Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anesthesia consists of separable and independent components, each of which involves distinct, but possibly overlapping, mechanisms at different sites in the central nervous system.
The potencies of general anesthetics correlate with their solubility in oil, indicating the importance of interactions with predominantly hydrophobic targets.
General anesthetics act by binding directly to amphiphilic cavities in proteins. Binding sites have been identified by a combination of site-directed mutagenesis and high-resolution structural analysis of anesthetic binding.
Mutations made to render putative protein targets insensitive to inhaled anesthetics have been expressed in mice but have not generated breakthroughs analogous to the success of this strategy with intravenous anesthetics.
The effects of inhaled anesthetics cannot be explained by a single molecular mechanism. Rather, several targets contribute to the component actions comprising the anesthetic effects of each anesthetic. However, these effects do converge on a limited number of states underlying the behavioral effects.
The immobilizing effect of inhaled anesthetics involves actions in the spinal cord, whereas sedation/hypnosis and amnesia involve supraspinal mechanisms that interact with endogenous memory, sleep, and consciousness pathways and networks.
Volatile inhaled anesthetics enhance inhibitory synaptic transmission postsynaptically by potentiating ligand-gated ion channels activated by γ-aminobutyric acid (GABA) and glycine, extrasynaptically by enhancing GABA receptors, and presynaptically by enhancing basal GABA release.
Inhaled anesthetics suppress excitatory synaptic transmission presynaptically by reducing glutamate release (volatile anesthetics) and postsynaptically by inhibiting excitatory ionotropic receptors activated by glutamate (gaseous and to some extent volatile anesthetics).
Inhaled anesthetics directly activate certain two-pore-domain potassium channels, which is likely to result in both pre- and post-synaptic effects.
There is as yet no comprehensive theory of anesthesia that describes the sequence of events leading from the interaction between an anesthetic molecule and its targets to the behavioral effects.
Despite the widespread clinical use of general anesthetics, our current understanding of their molecular, cellular, and network mechanisms is incomplete. This critical gap in the pharmacology of one of medicine’s most important drug classes not only impedes rational use of available anesthetics but also hinders the development of newer anesthetics that might selectively achieve the desirable end points of anesthesia with fewer adverse cardiovascular, respiratory, and possibly neuropathologic side effects. Although major progress has been made in understanding the pharmacology of the intravenous anesthetics by molecular genetic approaches, the actions of the inhaled anesthetics at the molecular and cellular levels are more enigmatic. It is still not possible to trace precisely the sequence of events that leads from inhaled anesthetic-target interactions, through ascending levels of biologic complexity, to the various behavioral effects that characterize the composite state of clinical anesthesia in humans. Nevertheless, investigations continue to reveal fundamental principles of action and have led to a framework for understanding anesthetic effects at different organizational levels.
The focus of this chapter is on the mechanisms involved in the principal therapeutic effects (anesthesia) and on the side effects of the inhaled anesthetics ( Fig. 19.1 ), a chemically and pharmacologically diverse group that includes the potent halogenated ether (isoflurane, sevoflurane, desflurane, enflurane) and alkane (halothane) volatile anesthetics and the inorganic gaseous anesthetics (nitrous oxide and xenon). This critical summary of the current state of knowledge begins with an historical overview and a review of the behavioral end points of anesthesia. We then trace, where possible, inhaled anesthetic effects through ascending levels of organization from molecules, cells, circuits, networks, and organs to mammalian behavior. We also briefly address studies of anesthetic effects in very simple model organisms, with anesthetic end points being identified that as yet bear uncertain relationships to those in mammals.
The first monograph reporting experimental work on anesthetic mechanisms, proposing a soon-to-be discredited lipid-elution theory of anesthetic action, was published only 6 months after Morton’s public demonstration of ether anesthesia in Boston’s Ether Dome. For decades thereafter, the phenomenon of anesthesia puzzled, inspired, and awed those who tried to understand it. An influential paradigm of anesthetic action formulated by Claude Bernard in the 1870s posited that anesthesia was a “unified” phenomenon―a unitary mechanism applicable to all forms of life. Although the anesthetized state could be brought about by a variety of agents, its essence was the same in all living creatures. In fact, Bernard thought that life itself was defined by susceptibility to anesthesia. Bernard also proposed a more specific theory of anesthesia, coagulation of protoplasm, which competed with a number of coexisting theories entertained by the scientific community. In a major work published in 1919, Hans Winterstein summarized the perplexing diversity of anesthetic theories by listing more than 600 references, the majority to original laboratory work—a convincing testimony to the interest of the scientific world in this phenomenon. Of note, the work of Meyer and Overton at the end of the nineteenth century had only a limited effect on the trajectory of research until the 1960s. Only then was the striking simplicity of the Meyer-Overton correlation ( Fig. 19.2 A ) of anesthetic potency with solubility in olive oil interpreted by the majority of researchers to indicate that lipids are likely to be the anesthetic target. This interpretation focused attention on anesthetic effects on the bulk physical properties of cell membranes, which were known to consist primarily of lipid molecules. Such nonspecific or “lipoid-based” anesthetic theories dominated the field from the 1960s to the 1980s.
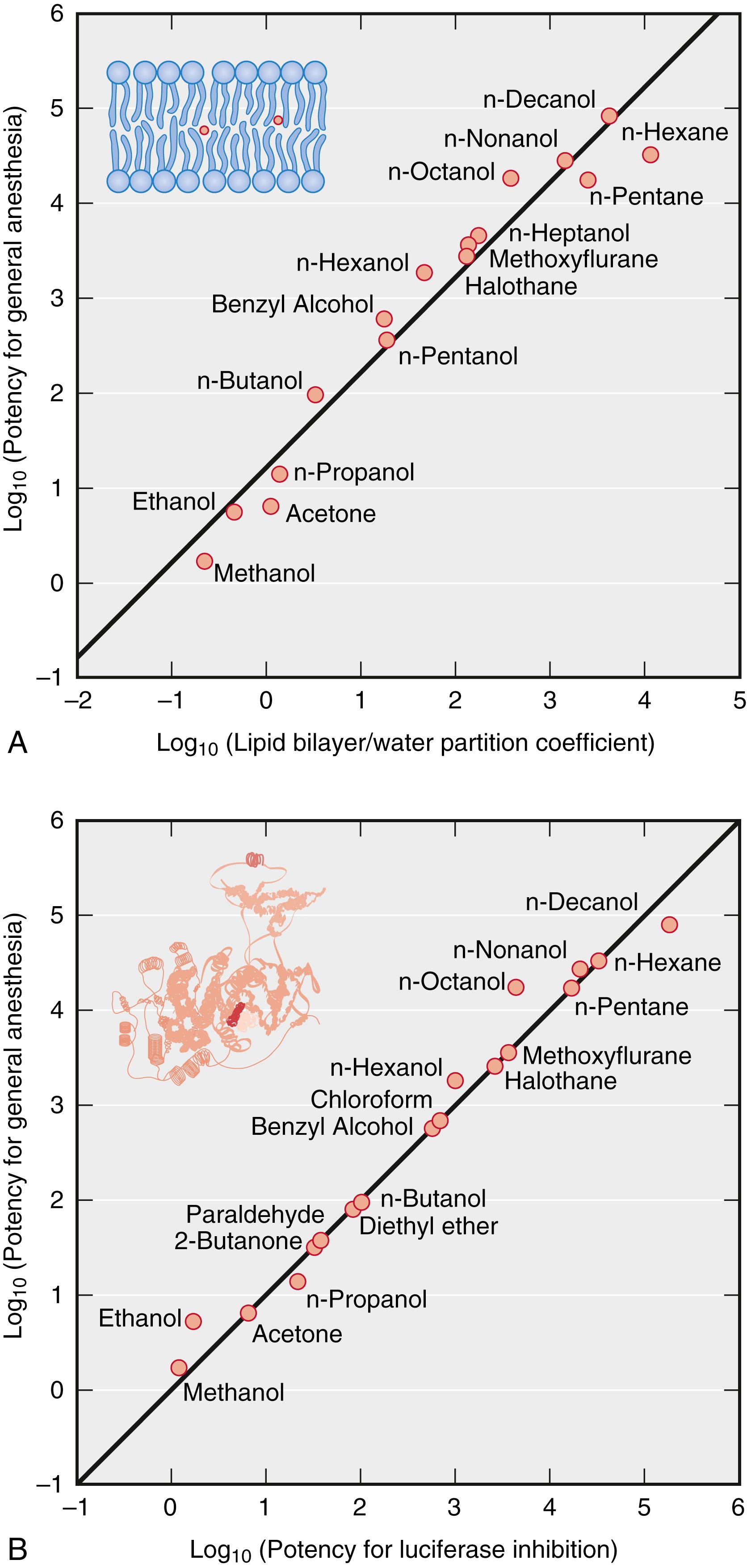
The potencies of inhaled anesthetics for immobilization were established in the classic studies in the 1960s of Eger and colleagues, who defined the minimum alveolar concentration (MAC) as the inhaled anesthetic atmospheric pressure required to prevent movement in response to a defined noxious stimulus in 50% of subjects. The concept of MAC evolved within a unitary paradigm of anesthetic action and also reflected the priorities of clinical practice. As a result, prevention of movement (immobility) became a universal yardstick for anesthetic effects, presumed to occur in the brain. Moreover, the simple elegance of the relationship between anesthetic potency and lipid solubility (see Fig. 19.2 A ) graphically illustrated Meyer and Overton’s conclusion that “All chemically indifferent substances that are soluble in fat are anesthetics. Their relative potency as anesthetics will depend on their affinity to fat on the one hand and water on the other hand, that is, on the fat/water partition coefficient.” This was interpreted as favoring lipids as the primary targets of anesthetics and a single nonspecific theory to explain anesthesia. The appeal of a single unified mechanism to explain anesthesia was (and remains) intellectually appealing. This focused the bulk of research efforts on delineating how anesthetic interactions with lipid membranes might lead to the behavioral changes observed under anesthesia―the nonspecific lipoid theory.
Because inhaled anesthetic concentrations reflect concentrations in the tissues after equilibration, which is most rapidly achieved for well-perfused organs such as the brain and heart, MAC is analogous to the plasma concentration for 50% effect (EC 50 ) for intravenous anesthetics. In clinical applications, MAC is usually expressed as volume percent (vol%), which varies considerably with temperature owing to the large temperature dependence of partitioning between the gas phase and condensed phases (be they water, lipids, or proteins), whereas the equivalent condensed-phase molar concentrations are much less dependent on temperature. The MAC concept provided researchers and clinicians with a universal standard whereby to measure a defined anesthetic end point (immobility), making meaningful comparisons of experimental results possible and accelerating clinical and laboratory research into anesthetic mechanisms. Today, a more nuanced understanding of MAC considers the structural and functional diversity of the physiologic targets for the different components of the anesthetic state.
Lipid-centered mechanisms of anesthesia prevailed in the two decades after definition of the MAC concept. Alternative targets were occasionally proposed but largely neglected by the scientific mainstream. Experimental inconsistencies of lipid targets, as well as evidence compatible with proteins as primary sites of action, were largely ignored. A shift from lipid- to protein-centered mechanisms, however, began in the late 1970s, owing largely to the discoveries of Franks and Lieb, who in an influential series of publications demonstrated that not only were lipids implausible targets but that protein targets were also compatible with the Meyer-Overton correlation (see Fig. 19.2 B )—a proof of concept that, within a few years, redirected the bulk of research efforts toward proteins. As a corollary of this reorientation, evidence against lipid-based theories was recognized. Examples include the cutoff in anesthetic potency in homologous series of long-chain anesthetic alcohols and the identification of hydrophobic drugs that do not obey the Meyer-Overton correlation. The enantiomeric selectivity of several anesthetics further strengthened the case for specific binding sites on proteins because stereoselectivity is difficult to reconcile with lipid targets. Today, there is widespread acceptance of the notion that lipid bilayers remain essentially unaffected by general anesthetics and that critical signaling proteins (e.g., ion channels or ligand-gated receptors) are the relevant molecular targets of anesthetic action. The exact identity of proteins contributing to specific anesthetic end points continues to be sought, with research addressing not only the “where” (target) but also the “how” (process) of anesthetic mechanisms.
At high concentrations in vitro, most inhaled anesthetics affect the functions of multiple proteins, several of which might be plausibly connected to the components of the anesthetic state or anesthetic side effects. However, when a specific anesthetic end point is considered, anesthetics are effective in vivo over a very narrow concentration range. This makes the concentration at which a relevant anesthetic effect is observed a critical consideration for deciding potential relevance. The mechanistic relevance of small effects observed in vitro at relevant concentrations is less clear; that is, what effect is too small to be considered relevant to anesthesia?
Whether anesthesia results from the sum of minor perturbations at multiple sites or from substantial effects on a small number of targets remains to be determined. This should be resolved as more sophisticated molecular genetics experimental techniques are applied to test the relevance of putative targets. There are two reasons for believing that the number of relevant targets may be small. First, the extreme steepness of anesthetic concentration-response curves means that, for a given end point, substantial effects on two or three targets would be sufficient to account entirely for the in vivo effect. Second, the stereoselectivity observed in vivo is comparable to the largest effects seen in putative targets in vitro, suggesting that only a small number of targets are likely to be involved. Set against this logic is the experimental evidence that a rather large number of plausible target proteins are affected, albeit usually to small extents, and determining which of these are relevant to the various anesthetic end points remains a challenge.
Along with progress in identifying the molecular mechanisms of anesthesia, our understanding of the nature of the anesthetic state has evolved. Whereas a drug-induced coma-like state of general anesthesia can be induced by inhaled anesthetics administered at appropriate concentrations (approximately 1.3 times MAC, equivalent to the EC 95 of an intravenous anesthetic), the use of such high concentrations can lead to short- and possibly long-term side effects. It is now clear that anesthesia consists of separable and at least partially independent components or substates, each of which involves distinct but possibly overlapping mechanisms in different regions of the central nervous system (CNS) and with variations in relative potencies between specific agents. Immobilization, the core measure of MAC, is mediated largely at the level of the spinal cord by inhaled anesthetics but not by barbiturates. On the other hand, the spinal cord is clearly not the primary site of such phenomena as amnesia, sedation, and unconsciousness, which rather are produced by anesthetic effects on cerebral cortical function ( Fig. 19.3 ). A functional separation between amnesia and sedation has been demonstrated for intravenous anesthetics, and it seems likely that this will apply to inhaled anesthetics as well. The state commonly referred to as “unconsciousness” is in itself heterogeneous, with evidence for distinct states of unresponsiveness and unconsciousness. These and similar findings have led to the concept that general anesthesia consists of multiple independent components that can be resolved experimentally and clinically.
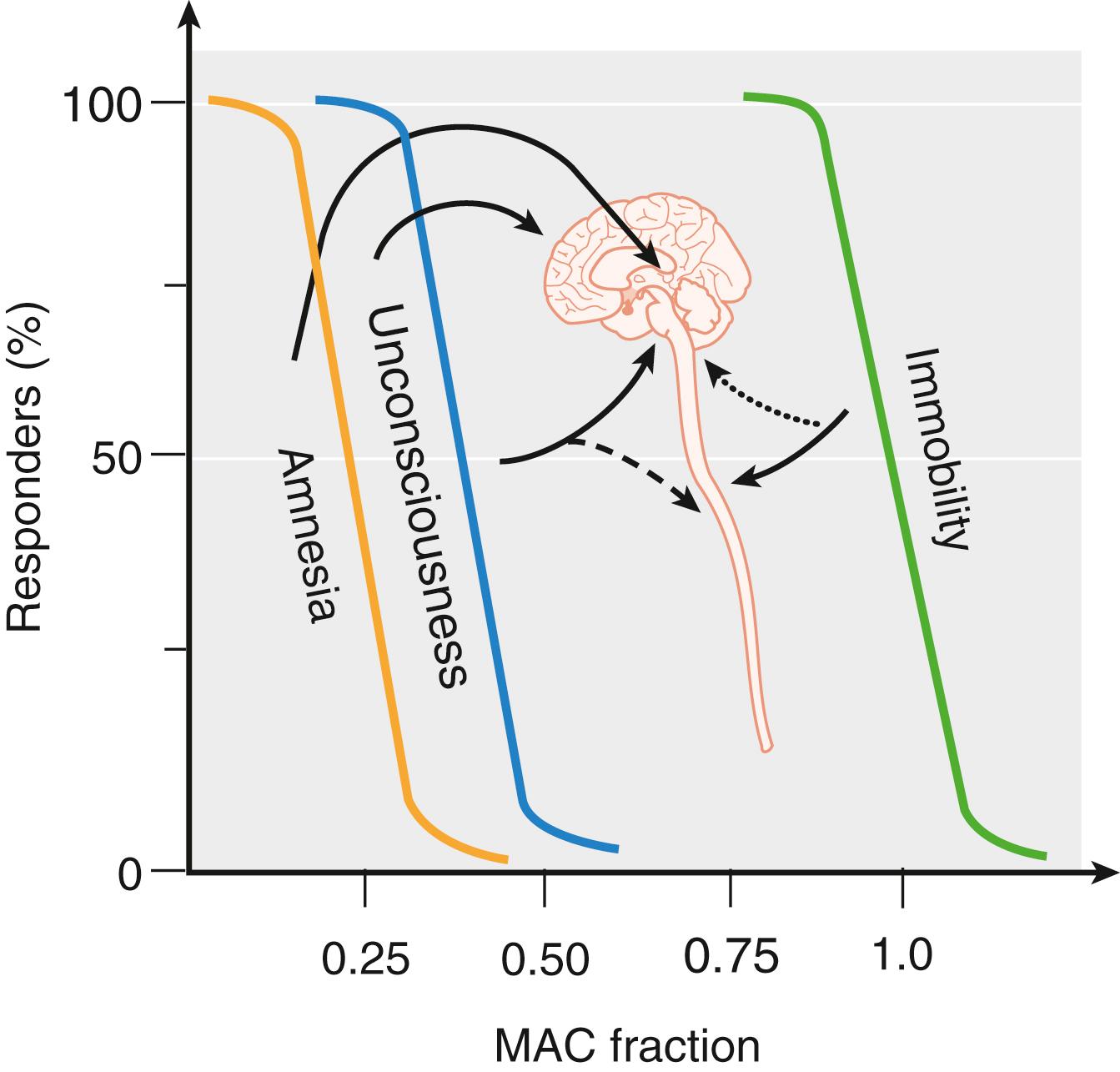
In principle, each component of anesthesia can be preferentially induced in a concentration- and agent-specific manner using individual cellular/molecular pathways in various regions of the CNS. For example, injections of pentobarbital into discrete sites in the mesopontine tegmentum induce a comatose state, whereas sedation induced by systemic administration of propofol can be reversed by microinjections of γ-aminobutyric acid (GABA) A receptor antagonists into the tuberomammillary nucleus, a sleep-regulating nucleus in the hypothalamus. Thus general anesthetics produce separate identifiable anesthetic substates via agent-specific actions at discrete anatomic sites in the CNS through different molecular targets. An important consequence of this complexity is that MAC, which is based exclusively on a motor response, might not proportionately reflect other components of anesthesia. Although this heterogeneity of anesthetic actions complicates a mechanistic understanding, it does open the possibility of developing substate-specific drugs.
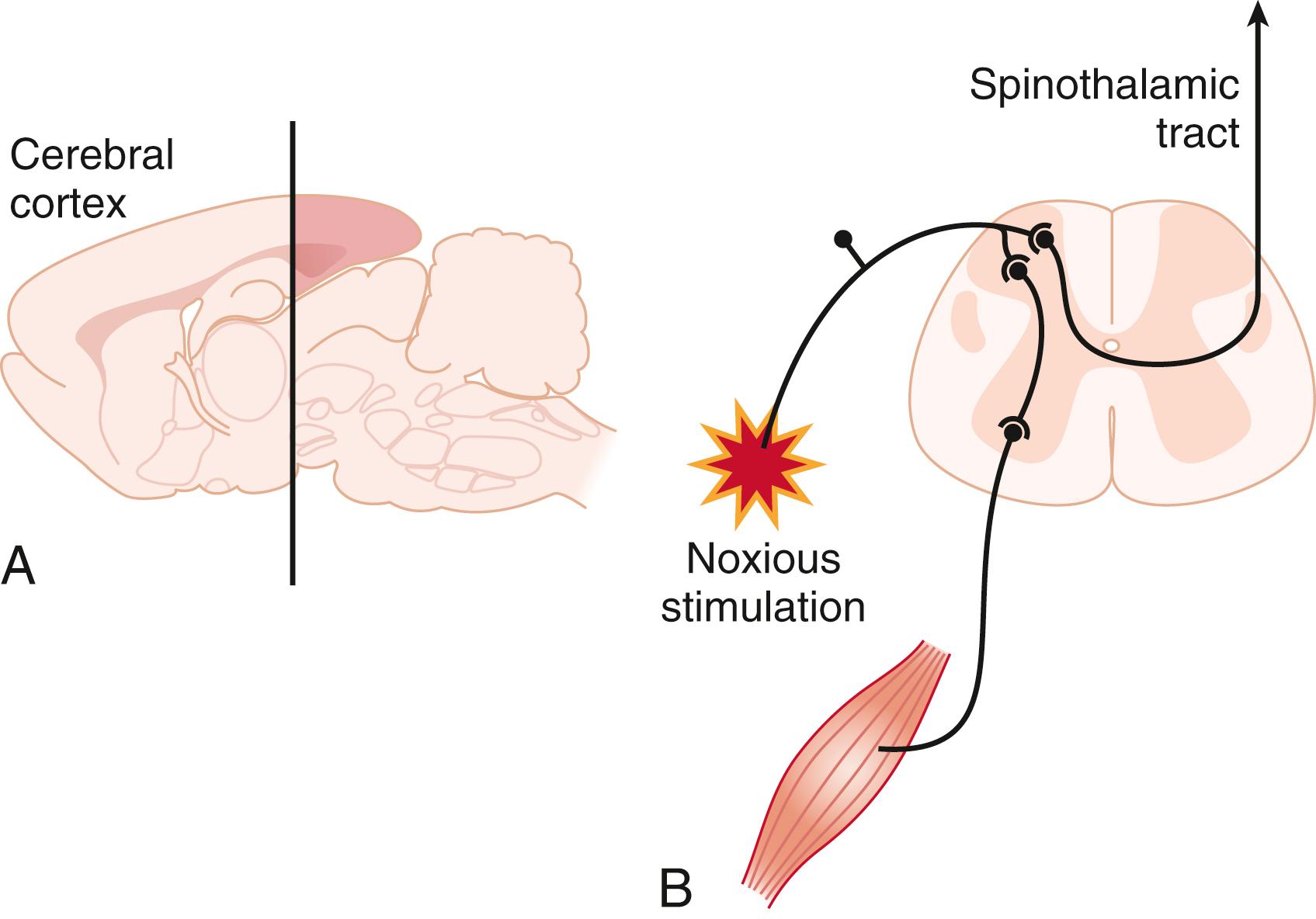
Electroencephalography as a monitor of brain activity has been applied both to the study of anesthetic mechanisms and as a monitor of the anesthetic state. Failure to find a correlation between quantitative electroencephalographic activity and immobility in response to noxious stimulation led to the somewhat radical (at the time) hypothesis that immobility was not a cerebral cortex–mediated phenomenon. Experimental demonstration that volatile anesthetics act on the spinal cord to suppress movement supported this hypothesis and was a major factor leading to the contemporary separation of anesthetic substates, of which immobility requires the highest drug concentrations (see Fig. 19.3 ). Taking advantage of the atypical blood supply of goat CNS, which allows separate experimental perfusion of the brain and spinal cord, Antognini and colleagues showed that immobility involves anesthetic effects at the spinal level because selective delivery of isoflurane or halothane only to the brain required 2.5-fold to 4-fold higher concentrations compared with delivery to the whole CNS. At the same time, experiments by Rampil and colleagues that used surgical separation of the forebrain and midbrain from the spinal cord in rats led to the conclusion that immobilization involves primarily suppression of the nocifensive withdrawal reflex arc at the level of the spinal cord ( Fig. 19.4 ).
In the 25 years since the identification of the spinal cord as the site of anesthetic-induced immobility, research has centered on pharmacologic, genetic, and complex network approaches. The conventional pharmacologic approach (“bulk” administration of agonists and antagonists into the CNS) to identify receptor-level contributions to isoflurane-induced immobility (isoflurane being the standard potent ether for experimental purposes) has severe limitations in the complex networks of the CNS. Nevertheless, it yielded at least one surprising insight―that actions at GABA A receptors appear not to be important for the end point of immobility, at least where inhalational agents are concerned. Anesthetic-resistant transgenic mice confirmed that GABA A receptors containing α1- or α3-subunits do not contribute to the immobilizing action of isoflurane. Perhaps less surprisingly, inhibition of central nicotinic acetylcholine receptors also plays no role in immobilization. A role for voltage-gated sodium (Na + ) channels was suggested by the finding that intrathecal administration of a selective inhibitor of Na + channels potentiates anesthetic immobility (reduces MAC), whereas an enhancer of Na + channel activity does the opposite.
In contrast, work in mutant mice suggests a potential role for tandem-pore domain potassium channels (K 2P ) in anesthetic-induced immobility. Global knockout mice lacking the TASK-1, TASK-3, and TREK-1 K 2P channels are less sensitive to volatile but not intravenous anesthetics, indicating a role for these channels possibly by a presynaptic mechanism. An important limitation is that global knockout results almost invariably in wide-ranging compensatory changes in the molecular landscape of the organism with unpredictable consequences for the phenomenon under investigation.
Work with ex vivo preparations that attempt to preserve parts of the complex spinal cord circuitry suggests that anesthetic inhibition of afferent (noxious sensory) input to the dorsal horn plays a subordinate role to the suppression of the efferent (motor) output from the ventral horn, although this may vary by specific agent. This motor output is coordinated by neuronal networks organized in so-called central pattern generators that control the activity of cholinergic motoneurons. Not unlike understanding the anesthetic effects on higher cognitive function, the key to understanding immobility will likely lie in resolving the effect of anesthetics on integrated spinal network activity after understanding the circuit physiology.
Compared with the other end points of anesthesia, research into the biologic basis of anesthetic-induced unconsciousness is relatively recent, but it has become an area of active investigation. Research is being conducted in animal models and human subjects and commercial interests are working to develop effective depth-of-anesthesia monitors. These efforts reflect increasing interest, and progress, in “consciousness science” in general. Moreover, anesthetics are themselves being used as research tools to help unravel the neuronal underpinnings of consciousness.
Loss of consciousness (or hypnosis) is a hallmark of the onset of anesthesia. However, what is commonly referred to as unconsciousness under anesthesia might be more accurately described as unresponsiveness, a condition that could also encompass states of self-awareness without environmental awareness (as in dreaming) or environmental awareness without recall (e.g. conscious amnesia combined with neuromuscular paralysis during induction of anesthesia).
Numerous theories have been advanced to explain anesthetic-induced unconsciousness. They can generally be divided into those that address “bottom-up” changes in the brain stem circuitry that controls arousal, versus “top-down” changes in the thalamocortical circuits that integrate information. Indeed, this distinction formed the basis for a recent suggestion that the level of consciousness reflects bottom-up processes whereas the content of consciousness reflects top-down processes―a notion with intuitive appeal.
One of the most influential theories has been the “integrated information theory of consciousness (IITC)” of Tononi, which emphasizes the need for simultaneous differentiation between brain states and their integration into a coherent whole. Drugs or diseases that suppress consciousness could act through either process. Other information-based approaches use symbolic analysis, transfer entropy, chaos theory, and more. The rich connectivity of the cerebral cortex and its hierarchical organization are especially suited to enable high levels of information integration in the human brain. Some brain areas present a “rich club” organization (i.e., highly connected nodes tend to be preferentially connected to other highly connected nodes), which has been suggested to be optimal for information integration. These hubs are promising targets for the hypnotic action of general anesthetic drugs.
Anesthetics might act by interfering with the operational synchronicity and coherence of corticothalamic networks. Consequent disruption of functional and effective connectivity has been observed during natural slow-wave and midazolam-induced loss of responsiveness. This breakdown of cortical connectivity, rather than pharmacologic deafferentation from the environment, could underlie loss of consciousness. Unconsciousness would then be characterized not by the absence but by the fragmentation of cortical processing.
Although the mechanism of “binding” (i.e., creating the unity of perception) is uncertain, synchronicity of neuronal activity in the 40- to 90-Hz range across functionally connected cortical areas (commonly referred to as 40-Hz- or γ-rhythm) is a viable candidate. Animal and human data implicate activity in the γ-band throughout the cortex as a network-level target of general anesthetics. Anesthetic actions on cortical information processing probably consist not merely of suppression of responses but of reduced complexity and variability reflected counterintuitively in the increased reliability and precision of evoked responses.
A consistent and intriguing observation has been that anesthetics suppress descending more than ascending neural connectivity. Within the framework of predictive coding, this indicates that unconsciousness is associated with a reduction in internally generated predictions more than a suppression of incoming sensory information. The molecular and cellular mechanisms underlying this effect remain undefined, but the preferential suppression by isoflurane of cortico-cortical responses in brain slices in vitro supports a top-down mechanism wherein the anesthetic acts directly on the thalamocortical circuitry.
By contrast, “bottom-up” theories attribute changes in consciousness to anesthetic modulation of subcortical arousal nuclei. An interesting theme has emerged from this line of research. There is substantial overlap between the centers whose activity is altered during natural slow-wave sleep and the state of general anesthesia. That is, many anesthetics may induce unconsciousness, at least in part, by “hijacking” neural sleep or arousal pathways.
Thalamic theories of anesthetic-induced unconsciousness incorporate aspects of both top-down and bottom-up mechanisms, reflecting not only the intermediate position of this structure in the hierarchical organization of the brain but also the different connection patterns of “sensory relay” versus higher-order “nonspecific” thalamic nuclei.
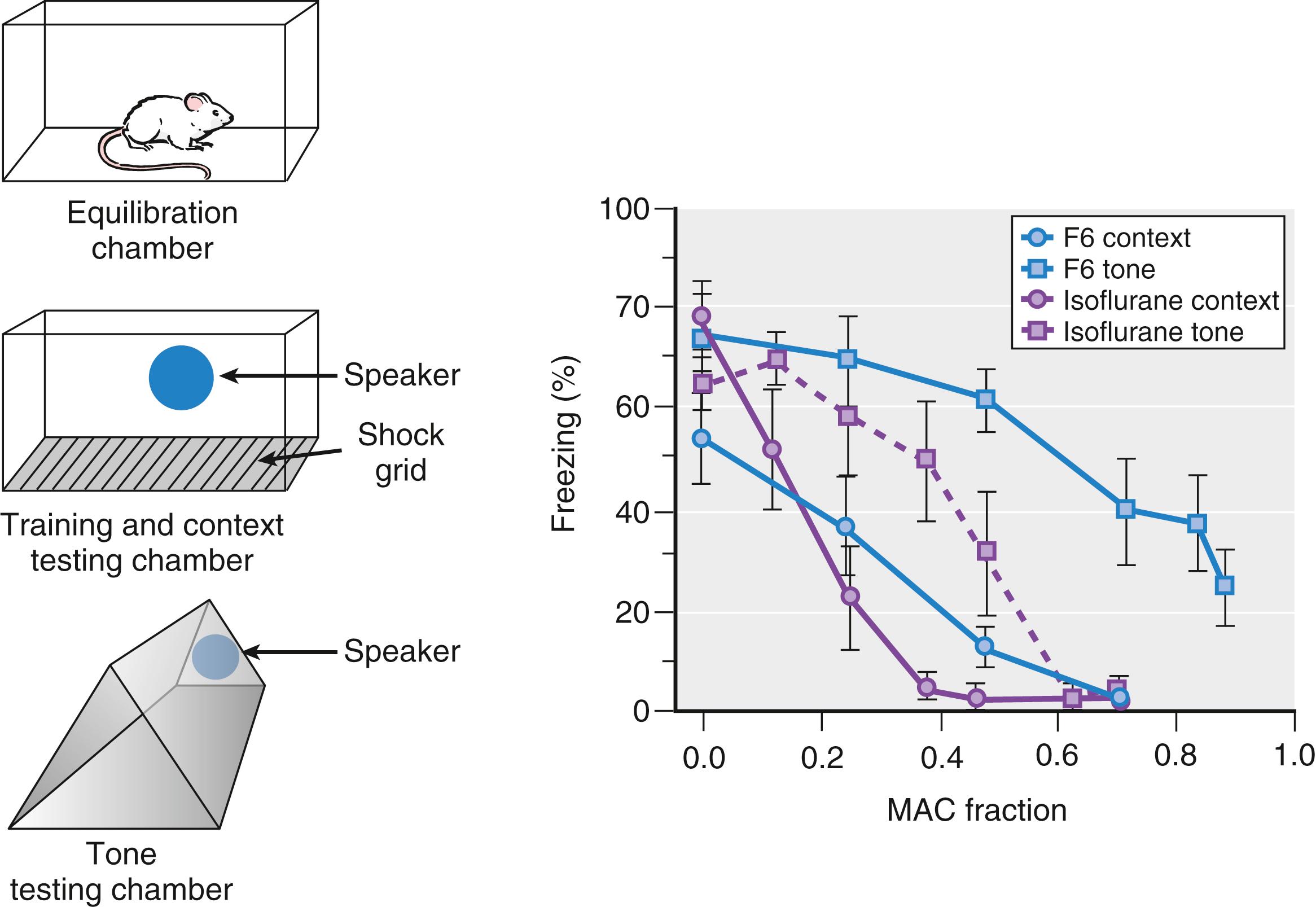
Anterograde amnesia, one of the core desirable anesthetic end points, is achieved at lower anesthetic concentrations (∼0.25 MAC) than those required for unconsciousness (∼0.5 MAC). Perhaps the closest analogue in rodents to explicit memory in humans is medial temporal lobe–dependentlearning of temporal and spatial sequences known as hippocampus-dependent spatial learning . Other learning paradigms, such as fear conditioning to tone, are by contrast independent of the hippocampus. Spatial learning can be tested by a variety of experimental paradigms, including fear conditioning to context ( Fig. 19.5 ). Isoflurane and the nonimmobilizer F6 both inhibit hippocampus-dependent learning at about half the concentration necessary for disrupting hippocampus-independent learning. Similarly, anesthetic concentrations that inhibit explicit memory in humans (memory that can be explicitly recalled as opposed to motor learning, classical conditioning, and so on) are similarly lower than concentrations that impair implicit memory (not subject to willful recollection). Taken together, these findings implicate effects on function of the medial temporal lobe, including the hippocampus, in the suppression of explicit memory by anesthetics. Effects on other structures, such as the amygdala, may be relevant to anesthetic impairment of implicit or other types of memory.
Because inhaled anesthetics affect multiple cellular targets even at amnesic concentrations, it is likely that anesthetic-induced amnesia arises from multiple cellular-level changes. A quantitative comparison of the degree of change in synaptic inhibition in the hippocampus produced by equiamnestic concentrations of isoflurane versus etomidate indicated that the enhancement of GABAergic inhibition can account for a substantial portion of isoflurane’s effect on memory. Other contributing targets may include nAChRs, HCN1 channels, and excitatory glutamatergic synapses, Conversely, it is also likely that suppression of learning and memory by drugs known to have different receptor affinities share common mechanisms at some level of integration. For example, θ-rhythms (4-12 Hz) are clearly important for hippocampus-dependent learning and memory. Benzodiazepines and cannabinoids slow and suppress hippocampal θ-rhythms in proportion to their ability to impair hippocampus-dependent learning. Isoflurane and the nonimmobilizer F6 have comparable effects on θ-rhythms at amnesic concentrations while having different receptor-level profiles and opposite effects on sedation. Thus alterations in neuronal synchrony may provide a common network-level substrate for memory impairment. The synchronization between amygdalar and hippocampal θ-rhythms that occurs during fear memory retrieval indicates that this principle might also apply to other forms of memory and their impairment by anesthetics. As with other components of the anesthetic state, the precise mechanisms of memory impairment by anesthetics and of memory itself remain to be fully elucidated.
Sedation (defined as a decrease in activity, alertness, arousal, and/or vigilance), which is on a behavioral continuum leading to hypnosis, is achieved at anesthetic doses similar to those that produce amnesia (<0.5 MAC). There is no clear mechanistic or clinical separation between sedation and hypnosis. By contrast, even though sedation can be difficult to separate from amnesia, for intravenous anesthetics there may be separate but overlapping substrates for these two end points. The mechanisms involved in these behavioral effects are likely to resemble those of less promiscuous drugs, for which genetic approaches have been informative. An amino acid knockin mutation (H101R) in mice that renders the α 1 GABA A receptor subunit insensitive to modulation by benzodiazepines produces resistance to the sedative and amnesic effects of benzodiazepines while maintaining other behavioral effects, among them anxiolysis. The α 1 subunit is abundantly expressed in the CNS, mainly in the cortical areas and thalamus. Volatile anesthetics have qualitatively similar effects on α 1 -containing GABA A receptors (but also those containing other subunits) at low concentrations. The observation that the nonimmobilizer F6, which is devoid of sedative properties, is amnesic but does not modulate benzodiazepine-sensitive α 1 -containing GABA A receptors, is compatible with a role for α 1 -containing receptors in volatile anesthetic-induced sedation, because few other targets are affected at purely sedative concentrations. Possible targets for the sedative effects of the gaseous anesthetics nitrous oxide and xenon, which do not affect GABA A receptors, include N -methyl-d-aspartate (NMDA) receptor antagonism and K 2P channel activation. Consistent with this distinct pharmacologic profile, nitrous oxide has strikingly different effects from those of benzodiazepines in behavioral tests aimed at evaluating sedation in mice.
Recognizing that there may be more than a superficial similarity between natural sleep and anesthetic-induced sedation and hypnosis, the effects of some anesthetics apparently share natural sleep mechanisms by directly activating discrete sleep-promoting nuclei in the hypothalamus. Indeed, the same neurons, or at least overlapping populations of neurons, that are activated by sleep deprivation are also activated during dexmedetomidine-induced sedation. Electroencephalographic patterns during natural slow-wave sleep and anesthesia show similarities, and recovery from sleep deprivation can occur under propofol and inhalational anesthesia, supporting this concept. Anesthetic effects on other cortical and subcortical structures may also contribute to anesthetic-induced sedation and hypnosis.
Specific criteria have been proposed to evaluate the relevance of the many potential molecular targets of anesthetics. These criteria include the following:
Reversible alteration of target function at clinically relevant concentrations. This criterion requires comparable in vivo and in vitro sensitivities and depends on the anesthetic end point under consideration. For example, targets involved in immobility must be sensitive to anesthetics near MAC, whereas targets mediating amnesia must be affected at a fraction of MAC. Recent evidence for persistent effects of inhaled anesthetics demonstrable in the absence of continued anesthetic exposure is challenging the notion of reversibility for certain effects.
Expression of the target in appropriate anatomic locations to mediate the specific anesthetic end point. For example, immobilization by inhaled agents appears to involve primarily actions in the spinal cord independent of actions in the brain.
Concordant stereoselectivity of anesthetic effects in vivo and on the target in vitro. Without a specific pharmacologic antagonist of anesthesia, correlation between the stereoselective actions of general anesthetics in vivo and in vitro is a useful test of pharmacologic relevance of putative molecular targets. Stereoselectivity data correlating in vivo potency and in vitro receptor actions implicate GABA A receptors as a target for the anesthetic actions of etomidate, pentobarbital, neurosteroid anesthetics, and isoflurane.
Appropriate sensitivity or insensitivity to anesthetic and nonanesthetic compounds. Anesthetic halogenated cyclobutanes together with structural analogs that do not produce anesthesia at concentrations predicted to be anesthetic by the Meyer-Overton correlation (nonimmobilizers) can be used to discriminate relevant volatile anesthetic targets in vitro. For example, the anesthetic F3 (1-chloro-1,2,2-trifluorocyclobutane), but not the structurally similar nonanesthetic F6 (1,2-dichlorohexa fluorocyclobutane), affect GABA A , glycine, AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), kainate, and 5-HT3 receptors; and Na + channels, consistent with possible roles in immobility, whereas both F3 and F6 affect neuronal nicotinic, M1 muscarinic, 5-HT2C, and mGluR5 receptors, indicating that these targets are not involved in immobility. F6 is interesting in that it lacks sedative and immobilizing effects but does possess amnesic effects, hence use of the more accurate term nonimmobilizer , making it a useful pharmacologic tool for discriminating targets for these actions.
Predictable effects of genetic manipulations targeted to putative molecular targets. The effects of targeted deletion of specific molecules implicated as anesthetic targets (knockout mutations) or genetic engineering to introduce specific mutations that modify anesthetic sensitivity (knockin mutations) in model organisms provide powerful approaches to test the roles of putative molecular targets of anesthetic action. This approach has been particularly successful in implicating specific GABA A receptor subtypes in the effects of the GABAergic intravenous anesthetics propofol and etomidate, where single amino acid substitutions in specific receptor subtypes eliminate anesthetic effects both in vitro and in vivo. Targeted mutations of putative anesthetic targets provide a bridge between in vitro observations and whole-animal experiments essential for demonstrating anesthetic end points. The existence of multiple targets and redundancy among ion channel subtypes makes this a more challenging experimental approach for inhaled anesthetics compared with intravenous anesthetics (discussed later).
A convergence of x-ray crystallography, molecular modeling, and structure-function studies indicates that inhaled anesthetics bind in the hydrophobic cavities formed within proteins. The lipophilic (or hydrophobic) nature of these binding sites explains their adherence to the Meyer-Overton correlation. An element of amphiphilicity (possessing both polar and nonpolar characteristics) is also required for effective interaction with these cavities, as indicated by improvements in the Meyer-Overton correlation with more amphipathic solvents (possessing both hydrophobic and hydrophilic properties).
Identifying inhaled anesthetic binding sites on plausible target proteins is difficult because of their low-affinity interactions, the paucity of atomic resolution structures of pharmacologically relevant target proteins, and the lack of specific antagonists. Consequently most anesthetic binding sites have been identified in well-characterized model proteins for which three-dimensional atomic resolution structures―such as luciferase and serum albumin ―are available but are not themselves relevant to anesthesia. These studies indicate that anesthetics bind in pockets with both nonpolar and polar noncovalent chemical interactions. Binding involves weak hydrogen bond interactions with polar amino acid residues and water molecules, nonpolar van der Waals interactions, and a polarizing effect of the amphiphilic binding cavity on the relatively hydrophobic anesthetic molecules. Occupation of a site, or sites, by an anesthetic provides a plausible mechanism for alteration of receptor and ion channel function by selectively binding to a particular conformation (e.g., an open or inactivated state of an ion channel). Studies of glycine, GABA A , and NMDA receptors provide convincing evidence for the existence of anesthetic binding sites in critical neuronal signaling proteins. It is likely that before long, high-resolution crystal structures of these receptors will be determined with inhaled anesthetic bound. However, because anesthetics act by binding only to certain transient conformational states, the relevance of necessarily static crystal structures will have to be assessed with care.
Structural studies using the more accessible prokaryotic homologues of eukaryotic ion channels have provided a powerful tool for the study of anesthetic binding sites in biologically plausible proteins. For example, both propofol and desflurane have been cocrystalized with Gloebacter violaceus , a bacterial homologue of eukaryotic inhibitory ligand-gated ion channels (glycine and GABA A receptors). Both anesthetics bind to a common preexisting site in the upper part of the transmembrane domain between the transmembrane segments of a single subunit ( Fig. 19.6 ). Molecular modeling based on structurally homologous proteins has also been used to identify putative anesthetic binding sites in the transmembrane domains of vertebrate GABA A and glycine receptors ( Fig. 19.7 ). These models suggest that different drugs may either bind in different orientations within a single amphiphilic cavity or occupy different cavities within the protein, causing similar functional effects. Refinement of these molecular models will continue to provide new insights in the molecular basis for general anesthetic action that can be experimentally tested. For example, potential sites of interaction of xenon and isoflurane with the NMDA receptor have also been identified using this approach. One site, which can contain up to three xenon atoms or one molecule of isoflurane, overlaps the known binding site for the coagonist glycine in the NR1 subunit. This suggests that two chemically dissimilar inhaled anesthetics inhibit NMDA receptors by direct competitive inhibition of coagonist binding.
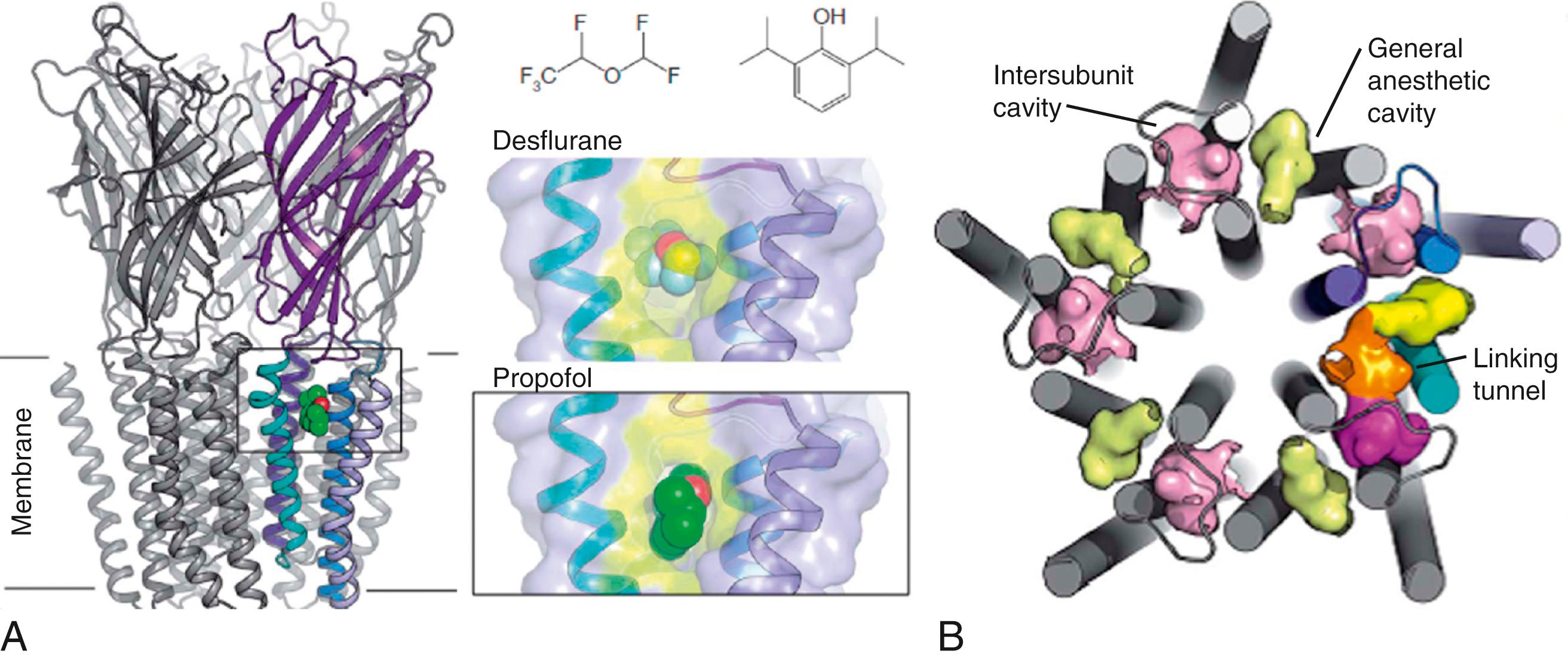
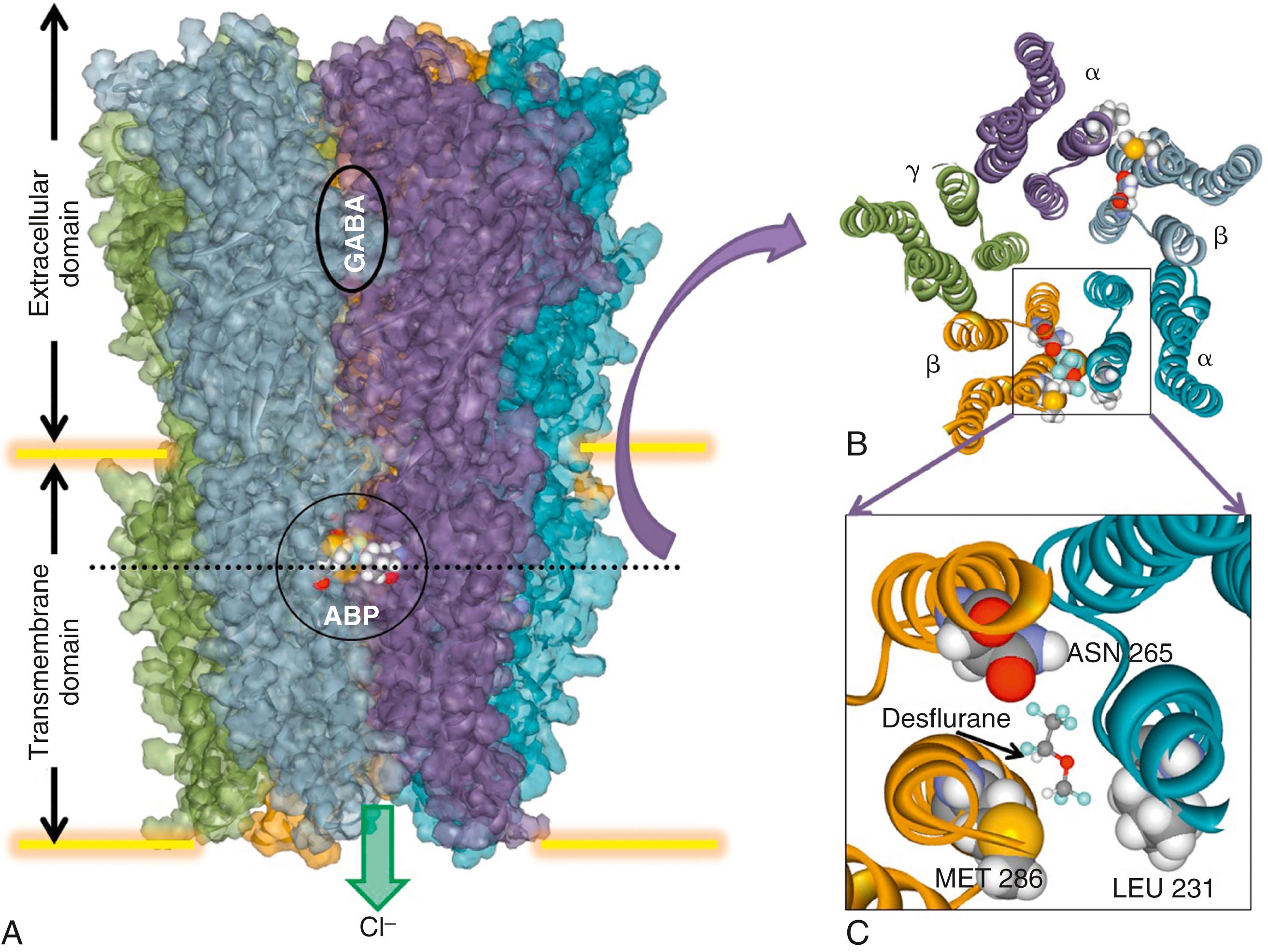
Ion channels have emerged as the most promising molecular targets for inhaled anesthetics. Neurotransmitter-gated ion channels―in particular GABA A , glycine, and NMDA-type glutamate receptors―are leading candidates owing to their appropriate CNS distributions, essential physiologic roles in inhibitory and excitatory synaptic transmission, and sensitivities to clinically relevant concentrations of anesthetics. Other ion channels that are sensitive to inhaled anesthetics include the hyperpolarization-activated cyclic nucleotide (HCN)-gated family of channels that give rise to pacemaker currents and regulate dendritic excitability, two-pore domain (K 2P ) “leak” K + channels that maintain resting membrane potential in many cells, and voltage-gated Na + and Ca 2+ channels.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here