Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Inflammatory disorders of the esophagus are extremely common. Among the most common is gastroesophageal reflux disease (GERD), a chronic condition, affecting as many as 40% of people in the Western world. Because of the ease and frequent use of upper endoscopy for diagnosing gastrointestinal (GI) illnesses, biopsy specimens procured from esophageal mucosa to evaluate the presence or absence of inflammatory diseases—particularly GERD and its attendant complication, Barrett’s esophagus (BE)—are commonly encountered by surgical pathologists. This chapter focuses on myriad inflammatory conditions that affect the esophagus, beginning with GERD.
GERD is an extremely common chronic condition, particularly in Western countries. Estimates based on an assumption that reflux-like symptoms are an indicator of the disease suggest a prevalence rate of 20% to 40%. , GERD is associated with considerable healthcare costs; in the United States in 2015 esophageal disorders accounted for $18.1 billion in health care expenditures, including $12.4 billion for acid-inhibition therapies. Clinically, GERD is often classified as erosive or nonerosive based on endoscopic or pathological features, and currently includes the concepts of proven and unproven GERD in clinical decision-making. One recent review defines GERD as “a family of syndromes attributable to, or exacerbated by, gastroesophageal reflux, evident symptomatically, endoscopically, or by physiological testing, which impart morbidity through troublesome symptoms and/or risk.” Using this definition, GERD phenotypes emerge including nonerosive reflux disease, GERD hypersensitivity, low- or high-grade esophagitis, Barrett’s esophagus, reflux chest pain syndrome, laryngopharyngeal reflux, and regurgitation dominant reflux. Risk factors associated with GERD include advanced age (especially after age 40 years), certain lifestyle habits (e.g., alcohol consumption), body mass index, and tobacco smoking, although the clinically relevant contributions of many of these factors are not entirely clear. Compared to females, males are at increased risk for erosive esophagitis, Barrett’s esophagus, and especially esophageal adenocarcinoma. The symptoms most often associated with GERD are heartburn, acid regurgitation, and dysphagia. Reflux symptoms are common in runners. Atypical or supraesophageal symptoms include asthma, chronic cough, chronic sore throat, pharyngitis, laryngitis, a globus sensation, and noncardiac chest pain. The clinical and pathogenetic aspects of GERD are discussed further in the section on BE.
In the traditional view, GERD may also be defined as “a condition in which a normal physiological event is affected by an imbalance between aggressive and defensive factors.” Aggressive factors include hiatus hernia, found in patients who have moderate to marked GERD; transient relaxation of the lower esophageal sphincter in GERD patients who do not have hiatus hernia; acid; pepsin; bile acids; alcohol; and acidic foods. Defensive factors include lower and upper esophageal sphincters, esophageal peristalsis, and restoration of normal esophageal pH following a reflux episode by salivary bicarbonate secretion, impaired in patients who have xerostomia or Sjogren’s syndrome. Expressed as the “burn hypothesis,” reflux esophagitis is caused by reflux of gastric or duodenal fluid into the esophagus, and refluxed gastric acid, bile, pepsin, and duodenal contents cause injury to the esophageal mucosa. Esophageal epithelial injury is greater if pepsin is present in addition to acid in refluxate. With ongoing reflux injury, surface esophageal cells die, triggering both an inflammatory response (infiltration of neutrophils) and a proliferative response (basal cell and papillary hyperplasia). The cause of reflux is multifactorial. Contributing factors in addition to those already stated above include delayed gastric emptying, increased gastric acid production, and bile reflux. , , Although the data have been somewhat conflicting, there is some evidence to support an association among high body mass index, the presence of hiatal hernia, and GERD. , Likewise, there is evidence to suggest that Helicobacter pylori infection may actually protect against the development of GERD and its complications. , , Specifically, corpus gastritis is associated with decreased acid secretion, and GERD is less common in patients with severe corpus gastritis. , When acid secretion returns to normal after the eradication of H. pylori , there is an increased risk of developing GERD. ,
Recent studies of the pathogenesis of GERD in animal models , , and in vitro indicate that exposing esophageal squamous epithelial cells to acids and bile salts alone can cause epithelial cells to secrete inflammatory cytokines (i.e., interleukin 8 [IL8] and IL1β) that cause inflammatory cells (T-lymphocytes and neutrophils) to migrate into the epithelium. This finding has led to the new inflammation hypothesis asserting that the inflammatory response, not the direct effect of acid, is the initial factor responsible for damaging esophageal mucosa. Evidence supporting the role of inflammation in GERD includes antiinflammatory symptoms reduction, reported by many patients affected by GERD, after commencing proton pump inhibitors (PPI) therapy, because PPI recently have been shown to have antiinflammatory effects on esophageal epithelium. Histological examination in animal models has demonstrated that infiltration of the submucosa by lymphocytes is an early manifestation of inflammation. Neutrophils involve the mucosal surface later in the course of disease progression. These findings provide further support for an alternative concept for the development of reflux esophagitis in humans.
The diagnosis of GERD has rested on the clinical impression of symptoms consistent with pathological reflux and the demonstration of excessive acid reflux. However, the association of symptoms with reflux is generally weak, and the use of additional modalities may add to the diagnostic accuracy. A schema to increase diagnostic accuracy for GERD is shown in Table 14.1 .
| Endoscopy | Ph or Ph-Impedance | High-Resolution Manometry |
|---|---|---|
| Conclusive Evidence for Pathological Reflux | ||
|
|
|
| Borderline or Inconclusive Evidence | ||
|
|
|
| Adjunctive or Supportive Evidence ∗ | ||
|
|
|
| Evidence Against Reflux | ||
|
||
∗ Increases confidence for pathological reflux if other evidence is borderline or inconclusive.
The role of esophageal biopsy in the diagnosis of GERD is debated. In the schema in Table 14.1 , for example, histomorphology is considered adjunctive, to be obtained only in cases not confirmed endoscopically or by pH or pH impedance. Other strategies suggest more liberal use of biopsy including to rule out eosinophilic esophagitis (see Eosinophilic Esophagitis section). However, a recent study concluded that in patients who have refractory GERD symptoms but do not have dysphagia, the prevalence of eosinophilic esophagitis is so low that obtaining biopsies may be considered unnecessary.
On endoscopic examination, patients with erosive esophagitis exhibit erosions, ulcers, strictures, or some combination of these. However, as many as 50% to 60% of all symptomatic patients with objective evidence of GERD have normal mucosa or only mild hyperemia at endoscopy. Furthermore, histologically inflamed esophageal mucosa (esophagitis) may appear normal endoscopically; and conversely, hyperemia does not necessarily indicate the presence of esophagitis microscopically. Because of these endoscopic/pathological discrepancies, biopsies are always warranted in symptomatic patients to document the presence of tissue injury and to exclude other entities such as infections, eosinophilic esophagitis (EoE) especially in patients who have dysphagia, BE, and other preneoplastic or inflammatory alterations. This approach is particularly important if empiric reflux therapy has failed.
Biopsies of grossly visible lesions in GERD characteristically reveal evidence of “active” esophagitis, a nonspecific injury pattern that can result from a variety of causes. Pinch biopsies obtained through standard endoscopes are often not adequate for evaluation of early histological changes resulting from reflux because they usually do not include the entire thickness of the mucosa and are difficult to orient. , Endoscopes with a large-caliber biopsy channel and jumbo biopsy forceps should be used to facilitate accurate histological diagnosis. Use of jumbo forceps does not increase the rate of complications related to endoscopy ; rather, it greatly improves the quality of the histological specimens.
Histologically, reflux changes are typically distributed over the distal 8 to 10 cm of the esophagus in a patchy fashion, and multiple biopsies are often necessary to consistently demonstrate histological abnormalities. However, changes are typically worse in the distal esophagus and decrease in intensity more proximally. Because biopsy specimens from the lower 1 to 2 cm of the esophagus, even in asymptomatic subjects, often reveal evidence of mild squamous hyperplasia, , diagnostic biopsy specimens should be obtained usually more than 2.0 cm above the level of the gastroesophageal junction (GEJ) to diagnose esophagitis reliably, and submitted specimens should state the esophageal site from which the biopsies were obtained. Furthermore, because of a high level of discordance between endoscopic and histological findings, it is recommended that all symptomatic patients undergo biopsy, regardless of the presence or absence of endoscopic abnormalities. Esophageal biopsies are considered more useful for establishing a diagnosis of GERD in infants than in adults.
Reflux esophagitis produces a characteristic, although nonspecific, tissue injury pattern. Features of untreated active esophagitis include basal cell hyperplasia, elongation of the lamina propria papillae into more than two thirds of the thickness of the mucosa, epithelial cell necrosis, increased intraepithelial inflammation (including eosinophils, neutrophils, and lymphocytes), lack of surface maturation (nucleated cells at surface of epithelium), distended pale squamous “balloon” cells, intercellular edema (acantholysis), and, in severe cases, surface erosions or ulcerations ( Figs. 14.1 to 14.3 ). Intercellular edema or dilated intercellular spaces reflecting increased paracellular permeability may be a useful marker of early injury in the absence of endoscopic evidence of injury. , Scoring several histological features of esophageal biopsies (elongated papillae, basal zone hyperplasia, dilated intercellular spaces, intraepithelial inflammatory cells, necrosis, and erosions) may be useful to distinguish nonerosive reflux disease biopsies from reflux hypersensitivity, functional heartburn, and control biopsies. , ,
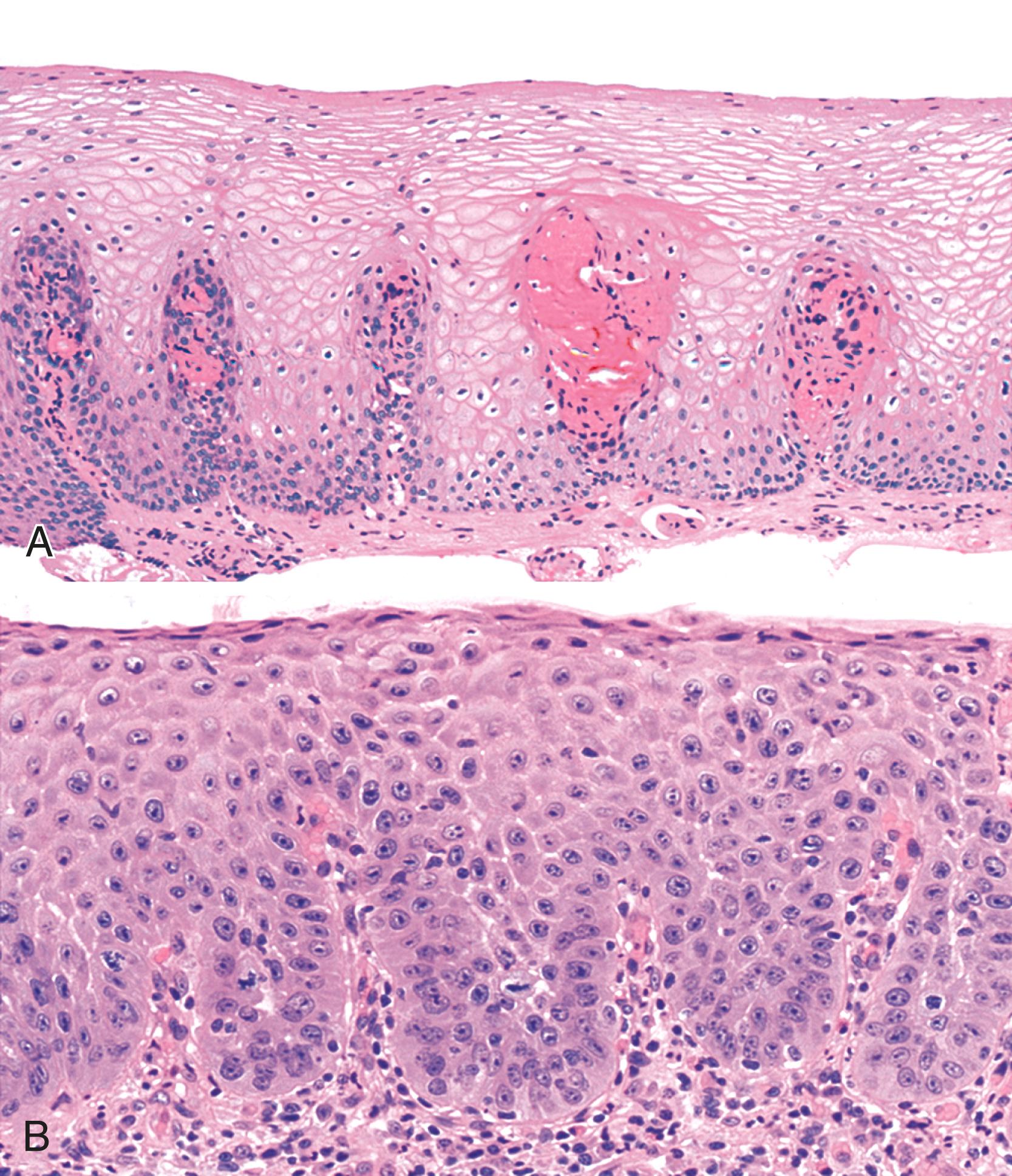
For many years, it was believed that the only true diagnostic criterion for esophagitis was the presence of intraepithelial inflammation. However, in 1970, Ismail-Beigi and co-workers found that some patients who had clinical symptoms of GERD but a normal or only minimally abnormal endoscopic appearance showed hyperplasia of the squamous epithelium, a finding that they postulated was an early histological manifestation of reflux-induced injury. Squamous hyperplasia was defined by (1) lengthening of the subepithelial lamina propria to more than two thirds of the thickness of the squamous epithelium and (2) expansion of the basal zone of the squamous epithelium to more than 15% of the thickness of the epithelium (see Fig. 14.1 ). Subsequent studies confirmed these initial observations and also demonstrated a significant positive correlation between the severity of reflux, as measured by 24-hour pH score (a composite quantitative evaluation of acid reflux) and the length of the lamina propria papillae. These histological features also occur in infants with GERD, but with poor correlations between pH score severity and histological severity. Increased mitoses, slight enlargement of basal and suprabasal nuclei, and prominent nucleoli and hyperchromatism are features often associated with true basal cell hyperplasia. True basal cell hyperplasia is best evaluated in well-oriented tissue sections that include at least three consecutive papillae cut longitudinally. Unfortunately, this orientation is rarely achieved in small pinch biopsy samples. Therefore caution should be taken not to overinterpret “mild” changes as evidence in favor of esophagitis.
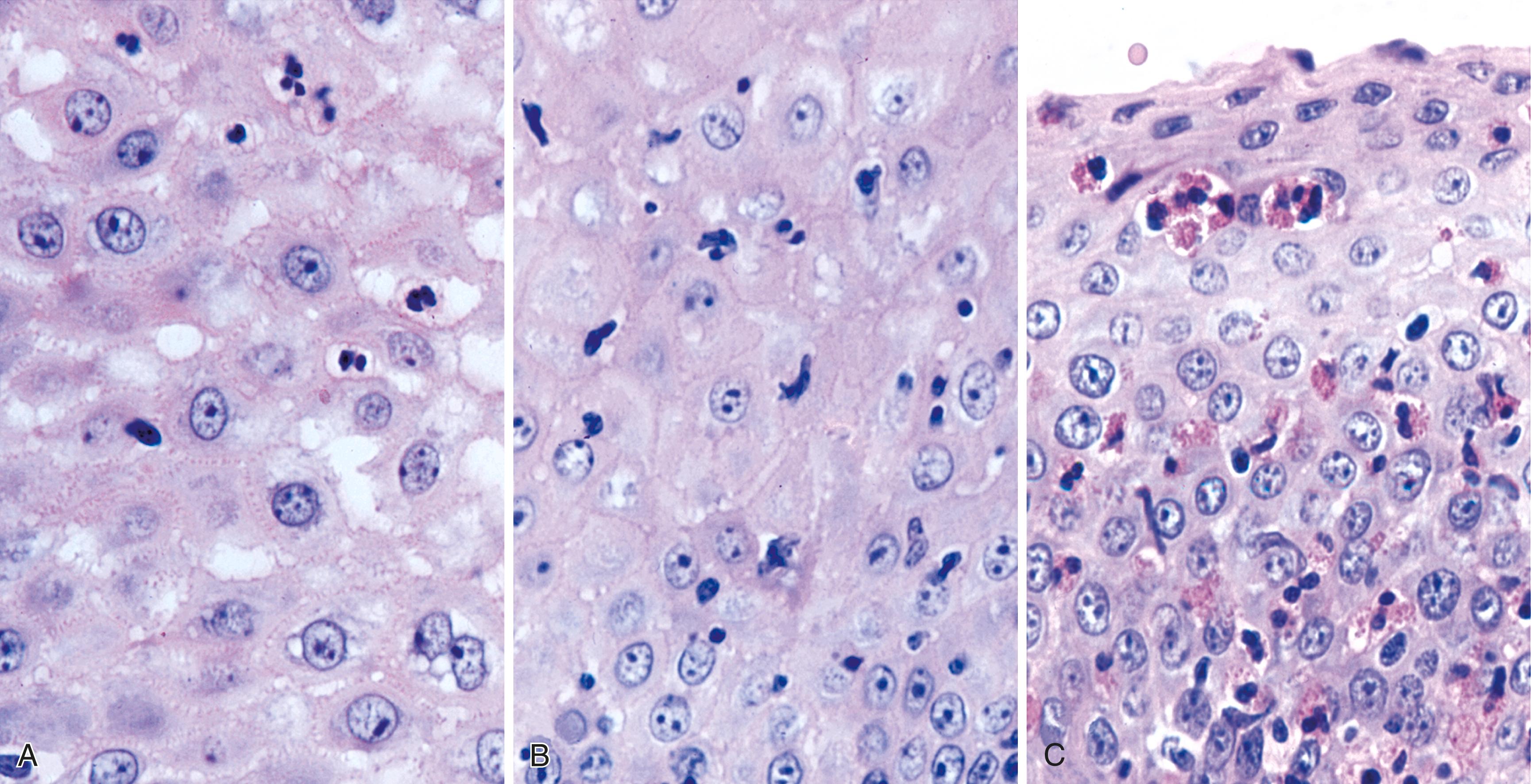
The principal inflammatory cells in patients with reflux esophagitis include neutrophils, eosinophils, and lymphocytes (see Fig. 14.2 ). However, depending on the phase of disease and the treatment status, none, one, or all of these inflammatory cells may be present in a single biopsy specimen. Therefore, a diagnosis of (reflux) esophagitis can be established in the absence of inflammation if the basal cell and lamina propria papillae changes are present, particularly in a patient who has begun treatment with antireflux agents.
Within the esophageal squamous epithelium, intraepithelial neutrophils always indicate a pathological process. However, intraepithelial neutrophils are not a sensitive indicator of reflux esophagitis, because they are present in fewer than 30% of GERD patients with documented reflux. They also are not specific for GERD. Therefore, the presence of a significant number of neutrophils, particularly in association with a surface erosion or ulcer, should prompt the pathologist to search for a viral or fungal ( Candida ) infection.
Increased numbers of eosinophils often are present in patients with esophagitis. However, because rare, isolated eosinophils may be found in the mucosa of normal adults, particularly in the distal 1 to 2 cm of the esophagus, and of normal children, they are not considered diagnostic of esophagitis if sparse in number and not associated with other features of esophagitis, such as those discussed previously. Eosinophils in the lamina propria are considered an even more sensitive indicator of GERD in infants.
Occasionally, large numbers of eosinophils are present in esophageal biopsy specimens of adult patients with putative reflux. , In this circumstance, causes of esophageal eosinophilia other than reflux, such as primary EoE, drug reaction (including Stevens-Johnson syndrome), pill-induced esophagitis, collagen vascular disease, and, very rarely, parasitic infection, should be excluded. Children with apparent GERD who have prominent eosinophilia may improve clinically when given an elemental diet, which suggests that certain food sensitivities may lead to reflux-like symptoms , (see Primary Eosinophilic Esophagitis).
Lymphocytes are considered a normal intraepithelial component of the esophageal squamous mucosa. , , These are largely T lymphocytes and have either a round or an irregular nuclear contour, particularly when deformed by adjacent squamous epithelial cells. Because of their “squiggly” appearance, intraepithelial lymphocytes may resemble granulocytes. These cells have been identified as CD8+ and TIA-1+ T lymphocytes and tend to be more prominent in the peripapillary epithelium. Normal esophageal mucosa contains roughly 10 to 12 lymphocytes per high-power field (HPF). , Although lymphocytes are present in increased numbers in patients with GERD, this finding in isolation has no independent diagnostic significance, because normal control subjects may also have increased numbers. In addition, other disorders, such as achalasia and Crohn’s disease, may be associated with increased numbers of intraepithelial lymphocytes. ,
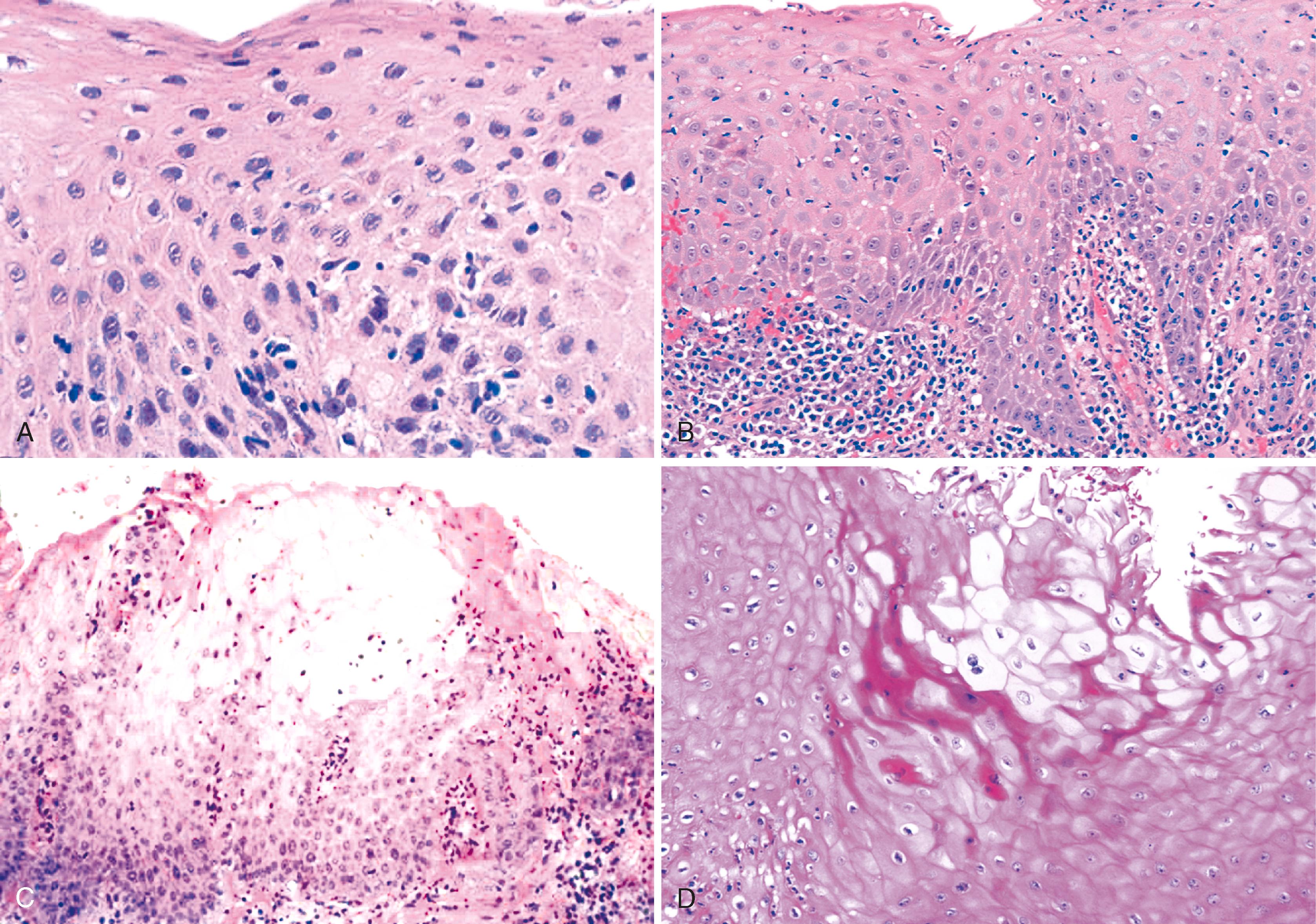
Dilation and congestion of lamina propria capillaries are additional characteristic features of reflux esophagitis, but this finding may also occur in specimens from normal controls, albeit in a mild fashion, possibly as a traumatic biopsy artefact. Other features that may be seen in GERD include ballooning degeneration of squamous cells, intercellular edema (acantholysis) that causes minor separation of individual squamous cells, multinucleation of squamous cells, increased mitoses, and decreased surface maturation. The presence of multinucleated (regenerative) cells may mimic herpetic esophagitis ( Fig. 14.4 ). Multinucleated regenerative squamous cells are distinguished from virally infected cells by the lack of characteristic nuclear inclusions and by their prominence at the base of the mucosa and adjacent to ulcers, rather than in the surface cells, which is characteristic of herpes.
The differential diagnosis of GERD includes infectious esophagitis, pill esophagitis, esophagitis caused by ingestion of corrosive agents such as lye, chemotherapy- and radiation-induced esophagitis, primary EoE, trauma, and systemic diseases such as collagen vascular disease, Crohn’s disease, Stevens-Johnson syndrome, various bullous diseases, lichen planus, and graft-versus-host disease. Many of these conditions share overlapping morphological features with GERD. Therefore, an accurate diagnosis of “reflux” esophagitis requires correlation with the patient’s clinical, endoscopic, manometric, and histological data. In the absence of clinical information, a diagnosis of reflux esophagitis cannot be established on the basis of biopsy findings alone. The histological features are essentially nonspecific. In fact, in this scenario, a “top line” diagnosis of “active esophagitis consistent with reflux” rather than “reflux esophagitis” should be used.
On occasion, marked reactive (pseudoepitheliomatous) hyperplasia related to reflux esophagitis may resemble squamous dysplasia histologically ( Fig. 14.5 ). The most helpful distinguishing feature is the presence of cytoarchitectural uniformity in cases of hyperplasia, compared with cytoarchitectural pleomorphism in cases of dysplasia or carcinoma.
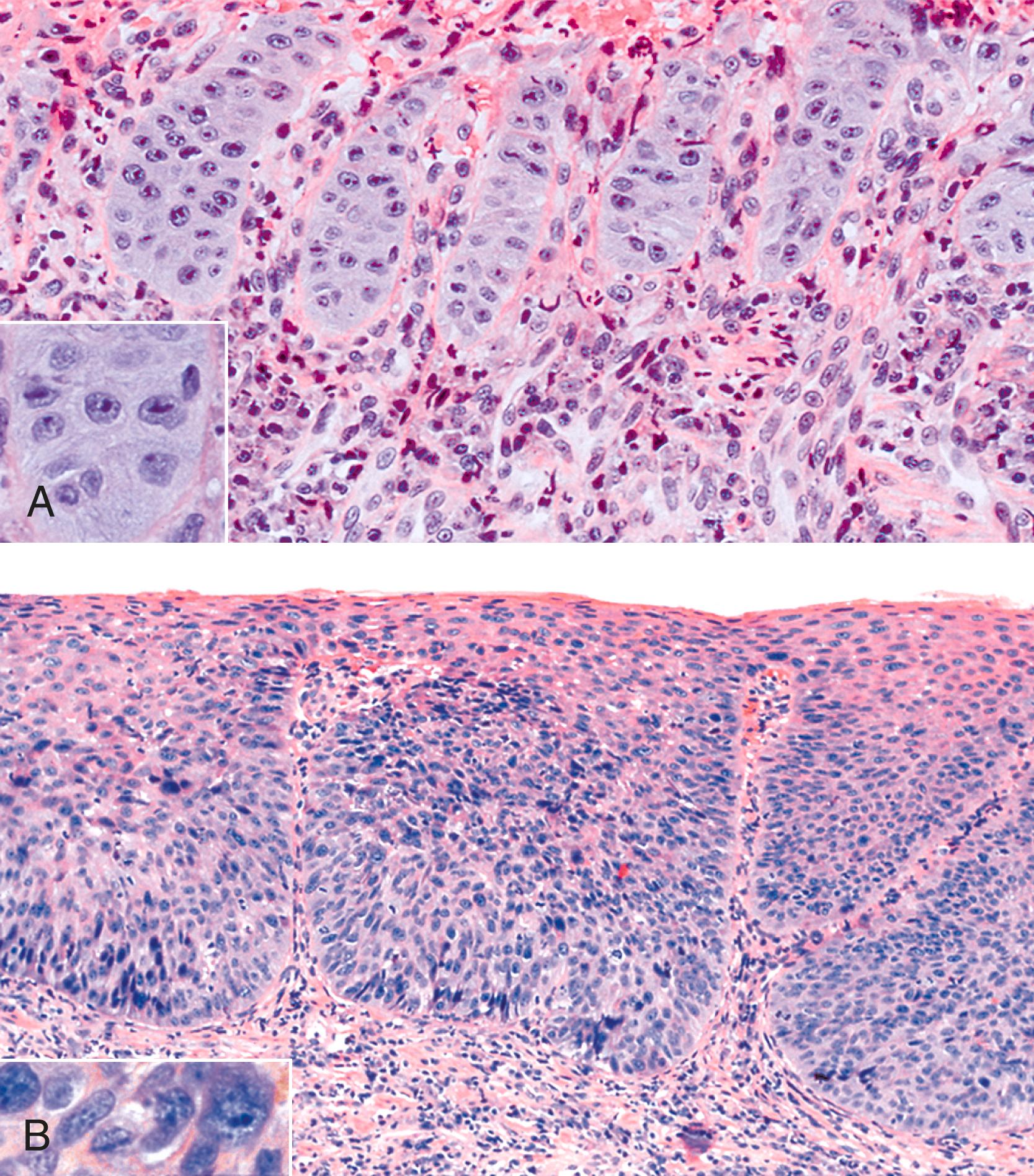
The mucosal architecture in hyperplastic lesions retains its uniformity, showing elongation of papillae that extend to roughly equal depths within the deep lamina propria and are usually of similar width. In contrast, dysplastic squamous epithelium typically displays architectural distortion with absent, sharply angulated, or markedly irregular papillae in terms of their length and width ( Table 14.2 ; see also Chapter 24 for details).
| Feature | Hyperplasia | Dysplasia |
|---|---|---|
| Papillae | Regular | Absent or irregular |
| Nuclear enlargement | ++ | +→+++ |
| Nuclear pleomorphism | +/− | +→+++ |
| Nuclear overlapping | − | +→+++ |
| Nuclear hyperchromasia | +/− | +→+++ |
| Nuclear membrane | Smooth | Irregular |
Cytologically, hyperplastic squamous epithelial cells are uniform and do not show loss of polarity or overlapping nuclei. The nuclei may be uniformly enlarged; however, they have smooth nuclear membranes, open chromatin, often prominent nucleoli, and increased mitoses but no atypical mitoses. Dysplastic squamous epithelial cells are typically more pleomorphic in size and shape, are more hyperchromatic, have irregular nuclear contours, and reveal nuclear overlapping and loss of polarity (see Fig. 14.5 ).
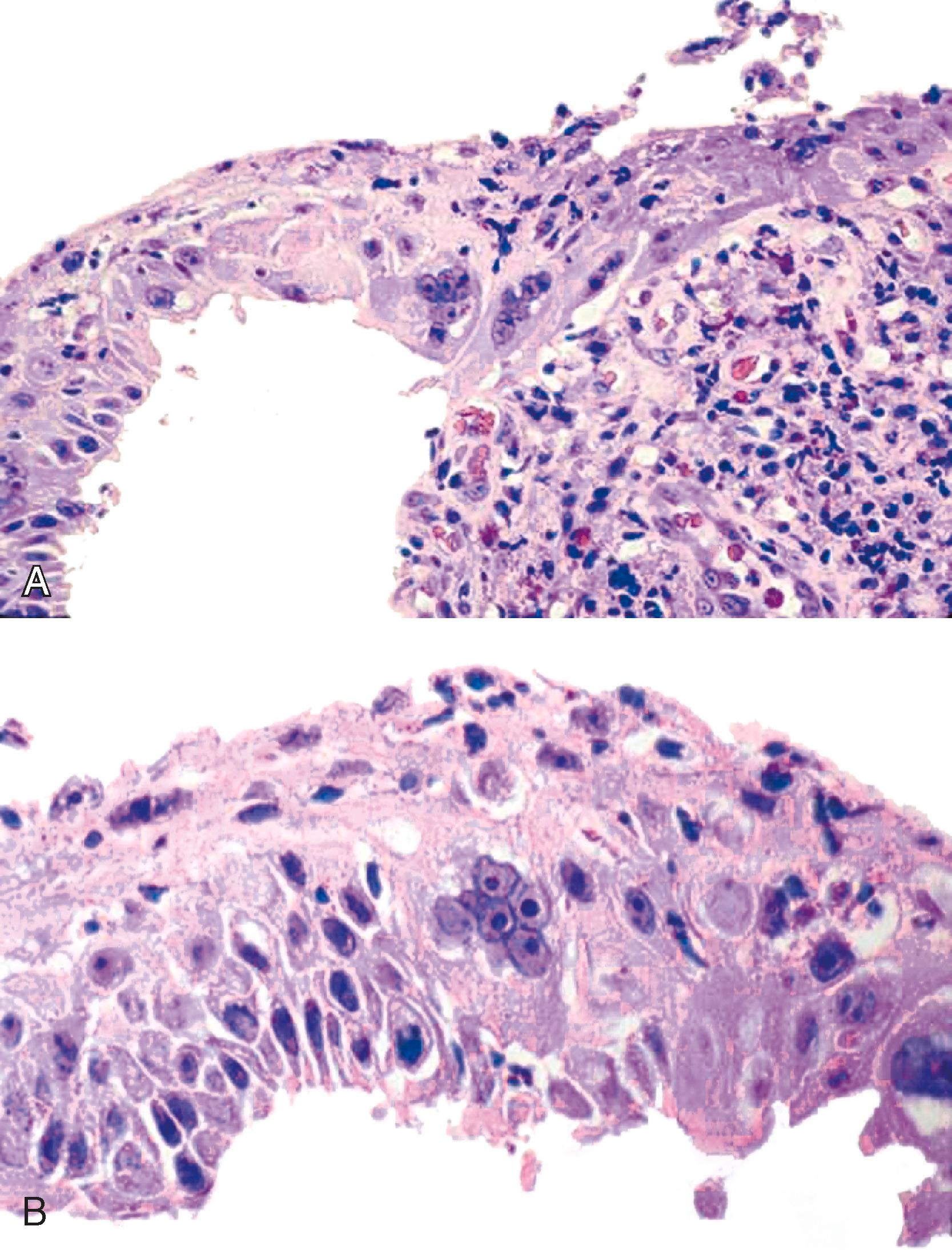
Granulation tissue within the base of erosions or ulcers may exhibit large atypical endothelial cells and fibroblasts , ( Fig. 14.6 ). They are usually distributed in a scattered fashion within otherwise typical granulation tissue. These cells do not form solid clusters of cells and have a normal, or even decreased, nucleus-to-cytoplasm (N:C) ratio. In contrast, carcinoma usually demonstrates groups or sheets of cohesive cells with overlapping nuclei and an increased N:C ratio. In difficult cases, immunohistochemistry for cytokeratins can be helpful in differentiating true carcinoma (positive) from reactive “pseudosarcomatous” alterations of the stromal cells (negative).
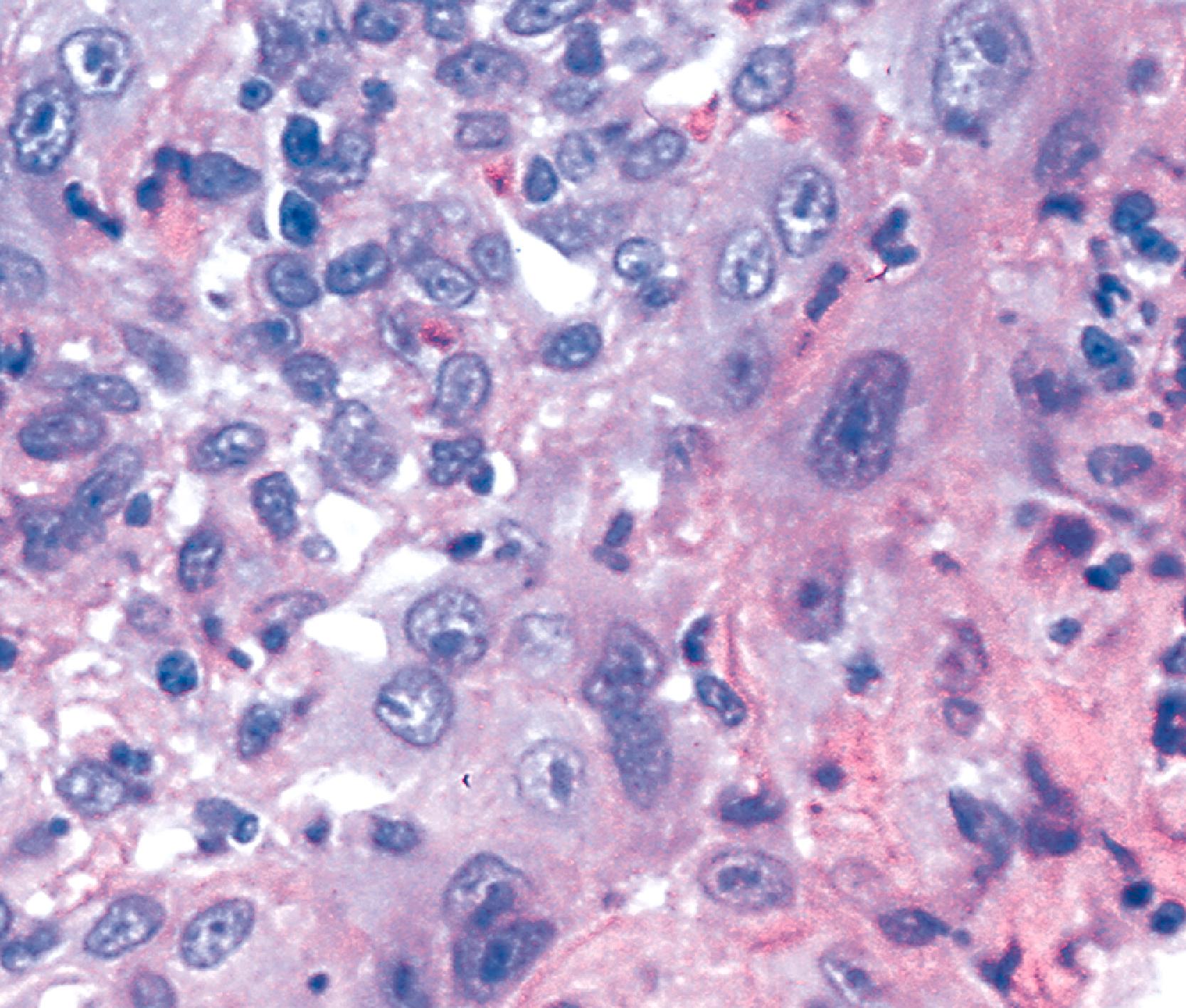
Rarely, the inflammatory exudate within the ulcer or erosion may contain abundant activated and atypical lymphocytes that can simulate lymphoma ( Fig. 14.7 ). In general, these cells are benign when confined to the surface exudate. The infiltrate should raise concern for a lymphoma if it involves the underlying tissue in a dense, confluent, and homogeneous manner.

The natural history of GERD in the general population remains uncertain because of the widespread use of acid inhibitors. GERD has multiple phenotypic presentations, and nonerosive reflux disease (NERD), erosive esophagitis, and BE may form a continuum. Most patients with GERD (50% to 70%) have normal mucosa on endoscopy and therefore are diagnosed with NERD. Some researchers have proposed that NERD is a discrete entity because of its unique physiologic characteristics, which include a more competent antireflux barrier. However, most authorities believe that GERD is a progressive disease that starts with NERD and progresses with time, to erosive esophagitis or BE or both, in selected high-risk individuals. Some data suggest that progression from NERD to erosive esophagitis occurs in as much as 30% of patients annually, but it is unknown whether NERD can progress directly to BE without an erosive phase. Between 1% and 22% of patients with erosive esophagitis progress to a more severe form of disease, whereas regression to a less severe form of disease occurs in 6% to 42% of patients, depending on whether acid inhibitors have been used. Erosive esophagitis is a risk factor for BE. , Barrett’s esophagus develops in between 1% to 13% of patients with erosive esophagitis annually. In a recent prospective, follow-up endoscopic study to evaluate the risk of BE in a Swedish general population (the Kalixanda study database), the incidence of BE was 9.9 per 1000 person-years, and the prevalence of BE in this GERD cohort increased from 3% to 8% during a 5-year follow-up period. In fact, progression of GERD to BE in the general population may be higher than previously recognized. , Interestingly, patients who have GERD have reduced risk of developing microscopic colitis, ulcerative colitis, or Crohn’s disease, especially GERD patients who have erosive esophagitis or Barrett’s metaplasia. The vast majority of patients with GERD have a recurrent but nonprogressive form of disease that is controlled adequately with acid inhibitor therapy.
Current medical forms of therapy, such as PPI and other acid inhibitors, are highly effective at relieving symptoms and repeat biopsies may show histological improvement or resolution of pre-therapy pathology. However, progressive disease develops in some patients despite ongoing therapy. In one study, as many as 82% of patients who showed healing of their esophagitis with omeprazole relapsed within 6 months after cessation of therapy. In a 10-year follow-up study of 101 patients with reflux esophagitis, significant morbidity related to GERD developed in almost 75%, showing quality of life scores significantly lower than those of a non–GERD control population. Surgical treatment is considered an option for patients with chronic GERD who are not responsive to medical therapy or have a defective LES. , Esophageal biopsy is indicated for patients who become refractory to therapy or who develop new symptoms to evaluate for emergence of complications such as neoplasms.
Although PPI are the current mainstay for GERD treatment, approximately 30% of GERD patients continue to experience symptoms while receiving once daily label-dose PPI. Use of a bile sequestrant along with a daily label-dose PPI significantly reduced heartburn symptoms compared to a placebo, and regurgitation symptoms were also reduced. A US expert panel of foregut surgeons and therapeutic gastroenterologists recently agreed that surgery was a viable option for GERD patients for whom PPI discontinuation becomes necessary or who want to discontinue PPI therapy. Laparoscopic fundoplication and magnetic sphincter augmentation were considered acceptable options for such patients, and transoral incisionless fundoplication was considered an option for such patients who do not have a hiatal hernia. For PPI-nonresponders, laparoscopic fundoplication was recommended as an appropriate surgical option if a hiatal hernia was present. Magnetic sphincter augmentation was considered appropriate for PPI-nonresponders with regurgitation-predominant disease who had or did not have a hiatal hernia, and transoral incisionless fundoplication was appropriate for PPI-nonresponders who did not have a clinically significant hiatal hernia. Laparoscopic fundoplication and magnetic sphincter augmentation were not considered appropriate surgical options for PPI-nonresponders whose impedance-pH study was negative.
Infections remain an important cause of esophagitis and can have significant implications in the immunocompromised host. Viruses and fungi cause most forms of acute infectious esophagitis. Bacterial esophagitis occurs in some patients with systemic or upper respiratory infection, but this condition is rarely sampled histologically.
Herpes esophagitis occurs primarily in immunosuppressed patients. Common causes of immunosuppression include prior chemotherapy, solid organ and bone marrow transplantation, and acquired immunodeficiency syndrome (AIDS). Herpes esophagitis also may occur in otherwise healthy young adults with normal immune function, who usually have a self-limited infection that resolves within 1 to 2 weeks. In a recent analysis of 46 patients with herpes esophagitis, 72% were immunocompromised and those with longer symptom duration were more likely to require treatment extension; in addition, 37% of patients had underlying esophageal disease and were more likely to have concomitant Candida esophagitis. Symptoms of herpetic esophagitis include odynophagia, dysphagia, epigastric pain, fever, and upper GI bleeding, but some patients are asymptomatic. Coexistent herpes labialis and oropharyngeal ulcers are seen in approximately one fourth of patients. , Herpetic ulcers in the esophagus may serve as a portal of entry for other pathogens and can be associated with herpetic pneumonitis. Endoscopically, herpetic ulcers are typically shallow, sharply punched-out lesions and are often surrounded by relatively normal-appearing mucosa.
Herpes simplex or varicella-zoster virus infects esophageal squamous epithelium. Accordingly, the characteristic inclusion bodies are limited to the squamous epithelial cells, typically accentuated at the superficial lateral margin of ulcers and erosions. In fact, biopsy specimens obtained distant from the immediate edge of the ulcer lesion may not be diagnostic. Microscopic diagnostic criteria include the presence of Cowdry A intranuclear viral inclusion bodies, ground-glass nuclei, nuclear molding, multinucleated giant cells, and ballooning degeneration of infected cells ( Fig. 14.8 ). , Cowdry A inclusions are eosinophilic to amphophilic round bodies separated by a clear zone from a thickened nuclear membrane. Ground-glass nuclei have a smooth, homogeneous chromatin pattern with a pale basophilic quality. Multinucleated giant cell changes in squamous epithelial cells may occur in reflux esophagitis and should not be confused with the cytopathic effects of herpesvirus infection. Reactive cells have prominent nucleoli and perinucleolar clearing, but nuclear inclusion bodies are not present. Large numbers of mononuclear cells, primarily aggregates of macrophages with convoluted nuclei, in the surface exudate adjacent to the infected epithelium have been noted as a characteristic finding in herpetic ulcers and should make the pathologist suspect herpesvirus infection. However, similar cells may be observed in nonherpetic ulcers throughout the GI tract in the absence of herpesvirus infection, so this finding is not specific. Herpes simplex type I is the most common cause of herpetic esophagitis, but on morphological grounds, this type cannot be distinguished from herpes simplex type II or varicella-zoster. Immunohistochemical staining and in situ hybridization, if clinically indicated, can help distinguish these three viral species.

On occasion, herpetic esophagitis may be difficult to distinguish from squamous dysplasia/carcinoma ( Table 14.3 ). The nuclear inclusions characteristic of herpetic esophagitis may be mistaken for macronucleoli typical of malignant cells. However, in herpes esophagitis, one usually sees a halo located between the nuclear inclusion and the nuclear membrane. In addition, the ground-glass chromatin pattern that is characteristic of virally infected cells shows little structure and is pale, in contrast to the variably granulated and darkly stained chromatin typical of malignant cells. Radiation-induced esophagitis also may be mistaken for herpetic esophagitis, because radiation can result in the formation of enlarged squamous cells with multiple nuclei, nucleoli, and pale chromatin. However, careful attention to the presence or absence of characteristic Cowdry A inclusions and ground-glass nuclei allows their distinction.
| Etiology | Endoscopy | Morphology |
|---|---|---|
| Herpesvirus | Superficial ulcers | Dense intranuclear eosinophilic inclusions (Cowdry A) Ground-glass chromatin Multinucleated syncytia of squamous cells Detached squamous cells with viral inclusions Macrophages in ulcer base |
| Cytomegalovirus | Linear ulcers Single deep ulcers |
Cytomegaly and nucleomegaly with single large nuclear inclusion or multiple amphophilic cytoplasmic inclusions in endothelial and stromal cells |
| Radiation esophagitis | Ulcer or stricture | Bizarre epithelial and stromal cells Degenerated and multinucleated squamous cells Preserved nucleus-to-cytoplasm ratio Rare mitotic activity Vascular intimal proliferation Stromal fibrosis and stellate fibroblasts |
| Squamous dysplasia or carcinoma | Mucosal erythema and friability, erosions, plaques, nodules | Nuclear hyperchromasia Nuclear pleomorphism, increased mitotic rate. Increased nucleus-to-cytoplasm ratio Lack of surface maturation Nuclear overlapping and loss of polarity |
Empiric treatment for herpetic lesions is often initiated on the basis of clinical suspicion, even in the absence of histological confirmation. Acyclovir (Zovirax) can be administered orally and helps initiate healing of active disease, but it does not prevent recurrences. Other medications, including famciclovir (Famvir) and valacyclovir (Valtrex), have also been shown to be effective therapy for herpes esophagitis. Intravenous medications may be indicated for severe disease.
Cytomegalovirus (CMV) is a member of the human herpesvirus family, with an estimated prevalence rate among adults ranging from 40% to 60% of all cases of infectious esophagitis in resource-rich countries. Like other herpesviruses, CMV is not normally cleared from the body but instead is kept in a state of latency by the immune system. Therefore, chronic CMV infection only rarely causes disease among immunocompetent persons, but it does represent a major cause of morbidity and mortality in immunocompromised patients (especially patients infected with the human immunodeficiency virus [HIV] whose CD4 counts are lower than 50 cells/μL), transplant recipients, patients with malignancies, and patients who have received immunosuppressive therapy.
CMV esophagitis, although less common than herpetic esophagitis, is not infrequently found in patients with AIDS. In one study, CMV infection was found either alone or in combination with Candida and herpes in 30% of AIDS patients. Similar to herpes simplex or varicella-zoster esophagitis, CMV esophagitis may also rarely occur in immunocompetent individuals.
Presenting symptoms may include odynophagia, nausea, substernal pain, and fever. Endoscopically, CMV esophagitis has a variable appearance. Most patients with CMV esophagitis have multiple, well-circumscribed ulcers, most often located in the middle to distal esophagus. Deep linear ulcers and shallow ulcers, erythema, diffuse erosive esophagitis, and an inflammatory exudate also may be seen in the setting of CMV esophagitis, but these findings lack diagnostic specificity. However, a recent retrospective study of endoscopic appearances of patients with herpes or CMV esophagitis identified a scoring system with 97% sensitivity and 89% specificity to discriminate CMV from herpes esophagitis. Viral culture is not used routinely because detection of the virus does not confirm active disease.
CMV-infected cells typically show marked cytomegaly and nucleomegaly with large ovoid intranuclear inclusions and thick marginated chromatin ( Fig. 14.9 ). The inclusion bodies may be brightly eosinophilic or deeply basophilic and are usually separated from the nuclear membrane by a halo. In addition, small eosinophilic to basophilic granular inclusions may be evident in the cytoplasm of infected cells. Cytoplasmic inclusions typically appear within minute vacuoles. CMV infects mesenchymal and columnar cells, but not squamous cells. Therefore, it is important for endoscopists to biopsy or brush the base of esophageal ulcers to optimize the chance of sampling diagnostic cells. No single technique is 100% sensitive in establishing a diagnosis of CMV esophagitis. A combination of diagnostic modalities is often used. If a careful search of well-prepared, routinely stained tissue sections or cytology preparations fails to reveal CMV inclusions, or at least suspicious cells, then additional ancillary immunohistochemical or in situ hybridization tests may be helpful. Immunohistochemistry may highlight infected cells without typical CMV morphology on routine stained sections and can be more sensitive than light microscopy. As previously mentioned, CMV esophagitis may coexist with herpes and Candida infection in both transplant recipients and patients with AIDS.

A variety of medications, such as ganciclovir, valganciclovir, foscarnet, and cidofovir, may be effective for the treatment of CMV esophagitis. The specific choice of therapy depends on the site and severity of infection, the level of underlying immunosuppression, the patient’s ability to tolerate and adhere to the treatment regimen, and the potential drug interactions.
Symptoms related to esophageal disease are common in AIDS patients. In most, a specific infectious agent such as Candida , herpes simplex, varicella-zoster, or CMV can be identified. , However, in some patients, no apparent cause for the esophagitis or ulcer can be found by standard techniques. Hybridization with specific DNA probes may reveal Epstein-Barr virus in some of these patients. In some HIV-positive patients, however, odynophagia and multiple discrete esophageal ulcers are observed in the absence of an identifiable pathogen. Ultrastructural studies in such patients have revealed the presence of viral particles consistent with retrovirus, which suggests that the esophagus may represent a primary direct target site of acute HIV infection itself. , Human papilloma virus (HPV) may cause esophageal ulcers, hyperkeratosis and solitary papilloma or papillomatosis. Koilocytosis, perinuclear clearing and giant and multinucleated cells are also features of HPV infection.
Fungal esophagitis is most commonly caused by Candida albicans or Candida tropicalis . Fungal infection occurs primarily in patients with some type of underlying disease (e.g., AIDS, other immunosuppressive disorders, diabetes) and in patients who have undergone treatment with broad-spectrum antibiotics, acid-suppressive therapy, , or inhaled or swallowed corticosteroids. It also may be found in otherwise healthy patients. Clinically, the presenting symptoms are dysphagia and odynophagia, but some patients remain asymptomatic, with the infection discovered incidentally at esophagoscopy performed for other reasons, especially in elderly patients.
At endoscopy, esophageal candidiasis typically appears as white plaques of fibrinopurulent exudate, which may be focal or confluent, overlying erythematous mucosa. These plaques can be scraped away to reveal ulceration underneath. This is in contrast to the white patches seen in eosinophilic esophagitis that cannot easily be dislodged from esophageal epithelium (see Eosinophilic Esophagitis).
Morphologically, pseudohyphae and budding yeast forms can be demonstrated in a background of active esophagitis and are easily identified within the ulcer slough and fibrinopurulent exudate ( Fig. 14.10 ). Because Candida organisms are part of the normal flora of the GI tract, confirmation of this diagnosis requires more than simply identifying budding yeast forms; pseudohyphae should be detected within tissue to document true infection. Pseudohyphae have a linear or ribbon-like appearance in which small indentations, rather than true septations, are typically noted along the long axis of the organisms. Yeast forms have a slight ovoid contour and frequently manifest budding from the tips. In immunosuppressed patients, who occasionally reveal only minimal inflammation, special stains (silver stain, periodic acid–Schiff [PAS]) should be used to detect small numbers of invasive fungal forms within the tissue. However, special stains cannot be used to speciate the type of Candida organisms. C. tropicalis is more virulent than C. albicans because of its increased potential for tissue invasiveness. Candida can colonize preexisting ulcers or any damaged mucosa, and the pathologist should consider the possibility of a dual infection or pathology. C. auris , first described in 2009 causing an ear infection, has been isolated from the oral cavity, oropharynx and nares, but has not yet been reported in the esophagus.

On occasion, the endoscopist may identify small white plaques that, although they resemble Candida esophagitis, represent glycogenic acanthosis, ectopic sebaceous glands or inflammatory exudate in EoE. Glycogenic acanthosis occurs as white mucosal plaques that measure 2 to 5 mm. Diffuse esophageal glycogenic acanthosis may occur as a rare manifestation of Cowden syndrome. Biopsy features include distention of squamous epithelial cells with glycogen, which is evident as pale-staining material that is PAS positive and diastase digestible. Ectopic sebaceous glands, which may also appear as small, pale yellow or white, punctate elevations of the mucosa, are rare but are easily recognized in biopsy material by their close resemblance to normal dermal sebaceous glands. White plaques in EoE tend to be smaller than those associated with Candida and are composed of exudate containing eosinophils and squamous epithelial cells. EoE plaques are typically more difficult to dislodge than are Candida -associated plaques.
Fluconazole (Diflucan) is the drug of choice because it is safe and well tolerated, although other drugs, such as itraconazole and ketoconazole, are also effective. , Amphotericin B is considered a second-line option and is reserved for severe cases and for patients for whom treatment with azole compounds has failed, such as patients who are infected with glabrata species of Candida which is more commonly azole-resistant than albicans species. Echinocandins (e.g., Caspofungin), a new class of antifungal agents that act on fungal cell walls, are being used in randomized trials for patients with Candida esophagitis refractory to azole compounds. , Lack of symptomatic response in patients with Candida esophagitis should prompt search for a co-infecting agent such as herpes virus.
Primary bacterial infections of the esophagus are exceedingly rare except in immunocompromised patients; most cases are secondary and occur in areas of ulceration. The most common infecting organisms are normal flora from the mouth and respiratory tract ( Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus viridans, and Bacillus species). A diagnosis of bacterial esophagitis can be established when sheets of confluent bacteria invade the subepithelial tissue. The number of bacteria appears to be inversely related to the intensity of the inflammatory reaction. Bacterial cultures of biopsy material are not performed routinely but may be helpful to further identify organisms that are detected on routinely stained tissue sections. Actinomyces may occur in areas of previous tissue injury; the infection is characterized by the presence of sulfur granules and intertwining thin branching actinomyces filaments within the subepithelial tissue. A distal esophageal microbiome analysis of patients receiving PPI, all of whom had either Barrett’s esophagus or GERD without Barrett’s esophagus, revealed Actinomyces was the only species significantly different in PPI users; it was more abundant in users independent of dose or duration of PPI use. Occasionally, one may encounter Mycobacterium tuberculosis, Mycobacterium avium-intracellulare, Histoplasma capsulatum, or Toxoplasma gondii in the esophagus. ,
The World Health Organization estimates that approximately 10 million people are infected with Trypanosoma cruzi worldwide. Approximately 100 million are at risk of contracting the infection, and these are mostly in Latin America. Humans are accidental hosts for T. cruzi. They are usually infected at night via contact with feces of blood-sucking triatomine insects. A second mechanism of transmission, responsible for as much as 10% of cases, is transfusion of whole blood or blood derivatives (except for lyophilized products). A third route of transmission is congenital. The likelihood of congenital transmission in children of chagasic mothers ranges from 1% to 10%. After 2 to 4 months, the acute clinical manifestations (inflammation of the eye, conjunctivitis, palpebral edema, periauricular satellite adenopathy, and generalized infection) disappear and the disease enters a period of clinical latency (“indeterminate” form). Studies performed in endemic areas have provided clinical and radiologic evidence of esophageal disorders in 7% to 10% of people with chronic T. cruzi infection, and megaesophagus in 3%. Dysphagia of solid and liquid food is the first and most important symptom of digestive disturbance and occurs in 98% of patients. It may begin slowly but leads to malnutrition and severe weight loss. Other clinical symptoms include regurgitation, pyrosis, hiccups, cough, and parotid gland hypertrophy. Esophageal pain often appears with deglutition as a consequence of spasmodic contraction of a hypersensitive esophagus. Esophageal involvement is progressive in some patients but not in others, and it evolves independently of heart involvement. The 2018 achalasia guidelines include a statement that there are only minor differences in the clinical presentation of idiopathic achalasia and achalasia caused by Chagas disease.
Destruction of neuron plexuses in the esophagus by the inflammatory reaction induced by T. cruzi is the main cause of dysperistalsis of the esophagus. In idiopathic achalasia, inhibitory neurons are lost selectively, whereas in Chagas disease both inhibitory and excitatory neurons are lost. This neuronal loss results in gradual narrowing of the distal esophagus with luminal enlargement of the proximal esophagus, sometimes termed chagasic megaesophagus (grade I to IV). Microscopically, distal muscular hypertrophy and mononuclear inflammatory infiltrates occur in the muscle layers. The submucosal and myenteric plexuses show an intense mononuclear infiltrate with associated neuronal injury ( Fig. 14.11 ). , A 2010 report indicates that patients with megaesophagus have a significantly decreased amount of S100-positive and glial fibrillary acidic protein (GFAP)-positive enteroglial cells when compared with seronegative controls and asymptomatic seropositive patients. A 2013 immunohistochemical study identified increased substance P, which has proinflammatory effects, and decreased vasoactive intestinal peptide, which has antiinflammatory effects, in sections of chagasic megaesophagus, suggesting that these alterations contribute to inflammation that ultimately leads to denervation. With disease progression, there is intense neuronal destruction and denervation of the organ, with loss of function.

Only two drugs are effective for the treatment of Chagas disease in the acute and chronic phases: nifurtimox and benznidazole. Both are almost 100% effective in curing the disease if treatment is begun soon after infection, at the onset of the acute phase, but their efficacy diminishes with time and duration of infection. Treatment protocols for the chronic phase of disease remain controversial. In the presence of megaesophagus, dilation of the esophagogastric junction may be performed by passing a dilator or air-filled balloon through an endoscope. Repeated dilations may be needed to prevent the development of recurrent strictures. Surgery is indicated in some cases. Cardiotomy is the most frequently performed procedure. Injections of botulin toxin into the esophagus have also been used in some patients but appear to be more effective in idiopathic achalasia than Chagas disease. , ,
Esophageal injury caused by prolonged direct mucosal contact with ingested, particularly large-sized, tablets or capsules occurs frequently. Commonly implicated agents include antibiotics (particularly tetracyclines and clindamycin), nonsteroidal antiinflammatory drugs (NSAIDs), potassium chloride, ascorbic acid, iron supplements, quinidine, and bisphosphonates. , , Symptoms include odynophagia, continuous retrosternal pain, and dysphagia. Elderly patients and women are affected most frequently. , Affected patients often report having ingested a pill with very little or no fluid before nighttime sleep. Most affected individuals do not have an abnormality of esophageal transit. However, in some reports, patients with quinidine-induced or potassium chloride–induced injury had a history of external esophageal compression, such as valvular heart disease with left atrial enlargement, or esophageal entrapment by fixed mediastinal structures and adhesions after thoracic surgery.
Pill esophagitis often results in discrete ulcers with normal or only mildly inflamed esophageal mucosa. Ulcers may be shallow or, more commonly, deep with extension into the muscularis mucosae. They are usually located at the junction of the proximal and middle thirds of the esophagus, the area where the aortic arch compresses the esophagus and peristaltic amplitude is relatively low. Patients with left atrial enlargement are especially susceptible to pill-induced esophageal injury.
The histological appearance of pill-induced esophagitis is typically nonspecific. However, prominent eosinophilic infiltration, spongiosis (dilated intercellular spaces), and necrosis of squamous epithelium should always raise the possibility of pill-induced injury, particularly if located in the upper esophagus. Crystalline stainable iron may be identified in cases of ferrous sulfate–induced disease, and polarizable crystalline material may be evident in cases of alendronate-induced injury ( Fig. 14.12 ). Some cases of pill esophagitis are severe and lead to perforation.

A variety of conditions can cause histological features similar to those found in pill esophagitis. Herpes esophagitis is often a major consideration; a clinical history of immunosuppression and identification of characteristic viral cytopathic inclusions is useful in this differential diagnosis. Other causes of infectious esophagitis, including CMV and Candida, may mimic pill esophagitis, both clinically and pathologically. Identification of CMV cytopathic effect or fungal elements is diagnostic. GERD is more common than pill esophagitis; however, it usually is not possible to distinguish these conditions on histological grounds in an isolated esophageal biopsy specimen and in the absence of clinical information. Features potentially helpful in the differential diagnosis are diffusely dilated intercellular spaces that were found more commonly in a study of 22 cases of pill esophagitis compared to 20 cases of reflux esophagitis, and reactive epithelial changes and papillary elongation that were more common in reflux compared to pill esophagitis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here