Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Disease results from a failure of homeostasis within the body, which causes impaired function. Failure of homeostasis itself can be due to a multiplicity of causes, extrinsic and intrinsic, many of which are discussed in other areas of this book, and are summarised in Tables 6.1 and 6.2 .
| Extrinsic factor | Agent | Example |
|---|---|---|
| Biological | Bacteria | Urinary tract infection |
| Viruses | Acquired immune deficiency syndrome | |
| Fungi | Candidiasis | |
| Protozoa | Malaria | |
| Helminths | Schistosomiasis | |
| Prion | Creutzfeldt–Jakob disease | |
| Chemical | Toxin | Bee sting |
| Inflammatory substances | Asthma | |
| Poison | Tobacco smoke | |
| Physical | Trauma | Fracture, laceration, crushed tissue |
| Temperature | Burn, frostbite | |
| Radiation | Cancer | |
| Environment | Dehydration |
| Intrinsic factor | Agent | Example |
|---|---|---|
| Biochemical | Endocrine | Diabetes |
| Nutritional | Obesity | |
| Metabolic | Phenylketonuria | |
| Cellular | Autoimmune | Rheumatoid arthritis |
| Degenerative | Alzheimer disease | |
| Uncontrolled cell division | Cancer | |
| Genetic | Single gene | Sickle cell disease |
| Multifactorial | Hypertension | |
| Structural | Congenital | Heart malformation, spina bifida |
| Acquired | Osteoarthritis |
This chapter deals with biological agents that are the agents of infectious disease, as well as the body's defence system, both immune and cellular, and the failure of cells to control their growth.
Biological agents, such as bacteria and viruses, cause infections by entering the host body and are important causes of injury and damage. Often, but not always, specific organisms cause particular diseases. The defence system deals with the body's attempt to resist and repair injury. It includes inflammation, wound healing, non-immunological defence and the immune system.
A disorder of cell growth, or neoplasia, is an abnormal proliferation of cells. Proliferation may be benign (non-progressive) or invasive (malignant).
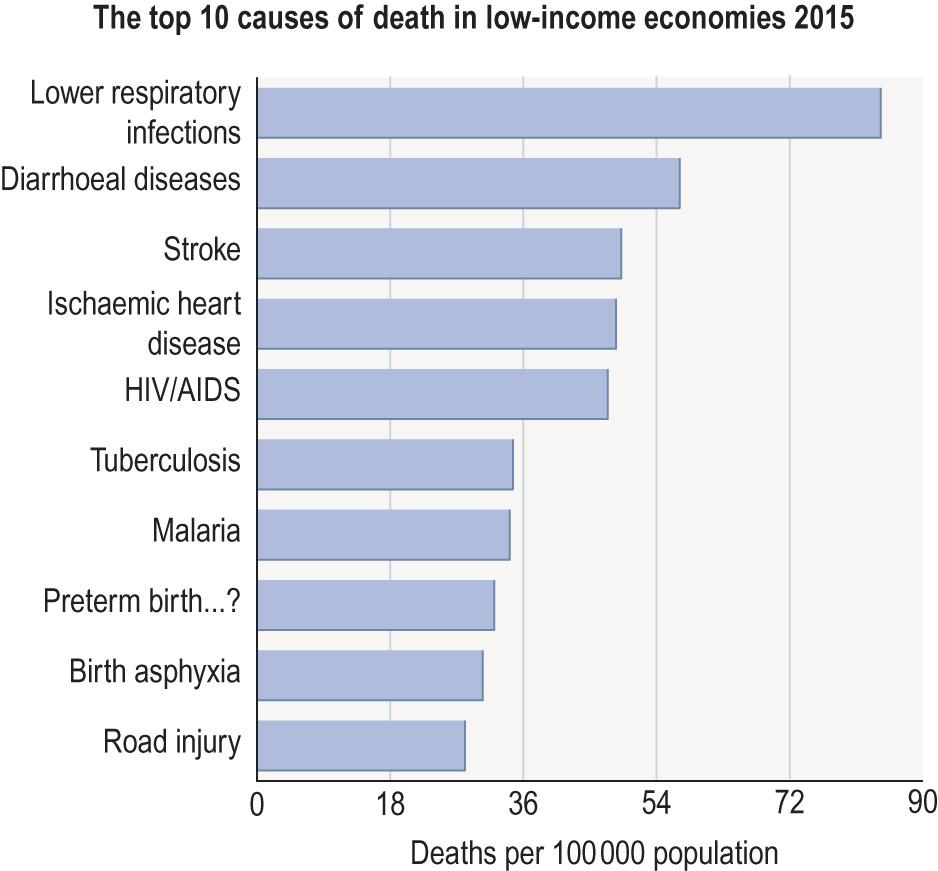
Infectious disease is the cause of approximately 15% of deaths worldwide, but in low-income countries this is much higher. Fig. 6.1 (from WHO factsheet 2017) shows the top 10 causes of death in low-income countries in 2015 and that five of these diseases are infective.
Organisms that cause infectious diseases can be grouped into the seven major categories: bacteria, viruses, fungi, protozoa, helminths, arthropods and prions. Many are microbes that need a microscope to be visualised. Each has its own classification system. Early classification divided organisms into prokaryotes and eukaryotes .
Prokaryotes:
Have no nuclear membrane
Have DNA in the form of:
A single circular chromosome, forming a nucleoid
Plasmids, which are extracellular circular DNA molecules of varying size
Have no membrane-bound organelles
Have cytoplasm rich in ribosomes
Transcription and translation can be carried out at the same time.
All bacteria are prokaryotes and they can be further subdivided into eubacteria (true bacteria) and archaebacteria (archaea). Archaea are prokaryotes, because they have no nucleus, but have introns within their genes and their ribosomal RNA (rRNA) sequence is similar to that found in eukaryotes. They were originally discovered in extreme temperature and chemical environments, but none are known pathogens and they will not be discussed further. Viruses have genetic material, but no other cellular characteristics, and depend on other cells for their survival. Prions are collections of protein molecules and are not considered living organisms. Fungi, protozoa and helminths are eukaryotes.
Eukaryotes:
Have a separate nucleus
DNA is carried on several chromosomes within the nucleus
Have membrane-bound organelles – mitochondria, ribosomes on endoplasmic reticulum, Golgi apparatus and lysosomes
Transcription requires movement of messenger RNA (mRNA) from the nucleus to the cytoplasm
Translation takes place on ribosomes.
Bacteria are a large group of ubiquitous unicellular microorganisms. They form much of the world's biomass and are vital components of the living world. Most, when they invade the human body, are destroyed by our immune system; some are beneficial, but others are harmful to humans and are described as pathogens . Each organism can be further classified according to various characteristics and described as a species, within a genus. For example, bacteria can be grouped according to how they stain, what their shape is, whether they need oxygen and how they group or reproduce. Information Box 6.1 shows general characteristics of bacteria.
Prokaryotes – contain DNA, and RNA
No nuclear membrane or membrane-bound intracellular organelles such as mitochondria, Golgi apparatus or endoplasmic reticulum
Have ribosomes for protein synthesis but these are different structurally from eukaryotic ribosomes
Have a plasma membrane, like eukaryotes
Most also have a cell wall, which gives bacteria their distinctive shapes
Divide by binary fission, but can exchange genetic material.
The main characteristics of bacteria are described in Information box 6.1 and the cellular features of bacteria are illustrated in Fig. 6.2 .
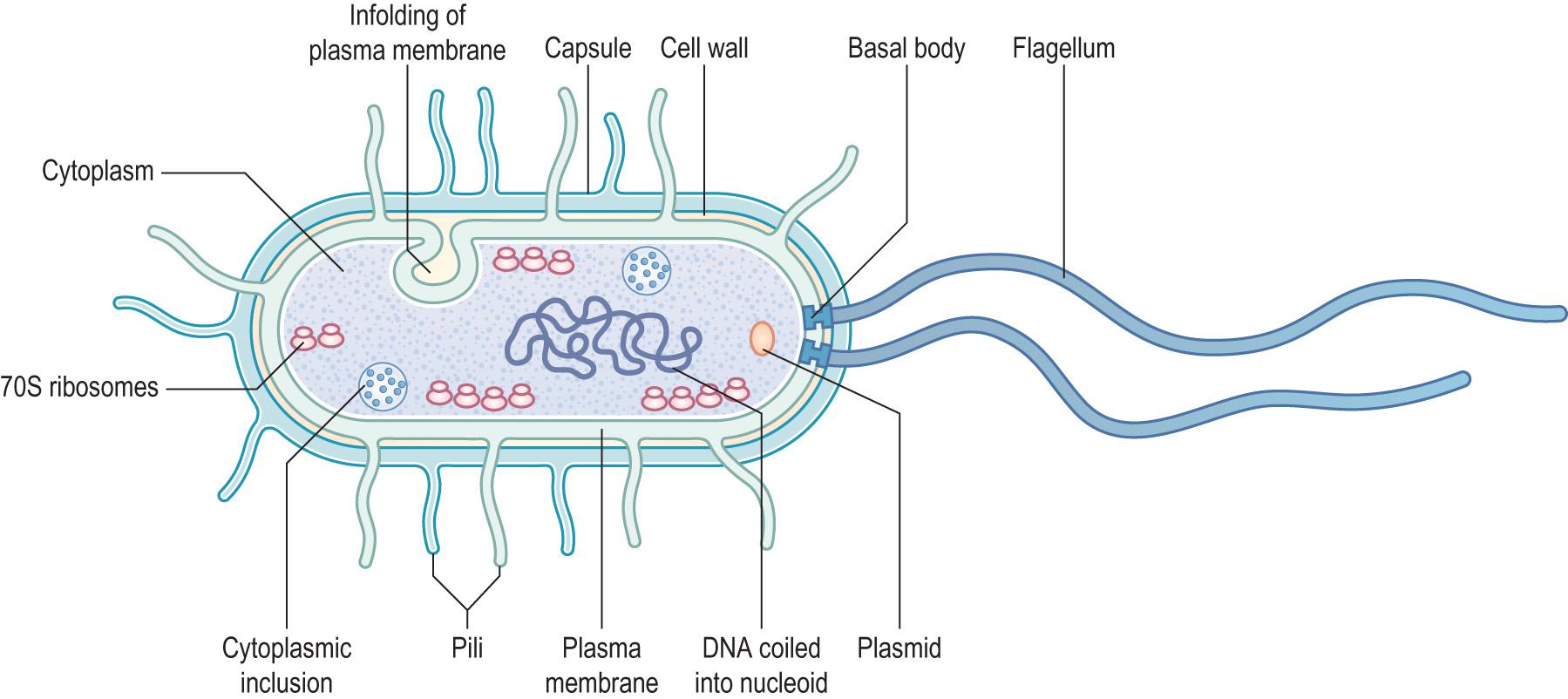
Within the bacterial cytoplasm are:
The genome : a single circular chromosome, plus or minus plasmids , which are independently replicating fragments of circular, double-stranded, DNA. The chromosome is located in the nucleoid , but there is no nuclear membrane. The genetic material is coiled and supercoiled, under the control of the enzymes DNA gyrase and DNA topoisomerase. The DNA lacks introns and extragenic sequences, such as are found in eukaryotes – it is just a continuous coding sequence of genes.
Bacterial mRNA is transcribed from DNA as in animal cells. There is no editing. Ribosomes can begin translation at one end of an mRNA molecule before the other end has been fully transcribed.
Ribosomes : often a string of ribosomes is joined by a single mRNA molecule. Bacterial ribosomes are different from eukaryotic ribosomes:
Bacterial ribosomes are 70S, and are composed of a 30S portion and a 50S portion
‘S’ refers to Svedberg units , which relate to how a particle behaves under ultracentrifugation
Eukaryotic ribosomes are 80S, although they do have 70S ribosomes in their mitochondria.
Granules – some bacteria have granules containing stored nutrients.
Mesosome – an invagination of the cell membrane, involved in cell division.
Other constituents – proteins, carbohydrates, messenger and transfer RNA, amino acids, etc.
This is a phospholipid bilayer with embedded protein molecules and structures, similar to eukaryotic membranes. The membrane has four important features:
Pores to control the entrance and exit of substances, such as nutrients, waste products and toxins
Respiratory enzymes on the inner surface
Enzymes involved in cell wall synthesis on the outer surface
Involvement in binary fission.
Entry and exit of molecules through the membrane is controlled by permeases through a variety of mechanisms:
Carrier-mediated down a concentration gradient
Phosphorylation-linked transport
Active transport.
This important structure surrounds the bacterium outside the plasma membrane. Animal cells do not have cell walls. The cell wall is strong, protects the bacterium from lysis in hypotonic solutions and from some physical trauma, and controls the access of some chemicals to the cell membrane. Different groups of bacteria have differently structured walls, determining the shape of the bacterium. They also cluster in different ways. Non-motile bacteria often stick to each other after replication.
If they always divide in the same plane, they will end up forming long chains, like streptococci
If they divide in different planes, they form clusters, e.g. staphylococci
Some bacteria stick together more firmly forming long filamentous threads, such as those seen in Actinomyces or Streptomyces cultures.
The main bacterial forms are:
Spherical (coccus) – cocci often occur in long chains ( streptococci ), in pairs ( diplococci ) or in clusters like grapes ( staphylococci )
Rod shaped (bacillus)
Comma shaped (vibrios)
Spiral (spirochaetes) – spirochaetes are long thin spiral-shaped bacteria with an outer membrane. Between the cell wall and the outer membrane are ‘internal flagella’: filaments running the length of the bacterium. They are motile through a spinning action and the filaments flex the bacterium to achieve this.
Gram staining, invented by a Danish scientist in 1884, remains the standard method for classifying bacteria dependent on the structure of the cell wall. Gram staining of a heat-fixed smear of bacteria is used to separate them into Gram positive or Gram negative . The process has four stages:
Primary staining with crystal violet (CV), which penetrates the cell wall and plasma membrane, staining the cells purple.
A mordant, Gram's iodine (I), is added and forms a complex with the crystal violet (CV-I).
Adding alcohol or acetone interacts with the cell membrane lipids, removing the outer layer and exposing the peptidoglycan layer. This layer is very thin in Gram-negative bacteria and the CV-I complexes are readily washed away. In contrast, the multilayered structure of Gram-positive bacteria retains the purple stain.
Counterstaining with basic fuchsin gives a red colour to the otherwise decolourised Gram-negative bacteria.
Some bacteria, e.g. Mycobacterium tuberculosis , responsible for tuberculosis, also have fatty acids and waxes within the cell walls, making it very difficult for materials to pass through the wall. Although this means that they are very slow dividing, it also means they do not take up the Gram stain. The Ziehl–Neelsen staining method uses an acid to allow fuchsin to penetrate the cell wall and colour the bacteria, making them visible under the microscope ( Clinical box 6.1 ).
Tuberculosis is an infectious, airborne disease caused by Mycobacterium tuberculosis (less commonly M. bovis ). Primary tuberculosis develops in response to the first infection by M. tuberculosis . This is usually subpleural, in the upper part of the lower lobe or the lower part of the upper lobe surrounded by the lobar fissure, known as the Ghon focus. The hilar lymph nodes draining the area may also be affected, and together with the primary lesion, are known as the Ghon, or primary, complex. A delayed hypersensitivity reaction, an immune response, then takes place over 3–8 weeks after the initial infection, with exudate formation, and aggregations of neutrophils, which are replaced by macrophages that react with T lymphocytes to form granulomas. The bacteria are not eliminated, but cell necrosis occurs to form the caseous (like soft white cheese) centre of the granuloma (tubercle) with variable amount of fibrosis. The caseous lesions heal completely, and may be calcified so that they are visible on X-ray. Some of these lesions still contain active tubercle bacilli that are dormant, but may be re-activated (usually years later) if the host immune system is compromised (e.g. in diabetes, immunosuppression, AIDS or malnutrition). Post-primary tuberculosis may then develop if the bacteria enter the bloodstream and disseminate to other foci in the body, causing infection. The commonest site of post-primary tuberculosis is the lungs. Other sites include lymph nodes, the brain, skin, gastrointestinal tract and kidneys. Diffuse blood-borne dissemination results in miliary tuberculosis, which is fatal without treatment.
A positive tuberculin test, an intradermal injection of a purified protein derivative of M. tuberculosis (tuberculin/PPD), indicates the development of cell immunity. The Mantoux test is used for testing in individual patients, and the Heaf test is used for population screening. The vaccine bacille Calmette–Guérin (BCG), a live attenuated vaccine made from a bovine strain of tuberculosis ( M. bovis ), is effective in reducing the risk of developing tuberculosis. The public health policy of vaccination for school children in the UK had almost eradicated the disease. The AIDS pandemic, however, has led to the re-emergence of tuberculosis, particularly in the developing world where poverty and malnutrition combined with limitation of access to medicines compound the problem.
Successful treatment of tuberculosis entails continuous self-administration of a combination of anti-tuberculous drugs, rifampicin and isoniazid (see Ch. 4 ), over at least 6 months. A lack of compliance, misuse of therapy and inadequate treatment have led to the emergence of multidrug-resistant tuberculosis (MDR-TB). Direct supervision in special clinics – directly observed therapy short course (DOTS) – improves compliance. Hospitalisation for treatment may sometimes be necessary for persistently uncooperative patients or those with severe disease or social indications.
Some bacteria form endospores – tough, spherical forms that resist extremes of temperature. Spore formation is triggered by adverse environmental conditions. In this form they remain dormant, with the ability to survive for many years.
Inhalation of endospores of Bacillus anthracis can lead to anthrax
Contamination of wounds with endospores from Clostridium tetani leads to tetanus .
Bacteria sometimes have extra material ( capsule ) outside the cells wall (Gram positive) or outside the outer membrane (Gram negative), composed of carbohydrates and/or proteins. This material hides the antigenic proteins, making them more resistant to host cell phagocytosis.
Flagella are whip-like structures that move, making the bacterium motile and allowing them to respond to chemical stimulants. They are completely different from eukaryotic flagella in both structure and function.
Pili (fimbriae) are long thin stiff structures, enabling bacteria to adhere to the cells of the host through specialised molecules, adhesins . Adhesins of Escherichia coli allow these bacteria to interact with fucose and mannose molecules on the intestinal epithelial cells. Although the pili are immunogenic, their antigens can change ( antigenic variation ), leading to avoidance of immune recognition.
Chlamydia are small Gram-negative bacteria, difficult to see, which can only divide within host cells. They have a ‘life cycle’ with two forms: the elementary body and the reticulate body . Both forms have a cell wall and an outer membrane. They are reminiscent of viruses, in that they have to replicate in a host cell, but they encode all of their own material, obtaining only nutrients from the host.
Rickettsia can also only replicate within a host cell, but they lack the special structures and life cycle of chlamydia. They are just small fastidious bacteria with Gram-negative structure.
Mycoplasma have no cell wall, are very small and of no definite shape.
The rate at which bacteria grow and divide depends mainly on the nutritional environment. Escherichia coli , in a rich nutrient, can divide several times in an hour; others, due to structural differences, may only divide once every 24 hours. Bacteria, when placed in a new environment, grow according to a characteristic pattern:
Lag phase – adjustment to the environment
Exponential phase – rapid growth with constant doubling rate
Stationary phase – induced as nutrients are depleted and toxic products accumulate
Death phase – cell growth declines and the cells die.
Bacteria divide by binary fission. The circular chromosome replicates by using a DNA-dependent DNA polymerase, with the help of DNA gyrase and DNA topoisomerase to facilitate uncoiling. The plasmids (if any) replicate independently of the chromosome. The chromosomes attach to the plasma membrane on opposite sides of the mesosome, and binary fission takes place, dividing the cytoplasm where the mesosome invaginates.
Bacteria do not form gametes, but DNA can be exchanged between them by various methods:
Conjugation : a conjugation tube forms between two bacteria, made by an outgrowth of the cell wall. Plasmids pass from one bacterium to the other along the tube.
Transduction : bacteriophage viruses (complex DNA viruses) infect bacteria and replicate in the usual viral fashion. The new virions may incorporate bacterial genes from the chromosome or from plasmids, and transfer them to other bacteria.
Transformation : bacteria can pick up naked DNA molecules and transcribe and translate the genes thereon. This is useful in the laboratory, but it is not certain if it occurs outside.
DNA can also move within the genome of a single bacterium: to a different place in the chromosome, from the chromosome to a plasmid, or from a plasmid to the chromosome. This is mediated by transposons : sequences of DNA that can loop out and in again to the main DNA strand. This ensures that any gene can be transferred by conjugation.
The origin of viruses is unknown, but because they are dependent on host cells it seems unlikely that they are some form of precursor to life in its cellular form. Information 6.2 shows general features of viruses.
Dependent on a living cell (obligate intracellular pathogen)
Nucleic acid core (DNA or RNA, and rarely both) with protein coat (the nucleocapsid)
10–300 nm in size
Circular, elongated or segmented
Surface binding protein to attach to cell.
Viruses are metabolically inert; although they have genetic material, they can only use this information to reproduce within host cells, where they are assisted by enzymes and ribosomes from the infected cell. Outside the cell, they are in the form of virus particles, or virions , and may lie within body fluids, within the body tissues or outside the body within the environment. Virions may sometimes be in the form of a nucleocapsid, but sometimes this may be surrounded by an outer envelope, which is normally a lipid bilayer of host origin. These different forms influence how viruses survive and are transmitted.
This may be DNA or RNA, single stranded or double stranded. It can be linear or circular and may, or may not, use enzymes that copy the viral genome once it enters the host cell. In addition, single-stranded RNA may be:
Sense – used directly as mRNA to translate viral protein
Antisense – the complementary strand must be produced within the host cell to be used as mRNA.
The capsid is a protein shell made up of numerous subunits ( capsomeres ) that give the virus a particular shape. There are three main shapes:
Helical – in which the capsomeres assemble around the genome to form a tubular capsid. Most human pathogenic viruses have an envelope, for example the paramyxovirus family.
Polyhedral – in which the capsid forms a geometrical shape with a central cavity. Icosahedral viruses have 20 faces and are a common form, for example the adenovirus . Sometimes these too are enveloped, such as the herpesvirus .
Complex forms are larger and more varied in their structure. The bacteriophage , for example, has an icosahedral head and helical tail and can have a hexagonal base with protein fibres coming from it. Other complex viruses are very different in form; the poxvirus may show an ovoid or brick shape.
Viruses enter the human body in a variety of ways:
Inhalation – through infected droplets
Ingestion – from contaminated food or drink, or from saliva
Inoculation – through injection, trauma exposure or insect bites
Transplacentally
Sexual intercourse.
Viruses normally infect only one or a small range of species and, once within the body, the continued success of the virus will depend on its ability to attach to the host cell and then replicate. Although replication varies, there are six basic stages ( Fig. 6.3 ) :
Attachment : specific receptors on the host cell bind with the viral capsid e.g.:
HIV binds to human T cells through its surface protein gp120 interacting with CD4 , a glycoprotein found on the surface of T cells
Influenza virus binds to sialic acid found on the surface of red cells and mucous membrane cells.
Penetration : this can happen in two main ways in human infections:
Fusion : attachment to the receptor causes a change in the viral envelope, allowing the membranes to fuse.
Viropexis : the virus is taken up in an endocytic vesicle and the virus enters the cytoplasm. If the virus has an envelope, this fuses with the vesicle membrane, as in the previously mentioned process, but this time from within the cell.
Uncoating : enzymes from the virus or host degrade the capsid and release the genomic material into the host cell cytoplasm.
Replication : this is a complex process and depends on the genomic material made available. The Baltimore classification proposes seven different schemes to deal with the different genomic structures. Important enzymes in these processes are:
DNA-dependent DNA polymerase , which makes a complementary strand of DNA, converting a single strand into a double strand. Some viruses use the human enzyme, others use their own.
DNA-dependent RNA polymerase , which uses viral DNA to produce mRNA, which can then be translated into proteins and other enzymes on host ribosomes. There are human and viral forms of this enzyme and different viruses will use different ones.
RNA-dependent DNA polymerase (reverse transcriptase) , which makes a complementary strand of DNA from RNA strands. Humans do not have this enzyme and so these viruses (e.g. retroviruses) must use their own.
RNA-dependent RNA polymerase makes a complementary strand of RNA. Again, humans do not have this enzyme, which must therefore be provided within the virion.
Assembly : some of the mRNA produced through the previously mentioned enzymes acting on the viral genomic material is translated on host ribosomes into viral proteins, which are assembled into new nucleocapsids with replicated viral genomic material. This may be followed by modification (or maturation) of the viral protein, which may take place after the virus has been released from the host cell, such as in HIV.
Release : although some viruses are released through lysis of the host cell, enveloped viruses bud off. New virions accumulate near the cell membrane, which then envelopes them and forms a bud that breaks off from the host cell, releasing the virions into the environment.
Viruses differ in how long they infect the host cell. Some are only present within the cell for a few days (such as that producing the common cold), others may be present for a long time producing a chronic infection (for example, hepatitis B, although more commonly this causes an acute infection, which then clears) and some may infect but not replicate for years, resulting in a chronic latent infection (such as HIV, or the chickenpox virus when it results in shingles). They also differ in their seriousness: Ebola, SARS (severe acute respiratory syndrome) and avian influenza are recognised as being very serious infections, whereas the common cold virus and herpes simplex virus type 1 (producing cold sores) are not serious human infections ( Clinical box 6.2 ).
HIV is the cause of the acquired immunodeficiency syndrome (AIDS). The infection may be sexually transmitted, vertically transmitted from mother to baby, acquired through transfusion of contaminated blood, blood products and organ donation, or via intravenous drug misuse. Symptoms include fever, lymphadenopathy, sore throat, mucosal ulcers, joint and muscle pains and occasionally a transient rash. There is usually an incubation period of 2–3 weeks followed by HIV conversion lasting approximately 6 weeks after exposure, before symptoms develop. Clinical manifestations of AIDS cover a wide spectrum, and are related to either the direct effects of HIV infection or to immunodeficiency.
HIV infection directly affects nearly all the body systems, including neurological complications, eye disease, skin and mucous membrane complications, haematological complications and diseases relating to the gastrointestinal, renal, respiratory, endocrine systems and the heart. Immunodeficiency increases the risk of all types of infections, including tuberculosis, candidiasis, herpes simplex, pneumonias (those caused by Pneumocystis jiroveci in particular), cytomegalovirus, toxoplasmosis and septicaemias. Tumours, such as lymphomas and Kaposi sarcoma, may also develop.
The introduction of highly active anti-retroviral therapy (HAART) has improved prognosis greatly to the extent that AIDS has become a chronic disease in the developed world.
Although very varied, viruses are distinct from all other organisms. In biology, a species is a population whose members can interbreed to form fertile offspring. Viruses do not undergo sexual reproduction; nevertheless, the concept of species is still used, differentiating between them according to structure and genome sequence. Groupings within a species are called strains or serotypes , produced as a result of mutations that occur when the viral material undergoes replication. Table 6.3 gives examples of the classification of various clinically important viruses.
| Species | Baltimore class | Shape | Envelope |
|---|---|---|---|
| Adenovirus | Double-stranded DNA (dsDNA) | Icosahedral | No envelope |
| Ebola, Marburg | Antisense single-stranded RNA (−ssRNA) | Complex | Enveloped |
| Hepatitis A, poliovirus | Sense (+) ssRNA | Icosahedral | No envelope |
| Hepatitis B | dsDNA and single-stranded DNA (ssDNA) | Icosahedral | Enveloped |
| Hepatitis C | +ssRNA | Icosahedral | Enveloped |
| Herpes simplex, Epstein–Barr virus, cytomegalovirus, varicella zoster | dsDNA | Icosahedral | Enveloped |
| Human immunodeficiency enzyme (HIV) | +ssRNA | Icosahedral | Enveloped |
| Influenza | –ssRNA | Helical | Enveloped |
| Measles, mumps | –ssRNA | Helical | Enveloped |
| Papillomavirus | ssDNA | Icosahedral | No envelope |
| Rabies | –ssRNA | Helical | Enveloped |
| Rotavirus | dsRNA | Icosahedral | No envelope |
| Rubella | +ssRNA | Icosahedral | Enveloped |
| Smallpox | dsDNA | Complex | Enveloped |
Both RNA and DNA viral infections are associated with certain cancers and can lead to a malignant transformation in cells. Sometimes it is believed that other agents ( cofactors ) may also be implicated, such as malaria influencing Burkitt lymphoma, or ultraviolet light stimulating skin cancer. Generally, but not always, the viral genome has been isolated within the cancer cells. Table 6.4 lists some viruses that are associated with particular human cancers.
| Virus | Cancer |
|---|---|
| Epstein–Barr | Burkitt lymphoma Nasopharyngeal carcinoma |
| Hepatitis B and C | Liver cancer |
| Human papilloma | Cervical cancer Skin cancer |
| HTLV-1 (human T cell lymphotrophic virus) | T cell leukaemia |
| HSV-2 (human simplex virus) | Cervical cancer |
Fungi are multinucleate or multicellular organisms and are eukaryotes, but quite distinct from plants and animals. Information box 6.3 shows general features of fungi.
Possess DNA and RNA and have a nuclear membrane
Have complicated membrane-bound intracellular organelles: mitochondria, Golgi, endoplasmic reticulum
Have a cell wall outside the cytoplasm, different from bacterial cell walls
Can grow as filaments ( hyphae ) forming a mesh ( mycelium ).
Can be syncytial – having multiple nuclei in the same cytoplasm
May grow as single cells ( yeasts ), which divide asexually
Replicate asexually ( budding ) and sexually, with gamete formation.
Fungal cells are eukaryotes, possessing a DNA genome, organised in linear chromosomes, with introns and extragenic material. There are no plasmids.
The chromosomes are in a nucleus, with a nuclear membrane and nucleolus.
There are ribosomes typical of eukaryotes, having mitochondria, endoplasmic reticulum, Golgi material, etc., within the cytoplasm.
Around the cytoplasm is a cell membrane , which differs from those of other groups in using ergosterol instead of cholesterol. This is the main feature exploited by antifungal therapy.
Outside the cell membrane is a cell wall ; although different from bacterial cell walls, it stains Gram positive. There is sometimes a capsule outside this.
There are two main forms that fungi can take: yeasts and mycelia. Some species can take both forms:
Yeasts are individual cells, which divide by budding or binary fission
Mycelia are long threads, in some cases divided by septa into cells, in others existing as syncytia.
There are over 250 000 species of fungi, which are classified by mycologists into four phyla according to the mode of sexual reproduction, or lack of it. Fewer than 200 species are pathogenic in humans. The mycologist's classification is not much use to the medical practitioner, and a strictly pragmatic classification is used instead, with the fungi being divided into groups depending on where the infection takes place:
Superficial infection of the skin or hair
Infection of the nails or subcutaneous layers of the skin
Systemic infections.
The first two groups generally produce mild infections. Systemic infections may be life-threatening and are often seen as opportunistic infections in patients who are immunocompromised.
Fungi enter the human body through inhalation, or through wounds, but others are part of the normal flora and only cause problems in individuals whose normal body defences are reduced. Table 6.5 lists some of the important fungal infections in humans.
| Type | Region of infection | Disease | Characteristics |
|---|---|---|---|
| Superficial | Hair cells, dead skin | Tinea nigra | Produces brown macules on the hands or feet |
| Cutaneous | Epidermis | Tinea (ringworm) | Raised red area of skin, often looking like a ring, rapidly spread by contact |
| Subcutaneous | Dermis | Mycetoma | Caused by actinomycetes entering into abrasions and producing a granulomatous disfiguring infection of the skin |
| Systemic | Internal organs | Histoplasmosis | Fungal infection usually affecting the lungs through inhalation |
| Opportunistic | Internal organs | Cryptococcosis | Produces a type of meningitis in immunocompromised people. Normally found in soil and harmless otherwise |
| Candidiasis | A yeast infection, generally a superficial infection of the skin or mucous membranes (eg. thrush) but systemic infections occur in the immunocompromised. | ||
| Aspergillosis | The fungus is found widely and used in industrial applications (such as production of citric acid) but produces an aspergillosis infection of the lungs in immunocompromised patients and others with poor respiratory function | ||
| Pneumocystis pneumonia | Produces pneumonia in immunocompromised people but rare otherwise |
Protozoa are single-celled animals, some of which cause infections in humans. Although the disease may be a direct consequence of the infection, frequently the symptoms are the result of the immune response to the infection. Information box 6.4 shows general characteristics of protozoa.
Are unicellular eukaryotic organisms, with DNA, RNA and a nuclear membrane
Possess complicated membrane-bound intracellular organelles: mitochondria, Golgi apparatus, endoplasmic reticulum, etc.
May form cysts with thick walls outside the plasma membrane, different from fungal or bacterial walls
May have complicated life cycles
Replicate asexually (binary fission), and sexually, with gamete formation.
Protozoa form the bulk of the biomass and play a vital role in ecology. They can grow up to 1 mm in size and are easily seen under a microscope. They are predators of bacteria and microfungi, absorbing food through their cell membranes and digesting the food in vacuoles. Protozoa have complex life cycles. Some alternate between growth, as trophozoites , and dormancy, as cysts . Cysts allow protozoa to survive extremes of environmental conditions outside the host.
Some protozoa are important parasites of humans, infecting them in an opportunistic fashion, or are only important in the immunocompromised. The AIDS epidemic has meant that new important human parasites have come to light. Parasitic protozoa can infect all major tissues and organs either as intracellular or extracellular parasites and are most prevalent in hot countries.
Although originally protozoa were classified according to their movement ability, as human parasites it is more useful to classify them according to their intracellular or extracellular location.
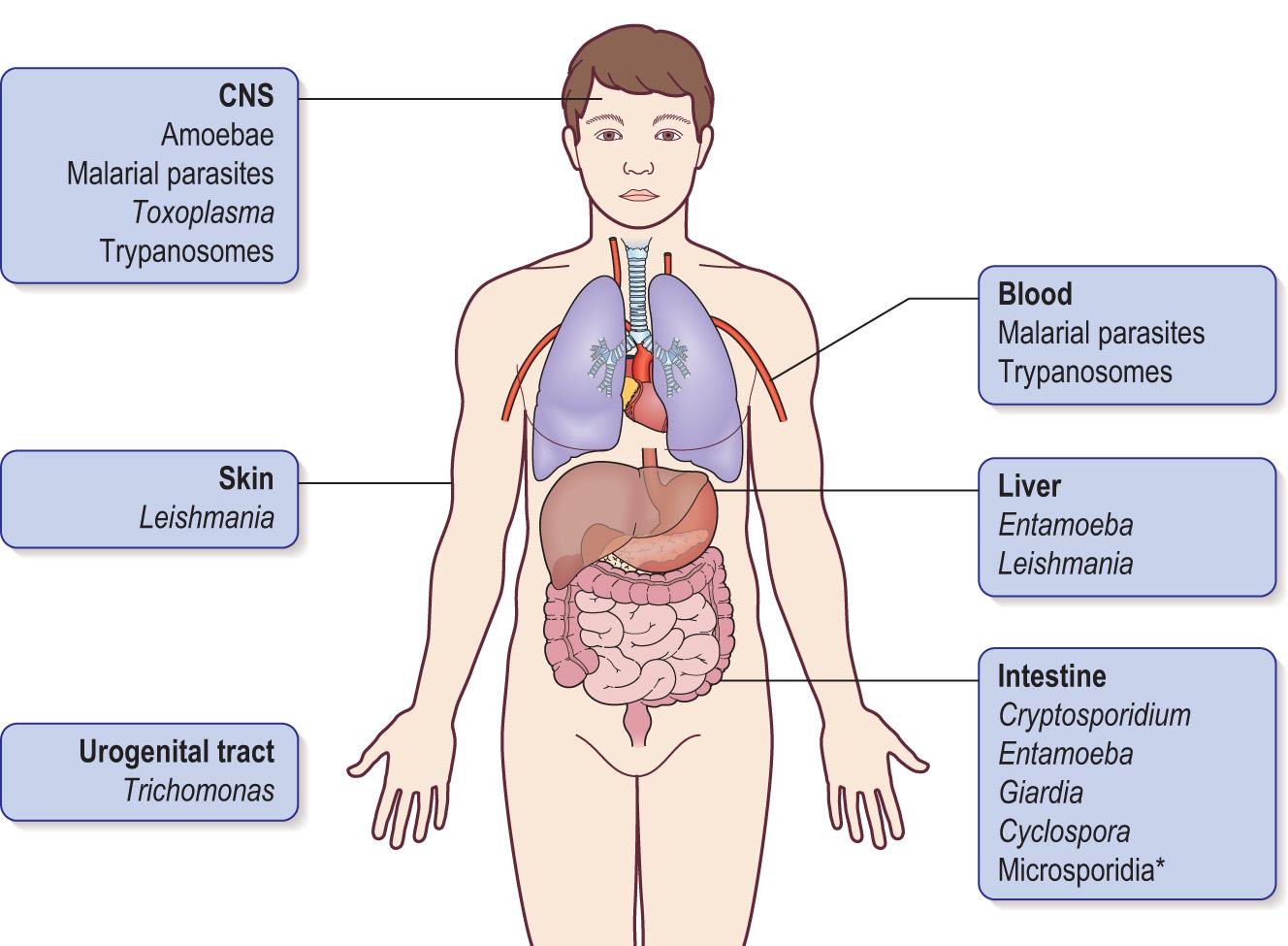
Intracellular parasites obtain nutrients from the host by direct uptake or by ingestion of cytoplasm. They infect a wide variety of cells – epithelial cells, red cells, muscle cells, brain cells and macrophages. They are normally transmitted by insects (e.g. causing malaria ), but can also be acquired through ingestion or in utero (e.g. Toxoplasma ).
Extracellular parasites obtain nutrients directly, or by ingesting host cells. This latter action can have serious implications, such as where the malarial parasite ruptures the infected human red cells. They are found in various locations – blood, intestine, urinogenital tract – and are normally transmitted by ingestion of cysts in contaminated food and water. Other mechanisms are possible: insect vectors transmit trypanosomes , and Trichomonas vaginalis is transmitted through sexual activity.
Fig. 6.4 shows the main sites of parasitic infections in the human body.
As single-cell organisms, protozoa vary in size from 2 µm up to 1 mm and have evolved in different ways in order to evade immune detection of their plasma membrane.
Intracellular species, when within the cell, are removed from attack by antibodies, complement and phagocytes. In order to survive within macrophages (such as in leishmaniasis) they have developed a range of mechanisms to evade or inactivate harmful intracellular enzymes, reactive oxygen species and nitrogen metabolites. Because their antigens may be expressed at the surface of the host cell, this has offered opportunities for therapeutic intervention.
Extracellular species evade recognition through changes in their cell membrane, or through their fight against body responses:
Amoebae consume complement at the cell surface
Malarial parasites have polymorphic surface antigens
Trypanosomes undergo repeated antigenic variation, changing their surface antigens.
In humans, reproduction of parasites is usually asexual, through binary division in trophozoite stages, which involves multiple divisions. Sexual reproduction is usually only seen within insect vectors but Cryptosporidium undergoes sexual and asexual reproduction in humans.
The complex nature of staged reproduction is illustrated by the life cycle of the malarial parasite ( Fig. 6.5 ) ( Clinical box 6.3 ). The life cycle of the malarial parasite involves a human and a mosquito host, which is prevalent in tropical regions. In 2010, malaria caused an estimated 655,000 deaths, of which 81% were in Africa and approximately 91% of these deaths in Africa were of children under 5 years old.
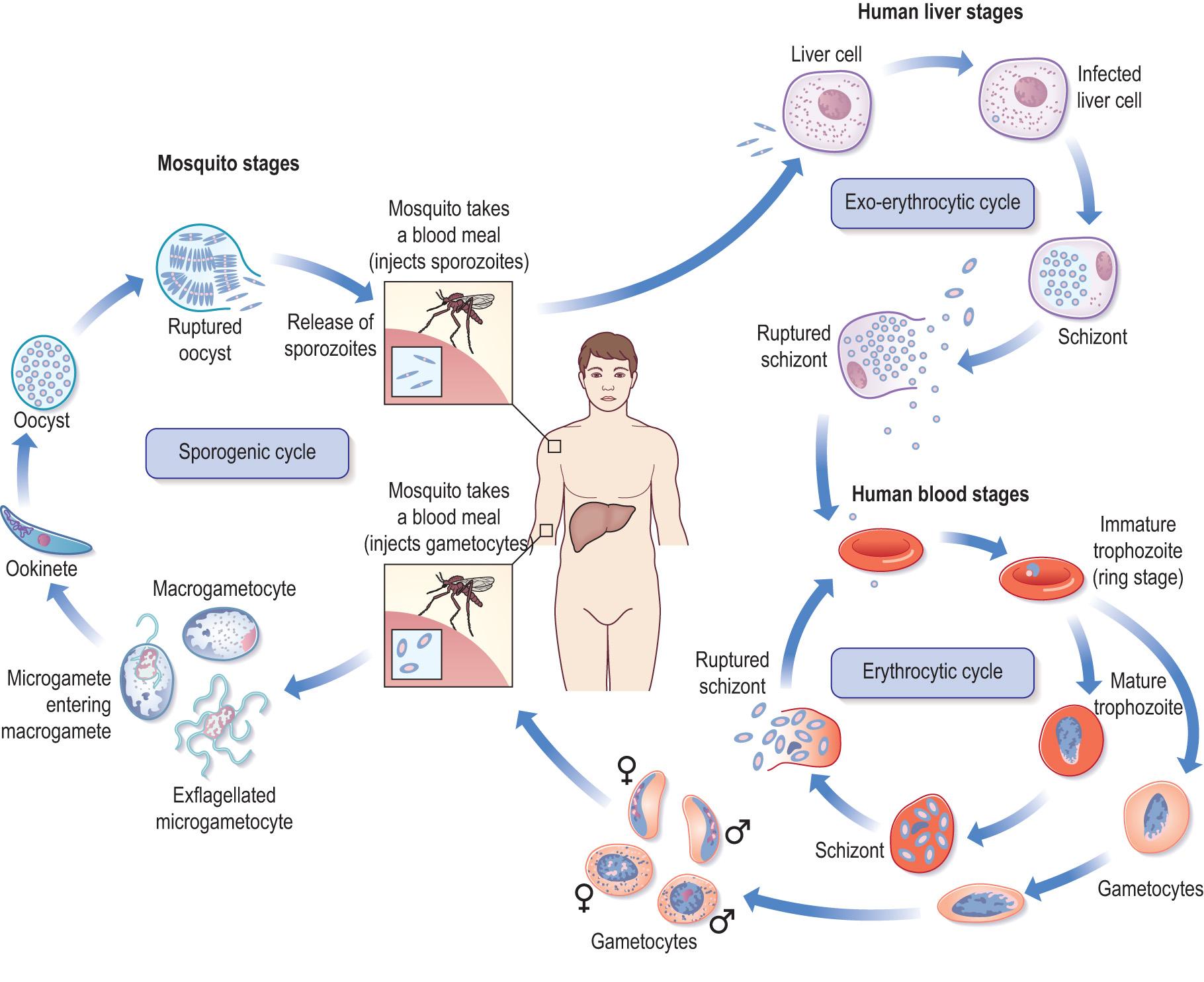
Malaria is a parasitic, protozoal infection caused by Plasmodium falciparum, P. vivax, P. ovale or P. malariae in humans. The disease is transmitted by a vector, the female anopheline mosquito, but may also be transmitted by transfusion of contaminated blood and blood products. When the mosquito bites an infected individual, gametocytes, the sexual form of the malaria parasite, enters the insect. During an incubation period of 1–3 weeks in the mosquito stomach, fertilisation takes place and the infective malaria sporozoites develop and migrate to the salivary glands to be inoculated into the next victim bitten by the mosquito. Malaria then develops in non-immune individuals with fever, general malaise and sometimes gastrointestinal symptoms. The fever is usually severe, with body temperature reaching up to 41°C, sweating and rigors.
Once the immature parasites have entered the circulation, those that are not destroyed by the immune system are taken up by the liver where they multiply in the hepatocytes (merozoites). The hepatocytes rupture after a few days to release merozoites into the bloodstream where they invade the red cells and multiply and form new merozoites. The red cells then rupture, releasing the new merozoites, which infect more red cells. Some species attack young red cells and reticulocytes. The subsequent course of the disease depends on the infecting Plasmodium species. Widespread organ damage may occur due to haemolytic anaemia resulting from the rupture of red cells, cytokine release and impaired microcirculation ( P. falciparum ). Cerebral, renal, metabolic, respiratory and endocrine complications may also occur. The genetic variation that resulted in the sickle cell gene evolved owing to the increased malaria resistance conferred by sickle cell disease (see Ch. 12 ).
Treatment of malaria with chloroquine or quinine is usually effective, but widespread resistant strains of Plasmodium have emerged and now, in the UK, artemisinin combination therapy is often used for treating uncomplicated falciparum malaria and IV artesunate for complicated falciparum malaria. Personal protection (malaria prophylaxis) when travelling to endemic areas is preferable. Vector eradication is beneficial, but not always feasible (e.g. use of insecticides). Protection from mosquito bites with treated bed nets is also effective.
In the human host:
During a blood meal, an infected Anopheles mosquito injects sporozoites into the human.
Sporozoites infect liver cells and grow into schizonts , which mature, rupture and release merozoites (exo-erythrocytic schizogony) .
Merozoites infect red cells and undergo asexual reproduction, producing ring stage trophozoites that mature again into schizonts, rupturing to release more merozoites ( erythrocytic schizogony ).
Rupturing of the red cell produces the clinical manifestations of the human disease. Some parasites differentiate into male and female gametocytes (sexual erythrocytic stage) .
In the mosquito host (sporogenic cycle):
During a blood meal, the Anopheles mosquito ingests gametocytes from an infected human host
In the mosquito stomach, the male and female gametocytes fuse to make zygotes
Zygotes become motile and elongate as ookinetes , invading the midgut, and develop into oocysts
Oocysts develop and grow, rupturing to release sporozoites , which travel to the mosquito salivary glands
Sporozoites are injected into the human host with mosquito saliva during a blood meal.
There are four main parasites that produce malaria, and the disease caused by each is usually characterised by the frequency of the fevers produced by the reproductive stages in the human. Fevers tend to occur at 2-day (tertian) or 3-day (quartan) intervals.
Plasmodium vivax ( tertian ):
Prevalent in Asia, Latin America and some parts of Africa
Has a dormant phase in which hypnozoites persist in the liver and result in relapses, weeks or years later
Generally, the disease caused is non-fatal, but it can result in splenomegaly (enlarged spleen), which can cause complications that lead to death.
Plasmodium falciparum ( tertian ):
The most dangerous malarial parasite, accounting for the majority of deaths.
Prevalent in sub-Saharan Africa.
Trophozoites and gametocytes are often seen in the peripheral blood, unlike in other species.
Individuals with the abnormal sickle cell haemoglobin are relatively protected from P. falciparum infection. Merozoites normally interact with red cells through two PfEMP-1 ( Plasmodium falciparum erythrocyte membrane protein 1) dependent interactions. These proteins are impaired in individuals with sickle cell haemoglobin, explaining why sickle cell disease has not been lost from the tropical world through natural selection.
Plasmodium ovale ( tertian ):
Rarer than P. vivax and P. falciparum, it is found in West Africa and the extremes of Southeast Asia
Like P. vivax it can produce hypnozoites in the liver, allowing relapses.
Plasmodium malariae ( quartan ):
Rare but found worldwide
Produces a benign or chronic long-lasting disease.
Helminths are eukaryotic parasitic worms that live within and obtain nutrients from their hosts.
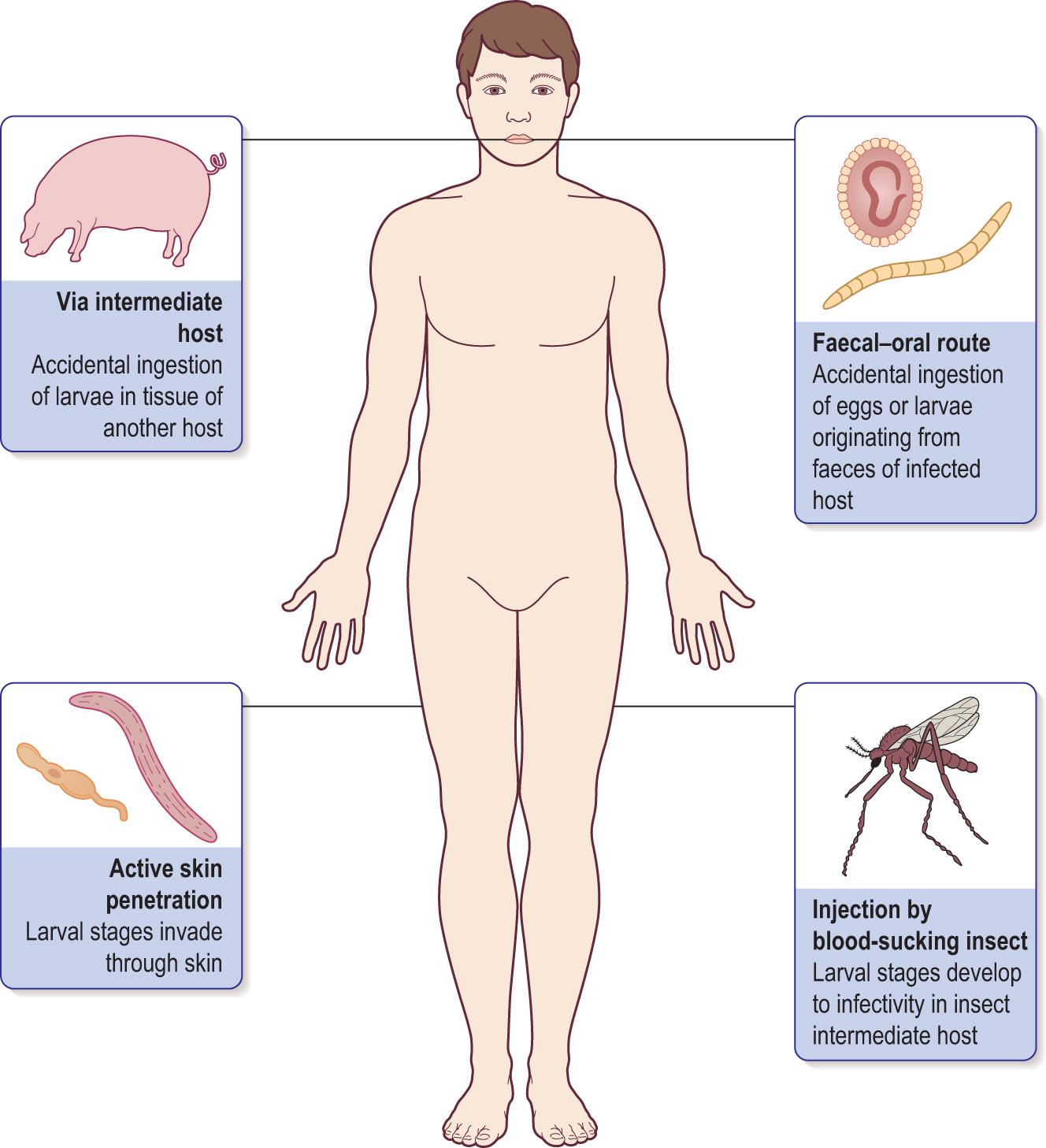
Worms are complex and often large organisms. Although the infecting larval forms are usually small (100–200 µm), the adults can be several metres in length. Infections are more common in warm countries, in children and in people working closely with animals because of the association with food. Intestinal infections, in particular, are seen worldwide, but worms can live in other tissues. Transmission can occur in four main ways ( Fig. 6.6 ) :
Faecal–oral – ingesting infective eggs or larvae from an infected human host
Intermediate – ingesting infective larvae from another infected host, such as eating uncooked infected meat
Active – when larval stages penetrate the skin
Bite – when insects suck blood, which is relatively uncommon.
Multicellular eukaryotic organisms, with DNA, RNA, and a nuclear membrane
Lack backbones, notochords, and jointed exoskeletons: they are ‘worms’ in common parlance
May form cysts with thick walls around the whole organism
May have complicated life cycles, with different forms in different hosts
Replicate asexually and sexually, with gamete formation.
There are three main groups of helminths important in human infections:
Tapeworms (Cestodes)
Flukes (Trematodes)
Roundworms (Nematodes).
Flukes and roundworms feed on human tissues and the contents of the host intestine. Tapeworms have no digestive system and must absorb digested nutrients from their host. Both tapeworms and flukes have complex plasma membranes with mechanisms to protect themselves from a host attack. They release large amounts of soluble antigenic material that plays an important role in the disease and subsequent immunity. Nematodes have a collagenous cuticle in the adult form, which makes them less vulnerable to immune attack.
Humans may also be infected by larvae from other hosts. Toxocara canis is a dog parasite that can also infect humans.
Most helminths replicate outside the host. In the intestine, sexual reproduction produces eggs that are released in the faeces and return to the human host as adults through faeces or injection.
Nematodes:
Can develop to maturity within a single host. The Strongyloides nematode also hatches its eggs within the intestine, producing an autoinfection
Have separate sexes
Some mature in the intestine: Ascaris, hookworms, Strongyloides, Trichinella
Some mature in deep tissues: filarial worms.
Flukes and tapeworms:
Must pass through an intermediate host or hosts
Flukes are mainly hermaphrodites that release larva from intermediate hosts, such as fish, crustaceans or vegetation, which are subsequently ingested
Schistosomes, whose larvae released from snails penetrate the skin, have separate sexes ( Clinical box 6.4 )
Schistosomiasis is an important helminth parasitic infection in humans. Also known as bilharzia , the disease is spread in water contaminated with infected freshwater snails. Common in many tropical developing countries, it particularly affects children who may be playing or swimming in the water. Although it is not fatal, it produces a chronic disease that damages other organs and can impair development in children, and can increase the risk of some cancers.
Tapeworms have replicated reproductive organs along their body ( strobila ) that break off when filled with mature eggs, passing out through the faeces.
Prions were named by the American neurologist Stanley Prusiner when he defined them as: ‘pro teinaceous in fectious particles that lack nucleic acid’. The unlikely concept of an infectious agent that lacks nucleic acid has meant that there continue to be scientists who believe that they could be acting with other agents, such as slow-acting viruses.
PrP C is a normal protein found in cell membranes and consists of amino acids in a mainly α-helical structure. There is some evidence that the protein is involved in the maintenance of long-term memory within the part of the brain known as the hippocampus . PrP SC is an atypical form in which a large proportion of the α-helical structure is replaced by a different type of secondary structure, the β-sheet. Its amino acid sequence is, however, no different from the normal form, although there are sequence differences in the protein between species. This sequence difference is thought to be the reason for resistance to cross-species infectivity.
An isoform of PrP C appears to catalyse its transformation into the abnormal form, PrP SC , resulting in a different structure and attracting, through aggregation, free proteins to form amyloid that accumulates and is deposited locally in the tissues. Over 20 different strains of the infective agent have been described, resulting in variable incubation periods and pathology. It is thought that these strains arise through a conformational change that occurs on crossing between the species ( Clinical box 6.5 ).
Five different human prion diseases have been identified, including Creutzfeldt–Jakob disease (CJD), variant CJD (vCJD) and kuru.
CJD : classic CJD is a fatal neurodegenerative disease that occurs sporadically in approximately 1 in 1 000 000 people each year, although inherited forms caused by a mutation in the prion gene also exist. It is more common with increasing age, and patients experience dementia and show early neurological abnormalities. Sections of brain tissue have a ‘sponge-like’ appearance. Transmission has been shown within human growth hormone, through corneal implants, and on surgical instruments.
vCJD : this was first described in 1996 and had a different clinical presentation from CJD, patients typically being young adults displaying marked behavioural symptoms and a marked accumulation of the abnormal protein.
There is strong evidence that the disease is causally linked to ongoing outbreaks of bovine spongiform encephalopathy (BSE, or ‘mad cow disease’) , which is a prion disease occurring in cattle. BSE began in the 1970s after cattle were fed with a bone meal food, probably from sheep infected with scrapie , another prion disease. BSE became more common in the UK when calves were fed with BSE-infected bovine material. By the end of 2005 more than 35 000 herds of cattle had been affected.
vCJD was linked to the BSE outbreak during which individuals ate beef contaminated with central nervous system material from infected cattle. Until the end of 2007, 166 patients have been identified in the UK, 163 of whom have died, with a peak in deaths in 2000. Although many individuals may carry the infection, only those who are homozygous for amino acid methionine at position 129 in the PRNP gene have so far been infected by this route. Although there are measures to prevent potentially infected tissues from entering the human and animal food chains, reducing the disease incidence, more recent cases have been associated with blood transfusions from individuals who themselves subsequently developed vCJD, suggesting that blood donation is a relatively efficient transmission route.
Kuru , meaning ‘trembling in fear’, was a fatal disease that occurred in the Fore people of Papua New Guinea in the 1950s and was associated with cannibalistic rituals in which the people ate human brain tissue. It is very similar to vCJD and is thought to have originated from scrapie-infected sheep.
An infectious agent may be able to live completely independently of its potential host, but usually it has to form some association with it. These associations are called symbiosis .
Parasitism is when one member of the association gains an advantage, and the other is harmed. The harm ranges from some disadvantage without overt disease, to overt disease, which may be lethal.
Commensalism is where one member of the association gains an advantage, and the other is left unaffected. Bacteroides species are present in large numbers in the large intestine.
Mutualism is where both members of the association gain an advantage, the popular meaning of symbiosis. Bacteroides infection in cattle rumen provides fatty acids as a nutrient for the host.
When an infectious agent causes disease, it is acting as a pathogen. However, there are many infectious agents that can exist in a commensal or even mutualistic relationship with their host, and only become pathogenic in some special circumstances.
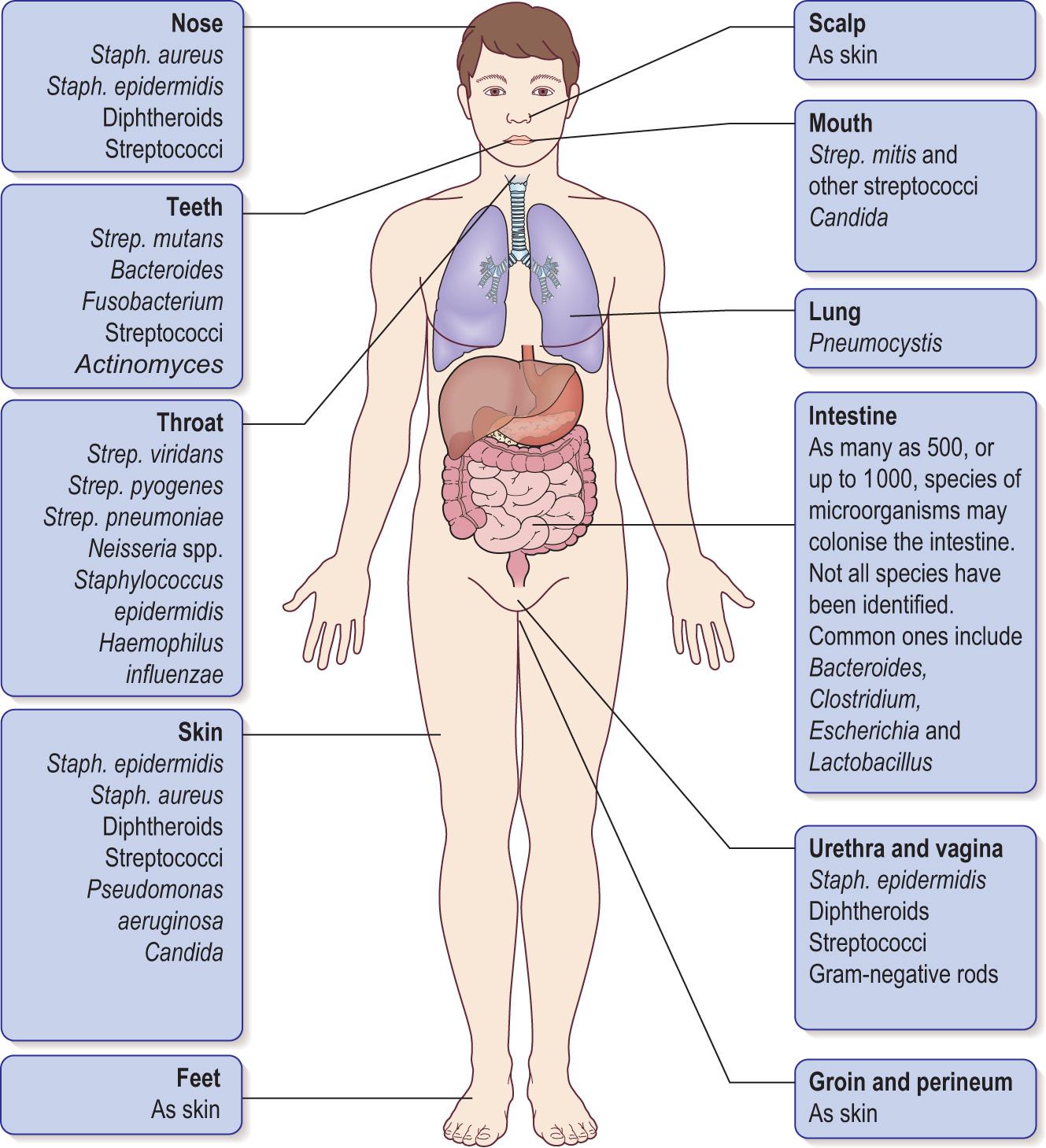
The normal flora consist of microorganisms, mostly bacteria, present on many of the body surfaces in the normal healthy individual.
The whole body is normally sterile immediately before birth, but the surfaces rapidly become colonised after delivery. The skin, mouth, upper respiratory tract, gastrointestinal tract, and genitourinary tract acquire a variety of microorganisms from the environment and from contact with other people. Bacteria form a major component of faeces. Secretions such as saliva, sebum and tears are normally sterile within the glands but become contaminated as soon as they reach the mucous membrane or skin surface. Blood, cerebrospinal fluid, lymph, bones, joints and all internal organs are normally sterile in health.
Establishment of microorganisms at particular sites depends on several factors, including exposure of the site, availability of suitable receptor sites and ability of organisms to adhere to target receptor sites, to compete for nutrients and to evade or withstand host defence mechanisms. Fig. 6.7 illustrates microorganisms found within the body as normal flora.
Normal flora offer humans several benefits:
Colonisation leads to resistance to more virulent bacteria.
Microorganisms may digest nutrients in the bowel (more important in animals).
Presence of the normal flora also resists other colonisation attempts through:
Competition for receptor sites involved in adhesion
Competition for essential nutrients for growth
Creation of unfavourable micro-environments that discourage colonisation.
Lactobacilli in the vagina produce acid from glycogen and maintain low pH that is unsuitable for many exogenous bacteria and Candida.
Production of inhibitory substances. Some staphylococci on the skin produce antibiotics, which inhibit other bacteria.
Different groups of bacteria that are adapted to live as normal flora are found in different sites: the mouth, the gastrointestinal tract, the nose and oropharynx, the skin, the vagina. There are no simple criteria, such as morphology, staining, biochemical characteristics or growth requirements, that distinguish normal flora from pathogens. The normal flora may be disrupted through a variety of mechanisms:
Suppression by antimicrobial agents allowing overgrowth with resistant organisms
Changes in general health or immunity
Hormonal changes
Local trauma.
Particular sites of normal flora may be affected in certain circumstances, allowing overgrowth by more virulent organisms:
Mouth:
Dietary changes
Reduction in salivary secretion
Dental disease, dental treatments and oral hygiene
Gastrointestinal tract:
Dietary changes
Gut disorders
Female genital tract:
Menstrual cycle
Pregnancy
Intrauterine contraceptive device
Skin
Use of soaps, cosmetics, antiseptics
Moisture – wet or dry
Respiratory tract
Viral infections
Secondary bacterial infections
Damage to ciliated epithelial cell function (through smoking, for example).
Sometimes organisms, which are part of a person's normal flora, can act as pathogens. This tends to occur when there is:
Breakdown in the local epithelium because of trauma (e.g. surgery) or other infection
Introduction to an unusual site (e.g. gut organisms in the urinary tract, possibly introduced by medical interventions, such as catheterisation)
Alteration in balance of normal flora (e.g. use of antimicrobial drugs can also lead to suppression of Lactobacillus in the vagina, encouraging Candida growth)
Immunodeficiency.
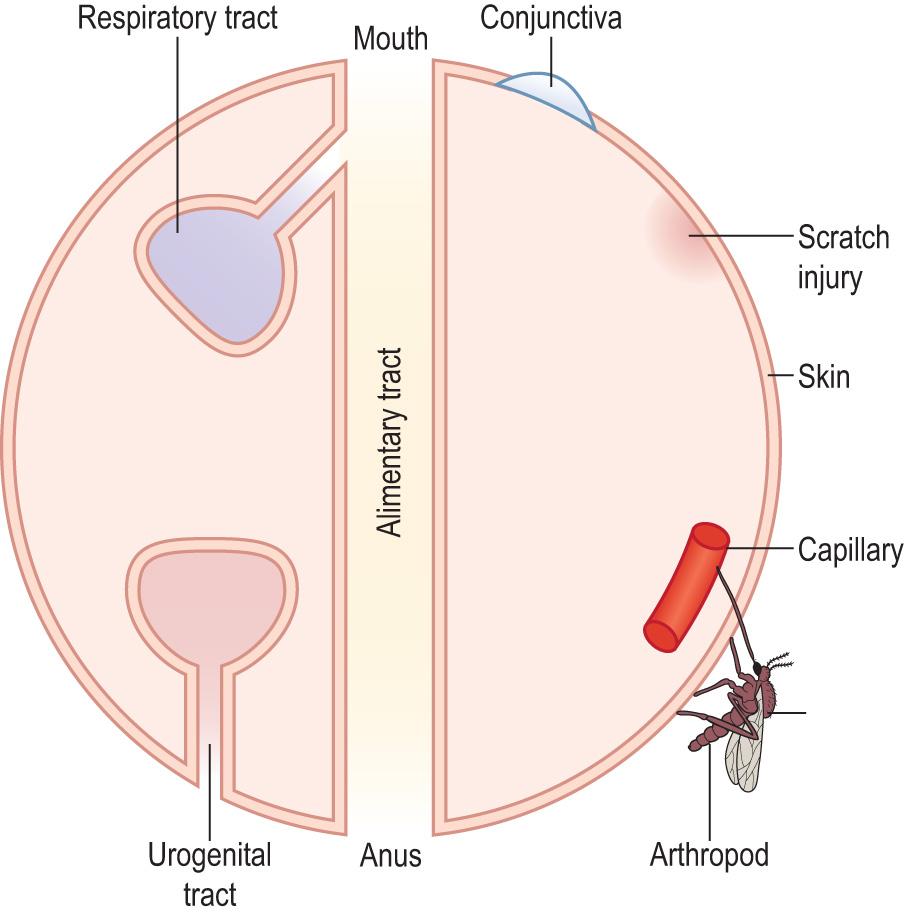
Microorganisms, in order to become pathogens, must attach to, or penetrate the body surface – a series of surfaces ( Fig. 6.8 ) that is extensive, offering considerable opportunities for its penetration. All these surfaces offer a portal for entry and all are covered by some kind of epithelium; the keratinised stratified squamous epithelium of the skin is the toughest. Epithelia have both innate and adaptive immune mechanisms in place and are capable of mounting an immunological response (see later). For example:
Immunoglobulin (Ig)A in mucous membranes blocks pathogenic adhesion
Lysozyme attacks bacterial peptidoglycan.
The epithelial surfaces are more easily breached if defence mechanisms are impaired. In untreated or uncontrolled diabetes mellitus, which impairs phagocyte activity, there is increased glucose available for pathogens, predisposing the patient to infection, especially of the skin and urinary tract. In AIDS, depletion of CD4 T lymphocytes and infection of dendritic cells allows opportunistic infections to gain access (see later). Other mechanisms that allow, or protect from, entry are described later.
The skin is a tough, multilayered membrane rendered waterproof by keratin, and more resistant to pathogenic invasion than internal membranes.
Sebum from sebaceous glands has antibacterial action
Clothing helps maintain integrity.
Some agents can penetrate intact skin:
Arthropods : some pathogens have adapted to a life cycle that is dependent on biting arthropods (e.g. mosquitos, flies, fleas, bugs, ticks and mites), penetrating the skin when they feed on human blood.
Needles : any mechanism in which needles are introduced into patients (taking blood, setting up intravenous access, blood transfusion) may introduce a pathogen. A patient may be infected locally by skin flora, staff may be infected, or the agent that is being transmitted may carry a pathogen. Good hygiene, screening of donated blood and education of intravenous drug users can reduce this.
Surgical wounds allow entry of any contaminating pathogens in much the same way: Staphylococcus aureus is a particular problem because of antibiotic resistance.
Abrasions and wounds : apparently intact skin often allows entry through minor cuts and abrasions leading to conditions such as warts, from the papillomavirus , or impetigo from S. aureus.
Animal bites enable a large variety of bacteria to infect the tissues. For example, rabies virus is present in the saliva of infected animals, and is passed on via bites.
Wounds of violence and war allow infection by a wide variety of contaminating bacteria. Foreign bodies in tissues are associated with local anoxia, encouraging the growth of anaerobic bacteria.
Intact skin can be penetrated by some larvae.
This is particularly vulnerable because it is a very large area of moist living cells, which are exposed to pathogens that may be present in inspired air, or through the lachrymal duct in the eye. The fact that the barrier between air and circulation in the alveoli is only one cell thick makes the potential for infection high. The respiratory system offers various defences:
Hairs in the nose trap large foreign particles
Sneeze and cough reflexes expel foreign bodies, excess mucus and infected secretions
Mucus, produced by goblet cells and subepithelial mucous glands, contains IgA and lysozymes and traps foreign particles
Cilia in the pharynx help remove particles so they can be swallowed (mucociliary escalator)
Alveolar macrophages phagocytose and destroy small particles.
Other environmental factors may break these defences:
Conditions that inhibit the mucociliary escalator – such as cystic fibrosis or smoking
Anatomical defects that allow mucus to collect, e.g. bronchiectasis
Foreign bodies that might block bronchi, e.g. peanut inhalation
Drugs that suppress the cough reflex
Inhalation of food or vomit in the unconscious person.
This is vulnerable because, in order to absorb nutrients, it has a very large surface area, which is constantly being exposed to the environment through food and drink that might be contaminated ( Clinical box 6.6 ).
Food poisoning (gastroenteritis) may be defined as ‘usually either infectious or toxic in nature, caused by agents that enter the body through the ingestion of food (or water)’. Diarrhoeal diseases are among the leading causes of death in children under the age of 5 years, particularly in the developing world. Bacteria cause gastroenteritis in three possible ways, sometimes a mixture of all three:
By adherence to specific receptors in the intestinal mucosa prior to invasion, to produce secretory diarrhoea as the direct result of adhesion.
By invasion of the intestinal epithelium to produce bloody diarrhoea with abdominal pain (dysentery). Common infecting agents include Campylobacter, Shigella, Salmonella and enteroinvasive Escherichia coli.
By producing toxins that cause excessive fluid loss into the intestinal lumen, causing profuse, watery diarrhoea. Organisms include Salmonella, Vibrio cholerae, verotoxin-producing E. coli, Bacillus cereus, Staphylococcus aureus, Clostridium difficile, C. botulinum and C. tetani.
The gastrointestinal tract defends itself through:
Stratified squamous epithelium in the mouth and oesophagus, which is less easy to penetrate than the simple epithelium found below the stomach
Low pH in the stomach and bile, both of which kill many pathogens
Mucus traps microbes and impedes their transport
IgA in saliva and digestive juices blocks the adherence of pathogens, and lysozyme attacks bacterial cell walls
Normal flora in the small and large intestine compete with pathogens
The vomiting reflex and diarrhoea void damaging contents through refection and increased turnover of gut epithelium.
Pathogens may pass up the urethra or vagina. Sexual intercourse is frequently involved. There are various defence mechanisms, which vary according to male or female anatomy:
In the urinary tract, the flow of urine, complete emptying of the bladder and functional integrity of the epithelium are important to resisting infection
In the vagina normal flora are important, competing with pathogens and maintaining an acid pH.
Most urinary tract infections come from the outside, via the urethra ( ascending ). Females are much more vulnerable because they have a shorter urethra and are more exposed through sexual intercourse, which can breach the intact epithelium. The entire urinary tract can also be infected through other routes: e.g. renal abscess, renal tuberculosis and bladder schistosomiasis offer opportunity for infection to enter the urinary tract other than the usual route, through the urethra.
The conjunctiva of the eye is formed of stratified, but not keratinised, epithelium and is protected by tears (containing lysozyme and IgA) and the cleansing action of the lids.
During childbirth , neonates' conjunctivae are vulnerable to infections of the mother's cervix or vagina, such as those caused by Neisseria gonorrhoeae or Chlamydia trachomatis
The naso-lachrymal duct offers a possible route of infection from the eye to the upper respiratory tract.
A variety of pathogens may cross from an infected mother to the foetus across the placenta, such as rubella and hepatitis B viruses (although the latter much more commonly causes infection during delivery).
Childbirth offers further opportunities for exposing a newborn infant to infections through a variety of portals described earlier and it may not always be possible to determine which one.
Pathogens are successful because they exploit the environments provided by a host, whether living within or outside cells. All pathogens need a supply of metabolic material, although viruses (which lack nuclei) need nuclear synthesis. This means that viruses can only live within host cells. Other organisms may flourish inside or outside the cell, or both, taking their nutrients from the cytoplasmic or extracellular fluid. The larger pathogens, such as nematodes , are almost always extracellular and some may gain their nutrients by ingesting host cells.
Intracellular pathogens , while being vulnerable to intracellular killing mechanisms, are protected from many of the host defence mechanisms, as well as from therapeutic agents. The pathogens may also live within the same cells that are responsible for the host immune reaction, reducing the ability of the host to mount a defence against the infecting organisms. To succeed, however, these organisms must have an extracellular phase as they pass between cells, and this offers opportunities for host and therapeutic attack. Any attack, intracellular or extracellular, makes the host cells vulnerable and many will die, leading to tissue damage.
Extracellular pathogens are exposed continuously to the host cellular defence mechanism. For this reason, they tend to be larger and more complex; they are able to move rapidly and reproduce faster than others. They may also have a structure that means, for example, that they are not vulnerable to mechanisms such as phagocytosis. The helminths (worms) are typical of these organisms.
Almost all pathogens have to adhere to host cells. The cells at the portal of entry are often the first target, but adhesion is essential later in dissemination round the body. Pathogens that are injected into the host do not have to adhere in order to invade, but they will probably have to adhere later.
Bacteria exhibit various different adherence mechanisms:
Non-specific adherence: hydrophobic molecules in walls, capsules and slime all adhere non-specifically to host cells
Specific adherence: many bacteria have adhesins, either on the cell wall, outer membrane or other wall structures, which bind to molecules on host cells
Carbohydrates, such as D-mannose, sialic acid and blood group carbohydrates
Proteins, e.g. fibronectin.
Viruses have docking proteins, e.g. haemagglutinins on the influenza virus. This may be at the portal of entry, or if they are injected in some way, they may travel to a site where they can bind.
Helminths may have specialised mouth parts, such as found in the tapeworm or the hookworm, which enable the organism to be retained within the bowel.
Some pathogens do not invade, but continue to adhere to the epithelium at the portal of entry, e.g. skin fungi, Vibrio cholerae . Others may invade the portal of entry, and spread no further, while others do spread further. Invasion may take the pathogen into the host's cells, or between the cells into the extracellular spaces, or both. It usually follows adhesion, but a pathogen may invade by being injected.
Bacteria : Bacteria may move between cells, through the intercellular junctions, e.g. Salmonella spp., while others may invade the cells to which they have adhered. This can lead to host cell involvement: (a) host cell actin polymerisation may be induced, leading to pseudopod formation and bacterial engulfment within a vacuole, and (b) the vacuole disintegrates and the bacterium lies free within the cytoplasm.
Viruses : all viruses must invade (infect) a cell to reproduce after adhesion via the docking protein. Many viruses enter cells on mucous membranes and remain localised to the epithelium with disease developing within a few days. There is little or no invasion of underlying tissue, and the virus is shed directly to the exterior. These local infections offer only short-term immunity. Influenza viruses, rhinoviruses and in the gastrointestinal tract, rotaviruses are all viruses of this type.
Fungi use enzymes to break down ground substance and matrix of epithelia and connective tissue.
Bacteria can be disseminated through:
Tissues, aided by enzymes such as collagenase, hyaluronidase and streptokinase
Blood through which bacteria can reach any tissue or organ. A bacteraemia is the presence of live bacteria in the blood; when the bacteria multiply in the blood this produces septicaemia.
( Clinical boxes 6.7 and 6.8 ).
Meningitis , an inflammation of the meninges, is an important public health issue that is often potentially preventable. It is more prevalent in developing nations. Viral meningitis is commoner than bacterial meningitis but is usually self-limiting in the UK, with the exception of Herpes meningo-encephalitis.
The main bacterial causes of acute bacterial meningitis in people who have not been immunised are:
Haemophilus influenzae B (in children under 5 years)
Neisseria meningitidis
Streptococcus pneumoniae
Other bacterial causes include Mycobacterium tuberculosis, Listeria monocytogenes (in the immunocompromised) and Group B streptococci and E. coli in neonates
Clinically, the classic triad of fever, headache and neck stiffness should give rise to suspicion of meningitis. A petechial rash may precede the symptoms, septicaemic shock may develop, and death may ensue. Bacteraemia may lead to multisystem/multiorgan infection.
The capsular polysaccharides of N. meningitides that inhibit destruction and clearance (phagocytosis) by the host defence mechanisms have been used to produce vaccines (meningococcal conjugate C vaccine), which were introduced into the UK routine vaccination of children programme in 1999. Haemophilus influenzae B (Hib) vaccine and a polyvalent pneumococcal vaccine are also available.
Some bacteria target specific organs or systems, but other bacterial infections can lead to multisystem disease. One example is E. coli, which has an enterohaemorrhagic form (serotype 0157:H7), also known as verotoxin-producing E. coli (VTEC). The enteroinvasive form of E. coli causes bacillary dysentery, but VTEC not only causes bloody diarrhoea but also secretes a toxin that affects vascular endothelial cells in the bowel and kidneys, when the patient may develop thrombocytopenic purpura and/or haemolytic uraemic syndrome (HUS). Administration of antibiotics may exacerbate HUS by increasing toxin production.
Viruses that have the capacity to invade subepithelial tissues may enter the lymphatic system.
If the virus is quickly inactivated by macrophages from the lymph nodes sinuses, the immune response is initiated, resulting in a regional lymphadenopathy , but the infection does not progress.
If the virus is not inactivated, particularly if it can survive or replicate in macrophages or lymphocytes, the particles are passed through the lymph nodes into the bloodstream.
The virus is likely to be distributed to distant parts of the body and establish infection in the reticuloendothelial system (the primary viraemia – an asymptomatic event during the incubation period).
Following a period of replication in distant sites, such as liver and spleen, large amounts of progeny virus may be released into the bloodstream, leading to the onset of the clinical effect of a systemic viral infection , which can spread to other organs.
If the virus lodges in skin capillaries, a rash may be a prominent feature, such as is seen in measles and chickenpox.
The nature of viraemia depends on the virus. Those carried in monocytes or lymphocytes are more protected and can be disseminated more widely.
As soon as a pathogen reaches the portal of entry, it encounters the defence system. The non-immune aspect includes the integrity of the epithelium, chemical defences and normal flora. Agents of the innate and adaptive immune systems may be present at the portal, and will be further encountered if the pathogen invades or is disseminated. Survival of the pathogen depends on circumventing the defence system, which it does in several ways ( Clinical box 6.9 ):
Stress survival:
Host defences may damage the pathogen by denaturing proteins.
Chaperonins (heat shock proteins) protect against denaturing of other proteins.
Free radicals , such as superoxide and hydroxyl radicals, and hydrogen peroxide damage and kill pathogens. They may be produced by the pathogen's aerobic metabolism, or by host defences.
Scavenge for nutrients:
Some nutrients, sugars, amino acids and fatty acids are freely available, but iron, in the form of Fe 3+ , is not. This form of iron is important for bacterial survival and its inaccessibility is part of the non-specific defence provided by the body.
Bacterial siderophores bind iron avidly and capture it for the bacterium.
Fungi use sophisticated mechanisms to acquire nutrients as saprophytes.
Shelter:
If the pathogen can enter host cells it will be protected from antibody attack. All viruses replicate within host cells, and some bacteria, fungi and protozoa shelter within cells.
Some survive within phagocytes, either by escaping from the phagosome into the cytoplasm, preventing phagosome-lysosome fusion (e.g. toxoplasmosis ), or by being tough and resisting the phagocytic enzymes and radicals (e.g. M. tuberculosis ).
When bacteria or virions are released from the cell they are fully exposed to the immune system, but viruses or bacteria that pass directly from one cell to another minimise this exposure.
Some pathogens survive in sites where the immune system is poorly represented, such as the lens of the eye, which can become infected in congenital rubella infection.
The pathogen is not completely protected from the immune system. The infected cell will present pathogen-derived antigens on class I human leucocyte antigen (HLA) molecules, and can be killed by CD8 T lymphocytes, destroying the shelter (see later).
Some viruses, however, can suppress the expression of HLA molecules (for example cytomegalovirus). Latent infections by viruses will not generate any viral proteins.
Stealth : sometimes the pathogen is fully exposed but avoids a lethal encounter through disguise or deception. This can be done in a variety of ways:
Shielding : bacteria, for example, may have structures outside their cell walls, such as capsules and slime, which prevent recognition and subsequent phagocytosis and complement activation that would destroy them.
Active action : bacteria may also possess protein A, which blocks binding of the Fc portion of the antibody to its receptor, thus blocking opsonisation , the process through which a pathogen is prepared for phagocytosis.
IgA proteases within bacteria can also cleave IgA, which mediates transport of immune complexes across epithelial cells.
Antigenic variation : some pathogens mutate and thus change their antigens. This may happen during an infection of a single individual, such as in African trypanosomiasis , or it may happen between outbreaks, such as in influenza , so that the pathogen remains one step ahead.
Antigenic mimicry , or adsorption of host protein: for example, S. aureus adsorbs fibrin and IgG.
Ineffective antibody : many pathogens elicit an antibody response, but it is ineffective because it is acting on unimportant determinants. Sometimes the antibody produced may even help the pathogen by enabling uptake into phagocytes where it can survive.
Forming a stronghold : pathogens may create some form of sanctuary where the immune system cannot reach them, or which is too strong to be destroyed. For example:
Coagulase, e.g. S. aureus, causes coagulation of plasma proteins around the bacterium, hindering the immune system
Abscesses are surrounded by fibrosis, isolating the contents
Some helminths form cysts that are too tough to be destroyed.
In order to invade the host, microorganisms have to evade normal protective physical or chemical barriers. One example is H. pylori, a Gram-negative spiral organism that causes gastritis and peptic ulcers, and is a predisposing factor for gastric cancers. Helicobacter pylori infects 50%–90% of the world population with the highest prevalence in developing countries with poorer hygiene. In order to attach to and colonise the human gastric mucosa, H. pylori has to survive in the extreme acidic environment and overcome the mucous secretions in the stomach. This is facilitated by its motility and the secretion of the enzymes urease and catalase.
Inhibition or death of cells of the immune system, and damage or death of other cells, can inhibit the defence system (see Clinical box 6.9 ).
Bacteria make a variety of toxins, which make the patient ill, and promote bacterial survival by killing cells of the defence system, or by killing other cells and disabling tissue function, which indirectly damages defence:
Exotoxins are substances produced by bacteria with a variety of functions
Pore-forming proteins lyse cells by assembling into pores in the cell membrane
Enzymes with phospholipase activity lyse cells by destroying part of the membranes
Toxins can enter cells and inhibit protein synthesis , e.g. diphtheria toxin
Toxins can also enter cells and deregulate their metabolism, e.g. cholera toxin stimulates adenylate cyclase in the gut epithelium, leading to massive electrolyte efflux, and copious watery diarrhea ( Clinical box 6.10 )
Microorganisms can produce disease by direct invasion of tissues or by producing toxins. The bacterium S. aureus is a good example.
Diseases produced by invasion include:
Skin infections such as impetigo, boils and cellulitis
Bone infections such as osteomyelitis
Brain abscesses, meningitis
Pneumonia, lung abscesses.
Diseases produced by the toxins include:
Staphylococcal food poisoning (enterotoxin B)
Scalded skin syndrome
Toxic shock syndrome (most commonly due to retained vaginal tampons).
Toxins stimulate nerves , e.g. staphylococcal enterotoxins that act on gut nerves, which signal to the brain to cause vomiting
Toxins also act as superantigens , activating many T lymphocytes, with the release of many cytokines, especially tumour necrosis factor (TNF), causing shock ( Clinical box 6.11 ).
Bacterial endotoxins in the bloodstream due to severe infection promote the release of pro-inflammatory factors, such as tumour necrosis factor (TNF), from phagocytic and non-phagocytic cells that mediate a systemic inflammatory response. At the same time, compensatory anti-inflammatory cytokines are released. This is part of the normal defence mechanism in response to the challenge of exogenous infection. However, when this response is disseminated due to overwhelming infection, shock and widespread tissue damage can occur, known as septic shock, which is clinically characterised by fever, hypotension and intravascular coagulation, and is potentially fatal. The initial overwhelming inflammatory response may be followed by immune suppression. Gram-negative bacteria that produce endotoxins are more likely to have this effect, mediated by the release of TNF.
Endotoxins are lipopolysaccharides from the cell wall, produced by Gram-negative bacteria in large quantities, particularly in Gram-negative septicaemia. They stimulate macrophages to release cytokines, especially TNF and interleukin (IL) 1. They also initiate intrinsic coagulation and alternative complement cascades that can lead to fever, hypotension, shock, disseminated intravascular coagulation (DIC), organ failure through poor perfusion and death.
Some bacteria and protozoans disable the phagocyte response by living within phagocytes.
Viruses often kill the cells which they infect, and if they are cells of the immune system, this will promote their own survival. For example, HIV infects CD4-positive T cells and causes profound immunosuppression.
For a microorganism to be successful, it must leave the body and be transmitted to a fresh host. Most microbes leave from the body surfaces, but some have to be extracted by vectors. Examples of exit mechanisms are the:
Gastrointestinal tract through faeces, vomit or anal intercourse
Respiratory tract through coughing and sneezing producing droplets
Genitourinary tract through sexual intercourse or in urine
Conjunctival fluids entering water, such as when swimming, or going onto the hand
Skin surfaces through touching or shedding of bacteria on skin
Through normally intact skin by insect vectors, needles, donating infected blood, blood splashing after wounding.
The ability for an organism to transmit its infection once it has exited the host depends on three main factors:
The number of organisms shed and the route of shedding
Its stability and survival outside the host within a wide variety of environments
The number of organisms needed to infect a fresh host.
Infections are transmitted through a variety of routes and these are the focus for many public health initiatives ( Fig. 6.9 ) . Transmission can also be described, not only by the route, but by the mechanism:
Vertical transmission occurs when infection is spread from mother to foetus, or breastfed infant:
The rubella virus, for example, if present in the mother's blood, crosses the placenta, infecting the foetus, which will not be protected because of the lack of ability to produce antibodies.
During delivery, abrasions and wounds from the birth process can breach the skin, or infectious agents can enter through the conjunctiva or mucous membranes. Hepatitis B and streptococcal infections are both examples of this type of transmission.
During breastfeeding, if the infectious agent is present within breast milk, this may infect the child. Human lymphotropic virus 1 (HTLV-1) and HIV can both be spread in this way.
Horizontal transmission occurs between individuals or between species, other than mother and offspring in utero or at or around the time of birth. The transmission may be:
Direct – when the transmitter and recipient are close. For example, through infected skin or other surface contact with a portal of entry, or inhaled droplet infection from a sneeze.
Indirect – where the transmitter and recipient can be distant from each other. Several mechanisms are possible and involve some form of vehicle, for example: food – such as Salmonella being transmitted through eggs; water – sewage can infect water, and cholera can be transmitted this way; fomites – any agents that retain an infective agent, such as cytomegalovirus surviving on toys; surgical instruments – before the days of sterilisation, or if sterilisation fails (prions are not inactivated by heat sterilisation), or through needle-stick injury; and transfusion of infected blood – the transmission of HIV to individuals with haemophilia through factor VIII concentrate prepared from pools of individuals, some of whom were later found to have HIV, is an example of how a large number of people were adversely affected by therapeutic intervention.
Vectors, such as mosquitos that transmit malaria or yellow fever, and the tsetse fly that transmits trypanosomes.
Airborne: such as dust from dried smallpox crusts, or water droplets from air conditioning transmitting legionnaires disease.
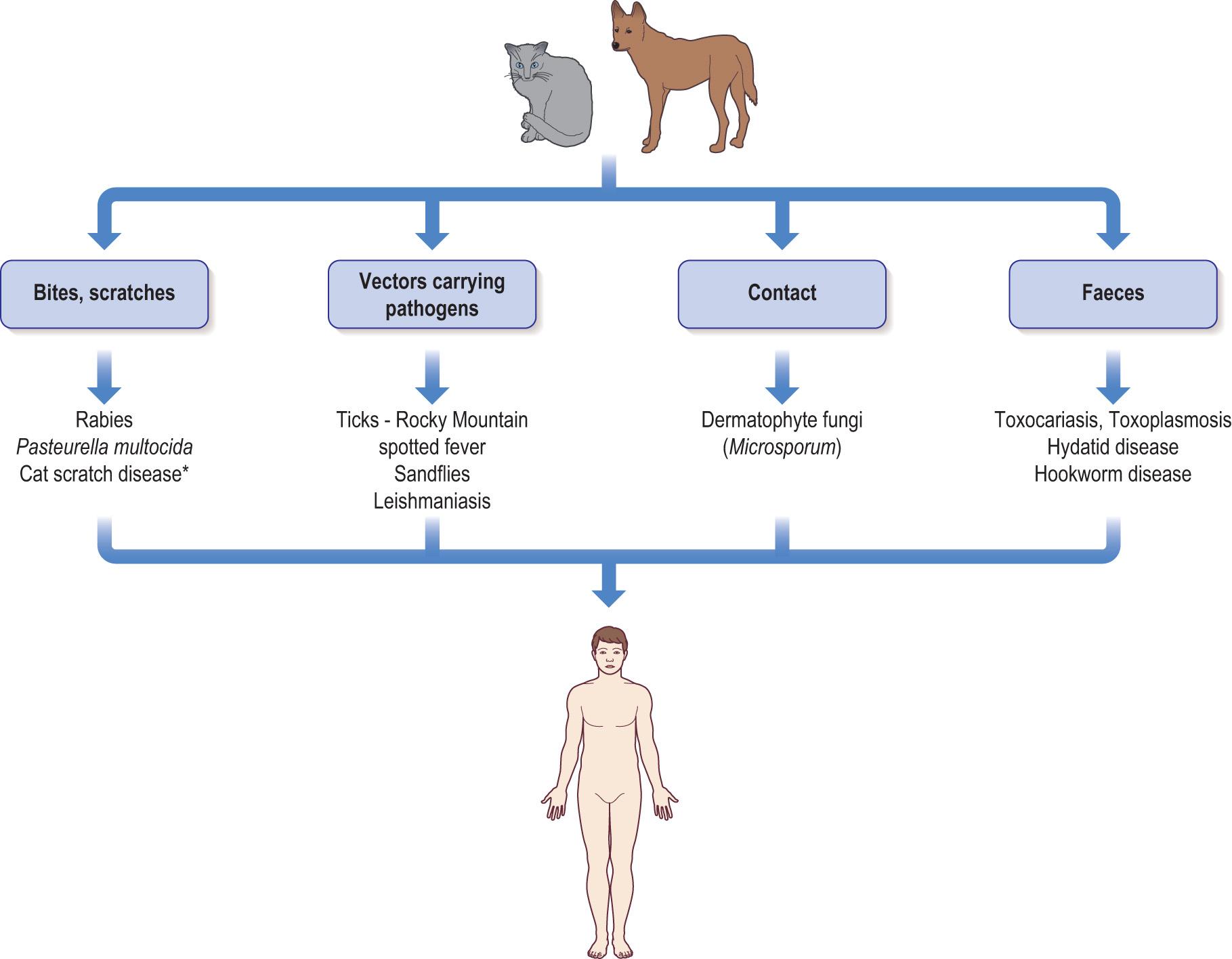
The disease experienced by a person will depend on the infecting agent, the status of the patient, the circumstances of the infection and its subsequent treatment. Treatment of microbial infections is too big a subject for this chapter and the reader should consult a specialist textbook on microbiology. Distinctions can be drawn between the various patterns, however, which help in describing what is observed.
Some viruses cause local infection at the portal and replicate, but do not spread
In some cases, there is limited replication at the portal and the infection spreads to the local lymph nodes for a further cycle of replication, spreading subsequently to a target tissue, resulting in a general infection .
Some virus infections cause local or general infections, but these do not persist
Those that persist with continuous viral production, lead to a chronic infection (e.g. HIV and hepatitis C infections)
Those that persist for long periods without virus production, but which can be reactivated, are called latent infections (e.g. herpes infection).
This is the time between infection and disease manifestation and can be short, medium, long or very long.
Sometimes diseases may first become evident as a result of a prodromal illness, or become evident because of the appearance of specific symptoms, which may or may not continue over time. Fever is a common feature of infections ( Clinical box 6.12 ).
In healthy human beings, inflammatory cytokines are released to combat bacterial or viral infections. These cytokines stimulate the hypothalamic thermoregulatory centre in the brain to increase prostaglandin (PGE 2 ) synthesis, leading to an increase in body temperature (fever). Fever has an inhibiting effect on bacterial and viral proliferation, and thus a beneficial effect on the course of infection. The use of salicylates and non-steroidal anti-inflammatory drugs to reduce fever is based on their inhibitory effect on prostaglandin synthesis.
A prodromal illness is one that produces non-specific symptoms, usually due to the release of cytokines, which usually occurs at the end of the incubation period of viral infections. For example, fever, malaise, myalgia, abdominal pain, anorexia and bowel disturbance may be observed several days before the patient becomes jaundiced in hepatitis B infection.
Acute disease starts suddenly, lasts a few hours or days, and then ends in death or the person recovers (such as the bubonic plague, or the common cold, respectively). Some diseases have a more insidious onset ( subacute ), while others are chronic , lasting many years. The same disease can produce all of these patterns. For example, osteomyelitis can have an acute onset, but can then last a long time and eventually lead to death. Pulmonary tuberculosis has an insidious onset but it too leads to a chronic disease, which without effective treatment culminates in death.
Self-limiting diseases are those in which the person recovers without treatment.
Human pandemics have been recorded throughout history. Many, but not all (e.g. the Black Death, which was caused by a bacterium, Yersinia pestis ) have been caused by viruses.
An epidemic is when the frequency of an infection or disease increases above the normal ( endemic ) levels.
A pandemic is a ‘global’ epidemic. The first recorded pandemic was thought to be smallpox in 430 BC, to be followed over the intervening years by outbreaks of bubonic plague (the Black Death) and, more recently, cholera. In the twentieth century, it was the viral infection influenza, which produced three pandemics. The first ‘Spanish’ flu was estimated to have killed over 40 000 000 people and was followed by ‘Asian’ flu in 1957 and ‘Hong Kong’ flu in 1968. Pandemics occur when a new virus emerges ( Clinical box 6.13 ).
Influenza is caused by a virus of the orthomyxovirus family, not to be confused with the common cold, which is caused by a rhinovirus. The clinical features and normal course of the two conditions are also different, where influenza is more debilitating and has potentially more long-lasting complications, such as the post-viral or chronic fatigue syndrome. After an incubation period of 2–3 days, influenza presents with an acute onset of fever, muscle pains, severe headache and sore throat. Secondary lower respiratory tract bacterial infection can occur due to Streptococcus pneumoniae and Staphylococcus aureus.
The influenza virus has two main forms, A and B. Influenza A has the ability to develop antigenic variants at irregular intervals and was the cause of the 1918 pandemic. A major change in the antigenic make-up of influenza A led to the emergence of the Hong Kong influenza type H3N2 in 1968, and the avian H5N1 strain in 1997 and ‘swine flu’ in 2008 (see also Pandemics).
Vaccination is the administration of antigenic material to a person in order to make them immune to a disease. It first became known about in Britain when Lady Mary Wortley Montagu wrote about the practice of people inoculating themselves with fluid from people suffering from smallpox when she was in Turkey, and used it on her children. Edward Jenner (1749–1823) is regarded as the founder of modern vaccination; milkmaids who had been exposed to cowpox (vaccinia) were resistant to smallpox infection and he tested this hypothesis by experimenting on a young boy, first infecting him with cowpox. Subsequent inoculation with smallpox failed to produce the latter disease. Vaccines can be made to provide protection against both viruses and some bacteria and there is a great deal of effort being made to produce vaccines to prevent malaria.
The best that can be hoped for is that vaccination should be used so that a disease is eradicated. Smallpox was reported as being eradicated in 1977 and polio is close to being eradicated. Sometimes vaccination is used to prevent the symptoms of a disease, such as avoiding toxins produced by tetanus, or attempting to block transmission of a disease, such as malaria.
Live attenuated : these are the most successful vaccines and consist of live virus particles that are less virulent (attenuated), perhaps by passing them through cell lines, or adapting the virus to work best at low temperatures. Polio (Sabin), measles, mumps, rubella and yellow fever are all examples of diseases that are treated with live attenuated vaccines.
Inactivated : inactivated viruses have been killed by a process, such as heat, or exposure to formaldehyde. The virus capsid is still recognised by the immune system, thus protecting the individual, but because there is no replication, an individual will need booster injections over time. Polio (Salk), rabies, hepatitis A and influenza are examples of these types of vaccine.
Subunit : These vaccines use parts of the virus, such as the capsid, or surface coat, or other viral proteins. This is not always very successful because the proteins are readily denatured and any antibodies that are produced may bind to the denatured protein, but not the viral protein itself. A development of this idea has led to recombinant vaccines in which the relevant protein gene is placed into another virus. Vaccination with the second virus will lead to expression of the harmful virus protein, stimulating antibody production that protects the patient from the harmful infection. Hepatitis B vaccination is an example of this type of vaccination.
Table 6.6 summarises the advantages and disadvantages of the different types of vaccine.
| Advantages | Disadvantages | |
|---|---|---|
| Live attenuated | Most successful as they produce all features of the infection in a mild form and protect over many years. Attenuated strains may pass into the population, further protecting people | May revert to a more virulent form |
| Produce severe disease in immunocompromised patients | ||
| Hypersensitivity to proteins from the attenuation process, such as chicken eggs | ||
| Danger of passing on animal viruses | ||
| Inactivated | Present no hazard as they are killed | Short acting |
| Subunit | Non-hazardous as they do not contain whole virus | May only stimulate B cells, producing only a primary response, rather than producing the longer antigenic memory that is the result of T cell activation. T cell activation may vary between people, depending on their inheritance of different polymorphisms of the class II human lymphocyte antigens (HLA-D) |
Everybody, to stay healthy, tries to keep other life forms outside. We are surrounded by a large variety of organisms, from viruses to insects, many of which try to subvert our cells, or live in our bowel, eat our food, frolic in our tissue spaces, breed in our bladders or brains, or lay their eggs under our skin. It has been known for a long time that such invaders are associated with disease. Koch, in 1890, defined four criteria that needed to be fulfilled to establish a causal relationship between a microbe and a disease ( Koch postulates ). He later abandoned the italicised part of the first postulate, because of the finding that asymptomatic carriers existed.
Koch postulates (the italicised part now having been abandoned):
The microorganism must be found in abundance in all organisms suffering from the disease, but not in healthy organisms
The microorganisms must be isolated from a diseased organism and grown in pure culture
The cultured microorganisms should cause disease when introduced into a healthy organism
The microorganisms must be reisolated from the inoculated, diseased experimental host and identified as being identical to the original specific causative agent.
To keep these infecting organisms away, the body has defence mechanisms, which may be classified as:
Non-immunological
Immunological – innate or adaptive.
The body has various barriers, which have been more fully discussed in the previous section. Some examples are given here.
The skin acts as a barrier, preventing microorganisms entering the body fluids
The upward flow of mucus in the bronchi, propelled by the cilia, washes out microorganisms (see Ch. 13 , the mucociliary escalator)
Complete emptying of the bladder and the inhibition of urine flow up the ureters both inhibit infection of the urinary tract (see Ch. 14 , the vesicoureteric reflux).
There is a low pH in the stomach and vagina. If the pH of the stomach rises, for example, infections of the bowel are more frequent.
The skin is constantly anointed with sebum , an oily secretion from the sebaceous glands, which contains chemicals that do no harm to the human host, but are not liked by many pathogens.
There is significant growth of bacteria in the lumen of the bowel, in the vagina and on the surface of the skin in the form of normal flora , which inhibit the growth of pathogens.
Destructive agents, in the form of specialised cells and molecules – lymphocytes, macrophages, antibodies and complement – form the body's immunological defence mechanisms. To be successful they must be able to distinguish friend from foe – to recognise non-self – and to be able to tell the difference between damaged and undamaged tissue.
Activation of some of these cells and molecules is called an immune response . Some other definitions that will be useful at this point are:
Immunogen – anything that provokes an immune response.
Antigen – anything that is recognised by the cells and molecules of the immune response.
Agents of the defence system recognise antigens through receptors and recognition sites. The part of the antigen that is recognised is called a determinant .
A receptor is a molecule or complex of molecules, which has at least one recognition site, the molecular feature recognised as being the determinant. Receptors are normally present on the surface of cells.
The immune system needs:
To detect the presence of non-self and, usually, to destroy and/or eliminate the invader
To leave undamaged, as much as possible, the body's own components ( self )
Sometimes to allow toleration of some non-self substances where an aggressive immune response serves no useful purpose.
It does this by acting through receptors and antigenic determinants and applying mechanisms that assist in destruction of the immunogen.
The recognition site on the receptor binds to the determinant not only because of their respective shapes but also because of electrostatic forces. There are different types of receptor that aid in the detection of the invader and subsequent healing processes.
Antigen receptors recognise determinants that are present on non-self substances, such as pathogen-associated molecular patterns (PAMPs) , which may be related to substances, such as lipoproteins, present in pathogen cell walls, but are scarce or absent on self – distinguishing self and non-self .
Scavenger receptors recognise determinants or features characteristic of dead or denatured material, but are scarce or absent on healthy tissue – distinguishing damaged and healthy tissue. They also act as pathogen recognition receptors (PRRs), triggering an immune response when bound.
Some elements of the defence system distinguish between these forms by recognising determinants characteristic of healthy self-tissues. Cells and molecules of the immune system also interact and cooperate with each other through specialised receptors and determinants, such as:
Receptors on macrophages for antibodies
Interactions within the complement cascade.
Receptors can be specific or non-specific.
A receptor is said to be very specific if it binds only to one particular kind of determinant. Some receptors are more specific than others.
A receptor that is non-specific might, for example, bind to a variety of determinants with strong negative charges, because of its strong positive charge, in spite of there being differences in their shapes.
Specificity , on the other hand, is a different concept and refers to the identity of the determinant, which it recognises. For example, the specificity of one receptor might be a determinant on S. aureus, and the specificity of another a determinant on tetanus toxoid. A cross-reaction occurs when a receptor binds to more than one kind of antigen. This may be because the same determinant is present on different antigens and the receptor is acting in a specific fashion, or because the receptor is acting in a non-specific way, binding to determinants of different shapes, as described earlier.
There are many different classes of receptors. For example, scavenger receptors A (SR-A) through to SR-F bind to different forms of low-density lipoprotein (LDL):
SR-A1 and SR-A2 on macrophages pick up excess LDL within blood vessels
SR-A and SR-B (also known as CD36) on macrophages bind to pathogens, such as E. coli , through collagen recognition sites. They act in two main ways:
The macrophage engulfs the bacteria in a phagosome. Lysosomes fuse and release their acidic destructive content.
They act as co-receptors with toll-like receptors (TLRs) binding to pathogen cell walls and releasing transcription factor (TF) , which travels to the nucleus to instruct production of cytokines that attract more immune cells.
Antigens are usually polypeptides or polysaccharides, sometimes with other chemical groups attached. The determinant size is probably only between 3 and 10 peptides or saccharides in length. Individual molecules may have several determinants recognisable by the receptors of the defence system. This allows tissues, cells (live or dead), cell fragments, molecular aggregates or single protein molecules (of eukaryotic, prokaryotic, viral or artificial origin) to be recognised, simply through the presence of a determinant that is foreign to the body.
Identification must be followed by destruction and elimination of the invader. The defence mechanism does this by acting through cells that use phagocytosis, lytic enzymes and strong oxidising agents, both inside and outside the cells. Some cells kill other cells by opening ion channels in their cell membranes. An important factor is that the destruction should be as localised as possible to minimise damage to healthy tissue.
Whilst it is important that self is not a target of destruction, there are many molecules that are foreign, but not dangerous. There is evidence that the defence system can specifically tolerate such potential antigens. For example, pollen antigens probably cross the lung mucosa but do not produce significant damage; an inappropriate aggressive immune response might also result in damage to self.
Cells that are part of the immune response communicate with each other through:
Cell surface molecules – cells touching each other may communicate through structures that allow them to fit or bind together to mediate communication
Secreted molecules, or mediators – such as hormones, cytokines or lymphokines:
Hormones are products of specialised endocrine cells
Cytokines are polypeptides
Lymphokines are cytokines secreted by, or acting on, lymphocytes.
In general, a cell must express a receptor for a mediator to be influenced by it, usually on the cell surface, but sometimes in the nucleus.
In autocrine activity, a mediator acts on the cell that secreted it
In paracrine activity, a mediator diffuses from the secreting cell to neighbouring cells and acts on them
In endocrine activity, the mediator is carried by the circulation all over the body, and acts on cells remote from those that secreted it.
The innate immune system is the first line of defence and involves several different types of cells: macrophages, dendritic cells and mast cells, and other substances, such as chemical mediators and complement proteins. These components cannot readily distinguish self and non-self, but nevertheless, provide elements that protect the body. For example, all lysozyme molecules are the same and have the same specificity of attack, which is different from other defending molecules, such as mannose-binding lectins (MBLs) . There are several other soluble factors that assist in the bodies' defence:
Lysosomes act by damaging bacterial cell walls, but do no harm to eukaryotic cells, which lack a cell wall.
MBL acts through recognition of particular carbohydrate patterns found on the surface of many pathogenic microorganisms.
C-reactive protein (CRP) binds to phosphorylcholine on bacteria, enhancing phagocytosis and assisting in complement binding.
The complement pathway is a cascade system of enzymes reminiscent of the clotting pathway and is triggered by certain foreign chemical configurations found in endotoxins, and bacterial and fungal cell walls, or by antigen/antibody complexes. It culminates in the activation of enzymes destructive to foreign organisms. It also produces chemotactic substances and adherent factors that promote the phagocytosis. It also acts to discriminate self and non-self as it is only stimulated by foreign material.
Chemotactic factors are chemical substances that direct the movement of cells – human or invading – to particular locations.
All body cells play their part in the innate immune system. Any cell that is damaged or undergoes necrosis may release mediators, or material such as heat shock proteins that signal the damage to neighbouring cells. Many cells that are virally infected signal the fact by releasing mediators ( interferons (IFNs)).
Basophils in blood and mast cells in tissues are motile and chemotactic, but not phagocytic. They are activated directly by physical and chemical stimuli, and also by nerves (collateral branches of afferent sensory nerves, which can detect damage through their receptors), complement and receptors, allowing them to interact with the adaptive immune system. Phagocytes such as neutrophils, monocytes/macrophages, dendritic cells and eosinophils deploy a variety of cell surface receptors, which can detect non-self and damaged tissues. Some are also used in cooperation with other parts of the immune system. Examples of the different types of receptors are:
TLRs – molecules that in various combinations in the cell wall, sometimes in heterodimers, recognise microbial molecules and components, such as cell wall lipoproteins, virus RNA and DNA (on the endosomes) and material released from damaged self-cells ( heat shock proteins ) and dead self-material. They are signalling receptors that bind to pathogenic material and form a cascade of events leading to production of transcription factors for various cytokines, including IFN, nuclear factor kappa enhancer of B cells (NF-κB) and activator protein-1 (AP-1) , for example.
Collectins – proteins that recognise sugar groups characteristic of microbes, e.g. the mannose receptor.
CD14 – a receptor for lipopolysaccharide, a component of Gram-negative bacterial cell walls. When an antigen engages these receptors, the cell will attempt to phagocytose and destroy it in the lysosomes. CD14 also assists TLRs.
C3b and Fc receptors – mediate cooperation with complement and antibodies, respectively.
Phagocytes can degrade the material that they phagocytose, and if they phagocytose microbes they are often able to kill them. These mechanisms involve enzymes, free radicals and exclusion of nutrients from the phagosome. The phagocytes, however, retain no memory of the attack to protect the body against repeat incursions. Secretion of mediators is an important function of phagocytes, mediating the recruitment of more phagocytes and inflammatory changes.
In summary, the innate immune system uses a relatively small number of receptors and recognition sites that detect components common on microorganisms, but that are not found within the human body. These mechanisms do not recognise all determinants and successful pathogens hide these. The body needs a more sophisticated defence mechanism to deal with these – the adaptive immune system .
When the innate immune system fails to resolve an infection, the adaptive immune system comes into action. In contrast, but in cooperation with the innate immune system, the adaptive immune system initiates and uses specific memory of the infection. This system is provided through the lymphocytes . There are different forms of lymphocytes: B lymphocytes, T lymphocytes and null lymphocytes. T lymphocytes can be further divided into:
CD4-positive (helper T cells) to promote the immune response
CD8-positive (cytotoxic T cells) to destroy pathogens.
Fig. 6.10 summarises the difference between these two systems of body defence. Any one lymphocyte, and its clone from division of a single naïve progenitor cell, will have receptors that are specific for an antigen, but different lymphocytes will have different specificities. After recognising an antigen, the lymphocyte will divide to form a clone, which is generally stable. The body may produce receptors on lymphocytes specific for self-components, but these are deleted or inactivated, probably through clonal deletion . Thus, lymphocytes are produced that have receptors for many different non-self specificities, producing a comprehensive defence system.
T lymphocytes originate in the bone marrow, but undergo further development in the thymus before they are mature. The receptors are formed of four subunits (two alpha and two beta chains) and consist of a constant region, which defines the T cell type, and a variable region, which recognises specific epitopes (antigen receptors) on pathogens, but which are not antibodies. The receptors on the surface of any single T lymphocyte all have the same specificity, as do the receptors on single B lymphocytes.
T cells are activated through the innate immune system. Macrophages that have engulfed pathogens will degrade the organisms and selected epitopes to present to the T cells. The receptors on T lymphocytes have highly variable regions known as complementarity determining regions (CDRs) . These regions interact with a major histocompatibility complex (MHC) – a peptide complex – to activate the lymphocytes. HLA refers to a subset of MHC genes that encode for cell surface antigen-presenting proteins.
CD stands for cluster of differentiation . This is a classification system for the different antigenic determinants found on cells.
Each cell surface molecule on a T lymphocyte must be recognised by a cluster of monoclonal antibodies developed in the laboratory before it is assigned a CD number.
The surface molecules are different on different types of cells and so act as markers of differentiation. The different CD complexes are given a number to differentiate them.
The MHC region is located on chromosome 6 and consists of approximately 140 genes, many having immunological functions. Proteins produced from fragmented microorganisms bind to various MHC molecules. The MHC molecule can be divided into three groups: class I, class II and class III.
MHC class I molecules are present on the surface of virtually every cell. They present antigen fragments to T lymphocytes and bind to CD8 receptors on cytotoxic T cells.
MHC class II molecules are found mainly on macrophages and B cells and present antigen to helper T cells, binding to the CD4 receptor.
MHC class III genes encode for other immune components, such as complement, or cytokines such as tumour necrosis factor (TNF) .
HLA antigens are classified into nine divisions:
HLA-A, HLA-B and HLA-C all belong to MHC class I
HLA-D consists of six genes, all belonging to MHC class II.
The HLA molecules, class I and II, are both two-chain glycoproteins. Class I molecules have a single transmembrane chain, whereas class II molecules have two transmembrane chains. Both classes have an antigen-presenting groove where oligopeptides bind ( Fig. 6.11 ) .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here