in FANCC and FANCA patients using retroviral vectors were disappointing. A central problem was suboptimal wild-type gene integration into FA cells in culture. Because of the apoptotic phenotype and the sensitivity to oxidative stress, FA cells die rapidly in vitro before efficient gene transfer is accomplished. Changing the tissue culture conditions (e.g., usage of low oxygen condition) and introducing lentiviral vectors that can infect noncycling human cells were deemed the solutions. A few clinical trials for FA-A were opened in the late 2010s. Rio and colleagues reported promising results demonstrating successful engraftment of CD34 + hematopoietic stem and progenitor cells carrying lentivector-mediated corrected FANCA gene in FA patients without conditioning. The procedure was well tolerated, hematopoiesis from CD34 + cells was improved, but clinical efficiency could not be assessed due to the small number of patients. One caveat: a successful FA gene therapy protocol may correct BM failure and possibly the propensity for MDS and AML, but the predisposition for cancer in other tissues will continue unchecked.
SDS is a multisystem disorder characterized by varying degrees of BM failure, a high risk of leukemia, and exocrine pancreatic insufficiency. Additional features may include short stature and skeletal abnormalities. SDS is considered a ribosomopathy, as almost all patients have mutations in genes involved in the last stages of pre-60S ribosome subunit maturation.
SDS has been reported among all ethnic groups. Older studies suggested a higher incidence in males. However, recent data suggest an equal distribution between genders as expected from a mainly autosomal recessive disorder. Based on data from the CIMFR, SDS is the third most common IBMFS with an incidence of 1 in 118,000 to 1 in 175,000 live births.
SDS is considered a ribosome-related disorder (ribosomopathy). The vast majority of patients with an SDS phenotype have mutations in genes involved in the last stages of pre-60S ribosome subunit maturation ( SBDS , DNAJC21 , EFL1 ). Some patients have a mutation in a gene related to the cotranslation protein-targeting pathway ( SRP54 ), which also cause severe congenital neutropenia phenotype.
The first identified SDS gene is the Shwachman-Boyden-Diamond-syndrome ( SBDS ) gene that is mutated in 85% to 90% of SDS patients. The described mutations in this gene include null and splicing alterations, but never biallelic null, suggesting that a complete absence of SBDS protein is lethal. The common SBDS mutations are composed of sequences that are homologous to its pseudogene, SBDSP , and are believed to result from genetic recombination events in which SBDSP1 acts as the donor. These recombinational events result in three common gene conversion mutations in exon 2 that account for 75% of SDS alleles: (1) a splice-site mutation, c.258+2 T>C, which may either cause premature truncation of the SBDS protein by frameshift (p.C84fs3) or use an alternative splice site; (2) a nonsense mutation, c.183_184TA>CT that introduces an in-frame stop codon (p.K62X); and (3) an extended conversion mutation, c.183_184TA>CT and c.258+2 T>C, that encompasses both mutations. In the Toronto database of 210 SDS families, 89% of unrelated SDS individuals carry a gene conversion mutation on one allele, and 60% carry conversion mutations on both alleles. The majority of patients are compound heterozygotes with respect to p.K62X and p.C84fsx3. Additional rare mutations in the SBDS gene have been identified in SDS patients. These include dozens of insertion, deletion, and missense mutations that have not arisen from gene conversion events. Most SBDS mutations alter the N-terminal domain of the protein and lead to markedly reduced protein levels.
DNAJC21 is the second gene identified to be associated with SDS. Biallelic mutation in DNAJC21 can be identified in about 10% of SDS patients. DNAJC21 -associated SDS is autosomal recessive. Both biallelic missense mutations and biallelic null mutations have been reported. Additional groups identified rarer SDS genetic groups with biallelic mutations in EFL1 and monoallelic mutations in SRP54. All reported patients with biallelic EFL1 mutations had at least one missense mutations.
SDS genes encode highly conserved proteins that are ubiquitously expressed. SBDS protein has three main domains (N-terminal, middle, and C-terminal) with predicted protein-protein, protein-DNA, and protein-RNA binding motifs. SBDS protein is essential for life because patients with homozygous null mutations have not been reported, and small levels of residual protein can usually be detected in SDS patients. Furthermore, a complete loss of the protein in mice causes developmental arrest before embryonic day 6.5 and early lethality. In contrast to SBDS , biallelic null mutations in DNAJC21 have been identified in SDS patients, indicating the complete loss of this protein is still compatible with life.
SBDS, DNAJC21, and EFL1 play a direct role in ribosome biogenesis. SBDS binds EFL1 on the 60 S large ribosome subunit, which through GTP hydrolysis causes release of the dissociation factor eIF6 and binding of 60 S to a mRNA-loaded 40 S subunit. Patient-related mutations in SBDS and in EFL1 have been shown to disturb this function. DNAJC21 is required for the release of the dissociation factor PA2G4 from the pre-60S ribosome subunit, which is critical for 60 S subunit maturation and association with 40 S. This function of DNAJC21 homolog in yeast (Jjjj1) is also required for the release of eIF6 homolog (Tif6) by SBDS homolog (Sod1).
Yeast strains deleted in SBDS and DNAJC21 homologs accumulate their targets in the cytosol (eIF6 and Arx1, respectively), grow poorly at low temperatures, accumulate halfmer ribosomes and have reduced levels of mature ribosomes, all hallmarks of dysfunctional 60 S ribosomal subunit biogenesis. Loss of SBDS or DNAJC21 in human cells results in markedly reduced global translation.
As the first identified disease-related gene, SBDS functions have been studied more extensively than other SDS genes. It has been shown that SBDS is critical for several cellular pathways, including cell survival, telomere maintenance, mitotic spindle stabilization, chemotaxis, and marrow stromal function, besides ribosome biogenesis.
The SBDS protein can be detected in human cell nuclei and cytoplasm. It concentrates in the nucleolus during G1 and G2. SBDS interacts with multiple proteins with diverse molecular functions; many of them are involved in ribosome biogenesis, such as RPL4, and DNA metabolism, such as RPA70.
SBDS is critical for cell survival. When SBDS is lost in SDS BM cells or in SBDS -knockdown K562 and HeLa cells, the cells undergo accelerated apoptosis. The accelerated apoptosis in BM cells and SBDS -knockdown cells seems to be through the Fas pathway and not through the Bax/Bcl-2/Bcl-XL pathway. SBDS deficiency in primary SDS cells and in SBDS -knockdown cells results in abnormal accumulation of functional Fas (transcript 1) at the plasma membrane level.
Interestingly, knocking down SBDS in CD34 + cells and in cell lines increased the levels of ROS, and antioxidants reduced Fas-mediated cell death and improved hematopoiesis from primary SDS cells. This suggests that SBDS promotes balanced levels of ROS, thereby protecting hematopoietic cells from cell death.
Patients with SDS have a defect in leukocyte chemotaxis. Consistent with this observation, the SBDS homologue in amoeba was found to localize to the pseudopods during chemotaxis. These observations suggest that the SBDS protein deficiency in SDS causes a chemotaxis defect in patients.
SBDS has been shown to colocalize to the mitotic spindle and bind microtubules and stabilize them. Its deficiency results in centrosomal amplification and multipolar spindles.
The pathophysiologic link between mutations in SDS genes and BM failure is still unclear. Colony forming assays have indicated a defect in CFU-GM, BFU-E, and CFU-GEMM colony formation in most patients, compatible with a defective stem cell origin of the BM failure. The BM phenotype is summarized in Table 30.3 . SDS BM is characterized by decreased numbers of CD34 + cells as well as an impaired ability for CD34 + cells to form multilineage hematopoietic colonies in vitro, confirming that they are intrinsically defective. A recent study showed decreased levels of multipotent HSCs, MPPs, CMPs, MEPs, and, GMPs, though GMPs were the relatively more preserved than other HSC/HPCs.
|
To a certain extent, the hematopoietic failure is likely related directly to reduced global protein synthesis. Indeed human myeloid cell lines feature reduced translation, which is accentuated during differentiation, at least toward erythroid cells.
Several studies pinpointed apoptosis through the FAS pathway as a central pathogenetic mechanism that lead to reduced numbers of hematopoietic cells. As mentioned above, patients’ BM cells overexpress FAS, and show increased patterns of apoptosis after preincubation with activating anti-FAS antibody. Induction of differentiation (at least toward the erythroid lineage) results in markedly accelerated apoptosis in SBDS-deficient cells, with only a minimal effect on proliferation.
Importantly, oxidative stress is also increased during differentiation of SBDS-deficient erythroid cells, and antioxidants enhance the expansion capability of both differentiating SBDS -knockdown K562 cells and colony production of SDS HSCs and progenitors. These studies indicate that when SBDS protein is deficient, several biologic pathways may be dysfunctional during hematopoietic cell development; this may be the cause of the high predilection for BM failure in patients with SDS.
A group from Boston used iPSCs from SDS patients and demonstrated an alternative mechanism for cell death. During differentiation of iPSCs to promyelocytes protease levels were increased and apoptosis was enhanced. Supplementing the culture media with protease inhibitors provided a rescue. Interestingly, TGF-β signaling pathway was also shown to be activated in SDS models (iPSCs, zebrafish, mouse) and in patient CD34 + cells, and inhibition of the pathway improved hematopoiesis.
When the averages of telomere lengths adjusted to age were compared with those of control participants, a tendency toward shortening of telomeres was found in patient leukocytes, which may reflect premature cellular aging. This may represent either an inherent defect in telomere maintenance or compensatory stem cell hyperproliferation.
In addition to an inherent hematopoietic defect, it has also been shown that the BM stroma is markedly defective in terms of its ability to support and maintain normal hematopoiesis, which may contribute to the hematopoietic defect and the high frequency of engraftment failure during HSC transplantation in patients with SDS.
The many clinical manifestations that occur in varying combinations have been reported in several publications and are shown in Table 30.4 . Most patients present in infancy with evidence of growth failure, feeding difficulties, diarrhea, and infections. Steatorrhea and abdominal discomfort are frequent. Approximately 50% of patients exhibit a modest improvement in pancreatic function and do not require further pancreatic enzyme replacement therapy. Hepatomegaly is a common physical finding in young children but typically resolves with age and does not have clinical significance.
| Feature | Patients (%) |
|---|---|
| Pancreatic insufficiency (decreased digestive enzymes) | 86–100 |
| Neutropenia | 81–100 |
| Thrombocytopenia | 24–70 |
| Anemia | 16–66 |
| Pancytopenia | 10–44 |
| MDS/AML | ≈30 |
| Delayed bone maturation | 100 |
| Low-turnover osteoporosis | ∼100 |
| Short stature | 50 (38- |
| Metaphyseal dysplasia | 44–77 |
| Rib cage anomalies | 32–52 |
| Hepatomegaly or elevated enzymes | <50 |
| Poor oral health (caries, ulcers, tooth loss) | >50 |
| Learning and behavioral problems | >50 |
Patients with SDS are particularly susceptible to bacterial and fungal infections, including otitis media, bronchopneumonia, osteomyelitis, septicemia, and recurrent furuncles. Overwhelming sepsis is a well-recognized fatal complication of this disorder, particularly early in life.
Short stature is a common feature of the syndrome; the 50th percentile of SDS charts for height is positioned on the 3rd percentile of regular charts, both for boys and girls. When treated with pancreatic enzyme replacement, most patients show a normal growth velocity yet remain consistently below the third percentile for height and weight, indicating an intrinsic growth defect. Although metaphyseal dysplasia is a common radiologic abnormality (44% to 77% of patients), particularly in the femoral head and the proximal tibia, in most patients it fails to produce any symptoms. Occasional patients have clinical joint deformities, resulting in pain, functional impairment, or cosmetic problems, necessitating surgery. Some patients present at birth with respiratory distress caused by thoracic dystrophy. Others may have asymptomatic short and flared ribs.
The majority of the patients have deficits in cognitive abilities at varying levels of severity. These include delayed language development, low intellectual ability, impaired visual-motor integration, and failure to achieve higher order language functioning and problem solving. About one-fifth of the children have behavioral challenges such as attention deficit hyperactivity disorder, pervasive developmental disorder, or oppositional defiant disorder.
Some additional clinical features are seen very infrequently in SDS. Endocrine abnormalities include insulin-dependent diabetes, growth hormone deficiency, hypogonadotropic hypogonadism, hypothyroidism, and delayed puberty. Cardiomyopathies have been noted in some cases. Urinary tract anomalies, renal tubular acidosis, and cleft palate also occur. Retinopathy has been reported in SDS patients with DNAJC21 mutations.
It is important to note that SDS phenotype might be mild and a diagnosis may be made only as part of a comprehensive genetic screening of adults with MDS. Nevertheless, to see if a subset of previously undiagnosed SDS patients presented for the first time with AML, 48 BM samples at remission were studied for mutations in SBDS, but none were found.
The spectrum of hematologic findings has been reported in several publications and is summarized in Table 30.4 . Neutropenia is present in almost all patients on at least one occasion. The neutropenia can be chronic or intermittent. Neutropenia has been identified in some SDS patients in the neonatal period during an episode of sepsis. Anemia is recorded in about half of the patients, but may fluctuate. RBC MCV and fetal hemoglobin are elevated in 60% and 75% of the patients, respectively, after the age of 1 year, which represents stress hematopoiesis. The combination of isolated neutropenia and high MCV or high HbF after the first year of life is seen in up to 28% of SDS patients and rarely in other IBMFSs. Reticulocyte responses are inappropriately low for the levels of hemoglobin in 75% of patients. Thrombocytopenia can be seen in about 40% of the patients and similar to anemia and neutropenia can also appear intermittently.
More than one lineage can be affected, and multi-lineage cytopenias have been observed in up to 65% of cases. Multi-lineage cytopenias can be due to profound severe aplastic anemia ( Fig. 30.2 ). However, BM biopsies and aspirates vary widely with respect to cellularity; varying degrees of BM hypoplasia and fat infiltration are the usual findings. BM with normal or even increased cellularity has also been observed, typically in young children. The severity of neutropenia does not always correlate with BM cellularity, nor is the severity of the pancreatic insufficiency concordant with the hematologic abnormalities.
SDS neutrophils may have defects in mobility, migration, and chemotaxis. There appears to be a diminished ability of SDS neutrophils to orient toward a gradient of N -formyl-methionyl-leucyl-phenylalanine. An unusual surface distribution of concanavalin A has also been reported that reflects a cytoskeletal defect in SDS neutrophils. Whatever the magnitude of the chemotaxis abnormality is in vitro in SDS, neutrophil recruitment into abscesses or empyemas ensues robustly in vivo.
Impaired immune function can be significant in SDS and contribute to recurrent infections even if adequate numbers of neutrophils are present. Patients have various B-cell abnormalities, including one or more of the following: low immunoglobulin G (IgG) or IgG subclasses, low percentage of circulating B lymphocytes, decreased in vitro B-cell proliferation, and lack of specific antibody production. Patients may also have T-cell abnormalities, including a low percentage of circulating T lymphocytes or subsets or NK cells, and decreased in vitro T-cell proliferation. Inverted CD4:CD8 ratios have also been described.
The exocrine pancreatic pathology is caused by failure of pancreatic acinar development ( Fig. 30.3 ). Pathologic studies reveal normal ductular architecture but extensive fatty replacement of pancreatic acinar tissue, which can be visualized by ultrasonography, CT, or MRI.
During the first 3 years of life, serum trypsinogen is typically reduced and can be used for diagnostic purposes. After the age of 3 years serum trypsinogen levels in SDS patients may normalize, which reduces its diagnostic usefulness at this stage. Serum isoamylase levels are reduced in SDS patients of all ages. However, normal children younger than 3 years have low isoamylase levels, so its measurement is not diagnostically useful at this age. Fecal elastase is another pancreatic enzyme that is reduced in SDS. Approximately 50% of patients exhibit a modest improvement in enzyme secretion with advancing age and normal fat absorption when assessed by 72-hour fecal fat balance studies. These patients do not require further pancreatic enzyme replacement therapy.
Pancreatic function studies using intravenous secretin or cholecystokinin can confirm the presence of markedly impaired enzyme secretion averaging 10% to 14% of normal but with preserved ductal function. Because of its invasive nature, this test has been replaced by measuring the levels of pancreatic enzymes in the serum.
Radiographs of the bone are useful as a screening diagnostic test for SDS. Osteopenia is seen in most patients but rarely results in clinical osteoporosis. Metaphyseal dysplasia has been reported in about 50% of the patients, particularly of the femoral heads, knees, humeral heads, wrists, ankles, and vertebrae. Rib-cage abnormalities can be found in 30% to 50% of patients. These include a narrow rib cage, short ribs, flared anterior rib ends, and costochondral thickening. Digital abnormalities such as clinodactyly, syndactyly, and supernumerary thumbs have been reported but are rare. Spinal deformities, including kyphosis and scoliosis, have been reported.
Patients with SDS do not have macroscopic brain malformations by MRI testing. However, they may have a decreased global brain volume (both gray matter and white matter) and a smaller posterior fossa, cerebellar vermis, corpus callosum, brainstem, and occipitofrontal head circumference compared with control participants. These anomalies might be the basis for the neurocognitive and neurobehavioral difficulties.
The French registry found cardiac anomalies in 11% of SDS patients. These include dilated and non-dilated cardiomyopathy, and structural malformations such as atrial septal defect, ventricular septal defect, coarctation of the aorta, and tetralogy of Fallot. Circumferential strain as measured by echocardiography was found to be decreased by the USA SDS registry, suggesting systolic dysfunction. Endocardial fibrosis and reduced left ventricular strain were also reported.
SDS is characterized by a high propensity to develop MDS and leukemia, particularly AML. The published crude rate for MDS/AML in patients with SDS ranges from 8% to 33%. In data from the CIMFR and the French Severe Chronic Neutropenia Registry, the cumulative risk of MDS/AML by the age of 18 and 20 years was 20% and 19%, respectively. The risk of leukemia in the French registry was 36% by the age of 30 years.
There is an increased frequency of BM clonal cytogenetic abnormalities as the sole evidence for a clonal disease in an otherwise hypocellular BM without excess blast counts or prominent multilineage cellular dysplasia. The incidence is roughly estimated to be 7% to 41% based on pooled published data; however, not all clones progress and some clones may not be detected on subsequent testing. Isochromosome 7q [i(7q)], an extremely uncommon finding rarely described in primary MDS or AML, was seen in up to 44% of SDS patients. This high occurrence suggests that it is a fairly specific marker for SDS and might be related to the mutant gene on 7q(11). Other chromosome 7 abnormalities are seen in 33% of SDS patients and include monosomy 7, i(7q) combined with monosomy 7 and deletions or translocations involving part of 7q. The prognostic significance of the cytogenetic changes requires prospective monitoring for clarification. Of the patients with i(7q), progression to advanced MDS with excess blasts or to AML has rarely been reported, but development of severe cytopenias and additional clones was described in three of four patients after long-term follow-up in the Canadian registry. Among a group of six patients with i(7q) from several hospitals in the United Kingdom, none progressed to advanced MDS/AML. In contrast, approximately 40% of patients with the other chromosomal 7 abnormalities progressed to either advanced MDS or to AML. Similarly, only rare cases with SDS patients and del(20q) evolve into advanced MDS/AML.
The pathophysiologic link between SBDS mutations and propensity to MDS and AML is unknown. Patients with SDS cells develop more frequent mutations than healthy subjects, possibly because of genomic instability due to mitotic spindle dysregulation or telomere shortening. It is also possible that impaired ribosome biogenesis and accelerated apoptosis cause a growth disadvantage for SDS BM cells, allowing for a growth advantage and expansion of malignant clones. Although molecular and cellular parameters do not distinguish SDS patients with transformation from SDS patients without transformation, it is remarkable that SDS BM demonstrates many features characteristic of MDS. These include impaired BM stromal support of normal hematopoiesis, increased BM cell apoptosis mediated by the Fas pathway, telomere shortening of leukocytes, increased BM neovascularization, high frequency of clonal cytogenetic abnormalities, and abnormal leukemia-related gene expression in BM progenitor cells, e.g. overexpression of the oncogenes TAL1 and LARG .
Similar to clonal evolution in FA, deep immunotyping analysis of early progenitors in SDS BM samples demonstrated relative selective survival of cells with GMP markers. Whole-exome and targeted sequencing of GMP-like cells in leukemia-free patients revealed a higher mutational load than in healthy controls and molecular changes that are characteristic of AML including increased G>A/C>T variants, decreased A>G/T>C variants, increased trinucleotide mutations at Xp(C>T)pT (X indicates any nucleotide), and decreased mutation rates at Xp(C>T)pG sites compared with other Xp(C>T)pX sites. Serial analysis of GMPs from an SDS patient who progressed to leukemia revealed a gradual increase in the mutational burden, enrichment of G>A/C>T signature, and emergence of new clones. Importantly, the molecular signature of marrow cells from a SDS patient with leukemia was similar to that of SDS patients without transformation. The predicted founding clones in SDS-derived AML harbored mutations in several genes, including TP53. The results suggest that GMP-like cells might represent a cellular reservoir for clonal evolution.
Monoallelic deletion of 20q10-11 and point mutations in EIF6 are commonly seen in SDS, and alleviate the SDS ribosome joining defect and translation by inactivating eIF6. In contrast, somatic monoallelic TP53 mutations, which are also frequently seen in SDS, decrease checkpoint activation and may progress to leukemia after developing a second mutation on the other allele, as demonstrated in one SDS patient and AML.
The vast majority of published cases of SDS-associated MDS/AML developed without previous G-CSF therapy. None of the six patients with SDS-associated MDS/AML from our institution were treated with G-CSF before transformation. However, it is still unclear whether G-CSF increases the risk of developing leukemia or promotes the expansion of existing malignant clones. Because G-CSF might increase neutrophil counts and prevent infections in SDS, a fraction of the reported patients with SDS-associated MDS/AML had been previously treated with G-CSF.
SDS patients with MDS/AML have common SBDS mutations, and no specific mutations have been shown to be associated with a higher risk of MDS/AML.
Several cases of solid tumors have been described in SDS. These include two cases of pancreatic ductal adenocarcinoma, one brain frontal lobe B-cell lymphoma, one dermatofibrosarcoma protuberans, and one breast cancer. However, more data are needed to determine whether the risk of solid tumors is higher than in the general population.
Despite the very similar function of SBDS and DNAJC21 in ribosome biogenesis, some important phenotypic differences between these two SDS genetic groups have been observed. (1) Many SBDS -associated SDS have neutropenia without prominent anemia and thrombocytopenia. By contrast, hematopoietic failure in DNAJC21 -associated SDS is often more global and affects more lineages and can be more severe. (2) Retinal disease is associated with DNAJC21 mutations, but not with SBDS mutations. ELF1 mutations are associated with multi-lineage cytopenia, pancreatic insufficiency, and skeletal dysplasia. Patients with a SRP45 mutation have a variable phenotype ranging from severe congenital neutropenia resembling ELANE-related disease to classical SDS.
The introduction of single gene analysis and later of comprehensive genomic testing has improved the ability to diagnose the disorder and particularly has helped identify cases with an atypical presentation. The diagnostic criteria have been summarized in an international consensus document and include having at least two of the following: (1) chronic BM failure, (2) exocrine pancreatic insufficiency, (3) positive genetic testing results or a first degree-relative with SDS. Several syndromes with overlapping features have to be excluded.
The syndrome of refractory sideroblastic anemia with vacuolization of BM precursors, or Pearson syndrome , is clinically similar to SDS but characterized by very different BM morphology. Severe anemia requiring transfusions rather than neutropenia is often present at birth and by 1 year of age in all cases. In contrast to SDS, the major BM morphologic findings are ringed sideroblasts with decreased erythroblasts and prominent vacuolation of erythroid and myeloid precursors. The disorder shares clinical similarities with SDS because of exocrine pancreatic dysfunction. Malabsorption and severe failure to thrive occur in approximately half of cases within the first 12 months of life. Qualitative pancreatic function tests show depressed acinar function and reduced fluid and electrolyte secretion. Approximately 50% of reported patients die early in life from sepsis, acidosis, and liver failure; the others appear to improve spontaneously with reduced transfusion requirements. At autopsy, the pancreas shows acinar cell atrophy and fibrosis, but fatty infiltration as seen in SDS is not a prominent feature. Patients may need pancreatic enzyme replacement. These patients have a diagnostic deletion of mitochondrial deoxyribonucleic acid (mtDNA). mtDNA encodes enzymes in the mitochondrial respiratory chain that are relevant to oxidative phosphorylation, including the reduced form of nicotinamide adenine dinucleotide dehydrogenase (NADH), cytochrome oxidase, adenosine triphosphatase (ATPase), mitochondrial transfer ribonucleic acids (tRNAs), and mitochondrial ribosomal RNAs. The degree of heteroplasmy affects the disease expression.
SDS shares some manifestations with FA such as BM dysfunction and growth failure, but patients with SDS can usually be distinguished because of malabsorption syndrome, fatty changes within the pancreatic body that can be visualized by imaging, and characteristic skeletal abnormalities not seen in patients with FA. In difficult cases with incomplete disease expression, the distinction relies on normal clastogenic stress-induced chromosome fragility testing and genetic testing of SDS and FA genes.
Atypical SDS cases with only little evidence of pancreatic changes can be difficult to distinguish from early-onset dyskeratosis congenita with no mucocutaneous manifestations. Telomere length testing showing very short telomeres (below 1% for age) is far more commonly seen in DC; nevertheless, when the diagnosis is unclear, genetic testing can help define the diagnosis.
Because of the carried clinical phenotype in SDS, the number of undiagnosed patients with mild or asymptomatic disease is unknown. Hence, the overall prognosis may be better than previously thought.
From a literature review, the projected median survival of SDS patients was calculated as 35 years. During infancy, morbidity and mortality are mostly related to infections, thoracic dystrophy, and malabsorption. Later in life, the major problems are hematologic or complications related to their treatment. Cytopenias tend to fluctuate in severity but do not fully resolve spontaneously. The most common cause of death in late childhood or adulthood is related to MDS/AML.
The impact of different SDS genetic groups on long-term outcome may vary, but needs to be further studied.
Patient management is ideally shared by a multidisciplinary team consisting of a hematologist and a gastroenterologist as core members and other subspecialists such as an orthopedic surgeon, a dentist, and a psychologist as required. All patients with DNAJC21 mutations should also have an eye examination by an ophthalmologist. The malabsorption component of SDS responds to treatment with oral pancreatic enzyme replacement with meals and snacks using guidelines similar to those for cystic fibrosis. Supplemental fat-soluble vitamins are also usually required. When monitored over time, approximately 50% of patients convert from pancreatic insufficiency to sufficiency because of spontaneous improvement in pancreatic enzyme secretion. This improvement is particularly evident after 4 years of age.
A long-term plan should be initiated for early detection of severe cytopenias that require corrective action or malignant myeloid transformation. There are currently no data about the cost effectiveness of a specific leukemia surveillance program in SDS. However, in a consensus document on SDS management it is generally recommended that surveillance should include periodic blood counts with differentials and blood smears every 3 to 4 months, a clinical evaluation by a hematologist every 6 months, and BM testing every 1 to 3 years. The latter includes aspirates for smears and cytogenetics analyses. Concomitant BM biopsies are recommended when the patient’s clinical status changes. Recently it has been shown that an SDS BM testing may reveal evidence of MDS before changes are seen in the peripheral blood counts.
G-CSF given for profound neutropenia has been very effective in inducing a clinically beneficial neutrophil response. A report from the Severe Chronic Neutropenia International Registry (SCNIR) and unpublished data from the CIMFR demonstrated that treatment with G-CSF results in a brisk neutrophil response in about 90% of the patients. The response can be sustained in some cases for over 10 years
A smaller number of patients have received androgens with or without steroids similar to their use in treating FA, and improved BM function has been observed in some. In the CIMFR database three patients were treated with androgens. Two had DNAJC21 mutations, one of them responded. One had SBDS mutation and responded. One patient on the CIMFR was treated with eltrombopag without improvement [unpublished data]. Anecdotal cases treated with cyclosporine or erythropoietin do not allow broad therapeutic conclusions. A few patients who were treated with corticosteroids with some hematologic improvement have been reported in the 1980s.
Anemia and thrombocytopenia are managed with transfusions of RBCs or platelets when symptoms appear or prophylactically for profound cytopenias. Antifibrinolytic therapy with tranexamic acid can also be given for mild mucosal bleeding. Broad-spectrum antibiotics are indicated for febrile episodes and severe neutropenia.
At present, the only curative option for severe BM failure and MDS/AML in SDS is allogeneic HSCT. The indications for HSCT include bone marrow failure (BMF) with severe or symptomatic cytopenias, MDS with excess blasts (5% to 29%), or AML. Published data are limited and derived from relatively small case series with a mix of sibling and MUD.
The European Group for Blood and Bone Marrow Transplantation (EBMT) Registry reported 74 transplanted SDS patients. The indications included BMF ( n = 61), MDS ( n = 7), and AML ( n = 6). Fifty-four percent were transplanted with myeloablative conditioning regimens that included busulfan or total-body irradiation and 46% with reduced intensity. The majority (68%) of the donors were unrelated, 24% were matched siblings, and 8% were parents. Overall survival at 3.5 years of those with BMF was 71% compared to 29% among patients with MDS/AML. Overall survival of patients with BMF remained 71% at 6 years post HSCT. Complications include graft failure (15%), acute GVHD grade I–IV (55%), chronic GVHD (20%), as well as relapse, infections, and organ toxicity. In this study the difference in overall survival between myeloablative (69%) and reduced intensity (57%) conditioning regimens was not significant ( P = .7). The overall survival of patients who received TBI was 38% and of those who did not receive TBI was 79%, but the difference did not reach statistical significance ( P = .1). The results of this study showed slight improvement from a previous publication from the same group in 2006.
In another retrospective multicenter study from the USA, 39 patients were transplanted either with reduced intensity ( n = 26) or myeloablative ( n = 13) conditioning from 2000 to 2017. Median age at transplant was 7 years (range from 0.4 to 51 years). Five-year overall survival of patients transplanted for BMF was 72% compared to 15% of those who were transplanted for MDS/AML (<0.001). Main causes of death among the patients with BMF were GVHD and graft failure. In contrast most common causes of death among the patients with MDS/AML were relapse and toxicity.
A note of caution is sounded regarding HSCT for SDS. Left ventricular fibrosis and necrosis without coronary arterial lesions have been reported in 50% of SDS patients at autopsy, suggesting that there may be an increased risk of cardiotoxicity as well as other problems with the intensive preparatory chemotherapy used in HSCT. Indeed, published data emphasized that complications are more common in SDS patients who receive chemotherapy or undergo HSCT than in patients with acquired aplastic anemia. Complications include toxicity (cardiac, neurologic renal, pulmonary, and venoocclusive disease), graft failure, and severe GVHD. The heightened HSCT-related toxicity can be explained by increased sensitivity to chemotherapy and radiation, resulting in massive apoptosis in various organs. The hypersensitivity might be related to shorter telomeres than in individuals in the general population, mitotic spindle destabilization, and to genomic instability. Increased risk of graft failure might be related to BM stromal defects that are not corrected by the allograft and might be aggravated by the conditioning regimen.
Reduced-intensity HSCT regimens have been proposed for patients with SDS, particularly if they have BMF without MDS/AML. Data from small studies utilizing Campath-1H, fludarabine, and melphalan or fludarabine, treosulfan, melphalan plus Campath-1H, or rabbit ATG demonstrated promising results. However, the larger and recent retrospective studies described above did not show superiority of this approach. The high risk of graft failure with both reduced intensity and myeloablative conditioning emphasize the need for innovative approaches to transplant SDS patients.
Several other clinical and basic research questions in SDS must be addressed. First, the various biochemical functions of SDS genes require further studies. How the protein products maintain normal hematopoiesis and protect from apoptosis as well as cancer is unclear. The natural history and risk factors for the development of complications need to be clarified. There is also a need to understand the mechanism for the heightened sensitivity of SDS patients to chemotherapy and irradiation and to develop effective and less toxic regimens for HSCT. Determining risk factors and molecular events during malignant myeloid transformation might prompt strategies for prevention and screening for complications. Further research is needed to develop new drugs that improve growth potential of HSCs and relieve the severity of the cytopenias. Clinical trials to explore efficacy and safety of agents that target pathways that were shown to be abnormal in SDS (e.g., translation, oxidative stress, and TGF-beta signaling) may help identify such drugs. Recently Ataluren, which promotes mRNA reading by the ribosome through premature stop codons, has shown to increase SBDS protein levels, improve CFU-GEMM and CFU-GM colony formation, and neutrophil maturation from SDS BM mononuclear cells as well as neutrophil survival. Ataluren has been approved for the treatment of patients with Duchenne muscular dystrophy and nonsense mutations, and might also be effective in SDS which will require evaluation in a clinical trial.
DC is an inherited multisystem disorder of the mucocutaneous and hematopoietic systems in association with a wide variety of other somatic abnormalities. Originally, it was considered a dermatologic disease, and was termed Zinsser-Cole-Engman syndrome after the first investigators who described it. The term “dyskeratosis congenita with pigmentation” was first proposed by Cole and colleagues, Rauschkolb, and Toomey in 1930. The traditional diagnostic ectodermal triad consists of reticulate skin pigmentation of the upper body, mucosal leukoplakia, and nail dystrophy. The skin and nail findings usually become apparent during the first 10 years of life, but the oral leukoplakia is commonly observed later. These manifestations tend to progress as patients get older. Age of presentation varies according to the genetic group.
Hematologic manifestations were reported in the 1950s, and in the 1960s/1970s BMF was recognized to be a major component of the syndrome and responsible for substantial morbidity and mortality. Indeed, the full diagnostic dermatologic triad is present in only about 46% of the patients, but BM failure of varying severity is reported in up to 90% of cases. With the recent advances in understanding the molecular basis of the disease, patients with hematologic abnormalities but without dermatologic findings have been identified that dramatically changed the historical definition of the disease. DC patients also have a predisposition to develop solid tumors and MDS/AML.
The estimated incidence of DC in childhood is about 4 cases per million per year. In older literature, most DC patients were reported as males. However, with better understanding and broadening of the genetic and clinical spectrum of the disease and with more autosomal cases being identified, the proportion of males is much lower.
DC is a telomere-related disorder (telomeropathy), and all the identified DC genes play a role in telomere homeostasis (see Table 30.1 ). DC genes encode components of the telomerase complex ( TERT , DKC1 , TERC , NOP10 , and NHP2 ), T-loop assembly protein ( RTEL1 ), telomere capping ( CTC1 ), the telomere shelterin complex ( TINF2 ), and the telomerase trafficking protein ( TCAB1 ); all of which are critical for telomere maintenance.
The X-linked recessive disease is a common form of DC. It was originally estimated to comprise as many as 75% of DC cases, but with the identification of more DC genes and more patients with autosomal dominant inheritance, the true incidence is approximately 30%. The X-linked disease is caused by mutations in DKC1 on chromosome Xq28. The encoded protein, dyskerin, is part of a complex (with TERC ) that stimulates the telomerase (TERT). It is also involved in rRNA biogenesis.
The autosomal recessive forms of DC are caused by biallelic mutations in RTEL1 , PARN , NOP10 , NHP2, TCAB1, CTC1, ACD ( TPP1 ), or TERT . Interestingly, several DC gene ( RTEL1 , PARN or TERT, ACD ( TPP1 )) mutations can be associated with a severe disease (Hoyeraal-Hreidarsson syndrome) when both alleles are mutated, or a milder disease when one allele is affected.
Several genes are mutated in families with autosomal dominant inheritance. TINF2 is probably the most commonly mutated gene in this group and accounts for approximately 11% to 25% of the DC families. The vast majority of TINF2 mutations are de novo, probably due to the severity of disease. Heterozygous mutations in TERT, TERC, PARN, ACD (TPP1), or RTEL1 also result in autosomal dominant disease. In autosomal dominant DC where family members from several generations are affected, younger generations tend to have longer telomeres and more severe disease than their ancestors.
DC is a complex disorder and several clinicogenetic sub-types have been described. Classical DC that includes BMF and typical extra hematological manifestations can be caused by mutations in any of the DC genes ( Table 30.3 ). DC with either hemizygous DKC1 mutations or a heterozygous TINF2 mutation or biallelic mutations in TERT , PARN, RTEL1, WRAP53 (TCAB1), or ACD (TPP1) can result in a severe form of DC called Hoyeraal-Hreidarsson syndrome . It is characterized by hematologic and dermatologic manifestations of DC in addition to cerebellar hypoplasia. Immune deficiency is common when this syndrome is caused by DKC1 mutations. Revesz syndrome is a combination of classical manifestations of DC and exudative retinopathy. It is caused by mutations in TINF2 and is an autosomal dominant form of the disease. Some cases of moderate/severe aplastic anemia without physical anomalies are caused by mutations in TINF2, TERT , and TERC . In contrast to cases with biallelic TERT mutations, heterozygosity for mutations in TERT is associated with a milder phenotype, late presentation, severe aplastic anemia without physical malformations, isolated pulmonary fibrosis, isolated hepatic fibrosis, or a combination of these clinical sequelae. Heterozygosity for mutations in TERC is associated with a milder phenotype, late presentation and severe aplastic anemia, or MDS without physical malformations . Coats plus syndrome is caused by mutations in the CTC1 gene. It is characterized by retinal telangiectasia and exudates, intracranial calcification, leukodystrophy, brain cysts, osteopenia, gastrointestinal bleeding, and portal hypertension caused by the development of vascular ectasias in the stomach, small intestine, and liver. Some patients with this disease have the additional manifestations of DC, which include sparse and gray hair, dystrophic nails, and anemia. Telomeres are short.
It is noteworthy that mutations in certain telomere-related genes can cause partial DC phenotype without BMF; for example, monoallelic mutations in NAF1 or ZCCHC8 causing pulmonary fibrosis and biallelic mutations in STN1 causing Coats plus.
The hallmark of DC is short telomeres. Most children have severely short telomeres (less than the first percentile for age). DC genes play a role in telomere length maintenance in several ways and can be divided into those who belong to telomerase complex ( TERT, TERC) , other telomere replication-related factors ( RTEL1 , CTC1 , RPA1 ), telomerase trafficking ( WRAP53 /TCAB1), TERC-associated factors that stimulate telomerase activity ( DKC1, NOP10, NHP2 ), TERC-maturation factors ( PARN ), shelterin complex ( TINF2, POT1, TPP1 )
Telomerase complex ( TERT, TERC) TERT encodes for the enzyme component of telomerase. Telomerase is a ribonucleoprotein polymerase that maintains telomere ends by synthesis and addition of G-rich telomere repeat TTAGGG at the 3′-hydroxy DNA terminus of telomeres using the TERC RNA as a template. TERC encodes for the RNA component of telomerase and has a 3′ H/ACA small nucleolar RNA -like domain. The H/ACA box at the 3′-end of TERC is essential for its stability and assembly into the telomerase pre-ribonucleoprotein complex.
Other telomere replication-related factors ( RTEL1 , CTC1 , RPA1 ). RTEL1 is a helicase that resolves DNA secondary structures. It is recruited to telomers by TRF2 and unwinds the telomeric t-loop, thereby allowing telomere replication. CTC1 is a member of the CTC1-STN1-TEN1 (CST) trimeric complex that helps synthesize telomeric C-strand by binding to the telomeric 3′ overhang and stimulating DNA polymerase a-primase. RPA1 binds to telomeric 3′ overhangs during late S-phase to unfold G-quadruplexes and facilitate telomerase activity. A gain of function mutation was identified in a patient with classic DC.
WRAP53 (TCAB1) is involved in telomerase trafficking to telomeres. TCAB1 interacts with the CAB box on TERC, promoting localized telomerase in Cajal bodies and trafficking it to telomeres. Mutations in the TCAB1 gene ( WRAP53 ) impair this trafficking activity and lead to misdirection of telomerase RNA to nucleoli, thereby preventing elongation of telomeres by telomerase.
Several DC genes encode TERC-associated factors that stimulate telomerase activity: DKC1, NOP10, NHP2. GAR1, TEP1, and NAF1 are also associated with TERC, but have not been shown to be mutated in DC as of December 2020. Dyskerin (encoded by DKC1 ) binds H/ACA classes of RNA. It binds to the 3′ H/ACA small nucleolar RNA-like domain of the TERC . This stimulates telomerase to synthesize telomeric repeats during DNA replication. Dyskerin is also involved in maturation of nascent rRNA. It binds to small nucleolar RNA through the 3′ H/ACA domain and catalyzes the isomerization of uridine to pseudouridine through its pseudouridine synthase homology domain. This might be the mechanism for impaired translation from internal ribosome entry sites seen in mice and human DC cells.
In the telomerase complex, the H/ACA domain of nascent human TERC forms a pre-ribonucleoprotein with dyskerin, NOP10, NHP2, and NAF1. Initially, the core trimer dyskerin-NOP10-NHP2 forms to enable incorporation of NAF1, and efficient reverse transcription of telomere repeats. NOP10 and NHP2 also play an essential role in the assembly and activity of the H/ACA class of small nucleolar ribonucleoproteins that catalyze the isomerization of uridine to pseudouridine in rRNAs.
Poly(A)-specific ribonuclease (PARN) is a TERC-maturation factor. It is one of the major mammalian deadenylases that trims single-stranded poly(A) tails of mRNAs, but also of oligoadenylated tails of H/ACA box snoRNAs and microRNAs. As such it trims excessive adenylate residues that are added to TERC by PARD5. In patient cells oligoadenylated TERC forms are more abundant in PARN-deficient patient cells than those of controls, although its total levels were slightly reduced.
The shelterin complex is composed of TINF2, POT1 , and TPP1 , which are mutated in DC, as well as TRF1, TRF2 , and RAP1 that as of December 2020 have not been shown to harbor mutations that cause DC. TINF2 protein binds to and protects telomeres by allowing cells to distinguish between telomeres and regions of DNA damage. In the complex, TINF2 binds to TRF1, TRF2, and TPP1.
In several acquired and IBMFSs besides DC, telomeres are shorter than in age-matched control participants. However, because the telomere maintenance pathway is profoundly impaired in DC, the telomeres in this disease are much shorter than in other disorders. Most children with DC have telomeres that are very short (lower than the first percentile of the normal range). Average telomere length in adults with DC is shorter than that of healthy subjects, but it is commonly at the low range of normal for age. Shortening of telomeres results in cellular senescence, apoptosis (“cellular crisis”), or chromosome instability. However, some cells may survive the crisis by harboring compensatory genetic mutations that confer proliferative advantage and neoplastic potential.
DC is a chromosome “instability” disorder of a different type than FA. Results of clastogenic stress studies of DC cells are typically normal. There is no significant difference in chromosomal breakage between patient and normal lymphocytes with or without exposure to bleomycin, DEB, MMC, or γ-radiation. This contrasts sharply with FA cells and distinguishes one disorder from the other. However, metaphases of cultured patient peripheral blood cells, BM cells, and fibroblasts show numerous spontaneous unbalanced chromosome rearrangements such as dicentrics, tricentrics, and translocations. These are probably caused by short telomeres.
Most studies of the pathogenesis of the aplastic anemia in DC have shown a marked reduction or absence of CFU-GEMM, BFU-E, CFU-E, and CFU-GM. Long-term DC BM cultures have shown that hematopoiesis is severely defective in all patients with a low frequency of colony-forming cells. The function of DC BM stromal cells is normal in their ability to support growth of hematopoietic progenitors from normal BM, but generation of progenitors from DC BM cells seeded over normal stroma is reduced, suggesting that the defect in DC is of stem cell origin. Telomerase is activated in HSCs and might be necessary for HSC self-renewal capacity and prevention of senescence. The BM failure in this disorder may be a result of a progressive attrition and depletion of HSCs. This is supported by studies showing reduced numbers of CD34 + /CD38 − cells in patient BMs. Alternatively, the BM dysfunction may represent a failure of replication, maturation, or both.
iPSCs from DC patients have been shown to have defects in telomere elongation during programming in a manner that is concordant with the mutated gene in the patients. In iPSCs from patients with heterozygous mutations in TERT , telomerase activity is directly affected. iPSCs from a patient with TCAB1 mutations featured mislocalization of telomerase from Cajal bodies to nucleoli. iPSCs from patients with mutant DKC1 display reduced telomerase activity because of impaired telomerase assembly. Extended culture of DKC1 -mutant iPSCs leads to progressive telomere shortening and eventual loss of self-renewal. In contrast, another group studied telomerase reactivation and TERC regulation during reprogramming and showed that reprogramming restores telomere elongation in DC cells despite genetic lesions affecting telomerase. This group showed that TERC upregulation is a feature of the pluripotent state and that several telomerase components are targeted by pluripotency-associated transcription factors.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here