Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Inflammation is a response of vascularized tissues to infections and tissue damage that brings cells and molecules of host defense from the circulation to the sites where they are needed, in order to eliminate the offending agents. Although in common medical and lay parlance inflammation suggests a harmful reaction, it is actually a protective response that is essential for survival. It serves to rid the host of both the initial cause of cell injury (e.g., microbes and toxins) and the consequences of such injury (e.g., necrotic cells and tissues) and initiates the repair of damaged tissues. The mediators of defense include phagocytic leukocytes, antibodies, and complement proteins ( Fig. 2.1 ). Most of these normally circulate in the blood, where they are sequestered from tissues and unable to cause damage. Infections and dead cells are typically in the tissues, outside the vessels. The process of inflammation delivers leukocytes and proteins to foreign invaders, such as microbes, and to damaged or necrotic tissues and activates the recruited cells and molecules, which then remove the harmful or unwanted substances. Without inflammation, infections would go unchecked, wounds would never heal, and injured tissues might remain permanent festering sores.
We start with an overview of some of the important general features of inflammation, then discuss the major reactions of acute inflammation and the chemicals that mediate these reactions. We continue with a discussion of chronic inflammation and close with the process of tissue repair.
Inflammation may be acute or chronic ( Table 2.1 ). The initial, rapid response to infections and tissue damage is called acute inflammation . It develops within minutes to hours and lasts for several hours to a few days. Its main characteristics are the leakage of fluid and plasma proteins (edema) and the accumulation of leukocytes, predominantly neutrophils (also called polymorphonuclear leukocytes). When acute inflammation achieves its desired goal of eliminating the offenders, the reaction quickly subsides, but if the response fails to clear the stimulus, it can progress to a protracted phase that is called chronic inflammation . Chronic inflammation is of longer duration and is associated with continuing tissue destruction and fibrosis (the deposition of connective tissue).
| Feature | Acute Inflammation | Chronic Inflammation |
|---|---|---|
| Onset | Fast: minutes to hours | Slow: days |
| Cellular infiltrate | Mainly neutrophils | Monocytes/macrophages and lymphocytes |
| Tissue injury | Usually mild and self-limited | May be significant |
| Fibrosis | None | May be severe and progressive |
| Local and systemic signs | Prominent | Variable, usually modest |
The external manifestations of inflammation, often called its cardinals signs, are heat ( calor in Latin), redness (rubor) , swelling (tumor) , pain (dolor) , and loss of function (function laesa) . The first four of these were described more than 2000 years ago by a Roman encyclopedist named Celsus, who wrote the then-famous text De Medicina ; the fifth was added in the late 19th century by Rudolf Virchow, known as the “father of modern pathology.” These manifestations occur as consequences of the vascular changes and leukocyte recruitment and activation, as will be evident from the discussion that follows.
Inflammatory reactions develop in steps (which can be summarized as the five R’s): (1) recognition of the offending agent; (2) recruitment of blood cells and proteins to the tissue site; (3) removal of the offending agent; (4) regulation of the reaction; and (5) repair of injured tissue. Each of these steps is described in detail in this chapter.
While normally protective, in some situations, the inflammatory reaction becomes the cause of disease, and the damage it produces is its dominant feature. Inflammatory reactions to infections are often accompanied by local tissue damage and pain. Typically, however, these harmful consequences resolve as the inflammation abates, leaving little or no permanent damage. By contrast, there are many diseases in which the inflammatory reaction is misdirected (e.g., against self tissues in autoimmune diseases), occurs against usually harmless environmental substances (e.g., in allergies), or is excessively prolonged (e.g., in infections by microbes that resist eradication such as Mycobacterium tuberculosis ). These abnormal reactions underlie many common chronic diseases, such as rheumatoid arthritis, asthma and lung fibrosis ( Table 2.2 ). Inflammation may also contribute to diseases that are thought to be primarily metabolic, degenerative, or genetic, such as atherosclerosis, type 2 diabetes, and Alzheimer disease. In recognition of the wide-ranging harmful consequences of inflammation, the lay press has rather melodramatically referred to it as “the silent killer.”
| Disorders | Cells and Molecules Involved in Injury |
|---|---|
| Acute | |
| Acute respiratory distress syndrome | Neutrophils |
| Glomerulonephritis, vasculitis | Antibodies and complement; neutrophils |
| Septic shock | Cytokines |
| Chronic | |
| Rheumatoid arthritis | Lymphocytes, macrophages; antibodies? |
| Asthma | Eosinophils; IgE antibodies |
| Pulmonary fibrosis | Macrophages; fibroblasts |
Inadequate inflammation is typically manifested by increased susceptibility to infections. Impairment of inflammation is caused by reduced production of leukocytes resulting from replacement of the bone marrow by cancers (e.g., leukemias), immunosuppressive agents used to treat graft rejection and autoimmune disorders, and many other conditions such as malnutrition. Inherited genetic disorders of leukocyte function are rare, but they provide valuable information about the mechanisms of leukocyte responses. These conditions are described in Chapter 5 in the context of immunodeficiency diseases.
Once inflammation has eliminated the offending agents, it subsides and also sets into motion the process of tissue repair . In this process, the injured tissue is replaced through regeneration of surviving cells and filling of residual defects with connective tissue (scarring) .
Of the myriad causes of inflammation, the following are the most frequent:
Infections, in which the products of microbes are recognized by the host and elicit different types of inflammatory reactions.
Tissue necrosis, which may be caused by ischemia (reduced blood flow, the cause of infarction in the heart, brain, and other tissues), trauma , and physical and chemical injury (e.g., thermal injury, irradiation, and exposure to toxins). Molecules released from necrotic cells trigger inflammation even in the absence of infection (so-called “sterile inflammation”).
Foreign bodies, such as sutures and tissue implants, also elicit sterile inflammation.
Immune reactions (also called hypersensitivity ) are reactions in which the normally protective immune system damages the individual’s own tissues. As mentioned earlier, autoimmune diseases and allergies are diseases caused by immune responses; in both, inflammation is a major contributor to tissue injury ( Chapter 5 ).
The first step in inflammatory responses is the recognition of microbes and necrotic cells by cellular receptors and circulating proteins. All tissues contain resident cells whose primary function is to detect the presence of foreign invaders or dead cells, to ingest and destroy these potential causes of harm, and to elicit the inflammatory reaction that recruits cells and proteins from the blood to complete the elimination process. The most important of these sentinel cells are tissue-resident macrophages and dendritic cells. These cells express receptors for microbial products in multiple cell compartments: on their surface, where they recognize microbes in the extracellular space; in endosomes, into which microbes are ingested; and in the cytosol where certain microbes may survive. The best known of these receptors are the Toll-like receptors (TLRs) ( Chapter 5 ). Activation of TLRs leads to the production of cytokines that trigger inflammation (discussed later). A different sensor system consists of cytosolic NOD-like receptors (NLRs) that, upon activation, recruit and activate a multiprotein complex (the inflammasome , Chapter 5 ) which generates the biologically active cytokine interleukin-1 (IL-1). NLRs recognize a wide range of stimuli, including microbial products and indicators of cell damage such as leaked DNA and decreased cytosolic potassium levels. The cytokine-induced inflammation then eliminates the stimulus that elicited the reaction (microbes and dead cell debris).
If microbes navigate the gauntlet of sentinels in tissues and enter the circulation, they are then recognized by a number of plasma proteins , such as antibodies and members of the complement system. These proteins can destroy circulating microbes and are recruited to tissue sites of infection, where they stimulate inflammatory reactions.
With this background, we proceed to a discussion of acute inflammation, its underlying mechanisms, and how it functions to eliminate microbes and dead cells.
Acute inflammation has three major components: (1) dilation of small vessels; (2) increased permeability of the microvasculature; and (3) emigration of the leukocytes from the microcirculation (see Fig. 2.1 ). Most of these changes happen in postcapillary venules at the site of infection or tissue injury. The walls of these vessels are capable of reacting to stimuli and are sufficiently thin to allow passage of fluid and proteins. Vasodilation slows down blood flow and sets the stage for the subsequent reactions, while increased vascular permeability enables plasma proteins to enter the tissue site. Transmigration moves leukocytes from their peaceful home inside the vessels into the maelstrom of infection or necrosis, where the cells perform their function of destroying noxious agents and cleaning up the damage. All these reactions are induced by cytokines and other molecules (collectively called inflammatory mediators ) produced at the site of infection or necrosis (described later).
Vasodilation is one of the earliest reactions of acute inflammation and is responsible for the externally visible redness (erythema) and warmth that accompany most acute inflammatory reactions. The most important chemical mediator of vasodilation is histamine, discussed later.
Vasodilation is quickly followed by increased permeability of the microvasculature and the outpouring of protein-rich fluid into the extravascular tissues. The escape of fluid, proteins, and blood cells from the vascular system into the interstitial tissue or body cavities is known as exudation ( Fig. 2.2 ). An exudate is an extravascular fluid that has a high protein concentration and contains cellular debris. Its presence implies that there is an increase in the permeability of small blood vessels, typically during an inflammatory reaction. By contrast, a transudate is a fluid with low protein content (most of which is albumin), little or no cellular material, and low specific gravity. A transudate is essentially an ultrafiltrate of blood plasma that is produced as a result of osmotic or hydrostatic imbalance across the vessel wall without an increase in vascular permeability and is usually not associated with inflammation ( Chapter 3 ). Edema denotes an excess of fluid in the interstitial tissue or serous cavities; it can be either an exudate or a transudate. Pus , a purulent exudate, is an inflammatory exudate rich in leukocytes (mostly neutrophils), the debris of dead cells, and, in many cases, microbes.
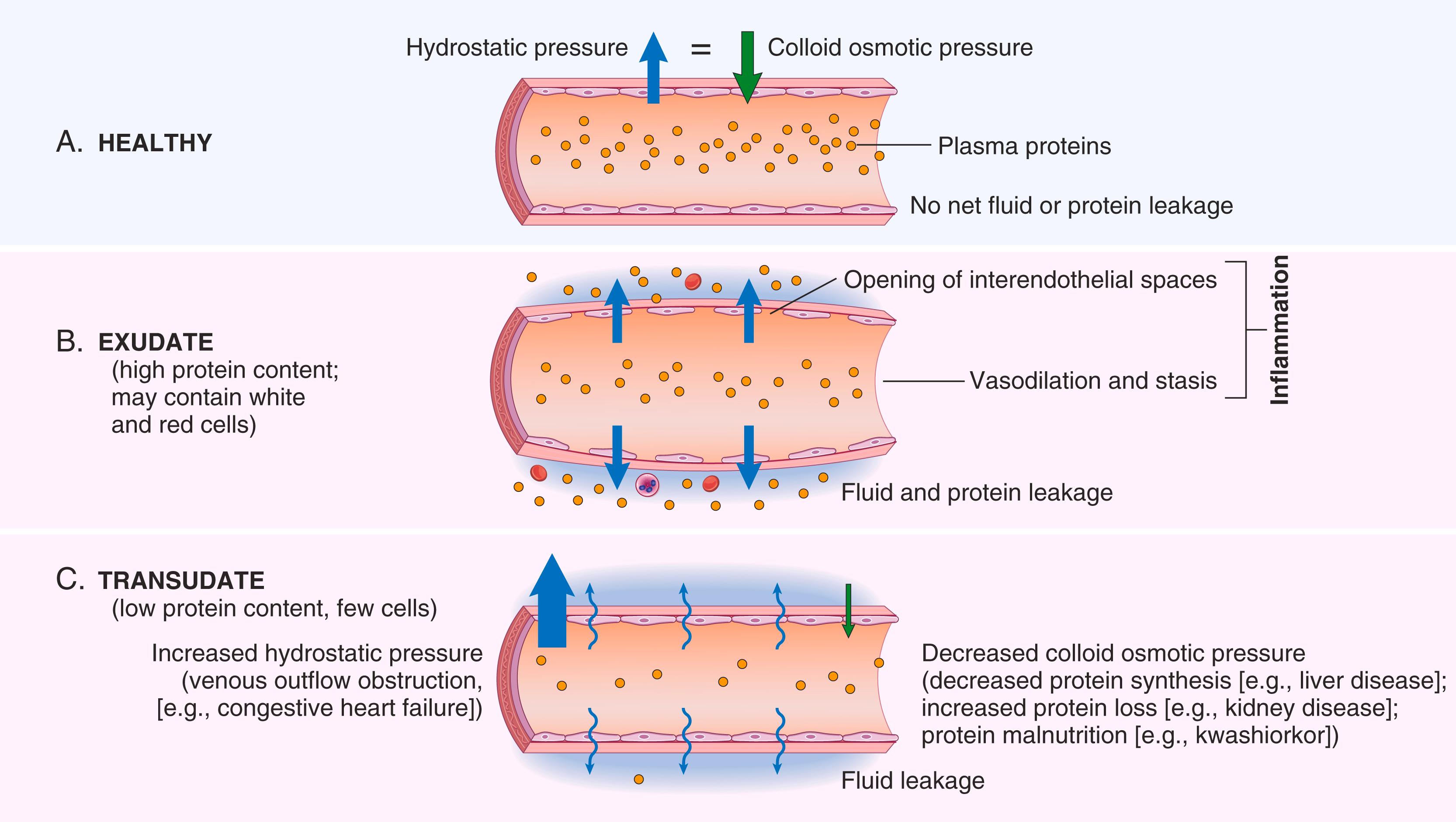
The principal mechanism of increased vascular permeability is the contraction of endothelial cells, which creates interendothelial openings. It is elicited by histamine, bradykinin, leukotrienes, and other chemical mediators. It occurs rapidly after exposure to the mediator (within 15 to 30 minutes) and is usually short lived. In unusual cases (e.g., in burns), increased vascular permeability may result from direct endothelial injury. In these instances, leakage starts immediately after injury and is sustained for several hours until the damaged vessels become thrombosed or are repaired.
The loss of fluid and increased vessel diameter lead to slower blood flow and higher concentration of red cells in small vessels, raising the viscosity of the blood. Involved small vessels become engorged with red cells, a condition termed stasis , which is seen histologically as vascular congestion and externally as localized redness of the affected tissue.
In addition to the reactions of blood vessels, lymph flow is increased and helps drain edema fluid that accumulates because of increased vascular permeability. The lymphatics may become secondarily inflamed (lymphangitis) , appearing clinically as red streaks extending from an inflammatory focus along the course of lymphatic channels. Involvement of the draining lymph nodes may lead to enlargement (because of increased cellularity) and pain. The associated constellation of pathologic changes is termed reactive or inflammatory lymphadenitis ( Chapter 10 ).
The journey of leukocytes from the vessel lumen to the tissue is a multistep process that is mediated and controlled by adhesion molecules and cytokines. Leukocytes normally transit rapidly through small vessels. In inflammation, they have to be stopped and then brought to the offending agent or the site of tissue damage, outside the vessels. This process can be divided into phases, consisting first of adhesion of leukocytes to endothelium at the site of inflammation, then transmigration of the leukocytes through the vessel wall, and finally, movement of the cells toward the offending agent ( Fig. 2.3 ).
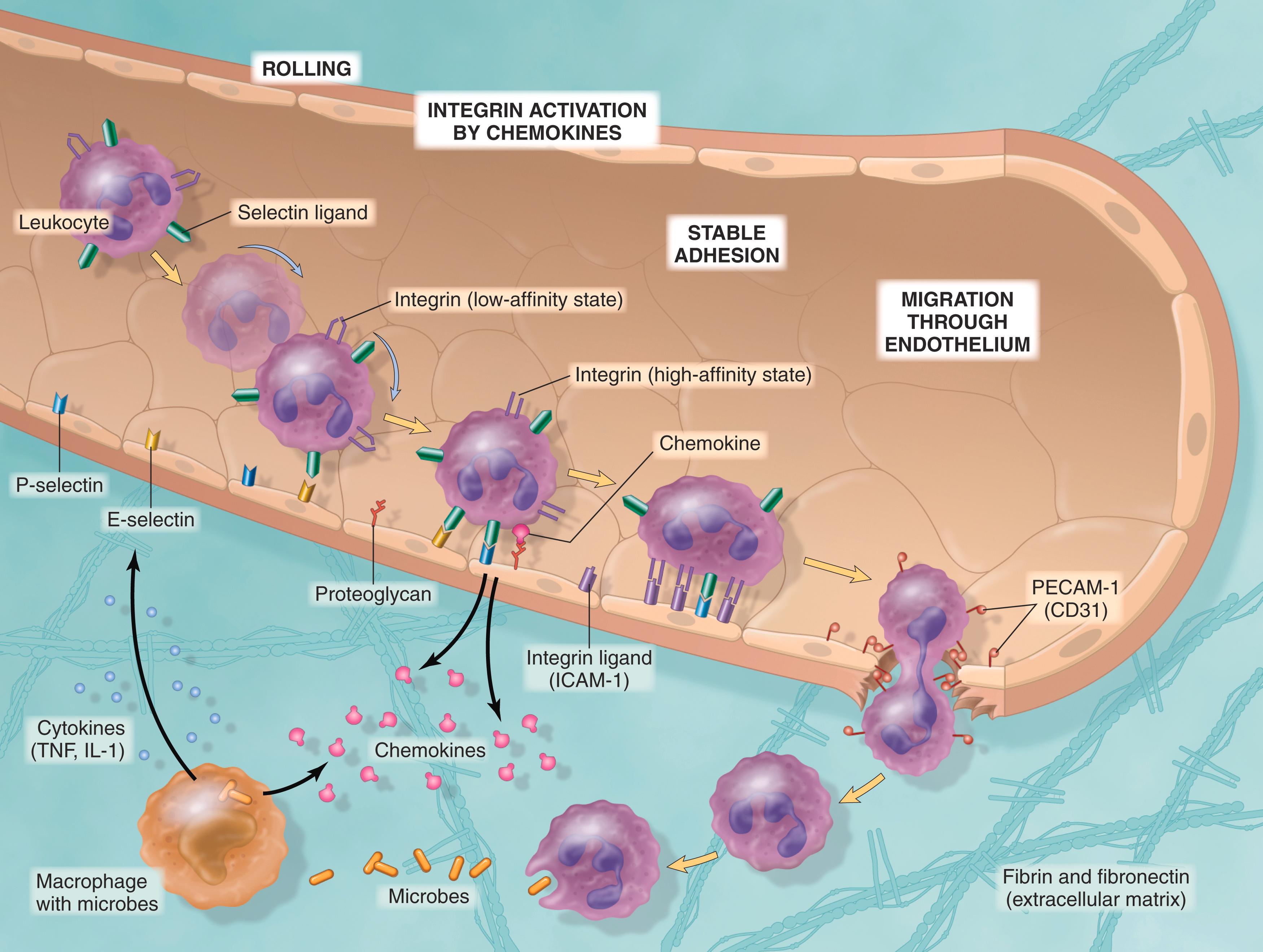
When blood flows from capillaries into postcapillary venules, under conditions of normal laminar flow, red cells are concentrated in the center of the vessel, displacing leukocytes toward the vessel wall. As the rate of flow slows early in inflammation (stasis), leukocytes, being larger than red cells, slow down more and assume a more peripheral position along the endothelial surface, a process called margination . By moving close to the vessel wall, leukocytes are able to detect and react to changes in the endothelium. When endothelial cells are activated by cytokines and other mediators produced locally, they express adhesion molecules to which the leukocytes attach loosely. These cells bind and detach and thus begin to tumble on the endothelial surface, a process called rolling . The cells finally come to rest at some point where they adhere firmly (resembling pebbles over which a stream runs without disturbing them).
The initial weak binding of leukocytes and their rolling on the endothelium are mediated by a family of proteins called selectins ( Table 2.3 ). Selectins are receptors expressed on leukocytes and endothelium that have an extracellular domain that binds carbohydrates (hence the lectin part of the name). The ligands for selectins are sialic acid–containing oligosaccharides attached to glycoprotein backbones; some are expressed on leukocytes and others on endothelial cells. Endothelial cells express two selectins, E- and P-selectins, as well as the ligand for L-selectin, whereas leukocytes express L-selectin. E- and P-selectins are typically expressed at low levels or not at all on nonactivated endothelium but are upregulated after stimulation by cytokines and other mediators. Therefore, binding of leukocytes is largely restricted to endothelium at sites of infection or tissue injury (where the mediators are produced). For example, in nonactivated endothelial cells, P-selectin is found primarily in intracellular membrane-bound vesicles called Weibel-Palade bodies; however, within minutes of exposure to mediators such as histamine or thrombin, P-selectin traffics to the cell surface. Similarly, E-selectin and the ligand for L-selectin, which are not expressed on normal endothelium, are induced after stimulation by the cytokines IL-1 and tumor necrosis factor (TNF), which are produced by tissue macrophages, dendritic cells, mast cells, and endothelial cells after encountering microbes or dead tissues. Selectin-mediated interactions have a low affinity with a fast off-rate, and they are easily disrupted by the flowing blood. As a result, the leukocytes bind, detach, and bind again to endothelium. These weak rolling interactions slow down the leukocytes sufficiently for them to recognize additional adhesion molecules on the endothelium.
| Family | Adhesion Molecule | Major Cell Type | Principal Ligands |
|---|---|---|---|
| Selectin | L-selectin | Leukocytes | Sialyl-Lewis X on various glycoproteins expressed on endothelium |
| E-selectin | Activated endothelium | Sialyl-Lewis X on glycoproteins expressed on neutrophils, monocytes, T lymphocytes | |
| P-selectin | Activated endothelium | Sialyl-Lewis X on glycoproteins expressed on neutrophils, monocytes, T lymphocytes | |
| Integrin | LFA-1 | T lymphocytes, other leukocytes | ICAM-1 expressed on activated endothelium |
| MAC-1 | Monocytes, other leukocytes | ICAM-1 expressed on activated endothelium | |
| VLA-4 | T lymphocytes, other leukocytes | VCAM-1 expressed on activated endothelium | |
| α4β7 | Lymphocytes, monocytes | MAdCAM-1 expressed on endothelium in gut and gut-associated lymphoid tissues |
Firm adhesion of leukocytes to endothelium is mediated by a family of leukocyte surface proteins called integrins (see Table 2.3 ). Integrins are transmembrane two-chain glycoproteins that mediate the adhesion of leukocytes to endothelium and of various cells to the extracellular matrix. They are normally expressed on leukocyte plasma membranes in a low-affinity form and do not adhere to their specific ligands until the leukocytes are activated by chemokines . Chemokines are chemoattractant cytokines that are secreted by many cells at sites of inflammation, bind to endothelial cell proteoglycans, and are displayed at high concentrations on the endothelial surface. When the rolling leukocytes encounter the displayed chemokines, the cells are activated and their integrins undergo conformational changes and cluster together, thereby converting to a high-affinity form. At the same time, other cytokines, notably TNF and IL-1 (also secreted at sites of infection and injury), activate endothelial cells to increase their expression of ligands for integrins. The combination of cytokine-induced expression of integrin ligands on the endothelium and increased affinity of integrins on the leukocytes results in firm integrin-mediated binding of the leukocytes to the endothelium at the site of inflammation. The leukocytes stop rolling, and engagement of integrins by their ligands delivers signals to the leukocytes that lead to cytoskeletal changes that arrest the leukocytes and firmly attach them to the endothelium.
A telling indication of the importance of leukocyte adhesion molecules is the existence of mutations affecting integrins and selectin ligands that result in recurrent bacterial infections as a consequence of impaired leukocyte adhesion and defective inflammation. These leukocyte adhesion deficiencies are described in Chapter 5 . Antagonists of integrins are approved for the treatment of some chronic inflammatory diseases, such as multiple sclerosis and inflammatory bowel disease.
After arresting on the endothelial surface, leukocytes migrate through the vessel wall, primarily by squeezing between interendothelial junctions. This extravasation of leukocytes is called transmigration or diapedesis . Platelet endothelial cell adhesion molecule-1 (PECAM-1, also called CD31), a cellular adhesion molecule expressed on leukocytes and endothelial cells, mediates the binding events needed for leukocytes to traverse the endothelium. After crossing the endothelium, leukocytes pierce the basement membrane, probably by secreting collagenases, and enter the extravascular tissue. The directionality of leukocyte movement within tissues is controlled by locally produced chemokines, which create a diffusion gradient that the cells migrate along.
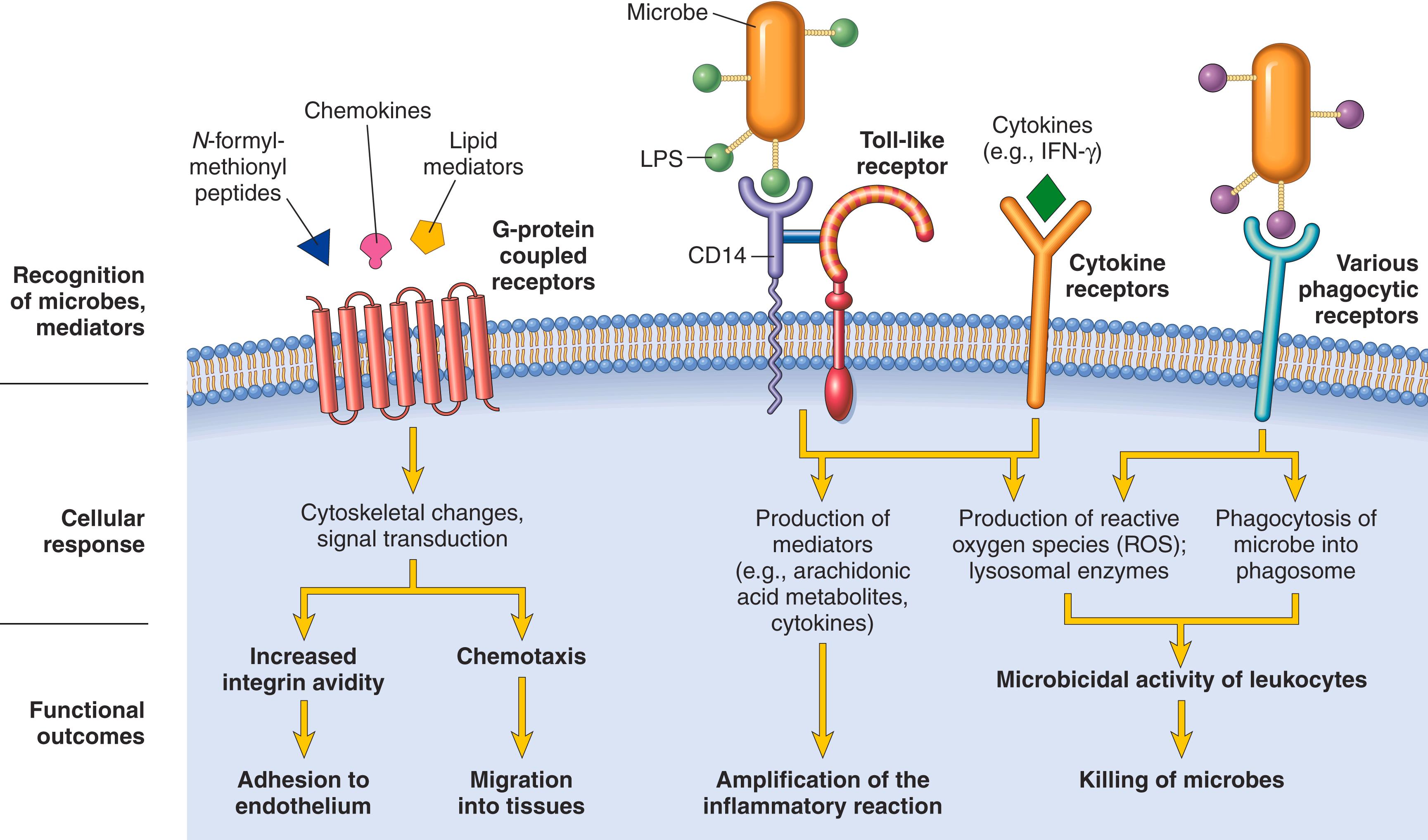
After exiting the circulation, leukocytes move in the tissues toward the site of injury by a process called chemotaxis , defined as locomotion along a chemical gradient. Among the many chemoattractants known, the most potent are bacterial products, particularly peptides with N -formylmethionine termini; cytokines, especially those of the chemokine family; components of the complement system, particularly C5a; and leukotrienes. These chemoattractants, which are described in more detail later, are produced in response to infections and tissue damage and during immunologic reactions. All of them bind to G protein–coupled receptors on the surface of leukocytes. Signals initiated from these receptors activate second messengers that induce the polymerization of actin at the leading edge of the cell and the localization of myosin filaments at the back. The leukocyte moves by extending filopodia that pull the back of the cell in the direction of extension, much as an automobile with front-wheel drive is pulled by the front wheels. The net result is that leukocytes migrate toward the inflammatory stimulus in the direction of the locally produced chemoattractants.
The nature of the leukocyte infiltrate varies with the age of the inflammatory response and the type of stimulus. In most forms of acute inflammation, neutrophils predominate in the inflammatory infiltrate during the first 6 to 24 hours and are replaced by monocytes in 24 to 48 hours ( Fig. 2.4 ). There are several reasons for the early preponderance of neutrophils: they are more numerous in the blood than other leukocytes, they respond more rapidly to chemokines, and they may attach more firmly to adhesion molecules that are rapidly induced on endothelial cells, such as P- and E-selectins. After entering tissues, neutrophils are short lived; they undergo apoptosis and disappear within a few days. Monocytes develop into macrophages in tissues that not only survive longer but may also proliferate, and thus they become the dominant population in prolonged inflammatory reactions.
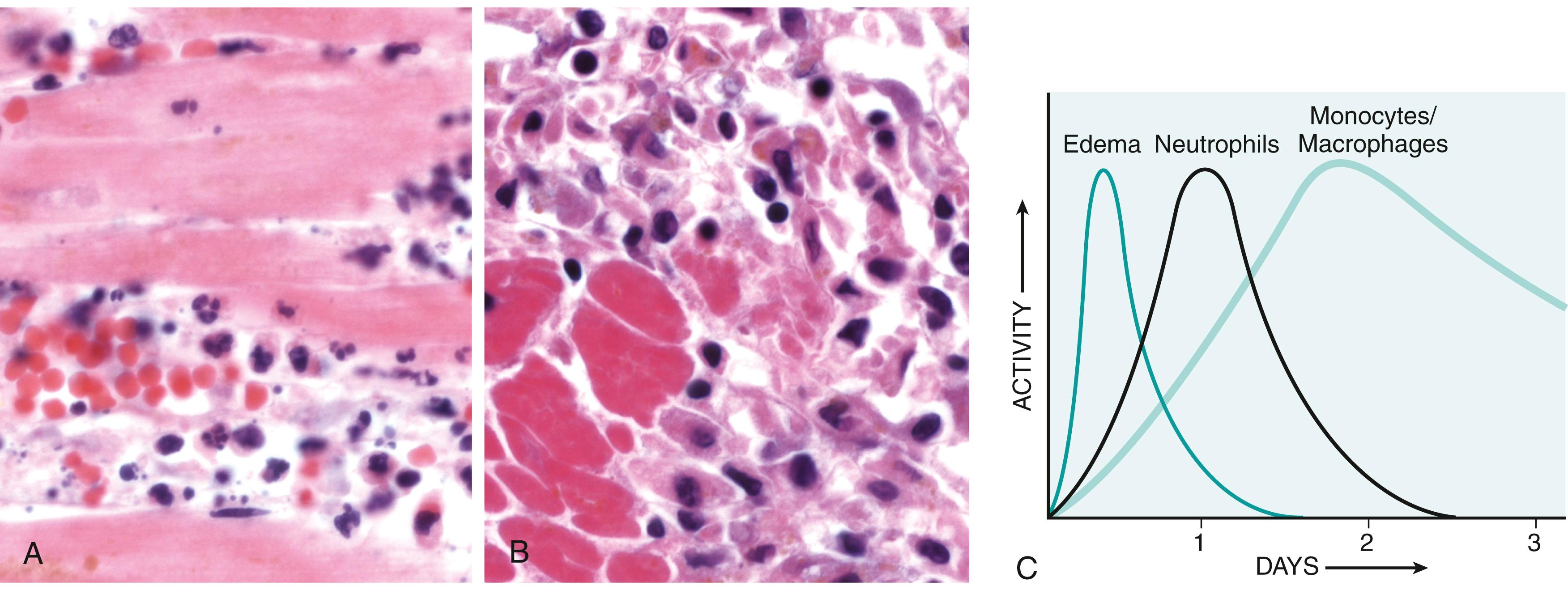
There are, however, exceptions to this stereotypic pattern of cellular infiltration. In certain infections—for example, those produced by Pseudomonas bacteria—the cellular infiltrate is dominated by continuously recruited neutrophils for several days; in viral infections, lymphocytes may be the first cells to arrive; some hypersensitivity reactions are dominated by activated lymphocytes, macrophages, and plasma cells (reflecting the immune response); and in allergic reactions and infections with certain parasites, eosinophils may be the main cell type.
The molecular understanding of leukocyte recruitment and migration has provided a large number of therapeutic targets for controlling harmful inflammation. As we discuss later, agents that block TNF, one of the major cytokines in leukocyte recruitment, are extremely useful therapeutics for chronic inflammatory diseases such as rheumatoid arthritis. Antibodies that block integrins were mentioned earlier.
Neutrophils and monocytes that have been recruited to a site of infection or cell death are activated by products of microbes and necrotic cells and by locally produced cytokines. Activation induces several responses ( eFig. 2.1 ), of which phagocytosis and intracellular killing are most important for destruction of microbes and clearance of dead tissues.
Phagocytosis is the ingestion of particulate material by cells. The body’s most important phagocytes are neutrophils and macrophages ( Table 2.4 ). Neutrophils are rapid responders but relatively short-lived. In inflammatory reactions, macrophages are derived from blood monocytes and can live for days or months. (As we will discuss later, some long-lived tissue-resident macrophages are derived from embryonic precursors that seed the tissues in early life and remain for years.) Macrophage responses tend to be slower but more long lasting.
| Neutrophils | Macrophages | |
|---|---|---|
| Origin | HSCs in bone marrow | HSCs in bone marrow (in inflammatory reactions) Stem cells in yolk sac or fetal liver (early in development, for some tissue-resident macrophages) |
| Life span in tissues | 1–2 days | Inflammatory macrophages: days or weeks Tissue-resident macrophages: years |
| Responses to activating stimuli | Rapid, short-lived, mostly degranulation and enzymatic activity | More prolonged, slower, often dependent on new gene transcription |
|
Rapidly induced by assembly of phagocyte oxidase (respiratory burst) | Less prominent |
|
Low levels or none | Induced following transcriptional activation of iNOS |
|
Major response; induced by cytoskeletal rearrangement | Not prominent |
|
Low levels or none | Major functional activity, requires transcriptional activation of cytokine genes |
|
Rapidly induced, by extrusion of nuclear contents | Little or none |
|
Prominent | Less |
Neutrophils and macrophages can ingest microbes following their recognition by phagocyte receptors, such as the mannose receptor (which recognizes terminal mannose residues found in microbial glycoproteins) and so-called “scavenger receptors.” The efficiency of this process is greatly increased if the microbes are coated (opsonized) with molecules called opsonins for which the phagocytes also have specific receptors. Opsonins include antibodies, the C3b cleavage product of complement, and certain plasma lectins. Following binding to phagocyte receptors the particle is ingested into a membrane-bound vesicle called the phagosome , which then fuses with lysosomes, resulting in discharge of lysosomal contents into the phagolysosome ( Fig. 2.5 ). During this process, neutrophils may also release granule contents into the extracellular space.
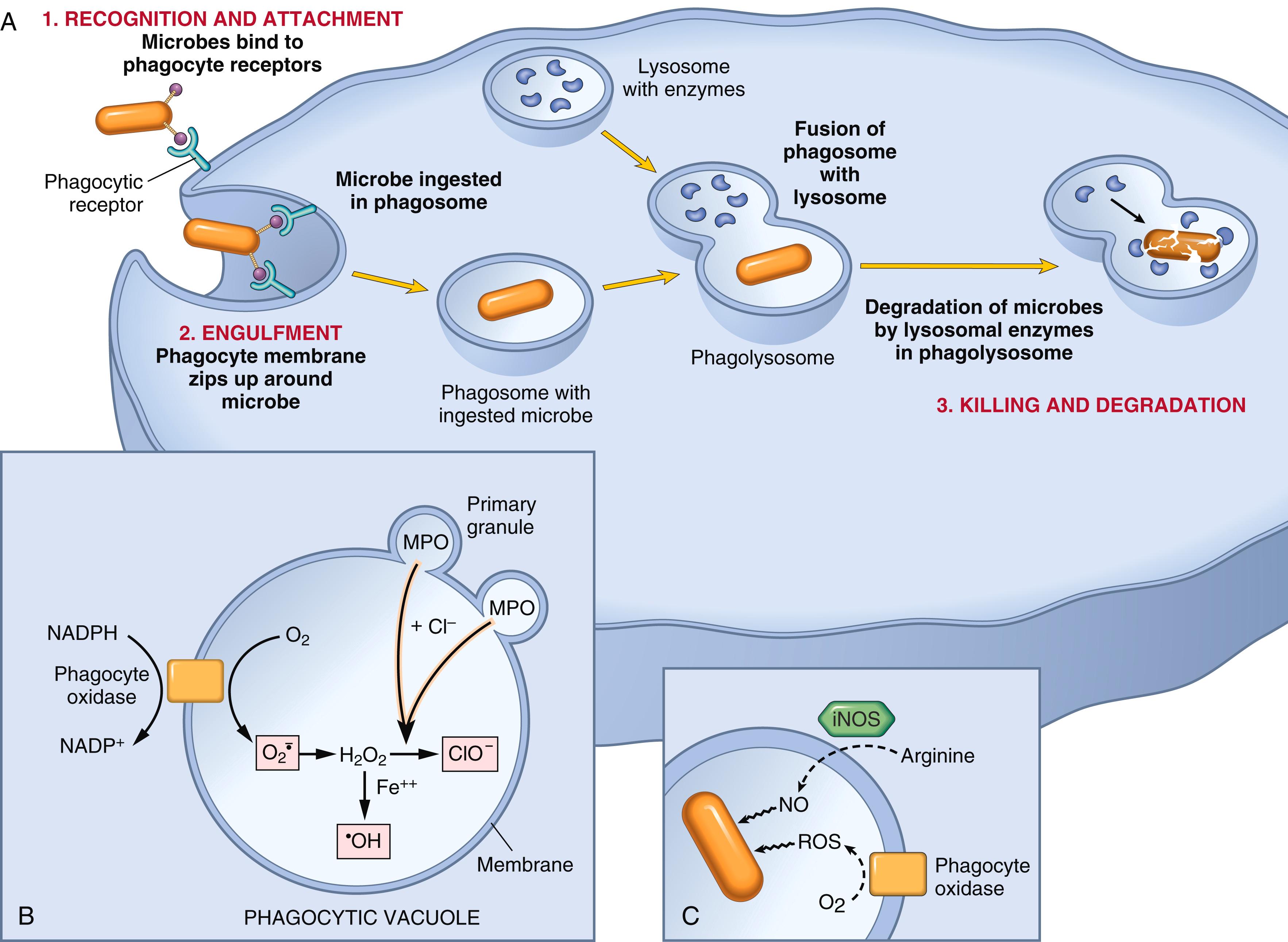
Killing of microbes and destruction of ingested materials are accomplished by reactive oxygen species (ROS, also called reactive oxygen intermediates ), reactive nitrogen species (mainly derived from nitric oxide [NO]), and lysosomal enzymes. All these substances are normally sequestered in lysosomes, to which phagocytosed materials are brought. Thus, potentially harmful substances are segregated from the cell’s cytoplasm to avoid damage to the phagocyte while it is performing its normal function.
These free radicals are produced mainly in the phagolysosomes of neutrophils. Upon activation of neutrophils, a multicomponent enzyme called phagocyte oxidase (or NADPH oxidase) is rapidly assembled in the membrane of the phagolysosome ( Fig. 2.5B ). This enzyme oxidizes NADPH (reduced nicotinamide-adenine dinucleotide phosphate) and, in the process, reduces oxygen to superoxide anion
, which is then converted to H 2 O 2 . H 2 O 2 is not able to efficiently kill microbes by itself. However, the azurophilic granules of neutrophils contain the enzyme myeloperoxidase (MPO), which, in the presence of a halide such as Cl − , converts H 2 O 2 to hypochlorite (ClO − ), a potent antimicrobial agent that destroys microbes by halogenation (in which the halide is bound covalently to cellular constituents) or by oxidation of proteins and lipids (lipid peroxidation). The H 2 O 2 -MPO-halide system is the most efficient bactericidal system of neutrophils. H 2 O 2 is also converted to hydroxyl radical (•OH), another powerful destructive agent. As discussed in Chapter 1 , these oxygen-derived free radicals bind to and modify cellular lipids, proteins, and nucleic acids and thus destroy cells such as microbes. The production of ROS coupled with oxygen consumption is called the respiratory burst . Genetic defects in the generation of ROS are the cause of an immunodeficiency disease called chronic granulomatous disease , described in Chapter 5 .
NO, a soluble gas produced from arginine by the action of nitric oxide synthase (NOS), also participates in microbial killing, especially in macrophages. Inducible NOS (iNOS) is upregulated in macrophages by transcriptional activation of the gene in response to microbial products and cytokines such as IFN-γ ( Fig. 2.5C ). NO reacts with superoxide
generated by phagocyte oxidase to produce the highly reactive peroxynitrite (ONOO − ). These nitrogen-derived molecules, similar to ROS, attack and damage the lipids, proteins, and nucleic acids of microbes.
Neutrophils have two main types of granules containing enzymes that degrade microbes and dead tissues and may contribute to tissue damage. The smaller specific (or secondary) granules contain lysozyme, collagenase, gelatinase, lactoferrin, plasminogen activator, histaminase, and alkaline phosphatase. The larger azurophil (or primary) granules contain myeloperoxidase, bactericidal factors (such as defensins), acid hydrolases, and a variety of neutral proteases (elastase, cathepsin G, nonspecific collagenases, proteinase 3). The contents of both types of granules are released when neutrophils are activated. Phagocytic vesicles containing engulfed material may fuse with these granules (and with lysosomes, as described earlier), allowing the ingested materials to be destroyed in the phagolysomes by the actions of the enzymes. Similarly, macrophages contain lysosomes filled with acid hydrolases, collagenase, elastase, and phospholipase, all of which can destroy ingested materials and cell debris.
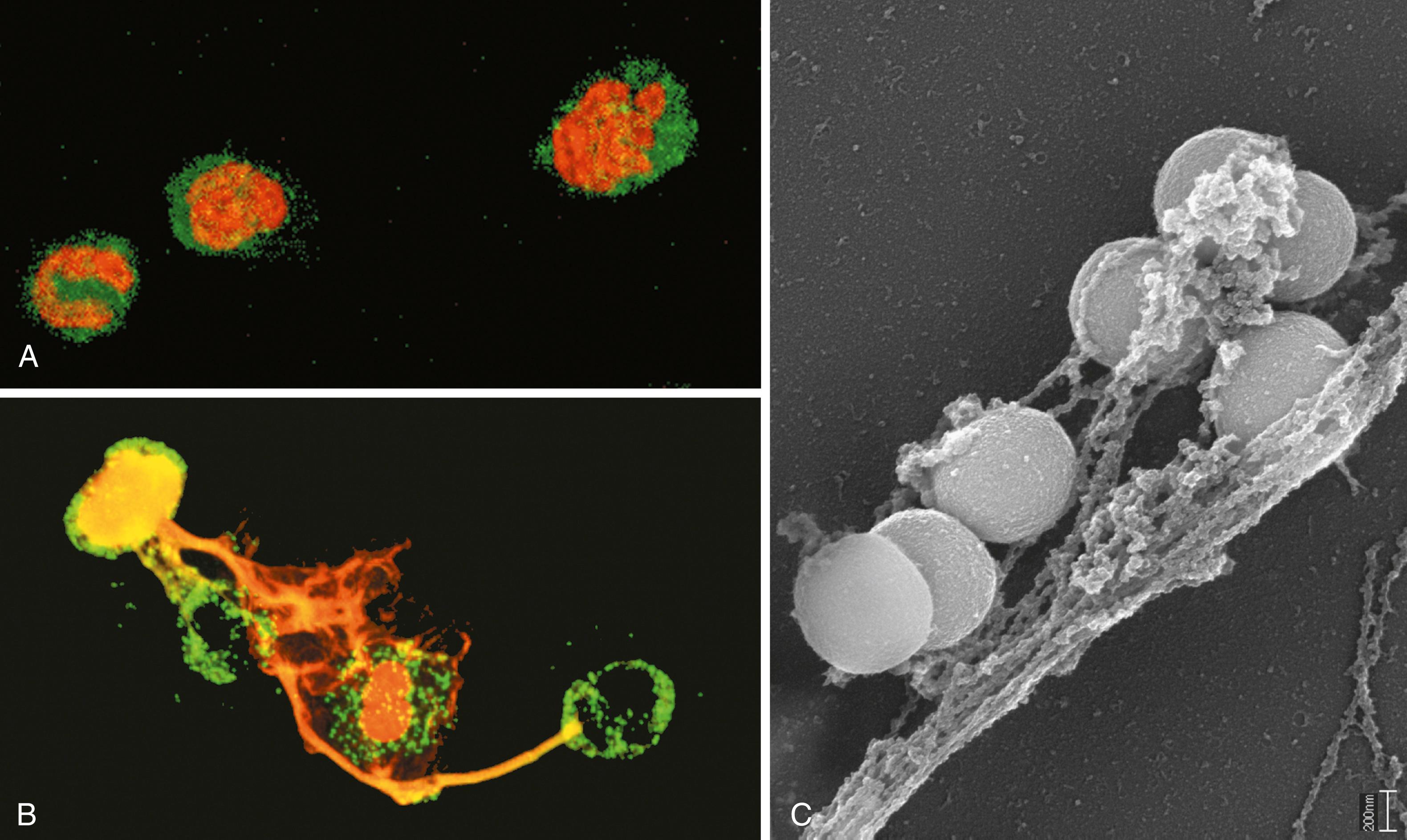
In addition to granule contents, activated neutrophils liberate chromatin components, including histones, which form fibrillar networks called neutrophil extracellular traps (NETs) ( eFig. 2.2 ). These networks bind and concentrate antimicrobial peptides and granule enzymes, forming extracellular sites for the destruction of microbes. In the process of NET formation, the nuclei of the neutrophils are lost, leading to death of the cells. NETs have also been detected in the blood during sepsis, as a consequence of widespread neutrophil activation.
Leukocytes are important causes of injury to normal cells and tissues. This happens during normal defense reactions against microbes, especially if the microbes are resistant to eradication, such as mycobacteria. It is also the basis of tissue damage when the response is inappropriately directed against self antigens (as in autoimmune diseases) or against normally harmless environmental antigens (as in allergic diseases).
The mechanism of leukocyte-mediated tissue injury is release of the contents of granules and lysosomes. To some extent, this happens normally, when activated leukocytes try to eliminate microbes and other offenders. The process is exaggerated if phagocytes encounter materials that cannot be easily ingested, such as antibodies deposited on indigestible flat surfaces, or if phagocytosed substances, such as urate and silica crystals, damage the membrane of the phagolysosome. The harmful proteases released by leukocytes are normally controlled by a system of antiproteases in the blood and tissue fluids. Foremost among these is α 1 -antitrypsin, which is the major inhibitor of neutrophil elastase. A deficiency of these inhibitors may lead to sustained protease activity, as is the case in patients with α 1 -antitrypsin deficiency ( Chapter 11 ).
While we have emphasized the role of neutrophils and macrophages in acute inflammation, other cell types also serve important roles. Some T cells, called Th17 cells , secrete cytokines such as IL-17 that recruit neutrophils and stimulate production of antimicrobial peptides that directly kill microbes. In the absence of effective Th17 responses, individuals are susceptible to fungal and bacterial infections. The skin abscesses that develop lack the classic features of acute inflammation, such as warmth and redness. Eosinophils are especially important in reactions to helminthic parasites and in some allergic disorders, and mast cells and basophils are critical cells of allergic reactions.
Once the acute inflammatory response has eliminated the offending stimulus, the reaction subsides because there is no further leukocyte recruitment, mediators are short lived and decline if they are no longer produced, and neutrophils have short life spans.
The inflammatory reaction is initiated and regulated by chemicals that are produced at the site of the reaction. The large number of mediators is daunting, but a basic understanding of the molecules is important because their identification has been the foundation for the development of many widely used and effective antiinflammatory drugs. We begin by summarizing the general properties of the mediators of inflammation and then discuss some of the more important molecules.
Mediators may be produced locally by cells at the site of inflammation or may be derived from circulating precursors that are activated at the site of inflammation.
Cell-derived mediators are rapidly released from intracellular granules (e.g., amines) or are synthesized de novo (e.g., prostaglandins and leukotrienes, cytokines) in response to a stimulus. The major cell types that produce mediators of acute inflammation are tissue macrophages, dendritic cells, and mast cells, but platelets, neutrophils, endothelial cells, and most epithelia also elaborate some inflammatory mediators.
Plasma-derived mediators (e.g., complement proteins) are produced mainly in the liver and circulate as inactive precursors that enter and are activated at sites of inflammation, usually by a series of proteolytic cleavages.
Active mediators are produced only in response to various stimuli, including microbial products and substances released from necrotic cells, which ensures that inflammation is triggered only when and where it is needed.
Most mediators are short lived. They quickly decay or are inactivated by enzymes, or they are otherwise scavenged or inhibited. These built-in control mechanisms prevent excessive reactions.
The principal mediators of acute inflammation are summarized in Table 2.5 and discussed next.
| Mediators | Sources | Actions |
|---|---|---|
| Histamine | Mast cells, basophils, platelets | Vasodilation, increased vascular permeability, endothelial activation |
| Prostaglandins | Mast cells, leukocytes | Vasodilation, pain, fever |
| Leukotrienes | Mast cells, leukocytes | Increased vascular permeability, chemotaxis, leukocyte adhesion, and activation |
| Cytokines (e.g., TNF, IL-1, IL-6) | Macrophages, endothelial cells, mast cells | Local: endothelial activation (expression of adhesion molecules) Systemic: fever, metabolic abnormalities, hypotension (shock) |
| Chemokines | Leukocytes, activated macrophages | Chemotaxis, leukocyte activation |
| Platelet-activating factor | Leukocytes, mast cells | Vasodilation, increased vascular permeability, leukocyte adhesion, chemotaxis, degranulation, oxidative burst |
| Complement | Plasma (produced in liver) | Leukocyte chemotaxis and activation, direct target killing (membrane attack complex), vasodilation (mast cell stimulation) |
| Kinins | Plasma (produced in liver) | Increased vascular permeability, smooth muscle contraction, vasodilation, pain |
The major vasoactive amine is histamine , which is stored as a preformed molecule in the granules of mast cells, blood basophils, and platelets. It is rapidly released when these cells are activated, so it is among the first mediators to be produced during inflammation. The richest source of histamine is the mast cell, which is normally present in the connective tissue adjacent to blood vessels. Mast cell degranulation and histamine release occur in response to a variety of stimuli, including binding of IgE antibodies to mast cells, which underlies immediate hypersensitivity (allergic) reactions ( Chapter 5 ); products of complement called anaphylatoxins (C3a and C5a), described later; and physical injury induced by trauma, cold, or heat, by unknown mechanisms. Antibodies and complement products bind to specific receptors on mast cells and trigger signaling pathways that induce rapid degranulation. Neuropeptides (e.g., substance P) and cytokines (IL-1, IL-8) may also trigger release of histamine.
Histamine causes dilation of arterioles and increases the permeability of venules. Its effects on blood vessels are mediated mainly via binding to histamine receptors called H 1 receptors on microvascular endothelial cells. Common antihistamine drugs that treat inflammatory reactions, such as allergies, bind to and block the H 1 receptor. Histamine also causes contraction of some smooth muscles, but leukotrienes, described later, are much more potent and relevant for causing spasms of bronchial smooth muscle, such as in asthma.
Serotonin (5-hydroxytryptamine) is a preformed vasoactive mediator present in platelets and certain neuroendocrine cells, for example, in the gastrointestinal tract. It is a vasoconstrictor, but its importance in inflammation is unclear.
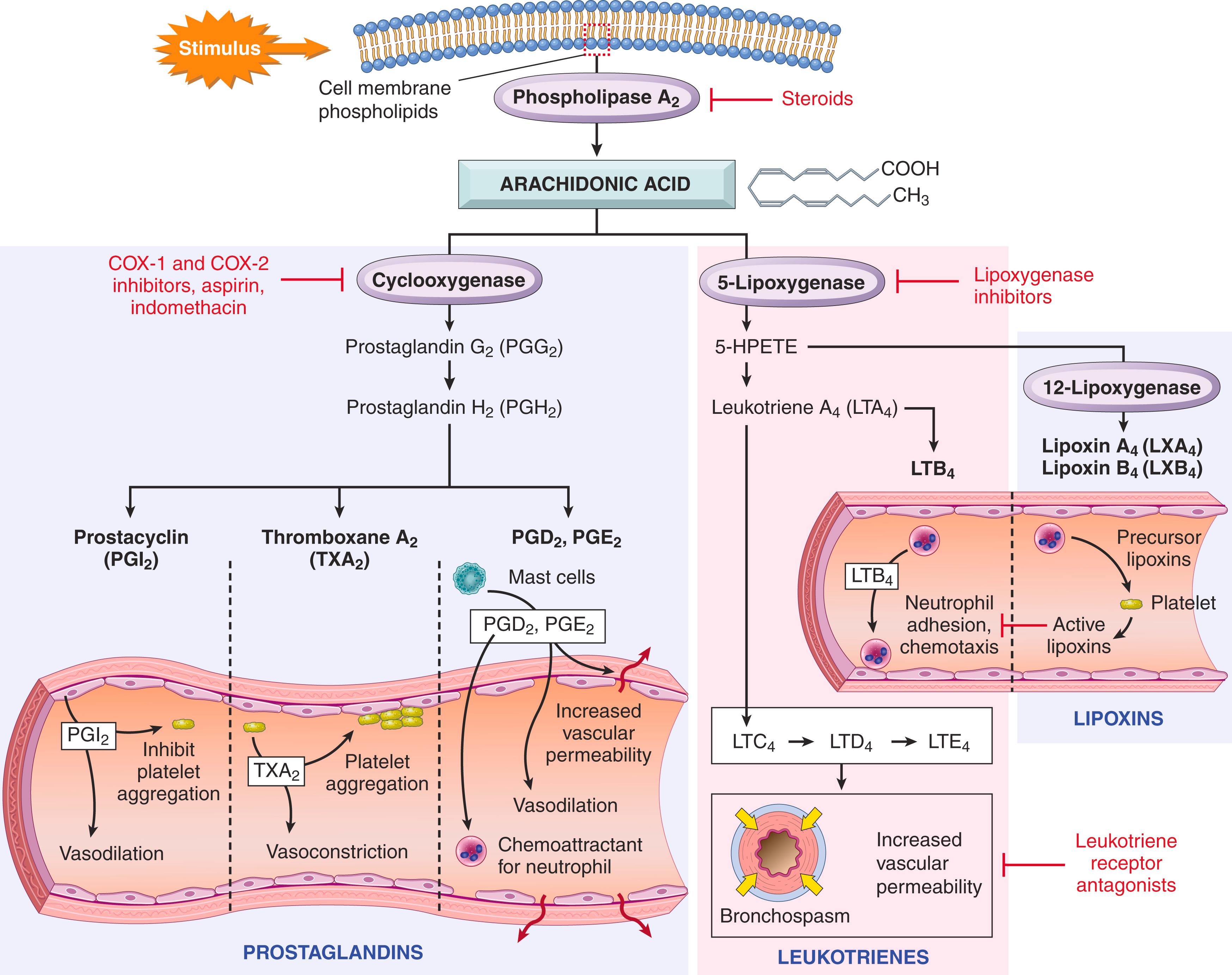
Prostaglandins and leukotrienes are lipid mediators produced from arachidonic acid (AA) present in membrane phospholipids that stimulate vascular and cellular reactions in acute inflammation. AA is a 20-carbon polyunsaturated fatty acid that is released from membrane phospholipids through the action of cellular phospholipases, mainly phospholipase A 2 , that are activated by inflammatory stimuli, including cytokines, complement products, and physical injury. AA-derived mediators, also called eicosanoids (from the Greek, eicosa , meaning 20, as they are derived from 20-carbon fatty acids), are synthesized by two major classes of enzymes, cyclooxygenases (which generate prostaglandins) and lipoxygenases (which produce leukotrienes and lipoxins) ( Fig. 2.6 ). Eicosanoids bind to G protein–coupled receptors on many cell types and can mediate virtually every step of inflammation ( Table 2.6 ).
| Action | Eicosanoids |
|---|---|
| Vasodilation | Prostaglandins PGI 2 (prostacyclin), PGE 1 , PGE 2 , PGD 2 |
| Vasoconstriction | Thromboxane A 2 , leukotrienes C 4 , D 4 , E 4 |
| Increased vascular permeability | Leukotrienes C 4 , D 4 , E 4 |
| Chemotaxis, leukocyte adhesion | Leukotriene B 4 |
Prostaglandins (PGs) are produced by mast cells, macrophages, endothelial cells, and many other cell types and are involved in the vascular and systemic reactions of inflammation. They are generated by the actions of two cyclooxgenases , called COX-1 and COX-2, which differ in where they are expressed. COX-1 is produced in response to inflammatory stimuli and is also constitutively expressed in most tissues, where it may have homeostatic functions (e.g., fluid and electrolyte balance in the kidneys, cytoprotection in the gastrointestinal tract). By contrast, COX-2 is induced by inflammatory stimuli and thus generates prostaglandins in inflammatory reactions but is low or absent in most healthy tissues.
Prostaglandins are named based on structural features coded by a letter, as in PGD, PGE, and others, and a subscript numeral (e.g., 1, 2), which indicates the number of double bonds in the compound. The most important prostaglandins in inflammation are PGE 2 , PGD 2 , PGF 2a , PGI 2 (prostacyclin), and TxA 2 (thromboxane A 2 ), each of which is derived by the action of a specific enzyme on an intermediate in the pathway. Some of these enzymes have restricted tissue distribution and functions.
PGD 2 is the major prostaglandin made by mast cells; along with PGE 2 (which is more widely distributed), it causes vasodilation and increases the permeability of postcapillary venules, thus potentiating exudation and resultant edema. PGD 2 is also a chemoattractant for neutrophils.
Platelets contain the enzyme thromboxane synthase, which produces TxA 2 , the major eicosanoid in these cells. TxA 2 is a potent platelet-aggregating agent and vasoconstrictor.
Vascular endothelium lacks thromboxane synthase and instead contains prostacyclin synthase, which is responsible for the formation of prostacyclin (PGI 2 ) and its stable end product PGF 1a . Prostacyclin is a vasodilator and a potent inhibitor of platelet aggregation and thus serves to prevent thrombus formation on normal endothelial cells. A thromboxane-prostacyclin imbalance has been implicated as an early event in thrombosis in coronary and cerebral arteries ( Chapter 10 ).
In addition to their local effects, prostaglandins are involved in the pathogenesis of pain and fever , two common systemic manifestations of inflammation (described later).
Leukotrienes are produced by leukocytes and mast cells by the action of lipoxygenase and are involved in vascular and smooth muscle reactions and leukocyte recruitment. The synthesis of leukotrienes involves multiple steps. The first generates leukotriene A 4 (LTA 4 ), which in turn gives rise to LTB 4 or LTC 4 . LTB 4 is produced by neutrophils and some macrophages and is a potent chemotactic agent and activator of neutrophils. LTC 4 and its metabolites, LTD 4 and LTE 4 , are produced mainly in mast cells and cause intense vasoconstriction, bronchospasm (important in asthma), and increased permeability of venules.
Lipoxins are also generated from AA by the lipoxygenase pathway, but unlike prostaglandins and leukotrienes, the lipoxins suppress inflammation by inhibiting neutrophil chemotaxis and adhesion to endothelium and hence the recruitment of leukocytes. Leukocytes, particularly neutrophils, produce intermediates in the lipoxin synthesis pathway that are converted to lipoxins by platelets interacting with the leukocytes.
Various other antiinflammatory AA-derived mediators have been described and given names such as resolvins because they resolve the active phase of acute inflammation. The role of these compounds in the inflammatory response is a topic of active study.
The importance of eicosanoids in inflammation has driven the development of antiinflammatory drugs, including the following:
Cyclooxygenase inhibitors include aspirin and other nonsteroidal antiinflammatory drugs (NSAIDs), such as ibuprofen. They inhibit both COX-1 and COX-2 and thus inhibit prostaglandin synthesis (hence their efficacy in treating pain and fever); aspirin does this by irreversibly inactivating cyclooxygenases. Selective COX-2 inhibitors were developed to target prostaglandins involved solely in inflammatory reactions. However, COX-2 inhibitors may increase the risk of cardiovascular and cerebrovascular events, possibly by impairing endothelial cell production of prostacyclin (PGI 2 ), which is antithrombotic, while leaving intact the COX-1–mediated production by platelets of thromboxane A 2 (TxA 2 ), which promotes platelet aggregation. COX-2 inhibitors are now used mainly to treat arthritis and perioperative pain in patients who do not have cardiovascular risk factors.
Lipoxygenase inhibitors. 5-lipoxygenase is not affected by NSAIDs. A pharmacologic agent that inhibits leukotriene production (zileuton) is useful in the treatment of asthma.
Corticosteroids are broad-spectrum antiinflammatory agents that reduce the transcription of genes encoding COX-2, phospholipase A 2 , proinflammatory cytokines (e.g., IL-1 and TNF), and iNOS.
Leukotriene receptor antagonists block leukotriene receptors and prevent the actions of the leukotrienes (zafirlukast). These drugs are used in the treatment of allergic asthma and allergic rhinitis.
Cytokines are proteins produced by many cell types (principally activated lymphocytes, macrophages, and dendritic cells, but also endothelial, epithelial, and connective tissue cells) that mediate and regulate immune and inflammatory reactions. By convention, growth factors that act on epithelial and mesenchymal cells are not grouped under cytokines. The general properties and functions of cytokines are discussed in Chapter 5 . Here the cytokines involved in acute inflammation are reviewed ( Table 2.7 ).
| Cytokine | Principal Sources | Principal Actions in Inflammation |
|---|---|---|
| In Acute Inflammation | ||
| TNF | Macrophages, mast cells, T lymphocytes | Stimulates expression of endothelial adhesion molecules and secretion of other cytokines; systemic effects |
| IL-1 | Macrophages, endothelial cells, some epithelial cells | Similar to TNF; greater role in fever |
| IL-6 | Macrophages, other cells | Systemic effects (acute-phase response) |
| Chemokines | Macrophages, endothelial cells, T lymphocytes, mast cells, other cell types | Recruitment of leukocytes to sites of inflammation; migration of cells in healthy tissues |
| In Chronic Inflammation | ||
| IL-12 | Dendritic cells, macrophages | Increased production of IFN-γ |
| IFN-γ | T lymphocytes, NK cells | Activation of macrophages (increased ability to kill microbes and tumor cells) |
| IL-17 | T lymphocytes | Recruitment of neutrophils and monocytes |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here