Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Changes in the demographics of patients who present in need of revascularization, advances in percutaneous and surgical revascularization techniques, and results from contemporary studies of percutaneous versus surgical revascularization have made it essential that patients be assessed as individuals prior to selection of a treatment strategy.
Risk stratification plays an important role in the assessment of patients undergoing revascularization.
Clinical tools used to assist the heart team in risk stratifying patients and deciding the most appropriate revascularization modality can be broadly divided into assessments based on clinical comorbidities, coronary anatomy, or a combination of the two.
Clinical tools based on the Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) trial have evolved from purely anatomic factors (anatomic SYNTAX score) to anatomic factors augmented by clinical variables (culminating in the development of the SYNTAX score II) and tools to assess a level of reasonable incomplete revascularization that would not have an adverse effect on long-term morbidity and mortality (residual SYNTAX score). Validation of many of these newly developed clinical tools is ongoing.
Clinical and anatomic factors have an impact on short- and long-term morbidity and mortality following surgical or percutaneous revascularization and must be considered by the heart team in open dialogue with the patient during the decision-making process.
Revascularization of patients with coronary artery disease (CAD) has progressed exponentially since Andreas Grüntzig performed the first balloon angioplasty in 1977. These developments, which have been fueled by new technology, have blurred the boundary between what was once considered exclusively surgical disease and what can be treated percutaneously. Consequently, there is a greater need than ever to tailor revascularization appropriately, taking into consideration a patient’s comorbidities, coronary anatomy, personal preferences, and individual perception of risk. This chapter will explore the increasing requirement for a more individualized assessment of patients undergoing revascularization, and it will review the clinical tools currently available to assist in this decision-making process.
A number of confounding factors have made it imperative that patients are assessed as individuals prior to the selection of revascularization strategy.
The demographics of patients presenting to tertiary care services in need of revascularization are constantly evolving. This has been largely the consequence of increased longevity of the general population, a lower threshold to investigate patients who present with symptoms suggestive of obstructive CAD, and increased resources that have made revascularization via percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) more accessible. Together with increased age, patients in need of revascularization are currently more likely to have comorbidities such as diabetes, hypertension, and hyerlipidemia. These factors are all implicated in accelerating the progression of CAD, and consequently patients are more likely to present with more extensive CAD. The Arterial Revascularization Therapies Studies (ARTS) parts I and II were separated by a period of 5 years, and despite both studies having the same inclusion criteria, patients in ARTS-II had a significantly greater incidence of risk factors and overall increased disease complexity ( Table 1.1 ).
| All-Comers Studies | ||||||
|---|---|---|---|---|---|---|
| SIRTAX | Leaders | Resolute | Arts-I | Arts-II | SYNTAX | |
| Years of Enrollment | 2003–2004 | 2006–2007 | 2008 | 1997–1998 | 2003 | 2005–2007 |
| Stent Type | DES | DES | DES | BMS | DES | DES |
| Demographics | ||||||
| Age, years (mean ± SD) | 62 ± 11 | 65 ± 11 | 64.4 ± 10.9 | 61 ± 10 | 63 ± 10 | 65 ± 10 |
| Diabetes, % | 20 | 24 | 23.5 | 19 | 26 | 26 |
| Hypertension, % | 61 | 73 | 71.1 | 45 | 67 | 69 |
| Hypercholesterolemia, % | 59 | 67 | 63.9 | 58 | 74 | 78 |
| Previous myocardial infarction, % | 29 | 33 | 28.9 | 44 | 34 | 32 |
| Left ventricular function, % (mean ± SD) | 57 ± 12 | 56 ± 12 | 61 ± 12 | 60 ± 12 | 59 ± 13 | |
| Lesion Characteristics (Per Patient) | ||||||
| Multivessel disease, % | 59 | 23 | 58.4 | 96 | 100 | 92 |
| Bifurcation lesions, % | 8 | 22 | 16.9 | 35 | 34 | 72 |
| Total occlusions, % | 19 | 12 | 16.3 | 3 | 17 | 24 |
| SYNTAX score (mean ± SD) | 12 ± 7 | 14 ± 9 | 15 ± 9 | – | 21 ± 10 | 28 ± 12 |
| Mean number of diseased lesions | 1.4 | 1.5 | 1.5 | 2.8 | 3.6 | 3.6 a |
| Procedural Characteristics (Per Patient) | ||||||
| Mean number of stents | 1.2 ± 0.5 | 1.3 ± 0.7 b | 11.9 ± 7.5 | 2.8 ± 1.3 | 3.7 ± 1.5 | 4.6 ± 2.3 |
| Total stent length, mm (mean ± SD) | 25.9 ± 15.5 | 24.7 ± 15.5 b | 34.4 ± 24.5 | 47.6 ± 21.7 | 72.5 ± 32.1 | 86.1 ± 47.9 |
Patient comorbidities must be taken into consideration when assessing patients for revascularization because they have the potential to significantly influence patient outcomes; moreover, they may have a different impact depending on the underlying revascularization strategy selected. Notably in patients enrolled in the ARTS-I and II studies, patient age was shown to be a significant independent correlate of major adverse cardiovascular and cerebrovascular events (MACCEs) who were treated with CABG. More recently, in the randomized all-comers SYNTAX trial, increasing age was shown to favor PCI over CABG when adjustments were made for other anatomic and clinical factors. In addition, other anatomic and clinical factors were shown to have an impact on long-term mortality, and thereby decision making between CABG and PCI (SYNTAX score II ), and this topic is discussed later under “SYNTAX-Based Clinical Tools.”
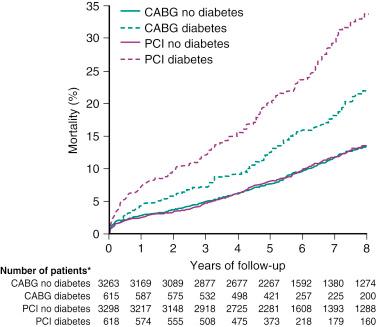
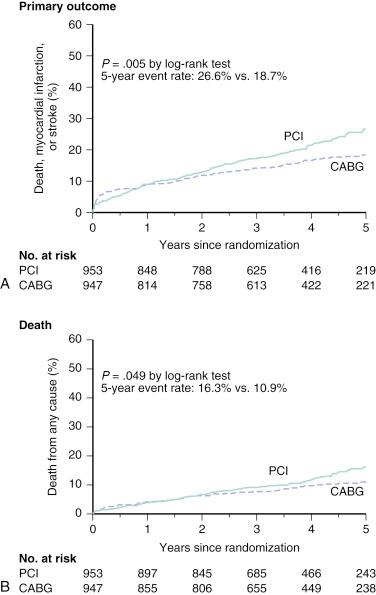
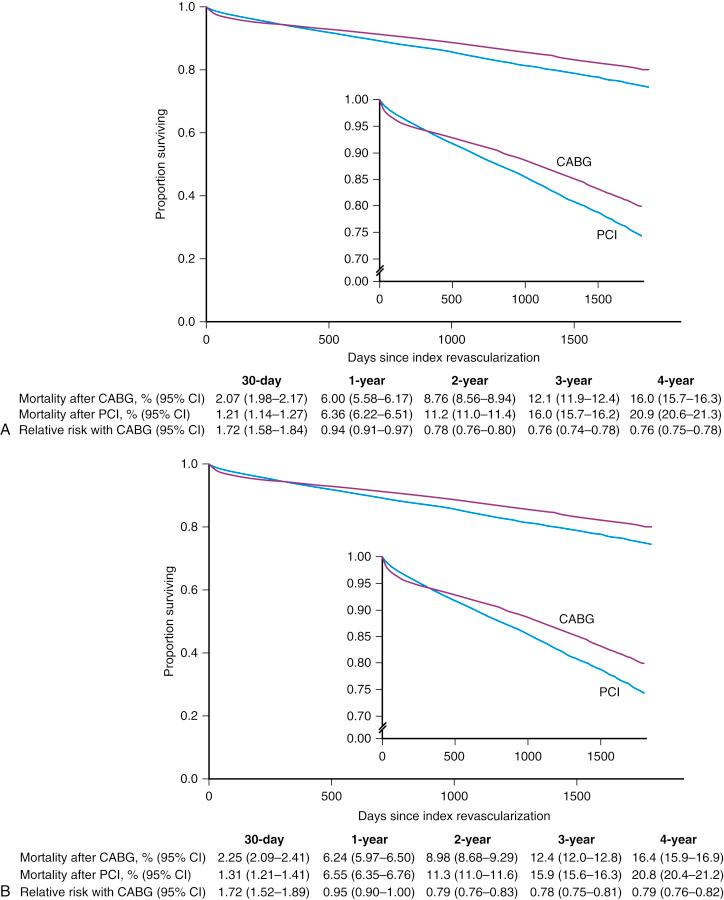
In a collaborative patient-level analysis of 10 randomized trials of patients with multivessel disease (MVD) treated with PCI using bare-metal stenting (BMS) and CABG, Hlatky and coworkers demonstrated comparable rates of 5-year mortality between both treatment groups in patients without diabetes. Notably, when patients with diabetes were viewed as a whole, mortality was significantly higher in those treated with PCI, even after multivariate adjustment ( Fig. 1.1 ). In the Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial, it was shown that in patients with diabetes and advanced CAD, CABG was superior to PCI in that it significantly reduced rates of death and myocardial infarction (MI) but at the expense of a higher rate of stroke ( Fig. 1.2 ). In addition, using the American College of Cardiology Foundation (ACCF) National Cardiovascular Data Registry (NCDR) and the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database, Weintraub and colleagues found that subjects who had elective intervention for MVD had a long-term survival advantage among patients who underwent CABG compared with PCI ( Fig. 1.3 ). Findings have been corroborated in the randomized, all-comers SYNTAX trial, as discussed later.
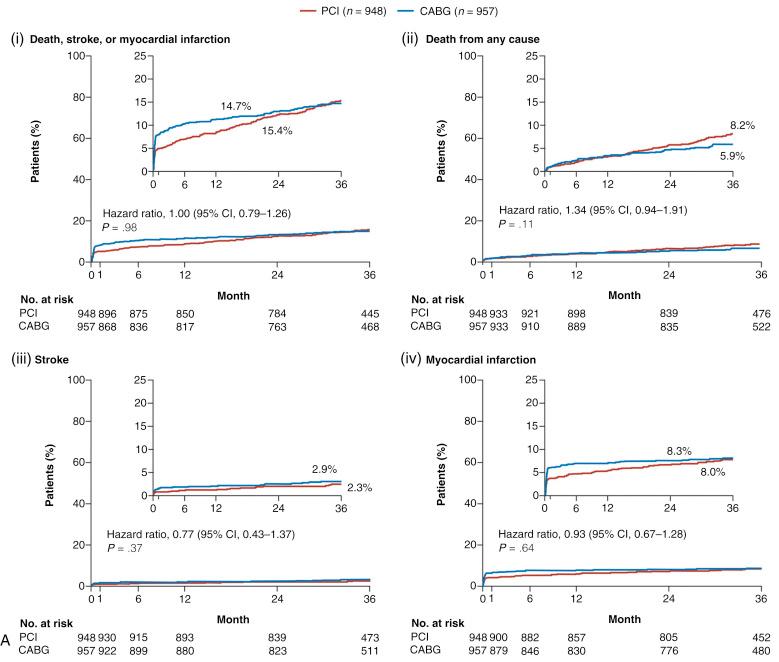
In addition, within the randomized Evaluation of the Xience Everolimus-Eluting Stent Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial, in appropriately selected patients with left main (LM) CAD (low-intermediate anatomic SYNTAX scores), PCI with everolimus-eluting stents was shown to be noninferior to CABG with respect to the rate of the composite end point of death, stroke, or MI at 3 years ( Fig. 1.4A ). Moreover, a substantially greater early benefit in quality of life (QOL) was evident with PCI at 1 month, with a similar QOL improvement at 36 months with both PCI and CABG (see Fig. 1.4B ). This corroborates findings originally made in the original landmark SYNTAX trial. Conversely, in the randomized NOBLE (Nordic-Baltic-British left main revascularisation study) and BEST (The Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease) trials investigating patients with unprotected LM and MVD, respectively, CABG was shown to offer superior long-term clinical outcomes compared with PCI with contemporary drug-eluting stents (DESs); notably in both trials, patients were not appropriately risk stratified and appropriately selected as occurred in EXCEL. Consequently, an urgent need exists for clinical tools that account for both anatomic and clinical factors and comorbidity to assist the heart team in decision making in regard to the most appropriate revascularization modality in patients with complex CAD.
The introduction in 2002 of DESs revolutionized the practice of interventional cardiology and was driven primarily through the dramatic reduction in rates of repeat revascularization. The favorable results observed with DES use promptly resulted in an expansion of the indications for PCI, such that bifurcation lesions, chronic total occlusions (CTOs), and MVD were no longer in the exclusive domain of surgical revascularization, and these were increasingly treated with PCI. Evidence of this expansion can be seen in the changing baseline lesion characteristics of patients enrolled in all-comers PCI trials such as the Sirolimus-Eluting and Paclitaxel-Eluting Stents for Coronary Revascularization (SIRTAX) trial, the Limus Eluted From a Durable Versus Erodable Stent Coating Study (LEADERS), the Clinical Evaluation of the Resolute Zotarolimus-Eluting Coronary Stent System in the Treatment of De Novo Lesions in Native Coronary Arteries (RESOLUTE), and in studies of complex three-vessel disease (3VD) and/or LM CAD, such as ARTS-I, ARTS-II, and the SYNTAX trial (see Table 1.1 ). Further evidence in support of this change comes from assessments of real world clinical practice, which indicate that approximately one-third of patients with complex CAD are currently treated with PCI. This practice has been coupled with the expanding use of PCI, driven largely through advances in PCI technology, with more deliverable newer-generation DESs, lower-profile balloons, new guidewires, adjunctive devices to aid stent delivery, crossing and reentry systems to aid total occlusion revascularization, functional assessment of lesions, intravascular ultrasound (IVUS) guidance to ensure adequate stent expansion, dedicated specialists for specific anatomic subsets including CTO operators with high successful revascularization rates, introduction of new adjunctive pharmacologic therapies, and the increasing availability of percutaneous extracorporeal circulatory support ( eFig. 1.1 ). From a technical perspective, a large subset of coronary lesions can currently be addressed with PCI; however, it is important to emphasize that the percutaneous approach to revascularization requires individual patient selection to ensure that it is appropriate.
Historically, and prior to the publication of the SYNTAX trial, randomized trials to compare CABG and PCI centered on two major patient groups: either isolated proximal left anterior descending (LAD) artery lesions or complex CAD (3VD and/or LM disease). Although results of these studies suggest no differences were found in the hard clinical outcomes of death and MI between patients treated with PCI or CABG at short- and long-term follow-up ( Table 1.2 ), there was profound selection bias in enrollment of patients prior to randomization. Specifically, between 2% and 12% of screened patients were randomized in most trials ( Table 1.3 ), with patients with lesser comorbidities, such as impaired left ventricular function or coronary anatomy (predominantly single- or double-vessel disease) often “cherry-picked” prior to randomization. Consequently, interpreting and extrapolating these results to routine and contemporary clinical practice has been challenging.
| First Author | Number of Patients (PCI/CABG) | POBA/BMS/DES (%) | Follow-Up (Months) | Death (PCI vs. CABG) | MI (PCI vs. CABG) | Stroke (PCI vs. CABG) | Repeat Revasc. (PCI vs. CABG) | MACCEs (PCI vs. CABG) |
|---|---|---|---|---|---|---|---|---|
| Isolated Proximal LAD | ||||||||
| Aziz | 1952 (1300/652) | 0/91/9 | 34 | 2.9% vs. 3.4% | 2% vs. 1.1% | 2.4% vs. 3.5% | 14.3% vs. 4.4% a | 21.4% vs. 11.1% a |
| Kapoor | 1210 (633/577) | 22/59/19 | 60 | 9.4% vs. 7.2% | NA | NA | 33.5% vs. 7.3% a | NA |
| Multivessel Disease | ||||||||
| Hlatky | 7812 (3923/3889) | 63/37/0 | 5.9 | 10.0% vs. 8.4% | 16.7% vs. 15.4% b | – | 24.5% vs. 9.9% a , b | 36.4% vs. 20.1% a |
| Daemen | 3051 (1518/1533) | 4/96/0 | 60 | 8.5% vs. 8.2% | 2.5% vs. 2.9% | 6.6% vs. 6.1% | 25.0% vs. 6.3% a | 34.2% vs. 19.6% a |
| Bravata | 9963 (5019/4944) | 56/42/2 | 60 | 9.3% vs. 11.3% | 0.6% vs. 1.2% a | 11.9% vs. 10.9% | 46.1% vs. 40.1% vs. 9.8% a , c | – |
| Trial | Number of Patients Screened | % Randomized | Stent | % 3VD | Proximal LAD | EF >50% | % Diabetes |
|---|---|---|---|---|---|---|---|
| MASS | 142 | 69 | – | – | 100 | 100 | 21 |
| ERACI | 127 | 9 | – | 45 | – | 100 | 11 |
| EAST | 392 | 4 | – | 40 | 70 | 100 | 25 |
| GABI | 359 | 4 | – | 18 | – | – | 10 |
| CABRI | 1054 | 3 | – | 40 | – | 100 | 12 |
| BARI | 1829 | 12 | – | 41 | 36 | 100 | 24 |
| SIMA | 121 | – | – | – | 100 | 100 | 11 |
| LAUSANNE | 134 | 3 | – | 0 | 100 | – | 12 |
| RITA | 1011 | 4 | – | 12 | – | – | 6 |
| TOULOSE | 152 | 3 | 29 | – | – | 14 | |
| AWESOME | 454 | – | + | 45 | – | – | – |
| ERACI-II | 450 | 2 | + | 56 | – | – | 17 |
| ARTS | 1205 | 5 | + | 32 | – | 100 | 19 |
| SOS | 988 | 5 | + | 38 | 45 | 100 | 14 |
| MASS II | 408 | 2 | + | 41 | – | – | – |
| Summary | 8826 | 5 | 35 | 41 | 100 | 16 |
The landmark SYNTAX trial represents the largest (and only) assessment of revascularization with PCI or CABG in all-comers with complex CAD. SYNTAX aimed to supply evidence to support the somewhat established but non–evidence-based practice of performing PCI in patients with complex CAD. In addition, SYNTAX also sought to identify which patients should be treated with CABG only. Through an all-comers design, SYNTAX addressed the limitations of the earlier CABG versus PCI trials, which were plagued by profound selection bias as previously discussed (see Table 1.3 ), and in doing so it was anticipated that the results would be more relevant to contemporary routine clinical practice. Specifically:
To ensure results were applicable to routine practice, the study was designed as an all-comers trial such that there were no specific inclusion criteria other than the need to have revascularization of de novo 3VD or unprotected LM CAD (in isolation or with CAD). Exclusion criteria were limited to prior revascularization, ongoing MI, and patients requiring concomitant cardiac surgery. In contrast to the earlier studies, 70.9% of eligible patients were enrolled.
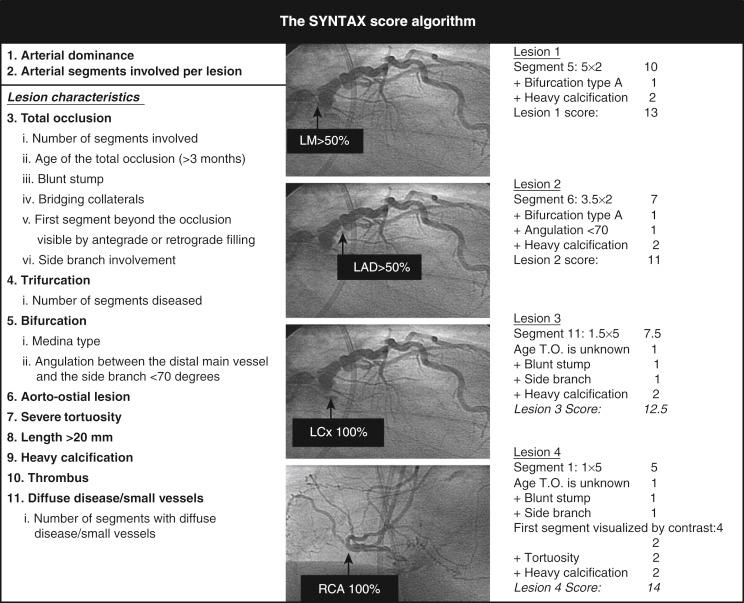
The previously indicated problem of reporting outcomes from all patients with complex CAD together, irrespective of disease severity, was addressed in the SYNTAX trial through the use of the anatomic SYNTAX score ( www.syntaxscore.com ; Fig. 1.5 ), which enabled CAD complexity to be objectively and prospectively quantified.
To ensure assessment of patients on an individual level, all patients eligible for enrollment were discussed by the heart team. An interventional cardiologist and cardiac surgeon carried out a careful and comprehensive review of the patient in terms of their anginal status, comorbidities, and coronary anatomy using the respective Braunwald score, European System for Cardiac Operative Risk Evaluation (EuroSCORE), and SYNTAX score (discussed under “SYNTAX-Based Clinical Tools”). The consensus reached from this meeting was subsequently used to allocate the patient into one of the three arms of the trial. In total, 3075 patients were enrolled into one of the following:
Randomized group ( n = 1800 [58.5%]; 897 CABG, 903 PCI): These patients had CAD and were equally suitable for revascularization with PCI or CABG. The mean SYNTAX score for this group was 26.1 and 28.8 in patients treated with CABG and PCI, respectively.
Nested CABG registry ( n = 1077 [35.0%]): These patients had CAD that was considered unsuitable for PCI, clearly reflected in the high mean SYNTAX score (37.8) for this group.
Nested PCI registry ( n = 198 [6.4%]): These patients were deemed unsuitable for CABG. The commonest reason for this decision was the presence of multiple comorbidities reflected in the mean EuroSCORE, which was 2 points higher in this group than the mean in the randomized group (5.8 vs. 3.8).
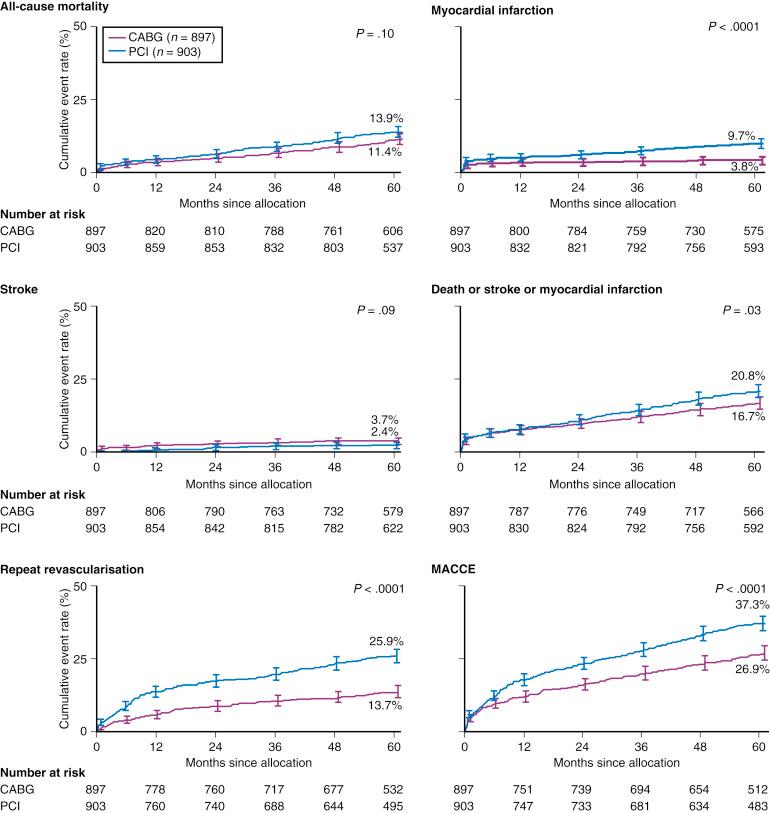
Overall, SYNTAX failed to meet the prespecified primary end point of noninferiority in terms of 12-month MACCEs, a composite of death, stroke, MI, and repeat revascularization (17.8% vs. 12.4%, P = .002). Final 5-year reporting of SYNTAX demonstrated significantly higher incidence of MACCE with PCI compared with CABG (26.9% vs. 37.3%, P < .0001; Fig. 1.6 ).
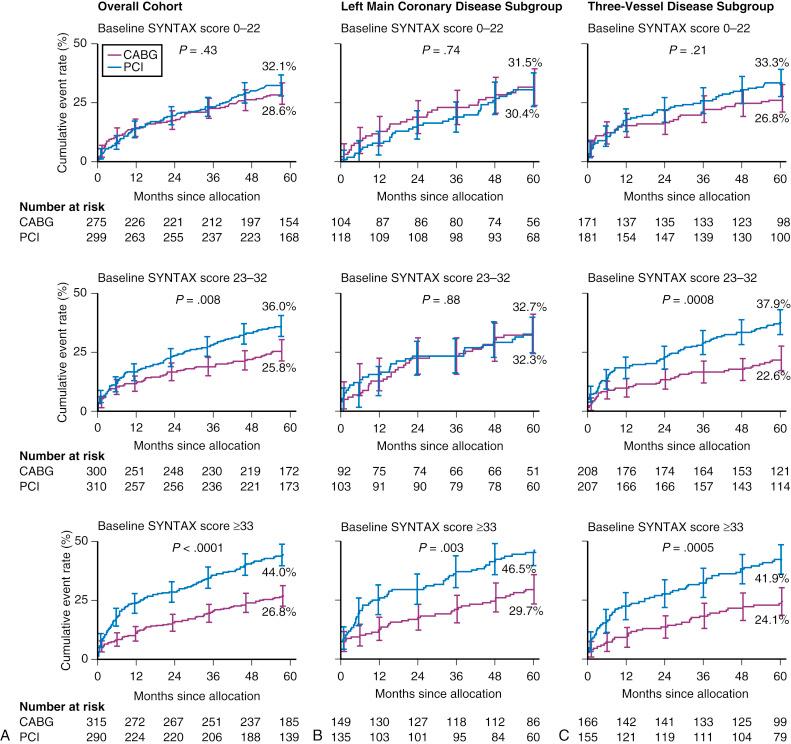
As indicated earlier, analyses of all patients irrespective of disease severity does not provide adequate information for clinicians who are faced daily with patients who display a wide variety of CAD complexity. To address this limitation of earlier studies, patient outcomes in SYNTAX were stratified according to tertiles of the anatomic SYNTAX score. As shown in Fig. 1.7 , clinical outcomes between patients treated with PCI and CABG in SYNTAX differed according to the presence of 3VD or unprotected LM CAD. With 3VD, a low SYNTAX score (<23) allowed for similar outcomes between CABG and PCI, whereas higher SYNTAX scores (particularly in the high SYNTAX score [>32] group) clearly favored CABG. With unprotected LM CAD, a low-intermediate SYNTAX score (<33) allowed for similar outcomes between CABG and PCI, whereas a high SYNTAX score (>32) clearly favored CABG. Furthermore, the SYNTAX score II, essentially the anatomic SYNTAX score augmented with clinical variables shown directly to affect decision making between CABG and PCI, was developed in the randomized, all-comers SYNTAX trial and allowed for the identification of higher- and lower-risk subjects in all tertiles of the anatomic SYNTAX score who had a long-term mortality that favored either CABG, PCI, or both revascularization modalities (discussed under “SYNTAX-Based Clinical Tools”).
The results of SYNTAX reiterate the importance of assessing patients when selecting a revascularization strategy. SYNTAX was able to identify those patients in whom either CABG or PCI was appropriate or in whom CABG or PCI was the optimal treatment. Considering the distribution of CAD in SYNTAX, overall one-third of patients with 3VD/LM disease were deemed to have CAD that could be treated safely and effectively with PCI or CABG, whereas in the remaining two-thirds, CABG remained the standard of care. Although these results helped further delineate the boundaries between a percutaneous and surgical revascularization approach in patients with complex CAD, the validation of the anatomic SYNTAX score and development of the SYNTAX score II notably facilitated a more objective assessment of patients by the heart team as discussed later under “SYNTAX-Based Clinical Tools.”
There is no disputing the need for and potential benefits of selecting a revascularization strategy following an individualized patient assessment or risk stratification. Risk stratification is performed routinely and subconsciously by physicians in everyday clinical practice and is in essence behind all clinical decisions made by a physician. Stratification of risk is vital when assessing patients for revascularization because this treatment is only considered appropriate when “the expected benefits, in terms of survival or health outcomes (symptoms, functional status, and/or QOL) exceed the expected negative consequences of the procedure.” However, it should be emphasized that the SYNTAX-pioneered heart team approach, consisting of at least an interventional/clinical cardiologist and a cardiac surgeon, carries a class I recommendation in international guidelines for assessing risk and is subsequently the most appropriate revascularization modality in patients with complex CAD.
Qualitative risk stratification is subjective and relies on a clinician’s experience. This subjective qualitative assessment also allows risk to be calculated and tailored to the expertise of the physician performing the procedure, as opposed to a clinician in another region who may use different techniques and who may have different equipment available. In addition, assessments of patient frailty can be made that are frequently not captured by conventional risk-scoring systems. This assessment does not require a calculator or computer and can be “computed” subconsciously very quickly. The major disadvantages of this method of risk assessment are its dependence on the operator’s prior experience, potential personal bias to undertake or withhold potential revascularization, and its high interobserver variability. In addition, influences of local practice often dominate clinical decision making, irrespective of the revascularization guidelines.
Quantitative risk stratification can be performed using a variety of risk scores that frequently incorporate clinical variables sourced from large patient registries, with the exception of the SYNTAX score II, which was developed in the all-comers randomized SYNTAX trial to reduce unavoidable (but often appropriate) selection bias inherent to all registries no matter their size. These risk scores largely incorporate objective variables to ensure adequate reproducibility of the score; however, those risk scores—such as the American College of Cardiology/American Heart Association (ACC/AHA) lesion score or the anatomic SYNTAX score/newly developed SYNTAX score II, which include angiographic variables—continue to have documented intraobserver and interobserver variability. However, these tools do provide a more objective assessment of the patient risk and suitability for the most appropriate revascularization modality, which may be modified by the heart team consensus. In addition to their role in the risk stratification of individual patients, these quantitative risk scores have increasing use in the wider context of overall health care. They can provide a vital measure of overall patient care and can help to identify future directions to further improve outcomes. Clinical governance and the increasing requirement to publicly report clinical performance and complications have also propelled the need to risk stratify patients, thereby allowing a useful comparison of performance to be made between clinicians and institutions against the standards dictated by regulatory authorities. In addition, calculation of risk using accepted risk scores may aid clinicians faced with an increasing need to be able to justify their clinical decisions to peers, regulatory bodies, and patients.
In comparison with the qualitative risk scores, the use of a finite number of variables results in these risk scores lacking the sensitivity to accurately predict risk in an individual, such that they are more apt at predicting risk for a population of patients with similar comorbidities. The number of variables included in the score must strike a balance between sufficient numbers to enable a meaningful prediction of risk to be calculated; however, the number must not be excessive so as to prevent use in routine practice. In addition, a minimal number of variables reduces the chances of colinearity between independent variables, which can result in redundant information being collected while also increasing the chances of “overfitting” the score and thereby reducing the overall applicability and accuracy of the results to conventional clinical practice.
The applicability of a risk score to contemporary practice must also take into consideration the time when the score was developed. Risk scores rely on large patient databases to derive appropriate weighting factors for variables in the score to enable the final calculation of risk. It follows that they are developed using retrospective information that may no longer be relevant in the era when the risk score is being used. For example, the EuroSCORE was developed in 1999; however, there have been calls for its recalibration because repeated evaluations indicate that it overestimates risk by a factor of 2 to 3, which has largely been attributed to improvements in surgical techniques and lower perioperative mortality in the decade following its construction. The updated EuroSCORE II currently addresses many of the limitations of the original EuroSCORE. The STS score is also derived from a large patient database and is periodically recalibrated to ensure its results are applicable to contemporary practice.
Numerous risk scores are available to assist clinicians in stratifying risk among patients undergoing revascularization. Some scores are appropriate for patients prior to the selection of a revascularization strategy, whereas some have been validated only in patients undergoing one form of revascularization. Nevertheless, the various risk scores can largely be categorized according to the variables—clinical, angiographic, or a combination of both—used in the overall estimation of risk. Tables 1.4 and 1.5 summarize the different risk scores used in contemporary CABG and PCI practice (excluding SYNTAX-based tools), and Table 1.6 summarizes SYNTAX-based clinical tools. A selection of these is described in more detail later.
| Risk Score | Number of Variables Used to Calculate Score | Validated in PCI/CABG | ||
|---|---|---|---|---|
| Clinical | Angiographic | PCI | CABG | |
| EuroSCORE | 17 | 0 | + | + |
| EuroSCORE II | 18 | 0 | − | + |
| ACEF | 3 | 0 | − | + |
| Society of Thoracic Surgery score | 40 | 2 | − | + |
| Anatomic SYNTAX score | 0 | 11 (per lesion) | + | + |
| Clinical Risk Score | Number of Variables Used to Calculate Risk | PCI Outcomes (Surgical Outcomes in Italics) | |
|---|---|---|---|
| Clinical | Angiographic | ||
| Anatomic Scores | |||
| ACC/AHA lesion classification a | 0 | 11 (per lesion) | Pre-DES era: predictive of angiographic success of PCI and prognostic effect on early and late clinical outcomes. Conflicting results were yielded in the DES era. |
| Myocardial Jeopardy Scores | |||
| Duke Jeopardy Score | 0 | Coronary tree divided into six segments: LAD, diagonal, septal perforating branches, LCx, OM, and PDA; a segment distal to ≥70% is considered at risk. Each segment is assigned 2 points with a maximum of 12 points. , b | |
| Myocardial Jeopardy Index (BARI) | 0 | Distal terminating portions of LAD, LCx, RCA, and major branch vessels (diagonals, OM, ramus, PDA and LV branches) assigned units of 1, 2, or 3 on the basis of length and vessel size. Septal perforators are arbitrarily assigned a maximum of 3 units. Extent of jeopardy defined by units jeopardized by ≥50% stenosis summated and divided by total LV territory. , b | |
| APPROACH lesion score | 0 | Based on principle from autopsy studies that the LAD generally subtends 41% of the LV, with the LCx and RCA supplying the remainder, dependent on vessel dominance. Score is calculated by percent of myocardium supplied by a vessel or its branches and jeopardized territories supplied by vessels with ≥70% stenosis (≥50% in the LMS); the maximum score is 100. b | |
| Clinical Scores | |||
| New Mayo Clinic Risk Score a | 7 | 0 | Procedural death and MACEs for PCI; score has been externally validated for death (in-hospital death with CABG). |
| Parsonnet Score | 14 | 0 | Independent predictor of long-term MACEs after LMS PCI in two registry populations (operative mortality after open-heart surgery) |
| EuroSCORE (additive or logistic) | 17 | 0 | Evidence for predicting death or MACCEs in high-risk tertiles for PCI (operative mortality for all forms of cardiothoracic surgery). |
| NCDR CathPCI Risk Score a | 8 | 0 | Developed from 181,775 procedures performed in Medicare patients; incidence of in-hospital and 30-day mortality after all PCI patient types internally validated in two separate cohorts. |
| ACEF score (age, creatinine, ejection fraction) | 3 | 0 | Predictor of cardiac death and MI at 1 year after PCI; inferior to the SYNTAX score at predicting overall MACEs and repeat revascularization in two separate populations (operative mortality in elective cardiac operations). |
| Combined (Anatomic AND Clinical) Risk Scores | |||
| EuroHeart PCI Score a | 10 | 6 | Developed from 46,000 patients from the Euro Heart Survey; in-hospital mortality in all PCI patient types; internally validated. The score has strong applicability for European practice. |
| New Risk Stratification Score (NERS) | 17 | Angiographic: 33 Procedural: 4 c |
6-month cardiac death and cumulative MACEs after unprotected LMS PCI; although internally validated, application to larger all-comers population is required (see text). |
| New York PCI Risk Score b | 8 | 1 | In-hospital death after PCI; developed based on data from 46,090 procedures in 2002 and validated from 50,046 procedures in 2003 ; excellent predictive ability in validation cohort (C-statistic 0.905). |
| The Texas Heart Institute Risk Score a | 8 | Angiographic: 2 Procedural: 1 d |
Predictors of in-hospital MACEs after PCI or CABG; developed in 9494 patients (BMS era) and validated in 5545 patients (DES era). |
| Mayo Clinic Risk Score a | 6 | 2 | In-hospital death, Q-wave myocardial infarction, emergent or urgent CABG or CVA after PCI; validated using the NHLBI registry. |
a Risk scores that include prediction of in-hospital mortality or MACEs. SYNTAX-based tools are shown in Table 1.6 .
b All myocardial jeopardy scores were validated in one population-based cohort consisting of more than 20,000 patients and were predictive of 1-year mortality in patients treated with PCI or medically.
c Need of intraaortic balloon pump, two-stent technique, intravascular ultrasound guidance.
| Year | Structure | Remarks | |
|---|---|---|---|
| Anatomic SYNTAX Score | 2006 | Score of angiographic variables (i.e., anatomic complexity); developed during the design of the SYNTAX trial as a tool to force the heart team to systematically analyze the coronary angiogram and agree equivalent anatomic revascularization (CABG and PCI) could be achieved | First reported to be useful for decision making between CABG and PCI in the SYNTAX trial in 2009 ; categories of anatomic complexity (low, intermediate, and high), no clinical variables, no individual predictions; adding a functional component shown to improve accuracy ; noninvasive multislice computed tomography anatomic SYNTAX score in development, with integration of a noninvasive functional component. |
| Development Phase: Augmenting the Anatomic SYNTAX Score With Clinical Variables and the Move Toward Individualized Decision Making | |||
| ACEF | 2009 | Age, creatinine, ejection fraction | Predicted individual in-hospital operative mortality post CABG; shown to be at least comparable to the EuroSCORE (composed of 17 variables) in predicting operative risk ; shown to aid in long-term predictions of mortality after PCI or CABG. |
| Clinical SYNTAX Score | 2010 | Amalgamation of SYNTAX score with modified ACEF score (creatinine replaced with CrCl shown to be more predictive of mortality ) | Similar to the SYNTAX score; categorized patient risk; could only identify a high-risk group in PCI-treated patients; provided little help in decision making between CABG and PCI; not individualized. |
| Global Risk | 2010 | Amalgamation of SYNTAX score with surgical EuroSCORE (composed of 17 variables) | Similar to the SYNTAX score; categorized patient risk; could identify a low-risk group with comparable outcomes with CABG and PCI in LM and 3VD patients; not individualized; patients with a high EuroSCORE were found to have a prognostic benefit in undergoing CABG compared with PCI irrespective of the SYNTAX score provided an acceptable threshold of operative risk was not exceeded. |
| Logistic Clinical SYNTAX Score | 2011 | Combination of age, SYNTAX score, CrCl, and LVEF shown to contain most of the prognostic data for 1-year mortality predictions after PCI | Individual 1-year mortality predictions in all PCI patients (STEMI, NSTEMI) irrespective of clinical presentation (except cardiogenic shock); not designed to help decision making between CABG and PCI; cross-validated in seven contemporary stent trials and more than 6000 patients and further externally validated. |
| End Result of This Process Leading to the Development of the SYNTAX Score II | |||
| SYNTAX Score II | 2012 | Augmenting SYNTAX score with clinical variables; based on the principle that age, CrCl, LVEF, and SYNTAX score contain most of the long-term prognostic data in CABG and PCI patients; additional variables added that directly influenced decision making between CABG and PCI | Individualized approach; threshold of the SYNTAX score in guiding decision making between CABG and PCI shown to alter based on the presence of other risk factors; validated in the DELTA Registry containing LM and 3VD (25% of the population) with almost a third (30%) with highly complex disease (SYNTAX scores ≥33); prospective validation studies are underway in the EXCEL trial (LM), and SYNTAX II trial is ongoing (de novo 3VD). |
| Use of the SYNTAX Score as an Objective Marker of Completeness of Revascularization | |||
| Residual SYNTAX Score | 2012 | Recalculation of the SYNTAX score after PCI | Developed and validated in the ACUITY and SYNTAX trials; a residual SYNTAX score greater than 8 was shown to have an adverse effect on long-term prognosis at up to 5-year follow-up; further, prospectively run validation studies are awaited. |
| Post-CABG SYNTAX Score | 2013 | Recalculation of the SYNTAX score after CABG with points deducted based on the importance of the diseased coronary artery segment (Leaman score ) that has a functioning bypass graft anastomosed distally | Pilot study in angiographic substudy of the SYNTAX trial demonstrated the feasibility of this approach in identifying subjects post CABG with an adverse long-term (5-year) prognosis ; validation studies are awaited. |
These risk scores incorporate only clinical variables and do not require any data from the angiogram. They offer the advantage of being able to be computed relatively quickly, usually at the bedside, and principally include variables that are not subject to user interpretation, thereby ensuring excellent reproducibility.
The EuroSCORE is an established risk score that uses 17 clinical variables used in cardiothoracic surgical practice for predicting operative mortality, and it has been validated in many populations around the world. In use since 1999, the score was derived from almost 20,000 consecutive patients from 128 hospitals in eight European countries. The additive EuroSCORE assigns an individual score to 17 clinical variables ( Table 1.7 ) with a low-risk tertile that ranges from 1 to 2, an intermediate-risk tertile from 3 to 5, and a high-risk tertile of 6 and higher. However, early validation studies suggested that the additive EuroSCORE underestimated risk in those at highest risk; this led to the development of the logistic EuroSCORE, which uses the same clinical variables and requires use of an online calculator (available at www.euroscore.org ) to quantify risk. However, the logistic EuroSCORE has been shown to potentially overestimate observed mortality, and its accuracy at predicting risk varies in different surgical subgroups.
| Patient Characteristics | Additive | Logistic β Coefficient | |
|---|---|---|---|
| Age | Per 5 years or part thereof over the age of 60 years | 1 | 0.07 |
| Sex | Female | 1 | 0.33 |
| Chronic pulmonary disease | Long-term use of bronchodilators or steroids for respiratory disease | 1 | 0.49 |
| Peripheral arteriopathy | Claudication, carotid stenosis >50%, previous or planned intervention on the abdominal aorta, limb arteries, or carotids a | 2 | 0.66 |
| Neurologic dysfunction | Severely affected mobility or day-to-day function | 2 | 0.84 |
| Previous cardiac surgery | Previous opening of the pericardium | 3 | 1.00 |
| Serum creatinine | Preoperatively greater than 200 μmol/L | 2 | 0.65 |
| Active endocarditis | Antibiotic therapy at time of surgery | 3 | 1.10 |
| Critical preoperative state | Preoperative cardiac arrest, ventilation, renal failure, inotropic support, intraaortic balloon pump use, ventricular arrhythmia a | 3 | 0.91 |
| Cardiac-Related Factors | |||
| Unstable angina | Rest pain that requires IV nitrates | 2 | 0.57 |
| Left ventricular function | Moderate (30%–50%) Poor (<30%) |
1 3 |
0.42 1.09 |
| Recent MI | Within 90 days | 2 | 0.55 |
| Pulmonary hypertension | Systolic pulmonary pressure greater than 60 mm Hg | 2 | 0.77 |
| Operation-Related Factors | |||
| Emergency | Operation performed before the start of next working day | 2 | 0.71 |
| Other than isolated CABG | Major cardiac procedure other than or in addition to CABG | 2 | 0.54 |
| Surgery on thoracic aorta | 3 | 1.16 | |
| Postinfarct septal rupture | 4 | 1.46 | |
| Constant β 0 | −4.79 | ||
In addition to the EuroSCORE’s assessment and validation in patients undergoing surgical revascularization, Kim and colleagues first demonstrated that the high-risk tertile of the additive EuroSCORE was an independent predictor of death/MI after unprotected LM intervention with sirolimus-eluting stents. Subsequently, Romagnoli and coworkers applied the additive EuroSCORE to predict in-hospital mortality in 1173 consecutive patients undergoing PCI in a single high-volume center and correlated the higher-risk tertiles of the EuroSCORE with in-hospital mortality; the study population also included patients who had undergone unprotected LM PCI. The EuroSCORE has since been evaluated in numerous studies of patients undergoing PCI, the majority of which specifically enrolled patients with LM disease. Notably, all studies, irrespective of disease severity, have demonstrated the EuroSCORE to be an independent predictor of mortality and/or MACCE at follow-up ranging from 1 to 3 years. Importantly, those studies that also included a surgical control group—such as the SYNTAX study, the Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization (MAIN-COMPARE) study, and the registry by Rodés-Cabau and colleagues —also demonstrated the EuroSCORE to be an independent predictor of MACCE in surgical patients. Only one study has examined the logistic EuroSCORE in PCI patients; however, little differences were found in stratifying risk when compared with the additive EuroSCORE.
Specifically in the SYNTAX trial, which represents the only randomized study to assess the EuroSCORE, the additive EuroSCORE was shown to be an independent predictor of MACCE at 1-year follow-up irrespective of the method of revascularization (odds ratio [OR]: 1.21; 95% confidence interval [CI]: 1.12 to 1.32; P < .001) in 705 patients undergoing LM revascularization. Similarly, at intermediate follow-up of 23 months, Rodés-Cabau and colleagues identified a EuroSCORE of 9 or higher as the best predictor of MACCE after PCI and CABG among 249 octogenarians with LM disease. In the MAIN-COMPARE registry, which enrolled more than 1500 patients with LM disease followed up for a median of 3.1 years, the EuroSCORE has been identified as an independent predictor of death, MI, and stroke irrespective of revascularization strategy. In addition, in the same registry, a EuroSCORE of 6 or higher has been shown to be an independent predictor of mortality following either PCI or CABG.
More recently, the EuroSCORE II ( Table 1.8 ) was developed to improve the risk prediction of the original EuroSCORE. EuroSCORE II was developed on newer data to reflect more contemporary surgical practice given that cardiac surgical mortality has decreased significantly in the last 15 years, despite patients being older and sicker, and that the previous additive and logistic EuroSCOREs were suggested to be representative of outdated surgical practices.
| Risk Factor | Coefficient | Standard Error | z | P ≥ | z | | [95% Confidence Interval] |
|---|---|---|---|---|---|
| New York Hospital Association (NYHA) | |||||
| II | 0.1070545 | 0.1463849 | 0.73 | 0.465 | [−0.1798547 to 0.3939637] |
| III | 0.2958358 | 0.141466 | 2.09 | 0.037 | [0.0185674 to 0.5731042] |
| IV | 0.5597929 | 0.1697565 | 3.30 | 0.001 | [0.2270763 to 0.8925095] |
| CCS4 | 0.2226147 | 0.1462888 | 1.52 | 0.128 | [−0.0641061 to 0.5093356] |
| IDDM | 0.3542749 | 0.145863 | 2.43 | 0.015 | [0.0683887 to 0.6401611] |
| Age | 0.0285181 | 0.0065954 | 4.32 | 0.000 | [0.0155914 to 0.0414448] |
| Female | 0.2196434 | 0.0953505 | 2.30 | 0.021 | [0.0327599 to 0.4065269] |
| ECA | 0.5360268 | 0.1106046 | 4.85 | 0.000 | [0.3192458 to 0.7528079] |
| CPD | 0.1886564 | 0.1232126 | 1.53 | 0.126 | [−0.0528358 to 0.4301486] |
| N/M mob | 0.2407181 | 0.1729494 | 1.39 | 0.164 | [−0.0982564 to 0.5796927] |
| Redo | 1.118599 | 0.1226272 | 9.12 | 0.000 | [0.8782539 to 1.3589440] |
| Renal Dysfunction | |||||
| On dialysis | 0.6421508 | 0.3083468 | 2.08 | 0.037 | [0.0378021 to 1.2464990] |
| CC ≤ 50 | 0.8592256 | 0.1446758 | 5.94 | 0.000 | [0.5756663 to 1.1427850] |
| CC 50–85 | 0.303553 | 0.1240518 | 2.45 | 0.014 | [0.0604159 to 0.5466901] |
| Active endocarditis | 0.6194522 | 0.2046001 | 3.03 | 0.002 | [0.2184433 to 1.0204610] |
| Critical | 1.086517 | 0.147657 | 7.36 | 0.000 | [0.797115 to 1.3759200] |
| Left Ventricular Function | |||||
| Moderate | 0.3150652 | 0.1036182 | 3.04 | 0.002 | [0.1119773 to 0.5181530] |
| Poor | 0.8084096 | 0.1498233 | 5.40 | 0.000 | [0.5147614 to 1.1020580] |
| Very poor | 0.9346919 | 0.2917754 | 3.20 | 0.001 | [0.3628227 to 1.5065610] |
| Recent MI | 0.1528943 | 0.136257 | 1.12 | 0.262 | [−0.1141646 to 0.4199531] |
| Pulmonary Artery Systolic Pressure | |||||
| 31–55 mm Hg | 0.1788899 | 0.1266713 | 1.41 | 0.158 | [−0.0693812 to 0.4271611] |
| ≥55 | 0.3491475 | 0.1676641 | 2.08 | 0.037 | [0.0205318 to 0.6777632] |
| Urgency | |||||
| Urgent | 0.3174673 | 0.1174178 | 2.70 | 0.007 | [0.0873326 to 0.5476020] |
| Emergency | 0.7039121 | 0.1719835 | 4.09 | 0.000 | [0.3668306 to 1.0409940] |
| Salvage | 1.362947 | 0.33706 | 4.04 | 0.000 | [0.7023221 to 2.0235730] |
| Weight of Procedure | |||||
| 1 non-CABG | 0.0062118 | 0.1463574 | 0.04 | 0.966 | [−0.2806434 to 0.2930670] |
| 2 | 0.5521478 | 0.1268137 | 4.35 | 0.000 | [0.3035975 to 0.8006980] |
| 3+ | 0.9724533 | 0.1463969 | 6.64 | 0.000 | [0.6855206 to 1.2593860] |
| Thoracic aorta | 0.6527205 | 0.221183 | 2.95 | 0.003 | [0.2192097 to 1.0862310] |
| Constant | −5.324537 | 0.1682446 | −31.65 | 0.000 | [−5.65429 to −4.9947830] |
The EuroSCORE II was shown to have improved calibration (actual mortality 4.18%, predicted 3.95%) compared with the original EuroSCORE (actual 3.9%, additive predicted 5.8%, logistic predicted 7.57%) while preserving discrimination (area under the receiver operating characteristic [ROC] curve of 0.8095). However, it should be noted that regular revalidation of the EuroSCORE II will need to be continued to identify calibration drift or clinical inconsistencies seen in previous versions.
In summary, while acknowledging that most of these studies have been nonrandomized observational studies, the findings do suggest that the EuroSCORE and EuroSCORE II are valuable tools in the individual assessment of risk prior to the selection of a revascularization strategy.
The new Mayo Clinic Risk Score (MCRS) was designed to replace the original MCRS by predominantly excluding angiographic variables, namely the presence of LM or MVD, and a few of the interaction effects of specific clinical variables (see Table 1.5 ).
The new MCRS is based solely on baseline clinical and noninvasive assessments and incorporates seven preprocedural variables (age, serum creatinine, left ventricular ejection fraction [LVEF], MI within the past 24 hours, preprocedural shock, congestive heart failure, and peripheral vascular disease). The risk score had a C-statistic (area under ROC curve) of 0.74 and 0.89 for major adverse cardiovascular events (MACEs) and procedural death, respectively, in the population from whom the risk score was derived. The risk score has since been validated for in-hospital mortality in the NCDR ; however, it has not been validated for MACEs. The new MCRS has also been demonstrated to be predictive of in-hospital mortality after CABG surgery.
Ranucci and colleagues demonstrated in a relatively simple risk score consisting of only three clinical variables—age, preoperative serum creatinine value, and LVEF—a risk score for assessing operative mortality risk in elective cardiac operations. Notably, despite the simplicity of the score, the clinical performance of the Age, Creatinine, and Ejection Fraction (ACEF) score appeared to be comparable with either the additive or the logistic EuroSCORE.
The ACEF score is calculated using the following formula:
From ACEF, a mortality risk can be calculated from a graphical relationship of ACEF with an operative risk or an equation ( Fig. 1.8 ). ACEF was developed from an initial dataset of 4557 patients and a subsequent validation series of 4091 patients from a single institution. The results demonstrated a similar accuracy and calibration for the prediction of in-hospital mortality with ACEF when compared with other more complicated surgical risk scores such as the EuroSCORE and the Cleveland Clinic Score. Subsequent validation studies have shown ACEF to have an accuracy level at least comparable with that of the EuroSCORE for operative mortality risk stratification in a series of 29,659 patients undergoing elective cardiac surgery.
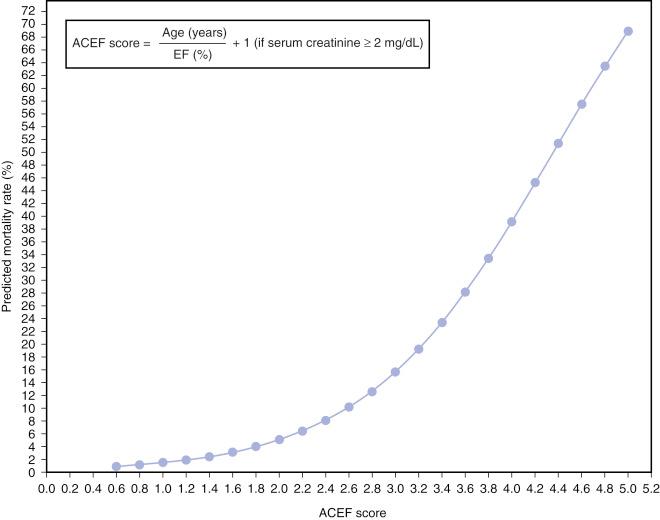
In addition, ACEF was applied to PCI patients from the all-comers LEADERS population at 1-year follow-up. Despite ACEF being demonstrated to be superior to the SYNTAX score alone as a predictor of cardiac death and MI after PCI, ACEF was found to be inferior to the SYNTAX score at predicting overall MACE rates and the risk of repeat revascularization. This reflects the observation that anatomic and clinical variables appear to be necessary requirements for a comprehensive risk score in predicting clinical outcomes after PCI.
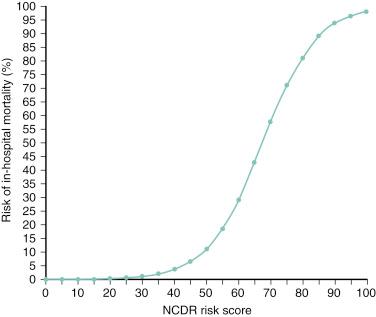
The NCDR CathPCI risk-prediction score is the most contemporary clinical risk score currently available. It incorporates information from nine clinical variables ( Table 1.9 ), which are assigned appropriate weighted values and are then added together to give a final score that can be translated into risk of in-hospital mortality ( Fig. 1.9 ). The score was developed using data from more than 180,000 patients from the voluntary U.S. NCDR database and was validated in more than 400,000 patients from the same database who underwent PCI between March 2006 and March 2007. Notably, the C-statistic for the prediction of in-hospital mortality was consistently greater than 0.90 for in-hospital mortality, whereas a lower but nevertheless adequate C-statistic of 0.83 was seen for 30-day mortality.
| Variable | Scoring Response Categories | |||
|---|---|---|---|---|
| Age | <60 | ≥60, <70 | ≥70, <80 | ≥80 |
| Weighted score | 0 | 4 | 8 | 14 |
| Cardiogenic shock | No | Yes | ||
| Weighted score | 0 | 25 | ||
| Prior CHF | No | Yes | ||
| Weighted score | 0 | 5 | ||
| Peripheral vascular disease | No | Yes | ||
| Weighted score | 0 | 5 | ||
| Chronic lung disease | No | Yes | ||
| Weighted score | 0 | 4 | ||
| GFR (mL/min) | <30 | 30 to 60 | 60 to 90 | >90 |
| Weighted score | 18 | 10 | 6 | 0 |
| NYHA Class IV | No | Yes | ||
| Weighted score | 0 | 4 | ||
| PCI Status (STEMI) | Elective | Urgent | Emergent | Salvage |
| Weighted score | 12 | 15 | 20 | 38 |
| PCI Status (no STEMI) | Elective | Urgent | Emergent | Salvage |
| Weighted score | 0 | 8 | 20 | 42 |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here