Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Infertility is considered to be a disease and affects approximately 7% of all U.S. couples of reproductive age—more than 7 million women in the United States.
A systematic evaluation of factors involved in infertility should be carried out rapidly, along with markers of ovarian reserve (antral follicle count, antimüllerian hormone); this will help frame the discussion with couples as to how best to proceed with treatment.
The prognosis for fertility after tubal reconstruction depends on the amount of damage to the tube and the location of the obstruction. Mild abnormalities of the proximal tube may be treated with selective catheterization/cannulation under fluoroscopy. Large hydrosalpinges (distal disease) are best treated by salpingectomy and in vitro fertilization (IVF).
In women with unexplained infertility, the use of controlled ovarian stimulation (COS) and intrauterine insemination (IUI) with clomiphene/IUI or gonadotropins/IUI yields monthly fecundity rates of approximately 8% to 10% (at least doubling the baseline rate) and should be the initial treatment for unexplained infertility. Use of gonadotropins does not offer a major advantage over clomiphene and carries more risks in terms of hyperstimulation and multiple pregnancies.
After three to six cycles of COS/IUI, IVF should be offered as the next step, and IVF should be the primary therapy in women around age 40.
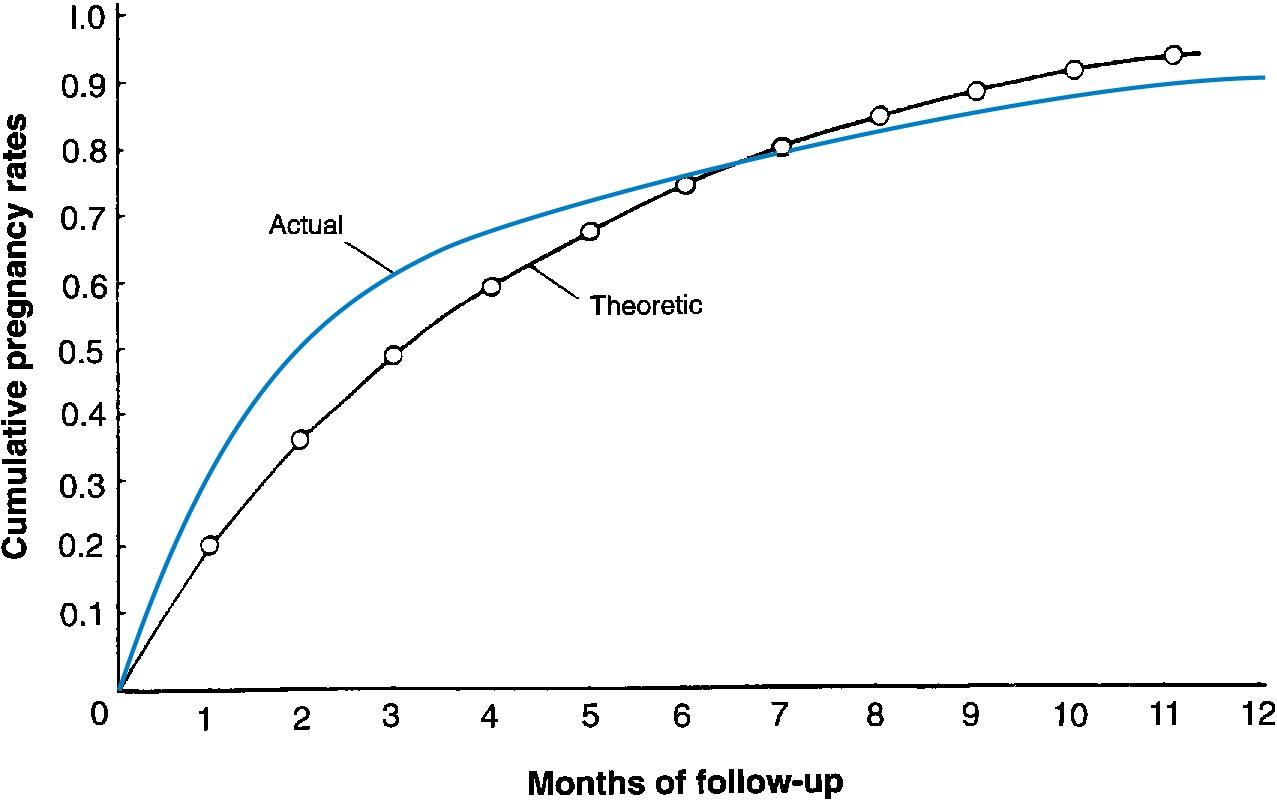
The term infertility is generally used to indicate that a couple has a reduced capacity to conceive compared with the mean capacity of the general population. In a group of normally fertile couples, the monthly ability to get pregnant, or fecundability , is approximately 20% (0.02). Analysis of data from presumably fertile couples who stop using contraception to conceive is depicted in Fig. 40.1 , with both the actual and theoretical time trends. ( ). The 20% is an average number and can be as high as 25% for the first 3 months of trying to conceive, but then decreases after that for the following months going forward ( ) ( Table 40.1 ). As time goes on, during the fourth, fifth, and sixth years of attempting to conceive, only 48%, 42%, and 37% of nonpregnant women conceive without treatment. These data are highly influenced by age, which will be discussed later.
| Couples Not Yet Having Conceived | |||
|---|---|---|---|
| Months without Conception | Proportion (%) | Mean Fecundability | Proportion (%) of Couples Who Will Conceive (within 12 mo) |
| 0 | 100.0 | 0.20 | 86.0 |
| 6 | 31.9 | 0.14 | 77.0 |
| 12 | 14.0 | 0.11 | 69.2 |
| 24 | 4.3 | 0.08 | 57.0 |
| 36 | 1.9 | 0.06 | 48.2 |
| 48 | 1.0 | 0.05 | 41.7 |
| 60 | 0.6 | 0.04 | 36.7 |
The definition of infertility is the inability of a couple to conceive after 1 year of trying. This timeline is relevant to help determine when an infertility investigation should begin. In women older than 35 years, this timeline should be after 6 months of trying. An early investigation is also warranted if any of the following is present: oligomenorrhea or amenorrhea; known tubal obstruction, uterine disease, or severe endometriosis; or known male factor infertility ( ).
The World Health Organization (WHO) has defined infertility as a disease and a significant cause of disability (warranting evaluation and treatment) (see www.who.int/reproductivehealth/topics/infertility/definitions ). Clearly, infertility is a cause of major distress for couples and should be assessed thoroughly and not neglected; and as with other disorders, counseling and support groups should be available in the clinical setting.
Approximately 6% to 7% of all reproductive-age women (15 to 44 years) in the United States are considered to be infertile according to statistics from the Centers for Disease Control and Prevention (CDC). The number of women in this age group who have ever used infertility services has been estimated to be 12% or 7.3 million women in the United States.
Data from both older and more recent studies have indicated that the percentage of infertile couples increases with increasing age of the female partner. Analysis of data from three national surveys in the United States has revealed that the percentage of presumably fertile married women not using contraception who failed to conceive after 1 year of trying steadily increased from ages 25 to 44 years ( ) ( Table 40.2 ). Data from a study of presumably fertile nulliparous women married to husbands with azoospermia who underwent donor artificial insemination revealed that the percentage who conceived after 12 cycles of insemination declined substantially after age 30 ( ) ( Table 40.3 ). This older, classic study used fresh semen; currently, only frozen donor sperm is used, which does not achieve as favorable pregnancy rates. Decreasing fecundability with age is even more pressing in this context. With in vitro fertilization (IVF), data from the Society for Assisted Reproductive Technology (SART) in the United States indicate that the percentage of deliveries per oocyte retrieval procedure is 43.4% in women younger than 35, 25.4% by ages 38 to 40, 14% by ages 41 to 42 years, and only 5% in women older than 42 years ( https://www.cdc.gov/art/artdata/index.html ).
| Age (yr) | Infertile (%) |
| 20-24 | 7.0 |
| 25-29 | 8.9 |
| 30-34 | 14.6 |
| 35-39 | 21.9 |
| 40-44 | 28.7 |
| Age (yr) | Pregnancy Rate (%) |
| <25 | 73.0 |
| 26-30 | 74.1 |
| 31-35 | 61.5 |
| 36-40 | 55.8 |
In general terms, approximately one in seven couples are infertile if the wife is 30 to 34, one in five is infertile if she is 35 to 40, and one in four is infertile if she is aged 40 to 44 years. Another way to interpret these data is to state that compared with women aged 20 to 24 years, fertility is reduced by 6% in the next 5 years, by 14% between ages 30 and 34, by 31% between ages 35 and 39, and to a much greater extent after age 40. Of interest is the finding that the most common diagnostic category among women undergoing IVF in the United States in the most recent survey cited previously is diminished ovarian reserve: 17% of cycles; which is a characteristic of older women undergoing treatment ( https://www.cdc.gov/art/artdata/index.html ).
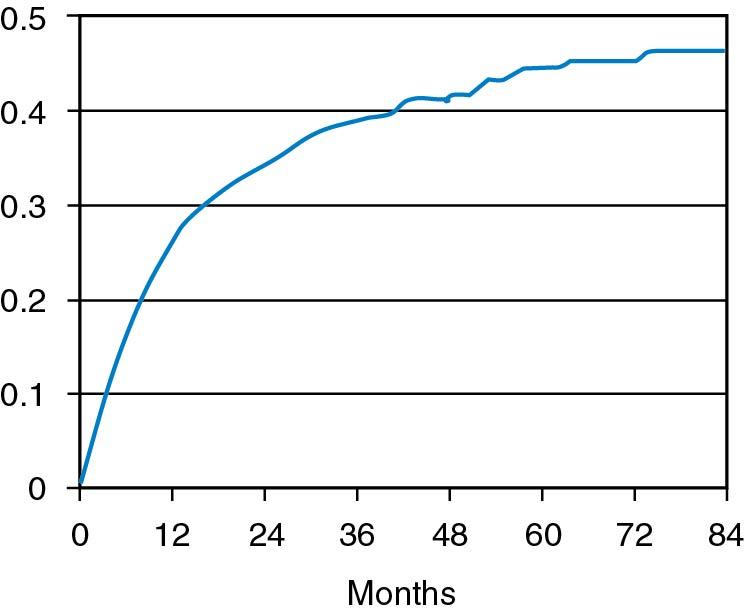
The exact incidence of the various factors causing infertility varies among different populations and cannot be precisely determined. Collins reported that among 14,141 couples in 21 publications, ovulatory disorders occurred 27% of the time; male factor, 25%; tubal disorders, 22%; endometriosis, 5%; other, 4%; and unexplained factors, 17% ( ). It has not been shown that other abnormalities, such as antisperm antibodies, luteal phase deficiency, subclinical genital infection, or subclinical endocrine abnormalities such as hypothyroidism or hyperprolactinemia in ovulatory women, are true causes of infertility. No prospective randomized studies have demonstrated that treatment of these latter entities results in greater fecundability than without treatment. If any of these do cause infertility, they do so infrequently. With current techniques of investigation, it is not possible to diagnose the cause of infertility in up to 20% of couples, and they are considered to have unexplained infertility . After a rigorous investigation, other reports have suggested this figure to be as low as 10%; however, it is unclear if subtle abnormalities, as noted, have much to do with infertility. Also, most couples with unexplained infertility are subfertile rather than infertile, and some couples are able to conceive without treatment , although it may take several years. A live birth rate among 873 infertile couples in several Canadian centers has been observed without treatment. The cumulative live conception rate was 38.2% at 3 years and 45% after 7 years ( ) ( Fig. 40.2 ). Among the total group with the diagnosis of unexplained infertility who received no treatment, one-third had a live birth during the first 3 years of observation without treatment.
The diagnostic evaluation of infertility should be thorough and completed as rapidly as possible. During the initial interview, the couple should be informed about normal human fecundity and how these probabilities decrease with increasing age of the female partner and with the duration of infertility. The various tests in the diagnostic evaluation and why they are performed should be thoroughly explained. The available therapies and prognosis for treatment of the various causes of infertility should also be included in the dialogue. The couple should be informed that after a complete diagnostic infertility evaluation, the cause for infertility cannot be determined in a large group of couples. For many couples, the reduced fecundability can be suggested to be age related. Methods to increase the fecundity of couples with a normal diagnostic evaluation, such as controlled ovarian stimulation (COS) and intrauterine insemination, and the possibility of IVF should be covered.
Each couple should be instructed about the optimal time in the cycle for conception to occur and should be encouraged to have intercourse on the day before ovulation. Unless the husband has oligozoospermia (oligospermia), daily intercourse for 3 consecutive days at midcycle should be encouraged. When ovulation is more precisely determined, as with luteinizing hormone (LH) monitoring (discussed later), intercourse should occur for 2 consecutive days around the LH surge. Because the egg disintegrates less than 1 day after it reaches the ampulla of the tube, it is best that sperm be present in this area when the egg arrives so that fertilization can occur. Because normal sperm retain their fertilizing ability for up to 72 hours, it is preferable to have sperm in the tube before the arrival of the oocyte.
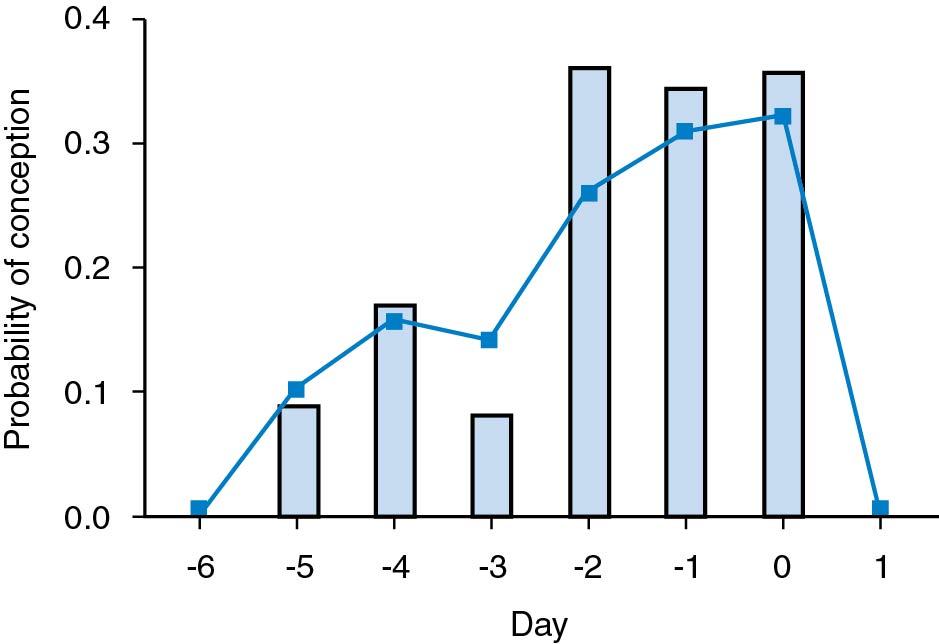
A study was performed by Wilcox and coworkers of fertile couples who stopped contraception to conceive and recorded the cycle day when they had sexual intercourse. Hormone analysis was performed to determine the day of ovulation. None of the women became pregnant in the group of couples who had intercourse after ovulation occurred . The pregnancy rate was approximately 30% if intercourse occurred on the day of ovulation, as well as 1 and 2 days before ovulation. The pregnancy rate was approximately 10% if coitus occurred 3, 4, or 5 days before ovulation. No pregnancies occurred when intercourse took place 6 days or more before ovulation ( ) ( Fig. 40.3 ). It is therefore considered optimal to perform insemination or have sexual intercourse on the day before ovulation.
Because peak levels of LH occur 1 day before ovulation, measurement of LH by urinary LH immunoassays is the best way to determine the optimal time to have intercourse or an insemination. Tests that measure LH in a random daily urine specimen are usually more convenient for planning natural or artificial insemination than tests that detect LH in the first morning urine specimen. Ovulation most commonly occurs on the day after the detection of LH in a random specimen (12 to 24 hours later ), and it occurs on the day when LH is detected in the first morning specimen, which contains urine formed during the prior night. Several types of commercial kits are available for determining peak LH, so women who find it difficult to determine a hormone change using one type can try another system or kit. Kits may vary in terms of sensitivity and variations may occur based on the dilution of the urine and hormonal conditions such as polycystic ovary syndrome (PCOS), which can give false positive tests. Basal body temperature (BBT) charts are not as precise for determining ovulation, with ovulation occurring over a span of several days of the thermogenic shift. In the last several years, multiple fertility apps have been launched, and a bracelet worn at night (Eva) has been advertised to provide information on a 5-day fertile window.
In some cases, women produce less than adequate amounts of vaginal lubricant. Various vaginal lubricants and chemicals, as well as saliva, used to improve coital satisfaction may interfere with sperm transport. Some men experience midcycle impotence because of the pressure of performing intercourse on demand. In such cases the intercourse schedule should be less rigorous. The couple should also be told that among fertile couples, there is only approximately a 20% chance of conceiving in each ovulatory cycle, even with optimally timed coitus, and that it takes time to become pregnant; thus the terms time and timing should be emphasized during the initial counseling session. Couples should also be advised to cease smoking cigarettes and drinking caffeinated beverages in excess. Cigarette smoking and caffeine consumption have been shown independently in several studies to decrease the chances of conception. The common practice of vaginal douching also reduces the chance of conception by approximately 30%.
All couples should have a complete history taken, including a sexual history, and a physical examination, which is often ultrasound based. After this initial evaluation, tests should be undertaken to determine whether the woman is ovulating and has patent fallopian tubes and if a semen sample of the male partner is normal.
Preliminary information that the woman is ovulatory is provided by a history of regular menstrual cycles. If the woman has regular menstrual cycles, a serum progesterone level should be measured in the midluteal phase to provide indirect evidence of ovulation and normal luteal function. Although serum progesterone levels vary in the normal luteal phase in a pulsatile manner, a serum progesterone level greater than 3 to 5 ng/mL suggests some ovulatory function; however, it cannot indicate the adequacy of normal ovulation. Progesterone levels of 10 ng/mL or higher are found during at least 1 day of the luteal phase of normal ovulatory cycles in which conception occurred ( ). Measurement of the daily BBT provides indirect evidence that ovulation has taken place but does not give precise information about ovulation timing as does the LH kit. The quality of ovulation cannot be determined accurately but may be suggested by a well-timed progesterone level. With the number of other methods such as phone apps and the bracelet, the use of the BBT, which is difficult for many women to do, has appropriately decreased.
Women with oligomenorrhea (menses at intervals of 35 days or longer) or amenorrhea who wish to conceive should be treated with agents that induce ovulation, regardless of whether they have occasional ovulatory cycles. Therefore for these women, direct or indirect measurement of progesterone is unnecessary until after therapy is initiated.
The endometrial biopsy is sometimes considered as a diagnostic method for the adequacy of ovulation and luteal function. We feel that an endometrial biopsy is not indicated in this setting of assessing ovulatory function . It is invasive and painful, and it does not provide accurate information in terms of “endometrial dating” of the luteal phase, as was carried out in the past ( ); however, an endometrial biopsy may be indicated as a subsequent test when assessing for endometrial receptivity and ruling out chronic endometritis in the setting of repeated implantation failure or recurrent miscarriage, as will be discussed in other chapters.
Although information about ovulation is being obtained, the male partner’s reproductive system should be evaluated by means of a semen analysis. Most urologists dealing with male fertility issues require obtaining two semen analyses. Abnormalities in the semen analysis (male factor) occurs in approximately 20% of couples with infertility as the sole factor and may be involved in 30% to 40% of cases overall . The male partner should be advised to abstain from ejaculation for 2 to 3 days before collection of the semen sample. It is best to collect the specimen in a clean (not necessarily sterile) wide-mouthed jar after masturbation. It is important that the entire specimen be collected because the initial fraction contains the greatest density of sperm. Ideally, collection should take place in the location where the analysis will be performed. The degree of sperm motility should be determined as soon as possible after liquefaction, which usually occurs 15 to 20 minutes after ejaculation. Sperm motility begins to decline 2 hours after ejaculation, and it is best to examine the specimen within this period. Semen should not be exposed to marked changes in temperature and, if collected at home during cold weather, the specimen should be kept warm during transport to the laboratory.
Parameters used to evaluate the semen include volume, viscosity, sperm density, sperm morphology, and sperm motility. The last parameter should be evaluated in terms of percentage of total motile sperm and quality of motility (rapidity of movement and amount of progressive motility). Sperm morphology is an extremely important parameter and is correlated to fertilizing ability. Using strict criteria (Kruger), only approximately 4% or more of the sperm in an ejaculate may be considered normal according to the WHO criteria ( ). It should be remembered that the sperm analysis is a subjective test and that there is a fair degree of variability from test to test in the same man. Also, the semen profile reflects sperm production that occurred 3 months earlier, which is important to note if there was illness at that time. Table 40.4 lists the parameters that are generally considered normal for a semen analysis, according to the WHO study. It is beyond our scope here to discuss fully the causes and diagnostic evaluation of semen abnormalities. In broad terms, the various causes of semen abnormalities are cited in Table 40.5 .
| Parameter | Value |
| Semen volume (mL) | 1.5 |
| Sperm concentration (million/mL) | 15 |
| Total number (million/ejaculate) | 39 |
| Total motility (%) | 40 |
| Progressive motility (%) | 32 |
| Normal forms (%) | 4 |
| Finding | Cause |
| A bnormal C ount | |
| Azoospermia | Klinefelter’s syndrome or other genetic disorder Sertoli-cell–only syndrome Seminiferous tubule or Leydig cell failure Hypogonadotropic hypogonadism Ductal obstruction, including Young syndrome Varicocele Exogenous factors |
| Oligozoospermia | Genetic disorder Endocrinopathies, including androgen receptor defects Varicocele and other anatomic disorders Maturation arrest Hypospermatogenesis Exogenous factors |
| A bnormal V olume | |
| No ejaculate | Ductal obstruction Retrograde ejaculation Ejaculatory failure Hypogonadism |
| Low volume | Obstruction of ejaculatory ducts Absence of seminal vesicles and vas deferens Partial retrograde ejaculation Infection |
| High volume | Unknown factors |
| Abnormal motility | Immunologic factors Infection Varicocele Defects in sperm structure Metabolic or anatomic abnormalities of sperm Poor liquefaction of semen |
| Abnormal viscosity | Cause unknown |
| Abnormal morphology | Varicocele Stress Infection Exogenous factors Unknown factors |
| Extraneous cells | Infection or inflammation Shedding of immature sperm |
When semen analyses were performed on a group of men whose wives had conceived within the past 4 months, approximately 75% had at least one abnormal characteristic and 25% had two abnormalities. These results confirm that there is normally a wide variability in the parameters used to characterize semen . Because the characteristics of semen may vary over time and undergo normal biologic variability, it is best to repeat the test at least once if an abnormality is found. If abnormalities persist, the male partner should have a urologic examination . It is important not to miss a rare abnormality, such as a testicular tumor; in addition it has been appreciated that male factor infertility is associated with other medical conditions and subsequent problems ( ).
The comprehensive evaluation should include a history of physical examination (occasionally with ultrasound); hormonal evaluation (LH, follicle-stimulating hormone [FSH], testosterone, estradiol, prolactin [PRL], and thyroid-stimulating hormone [TSH]); and genetic abnormalities (karyotype and defects such as cystic fibrosis mutations and Y-chromosome microdeletions), particularly with severe sperm abnormalities ( ).
Aspects of the woman’s medical history that should be highlighted include the following: any pregnancy complications if previously pregnant; previous pelvic surgery of any type; significant dysmenorrhea; dyspareunia or sexual dysfunction; abnormal cervical cytologic test results or procedures to treat cervical abnormalities; and use of medication, drugs, and tobacco. Family history should be explored for genetically related illnesses, birth defects, and, most importantly, the history of age of menopause in female family members. Finally, any symptoms suggestive of endocrine disorders should be solicited (e.g., weight changes, skin changes).
The physical examination should focus on extremes of body mass, skin changes, thyroid abnormalities, breast secretion, abnormal pain on abdominal or pelvic examination, and assessment of the vagina and cervix. In addition, if available, vaginal ultrasound performed at the same time may be extremely valuable in picking up abnormalities of the uterus (e.g., fibroids) endometrial thickness, pelvic masses, and ovarian morphology (e.g., polycystic appearance, unusually small). These may provide a guide for further testing.
In a healthy woman, a complete blood cell count (CBC), blood type, Rh factor, and rubella status are needed, together with the record of a Papanicolaou (Pap) test obtained within 3 years. Increasingly it is recommended (although not mandatory) to screen for genetic carrier status. Comprehensive screening for carrier status, including fragile X and other abnormalities such as cystic fibrosis, is easily carried out at the time of routine blood testing. Several laboratories can do such screening. Infectious disease screening (for chlamydia and gonorrhea) is carried out routinely in most practices at the time of the Pap test. Further infectious disease screening (e.g., syphilis, human immunodeficiency virus [HIV], hepatitis) is warranted, particularly for couples undergoing insemination or IVF.
In most women, and particularly in women older than 35 years, serum FSH and estradiol (E 2 ) levels should be obtained on cycle day 2 or 3. Elevated FSH values (>10 mIU/mL) suggest decreased ovarian reserve, which reflects the pool of viable oocytes remaining in the ovary. Levels greater than 20 mIU/mL afford a particularly poor prognosis; however, although FSH levels tend to fluctuate from cycle to cycle, once the FSH level has been elevated in a given cycle, the overall prognosis is reduced. E 2 levels, if elevated on days 2 and 3 (>70 pg/mL), do not allow for a valid interpretation of FSH values and may independently suggest a decreased prognosis regarding ovarian reserve.
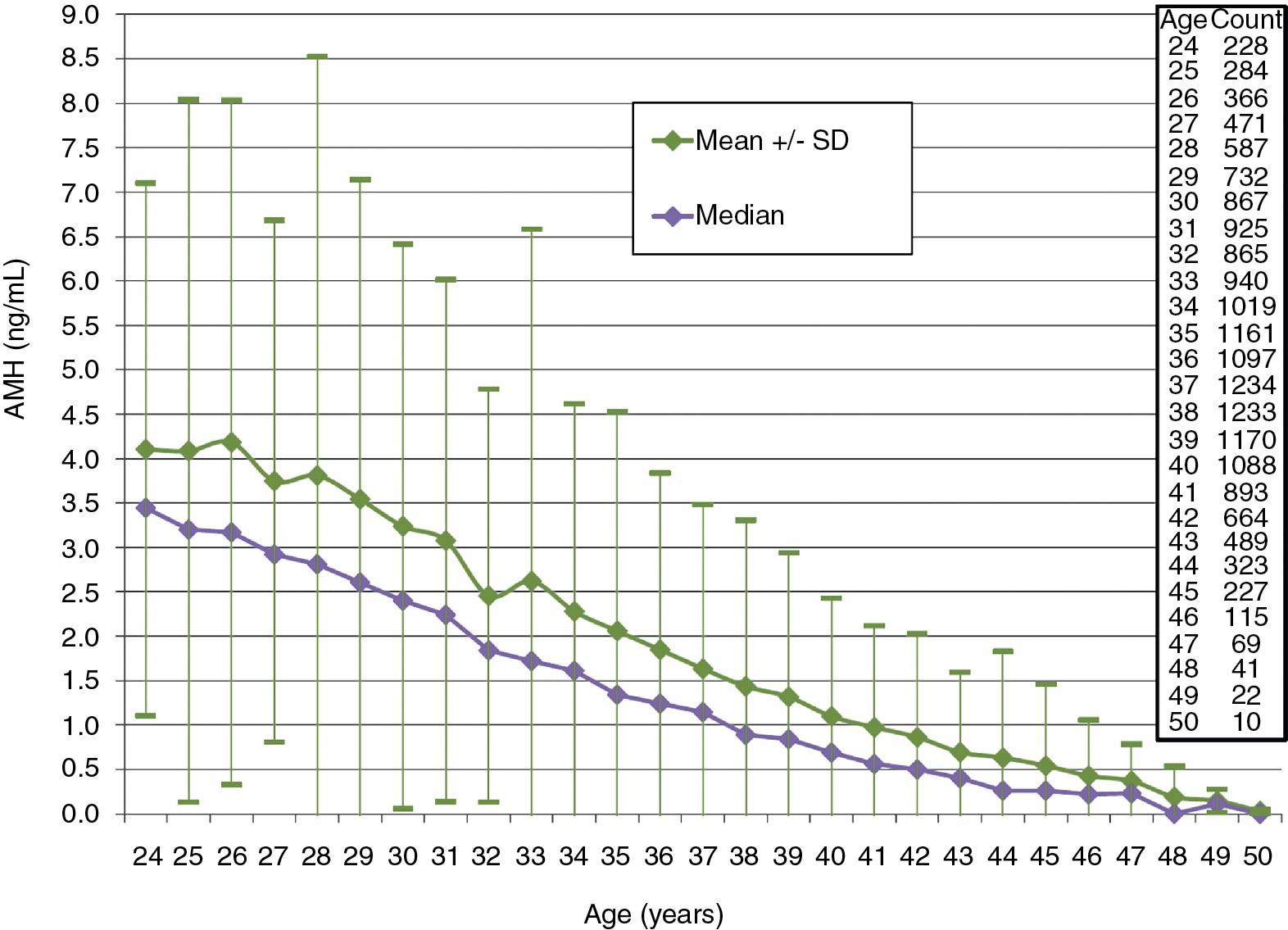
Antimüllerian hormone (AMH) has become a valuable standard for assessing ovarian reserve . AMH, which is produced by the granulosa cells of small growing follicles, physiologically suppresses FSH stimulation of sustained follicular growth. Levels are highest in young women and lower with reproductive aging; various nomograms by age have been established ( ) ( Fig. 40.4 ). Serum AMH decreases with aging, and when levels reach 0.05 ng/mL (essentially undetectable levels), menopause occurs within 4 to 5 years. Levels are higher in women with PCOS ( ). The biggest concern with measurement of AMH is differences between assays because there is no international standard; however, in general, higher levels (>2 ng/mL) suggest a larger cohort of small available follicles and low levels (<0.5 ng/mL) suggest a decreased ovarian reserve. Values are of concern if they are less than 1 ng/mL. The level of AMH also reflects the sensitivity of the ovary to gonadotropic stimulation, and thus the choice of treatment when ovarian stimulation is desired. Unlike FSH, AMH values are fairly constant and stable throughout the menstrual cycle, particularly in the low ranges. Higher values, however, exhibit more variability in the early to midfollicular phase. It is now established that use of oral contraceptive pills decreases values by 15% to 20%.
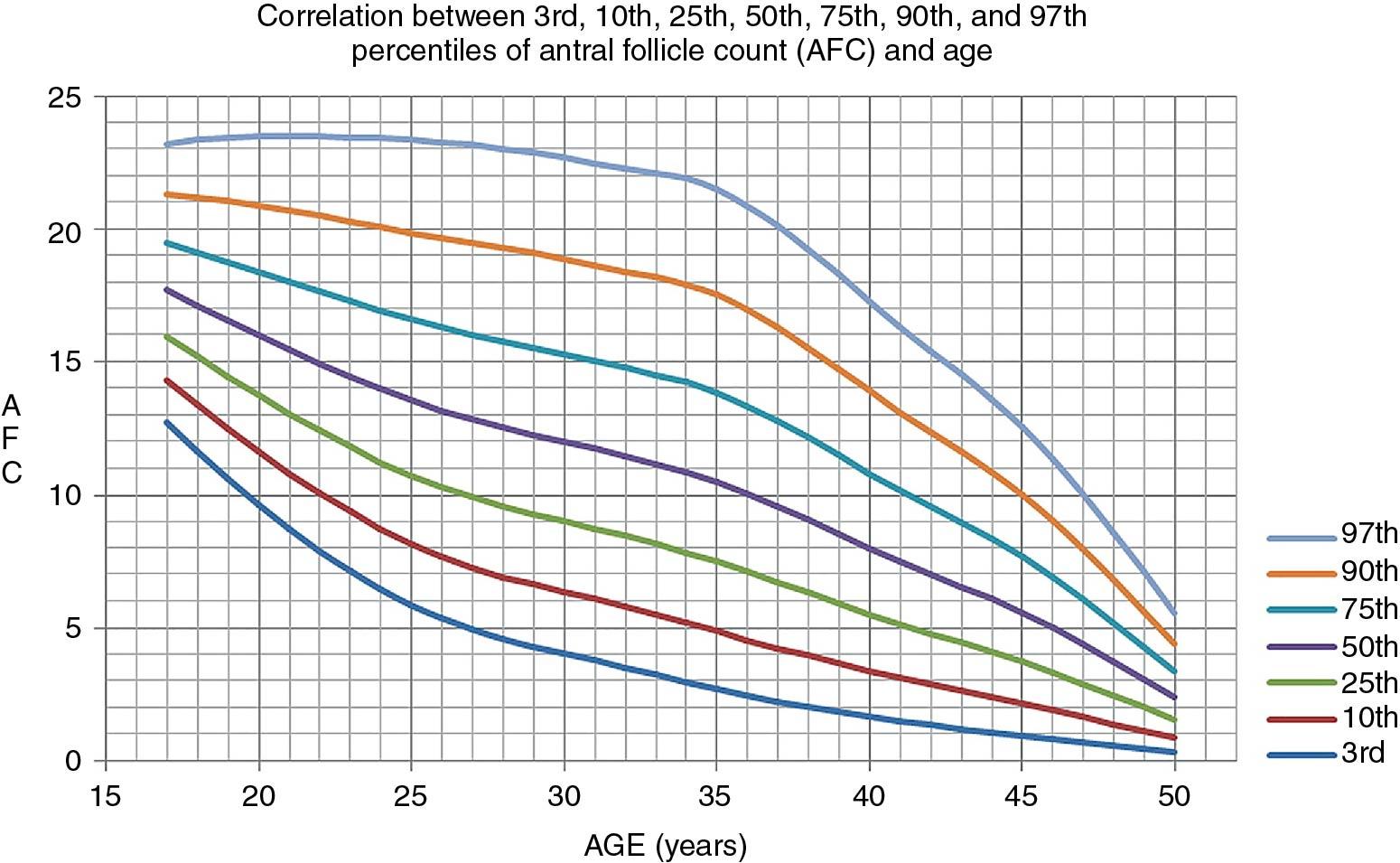
It is most common to carry out a pelvic ultrasound evaluation as part of the investigation. By so doing, significant pathologic conditions such as fibroids, endometriosis, and other changes can be uncovered. In addition polycystic ovaries, which are prevalent, can be appreciated; and finally an antral follicle count (AFC) can be obtained , which is similar in value to the measurement of AMH, in the assessment of ovarian reserve. An age-related nomogram for AFCs has also been reported ( ) ( Fig. 40.5 ). For standardization it has been suggested that the AFC be obtained on cycle days 2 to 4, although an AFC can be obtained at other times in the cycle as long as all follicular structures over 10 mm are not counted.
Some specialists obtain antibody titers for Chlamydia trachomatis, which if elevated may signify the possibility of tubal disease. It has been suggested that if the immunoglobulin G (IgG) antibody titer is greater than 1:32, 35% of patients have evidence of tubal damage. Whether this type of evaluation is routinely warranted as a focus for the infertility investigation continues to be debated.
Although not proven to be of major benefit in normal ovulatory women, most clinicians will measure TSH and PRL at the screening visit. TSH values in the normal range (<4.4 μU/mL), but higher than 2.5 mIU/mL are often considered to be abnormal in women presenting with infertility. This is because normal values in the first trimester of pregnancy should be less than 2.5 μU/mL; however, there is no evidence that values between 2.5 to 4 μU/mL affect fertility status or outcomes of pregnancy ( ). It is common to have slightly elevated PRL levels at the initial visit, which normalize when retested in the fasting state.
If an abnormality is found in one of the first two noninvasive diagnostic procedures (documentation of ovulation and semen analysis), it should be treated before proceeding with the more costly and invasive procedures, unless there is a history or findings suggestive of tubal disease. For example, if the woman has oligomenorrhea and does not ovulate each month, after a normal semen analysis is observed, ovulation should be induced with clomiphene citrate for two to three cycles before performing the other diagnostic measures. Provided that no other infertility factors are present, most anovulatory women (80%) conceive after induction of ovulation with therapeutic agents, and half the couples will conceive during the first three ovulatory cycles ( ).
If these initial diagnostic tests are normal, the more uncomfortable and costly hysterosalpingography (HSG) should be performed in the follicular phase of the next cycle.
It is best to schedule the HSG during the week after the end of menses to avoid a possible pregnancy and also get better definition of the uterine cavity when the endometrium is still thin. The HSG should be avoided if there has been a history of salpingitis in the recent past or if there is tenderness on pelvic examination. As noted, most practices routinely screen for chlamydia and gonorrhea during the initial examination; however, we routinely prescribe prophylactic antibiotics at the time of HSG: doxycycline (100 mg twice daily for 3 days, starting 1 day before the procedure), but this recommendation is not universally followed. If a hydrosalpinx is seen with HSG, doxycycline should be continued for 1 week. The examination should be performed with use of a water-soluble contrast medium and image-intensified fluoroscopy. A water-soluble contrast medium enables better visualization of the tubal mucosal folds and vaginal markings than an oil-based medium. It is important to be able to evaluate the appearance of the intratubal architecture to determine the extent of damage to the tube ( Fig. 40.6 , A and B ). Although it has been debated for several years, a large randomized trial in the Netherlands concluded that an oil-based contrast resulted in a higher ongoing pregnancy rate and live birth rate compared with water-based contrast ( ). An approach that has been advocated is to perform the test with a water-based medium, but to carry out tubal flushing with an oil base at the time of the procedure. It has been shown than flushing with oil contrast improves clinical pregnancy rates ( ).
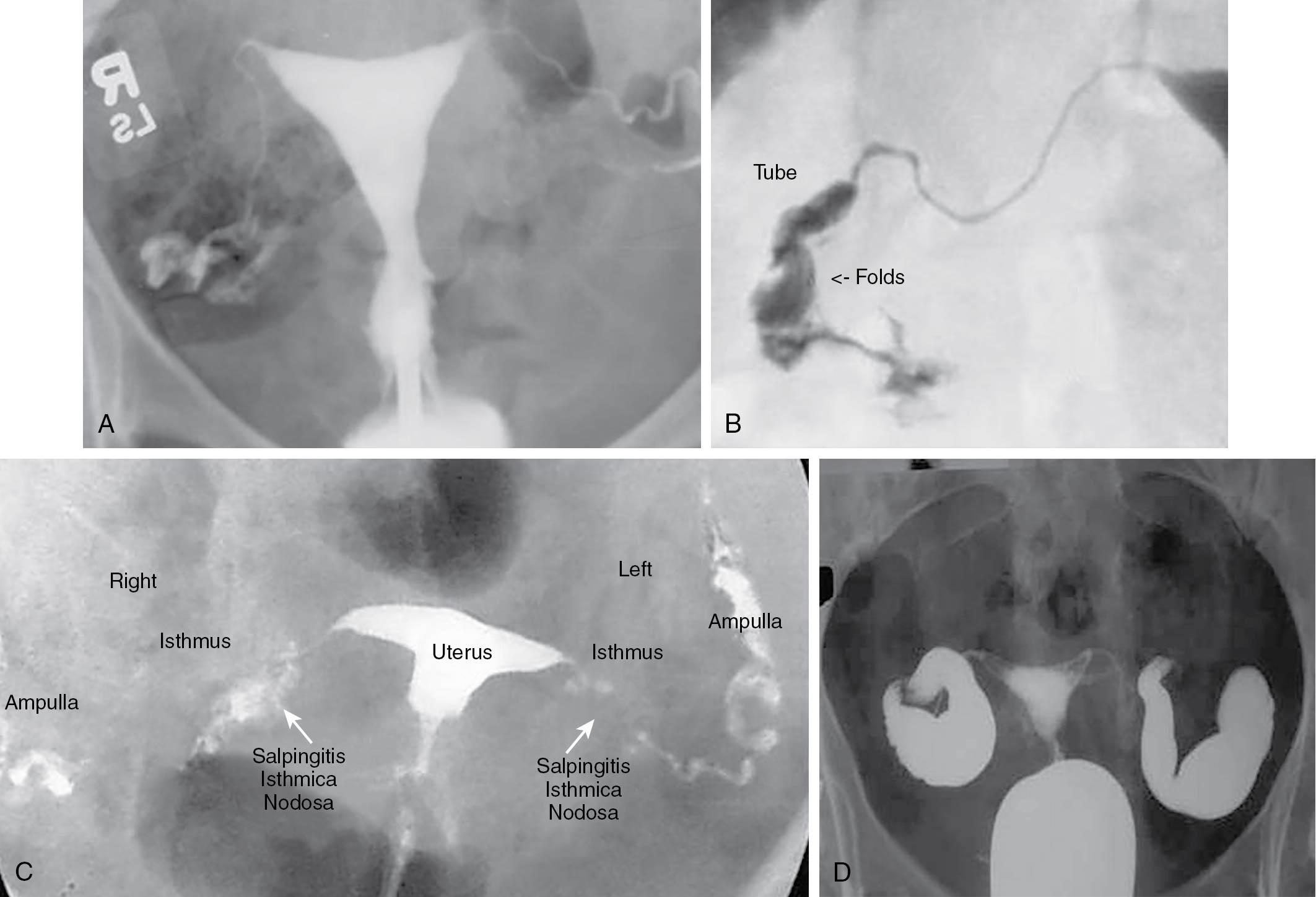
The diagnostic HSG provides important information about the magnitude of the disease process, if present, and provides information about the uterine cavity that cannot be obtained by laparoscopic visualization. The procedure can also determine whether salpingitis isthmica nodosa is present in the interstitial portion of the oviduct ( Fig. 40.6 , C ). When an HSG shows lack of patency in one tube, this has been shown to be falsely positive, approximately 50% of the time at laparoscopy. Therefore it is not necessary to perform tubal reconstructive surgery on a woman with one patent tube. The finding of a normal endometrial cavity at the time of HSG obviates the need for hysteroscopy. If severe tubal disease, such as a large hydrosalpinx, is found at the time of HSG ( Fig. 40.6 , D ), based on success rates, it is preferable for the couple to undergo IVF–embryo transfer (ET) than for the woman to have tubal surgery. If the hydrosalpinx is large and clearly visible on ultrasound, it is preferable to perform laparoscopic salpingectomy before IVF-ET because the pregnancy rate with IVF-ET may be decreased by as much as 40% ( ).
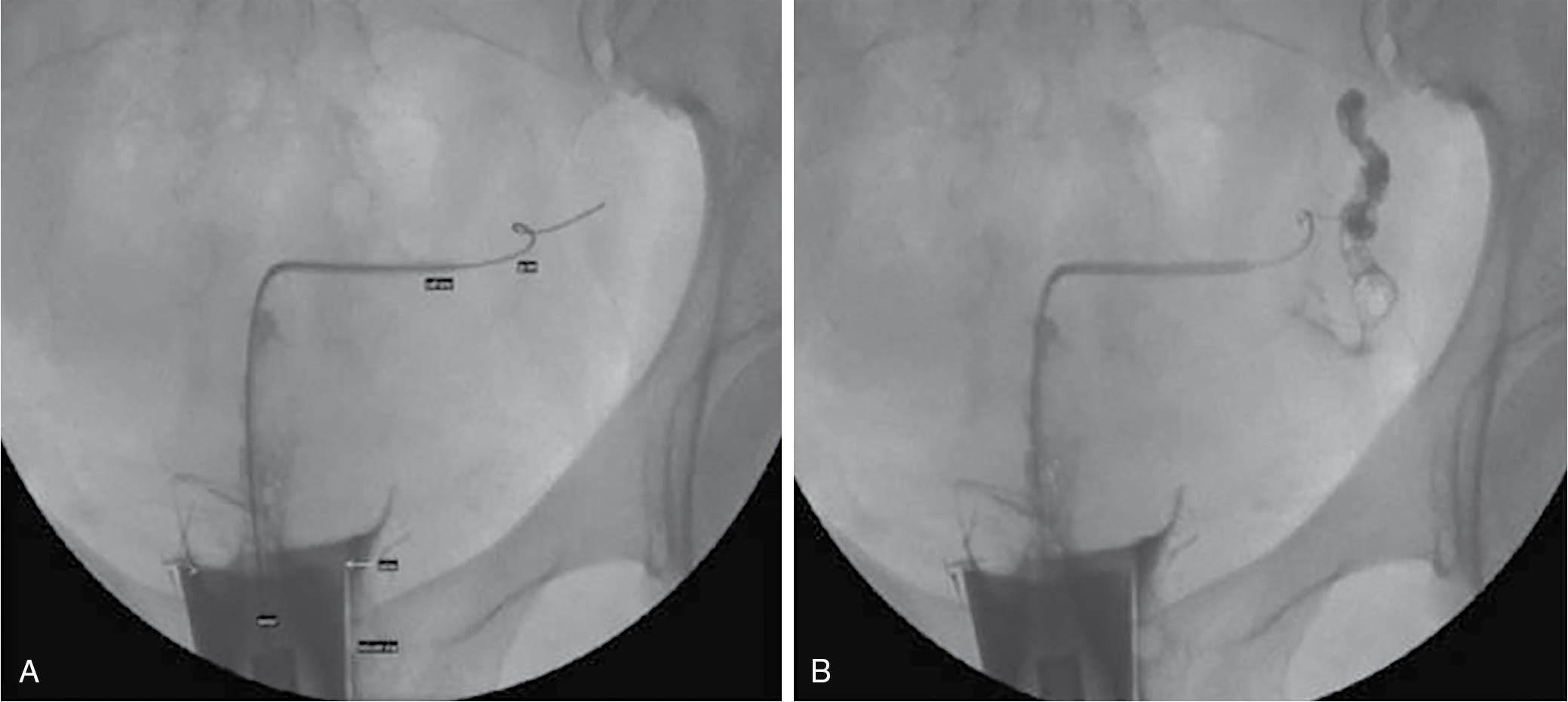
Quite often one or both tubes may show proximal obstruction. This may be the result of uterine spasm (contractions) caused by the discomfort of the procedure, or because of true obstruction; the latter may be only a mild obstruction caused by tubal debris. It has become common to attempt fluoroscopic-controlled selective cannulation of the proximal tube, either immediately or at a subsequent visit ( ) ( Fig. 40.7 ). This can be successful in up to 90% of cases, and if successful, it alleviates either unnecessary concern or the need for laparoscopy or IVF.
When the extent of tubal disease is unclear or the couple prefers not to undergo IVF-ET, diagnostic laparoscopy should be carried out in the follicular phase of the cycle. In general, the goal should be to have all tubal reconstruction carried out laparoscopically (discussed later).
Although important from a physiologic standpoint (cervical mucus being important for sperm transport), the postcoital test (PCT) is now rarely indicated as a necessary part of the infertility investigation. It is a very subjective test. A normal PCT is one in which at least five motile sperm are visible in normal cervical mucus obtained from the upper canal just before ovulation. A suboptimal test can be the result of technique, timing of the test, and problems with cervical mucus or with sperm. Although a good PCT has been correlated with a better prognosis for pregnancy, sperm have been recovered at laparoscopy when there was a poor PCT. Moreover, because the suggested treatment for a poor PCT is intrauterine insemination after ovarian stimulation, this is the exact next step taken, even if the PCT is normal, in the setting of unexplained infertility. Occasionally, as may happen with an orthodox Jewish couple, a semen analysis cannot be obtained. Here, a PCT provides a surrogate for visualizing motile, normal-appearing sperm.
In the past, this was an obligatory final step in the infertility investigation when all other test results were normal. Data have shown that in 20% to 40% of cases, some minor abnormalities may be found (e.g., endometriosis, adhesions), which may have a bearing on fecundability. Obviously, if there is something suspicious on ultrasound or examination, there has been prior pelvic surgery or appendicitis, or there is pelvic pain or dyspareunia, the index of suspicion is increased. The probability that peritubal adhesions of sufficient severity to cause infertility will be found at the time of laparoscopy is less than 5% in a woman with no history of salpingitis or symptoms of dysmenorrhea, a normal bimanual pelvic examination, and normal antibody titers (if obtained) ( ). Provided the woman is younger than 40 years and is having ovulatory cycles, and there is an acceptable semen analysis and an age-appropriate maker of ovarian reserve such as AMH, several cycles of COS and intrauterine insemination may be undertaken before performing diagnostic laparoscopy or going directly to IVF-ET . At this juncture, a decision can be revisited about whether performing a laparoscopy should be considered, although many couples usually prefer to proceed with IVF-ET, particularly if they have insurance coverage for IVF.
Although suggested for many years, it has never been established that luteal phase defects cause infertility. This pertains to women who are completely normal on evaluation and have regular cycles. Women who are oligoovulatory and have longer cycles and women with elevated PRL levels can have luteal inadequacy that is corrected by lowering of PRL levels and/or ovulation induction.
The diagnosis of luteal deficiency used to be made on finding serum progesterone levels consistently less than 10 ng/mL 1 week before menses or finding consistent evidence for a histologic delay (>3 days) in the pattern of the normal secretory endometrium, indicating an inadequate effect of progesterone on the endometrium. This endometrial defect had to be found in two consecutive cycles. Erroneous diagnoses of this entity occur because of cycle variability and the subjective interpretation of histologic dating criteria. There is at least a 10% disagreement of more than 2 days when the same observer dated the specimens on two separate occasions, and even more interobserver variability. As noted earlier, studies have confirmed the lack of efficacy of the endometrial biopsy ( ). Routine endometrial biopsies for the diagnosis of infertility should not be carried out.
As noted regarding the PCT, ovarian stimulation with intrauterine insemination (IUI) is often the first empirical treatment for unexplained infertility. This essentially treats the luteal inadequacy, if it exists, preempting the need for invasive and imprecise endometrial biopsies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here