Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Infectious endocarditis (IE) is a rare disease with a yearly crude incidence of 1.5–11.6 episodes per 100,000 persons (3–9/100,00 in developed countries), increasing dramatically with advanced age and male sex and with varying epidemiology between high- and low-income countries. , Despite advances in early diagnosis and treatment, mortality of IE at 1 year has not improved over the last decades, being ≈30%. In low-income countries rheumatic heart disease remains the main risk factor for IE, but in high-income countries the epidemiologic profile has changed. Patients with IE are old, frail, with comorbidities and risk factors including diabetes, cancer, immunosuppression (including human immunodeficiency virus [HIV]), hemodialysis, prosthetic valve replacement, presence of cardiac implantable electronic devices, central venous catheters, intravenous drug use, and degenerative or congenital heart disease.
IE is presently classified by the setting and source of infection into healthcare-associated infectious endocarditis (HAIE) and community-acquired infectious endocarditis (CAIE) and as native valve endocarditis (NVE) and prosthetic valve endocarditis (PVE). HAIE includes patients hospitalized for more than 48 hours before the symptoms of IE develop or patients with extensive healthcare contact defined as (1) home-based nursing or intravenous (IV) therapy, hemodialysis, or IV chemotherapy less than 30 days before onset of IE symptoms; (2) hospitalization less than 90 days before onset of IE; (3) residency in a nursing home or a long-term care facility; or (4) history of any interventional procedure during the previous 4–8 weeks. , The definition of HAIE applies both to NVE and PVE. Early PVE (now defined as presenting <1 year postsurgery) has a portion included in the HAIE definition (those presenting 60 days postsurgery). , IE in high-income countries is >25% healthcare associated, carrying a significant in-hospital mortality rate of 15%–20%, with a 1-year mortality rate approaching 40%.
Gram-positive cocci (staphylococci, streptococci, and enterococci) comprise 80%–90% of the microbial etiology of IE, whereas rare (<5%) causes include HACEK species ( Haemophilus spp., Aggregatibacter [formerly Actinobacillus ] spp., Cardiobacterium spp., Eikenella corrodens, and Kingella spp.); aerobic gram-negative bacilli; fungi ( Candida spp. and Aspergillus spp.); Bartonella spp.; Brucella spp.; Coxiella burnettii; and Tropheryma whipplei . In 10% of cases, IE is culture negative, and serology or polymerase chain reaction (PCR) assays in blood or on surgically removed heart valves/vegetations may contribute to the diagnosis. Staphylococcus aureus is responsible for 35%–40% of NVE and 50% of HAIE, replacing streptococci as the most common pathogen in developed countries, and streptococci are the cause of 30%–40% of CAIE (both NVE and PVE). Group D streptococci (e.g., Streptococcus gallolyticus, formerly Streptococcus bovis ) are isolated in 15% and should be notable for causing IE associated with an underlying colonic tumor (usually undiagnosed). Enterococci (mostly faecalis ) are implicated in 10% of IE, being more common in HAIE and transcatheter aortic valve replacement (TAVR) IE. Coagulase-negative staphylococci predominate (>60%) in IE in the presence of prosthetic valves, cardiac implanted electronic device (CIED), or venous catheters except for Staphylococcus lugdunensis, which can also be the cause of community-acquired NVE.
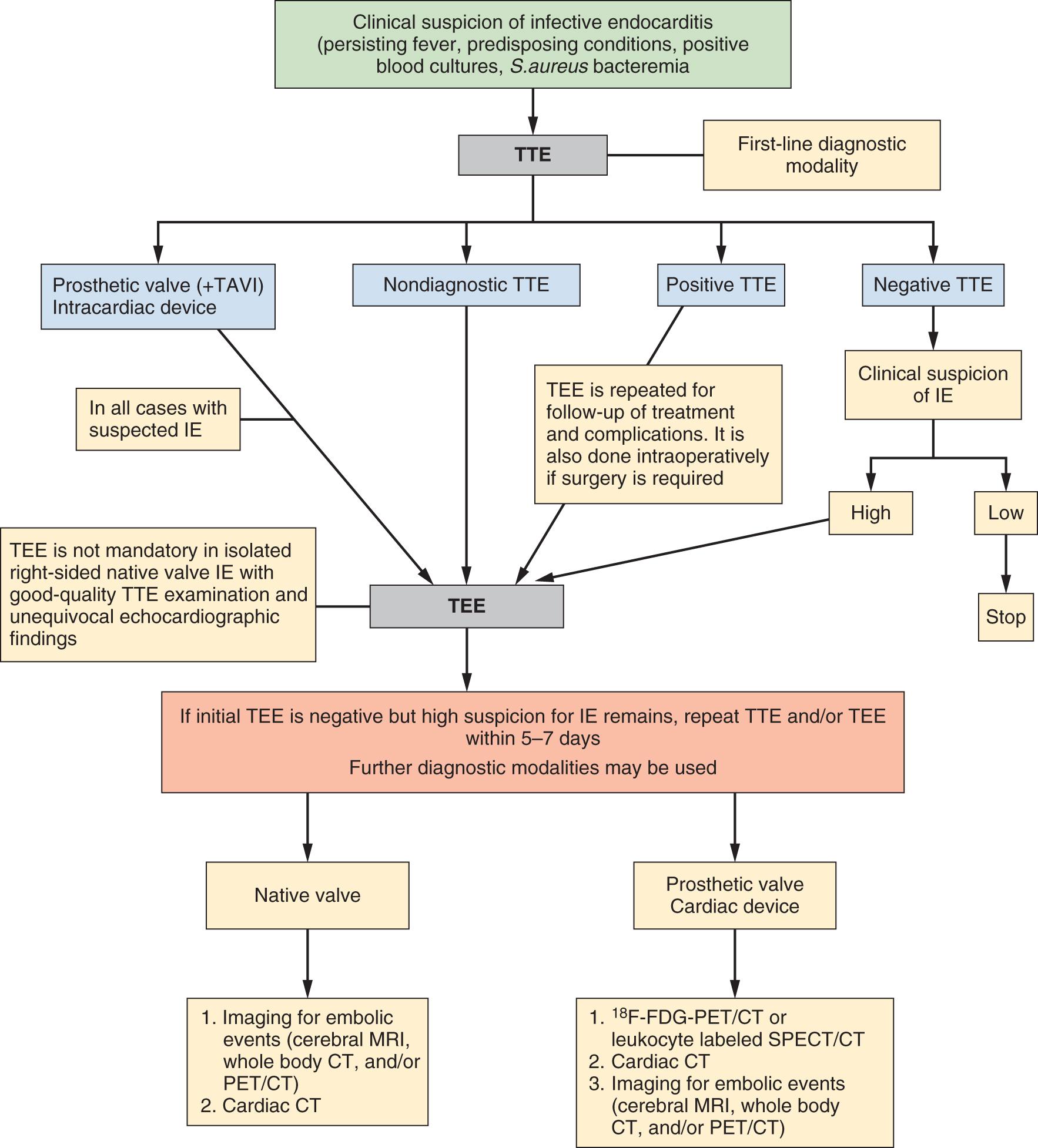
Rapid and accurate diagnosis of IE remains a challenge and affects disease outcome. Fever is present up to 90%, a heart murmur up to 85%, and an embolic event up to 25% at the time of diagnosis. Other signs are less common: hematuria, splenomegaly, splinter hemorrhages, Janeway lesions, Roth spots, conjunctival hemorrhage, sepsis, meningitis, unexplained heart failure, septic pulmonary emboli, stroke, acute peripheral arterial occlusion, and renal failure. Cerebral complications are severe and frequent in IE, occurring symptomatically in 15%–30% of patients, and there is evidence that additional clinically silent cerebral embolism may occur in 35%–60%. , Diagnosis of IE relies on clinical, microbiologic (blood cultures), and imaging findings (the first-line modality is transthoracic echocardiography [TTE] and transesophageal echocardiography [TEE]), incorporated in the modified Duke diagnostic criteria, which have an overall 80% sensitivity and specificity, which decrease in PVE and cardiac device–related IE. A definite diagnosis requires two major, one major with three minor, or five minor criteria , ( Table 117.1 and Fig. 117.1 ).
| Major Criteria | Minor Criteria |
|---|---|
|
Definite IE
Possible IE
Rejected IE
|
|
Treatment of IE aiming at microbial eradication necessitates the use of bactericidal combination regimens for prolonged periods. In the 2015 guidelines (1) aminoglycosides are no longer indicated in staphylococcal NVE; (2) when used in streptococcal, enterococcal IE, or staphylococcal PVE, they should be given in a single daily dose to reduce nephrotoxicity; (3) rifampin should be used in IE with foreign material in place (PVE) after 3–5 days of effective antibiotic therapy (to prevent an antagonistic effect against planktonic/replicating bacteria) and after bacteremia has been cleared; (4) daptomycin used in methicillin-resistant S. aureus (MRSA) IE should be used in high doses (≥10 mg/kg per day) and in combination with a second antibiotic to increase activity and prevent emergence of resistance; (5) in methicillin-sensitive S. aureus (MSSA) IE treatment with beta-lactams is preferred (vancomycin is inferior to beta-lactams); and (6) treatment duration is evaluated from day 1 of effective treatment (i.e., negative blood cultures in case of previously positive ones). In the case of new prosthetic valve implantation in the course of IE, if the removed valve is culture positive, a new full treatment course starts postsurgery. , Indications for cardiac surgery during the course of IE are heart failure (severe regurgitation or obstruction of the affected valve), evidence of uncontrolled infection (persisting bacteremia despite treatment and control of metastatic foci, abscess or fistula formation, infection by fungi or multiresistant organisms), and prevention of embolism (vegetation size, mobility, and embolic events). A summary of antibiotic treatment recommendations is shown in Table 117.2 .
| Microorganism | Treatment Regimen | Duration | Comments |
|---|---|---|---|
| Penicillin-susceptible streptococci (MIC ≤0.125 mg/L) | Penicillin G 12–18 million U/day IV in 4–6 doses or continuously or Amoxycillin or ampicillin 100–200 mg/kg/day IV in 4–6 doses or |
2 weeks | Short-duration treatment only in normal renal function and with monitoring of aminoglycoside levels |
| Ceftriaxone 2 g/day IV or IM in 1 dose | In PVE 6-week duration is recommended | ||
| plus | |||
| Gentamicin 3 mg/kg/day IV or IM in 1 dose or netilmicin 4–5 mg/kg/day IV in 1 dose | |||
| Penicillin G 12–18 million U/day IV in 4–6 doses or continuously or | 4 weeks | ||
| Amoxycillin or ampicillin 100–200 mg/kg/day IV in 4–6 doses or | |||
| Ceftriaxone 2 g/day IV or IM in 1 dose | |||
|
4 weeks | Vancomycin levels should be monitored | |
| Relatively resistant to penicillin streptococci (MIC 0.250–2.0 mg/L) | Penicillin G 24 million U/day IV in 4–6 doses or continuously or | 4 weeks | In PVE 6-week duration is recommended |
| Amoxycillin or ampicillin 200 mg/kg/day IV in 4–6 doses or Ceftriaxone 2 g/day IV or IM in 1 dose |
If beta-lactam allergy, change beta-lactam to vancomycin | ||
| Plus | 2 weeks | ||
| Gentamicin 3 mg/kg/day IV or IM in 1 dose | |||
| Enterococci and streptococci with penicillin MIC>2 mg/L | Amoxycillin or ampicillin 200 mg/kg/day IV in 4–6 doses Plus |
6 weeks 2–6 weeks |
|
| Gentamicin 3 mg/kg/day IV or IM in 1 dose | 6 weeks | ||
| If high-level resistance to gentamicin | Ampicillin 200 mg/kg/day IV in 4–6 doses plus ceftriaxone 4 g/day IV or IM in 2 doses | 6 weeks | |
| If allergy or resistance to beta-lactams ( Enterococcus faecium ) | Vancomycin 30 mg/kg/day IV in 2 doses plus gentamicin 3 mg/kg/day IV or IM in 1 dose | 6 weeks | Consultation with ID physician recommended |
| If resistance to beta-lactams, vancomycin and gentamicin | Daptomycin 10 mg/kg/day IV plus ampicillin 200 mg/kg/day IV in 4–6 doses or ertapenem | 8 weeks | |
| linezolid 600 mg BID IV | |||
| MSSA NVE PVE |
Flucloxacillin or oxacillin 12 g/day IV in 4–6 doses Flucloxacillin or oxacillin 12 g/day IV in 4–6 doses with rifampin 900–1200 mg/day IV or PO in 2–3 doses plus |
4–6 weeks ≥6 weeks |
Rifampin has been suggested to start 3–5 later |
| Gentamicin 3 mg/kg/day IV or IM in 1 dose | 2 weeks | ||
MRSA or beta-lactam allergy
|
Vancomycin 30–60 mg/kg/day IV in 2–3 doses or
|
|
Daptomycin is superior to vancomycin for MSSA and MRSA bacteremia with vancomycin MIC >1 mg/L. Some experts recommend combination of daptomycin with fosfomycin or cloxacillin. |
| HACEK | Ceftriaxone 2 g/day IV in 1 dose |
|
|
| or ampicillin plus gentamicin if beta-lactamases are not produced |
|
Ciprofloxacin is a not well-validated alternative |
Data emerging from the International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) recognize that 34% of NVE is healthcare–associated (HANVE), consistent with the contemporary high incidence of healthcare–associated infection. , Almost 50% were acquired outside of the hospital, and compared with CAIE, patients with HANVE more often have comorbid conditions (e.g., diabetes mellitus, cancer, or long-term immunosuppressive therapy). Fever is the most common presenting feature, but physical signs of IE present more rarely in HANVE, suggesting a more acute course. Non-nosocomial acquisition of HANVE is most often dependent on hemodialysis or an intravascular catheter (54%), whereas patients with nosocomial acquisition more often have a preexisting valvular disease or undergo a nondental invasive medical procedure. The mitral valve is most frequently involved, followed by the tricuspid and aortic valve. Staphylococci represent the major pathogens in HAIE. S. aureus is responsible in >50% of cases, 91% in the presence of an intravascular device. In the ICE-PCS study, S. aureus was methicillin-resistant (MRSA) in 47%. The second most common bacteria were enterococci (15%), followed by coagulase-negative strains of staphylococci (13%). MRSA is more prevalent in infections acquired during hospitalization (57% vs. 41%). Among coagulase-negative strains, S. lugdunensis deserves attention because it behaves like S. aureus with high virulence, has a 50% probability of complicated infection when isolated in blood, and has an aggressive course when it is the cause of IE.
Gram-negative bacilli are rare causes of HANVE despite the fact that they cause lethal bacteremias in hospitals, probably as a result of their decreased ability to adhere to heart valves and susceptibility to bactericidal action of serum. , Recently, cases of IE caused by multidrug-resistant MDR and extensively drug-resistant (XDR) gram-negatives (e.g., Pseudomonas, Acinetobacter, Escherichia coli, and Burkholderia cepacia ) have been published with poor outcomes despite aggressive management because of a lack of effective treatment options.
Fungal IE is a rare infection, comprising <2% of IE cases, with a mortality rate exceeding 30%. An increasing frequency of fungal endocarditis has been observed in recent years, attributed to the extensive use of vascular lines, noncardiac surgery, and increased numbers of immunocompromised patients. , Fungi most commonly associated with IE are Candida ( albicans and non- albicans, 50%–80%) and Aspergillus spp. (20%–25%). In contradistinction to Candida spp., in which blood cultures in cases of IE are positive in 83%–95% of cases, blood cultures are positive in only 11% or less of patients with Aspergillus spp. In cases of fungal endocarditis, prolonged symptoms before hospitalization and the embolization of major arteries are classic findings. However, the diagnosis is delayed or missed in 82% of patients. For early diagnosis of fungal endocarditis, it should be considered in the differential diagnosis and echocardiography performed, which demonstrates large, bulky vegetations. Peripheral blood cultures should be obtained and accessible embolic specimens subjected to histologic examination. ,
HANVE has higher mortality compared with community-acquired NVIE (25% versus 13%), and factors independently associated with increased risk of death are increased age (>60 years old), diabetes, S. aureus infection, paravalvular abscess, stroke, heart failure, and new conduction abnormality. Cardiac surgery during the IE episode is found to be associated with a lower mortality, and therefore early surgical intervention is often mandatory. In fungal endocarditis, the removal of the infected valve is indicated, and postsurgery suppressive therapy for 2 or more years along with close follow-up are required to detect relapses. ,
Special consideration should be given to chronic hemodialysis (HD) patients, in whom IE is significantly more common (16–18 times) and causes greater morbidity and mortality. In this group of patients, IE is the second leading cause of death after cardiovascular disease, and it has been proposed to be added as a special category in classification by acquisition. , In the ICE-PCS study, 63% of HANVE cases were HD patients. S. aureus was the pathogen in 75%–80% of cases, half of which were MRSA. Fever may not be present, and blood cultures may less often be positive, complicating a diagnosis by the Duke criteria. Mortality remains high: 30% during the first month, about 65% during the first year, and reaching more than 70% if cardiac surgery is indicated. Age older than 65, diabetes as the cause of renal failure, mitral involvement, large vegetations, septic emboli, and infections caused by MRSA or vancomycin-resistant enterococci (VRE) have been identified as risk factors for mortality.
For MSSA, anti-staphylococcal penicillins should be the treatment of choice, whereas in cases of MRSA with minimum inhibitory concentration (MIC) >1 mg/L to vancomycin, antimicrobial choices include high-dose daptomycin in combination with another antibiotic. If vancomycin is indicated, drug levels should be followed, with trough levels of 25–30 mg/L required for efficacy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here