Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anterior lamellar keratoplasty includes techniques whereby the diseased corneal stroma is partially or totally replaced by donor tissue, provided the endothelium is still healthy.
Anterior lamellar keratoplasty induces a low immunologic insult. This allows for larger grafts without increased risk of rejection, as in extreme ectatic disorders, and allows the surgeon to perform a successful transplant in the inflamed eye.
Deep anterior lamellar keratoplasty is the gold standard technique to restore transparency and curvature in corneal stromal diseases when the endothelium is healthy.
Manual dissections may be considered safer techniques in cases with a history of hydrops, radial keratotomy, and perforating wounds.
Early therapeutic deep anterior lamellar keratoplasty can be considered to treat dangerous infectious keratitis that is poorly responsive to medical treatment.
Techniques for anterior lamellar keratoplasty (ALK) were established during the 1800; however, the initial difficulty of achieving good visual outcomes and the introduction of topical corticosteroids gave way to penetrating keratoplasty (PK) as the mainstay of corneal transplant surgery from the mid-1950s until recently. In the past quarter of the century, techniques of selective lamellar keratoplasty (LK) have evolved, leading to a fundamental change in the paradigm for corneal surgery. The focus has shifted away from full-thickness grafts to anatomically targeted procedures, replacing only the diseased layer and providing improved visual outcomes, better graft survival, and fewer complications. These procedures include deep anterior lamellar keratoplasty (DALK) to address stromal disease; a variety of endothelial keratoplasty (EK) procedures, including Descemet stripping endothelial keratoplasty (DSEK), Descemet stripping automated endothelial keratoplasty (DSAEK), and Descemet membrane endothelial keratoplasty (DMEK) for endothelial disease; as well as ocular surface stem cell transplantation for epithelial diseases due to limbal stem cell deficiency (LSCD). ,
This chapter will discuss LK for stromal diseases with healthy endothelium and includes several different procedures ( Table 118.1 ).
| Central LKs | Superficial anterior lamellar keratoplasty Anterior Lamellar Keratoplasty
Deep Anterior Lamellar Keratoplasty |
| Peripheral LKs | Lamellar crescentic keratoplasty “Banana” graft D-shaped or C-shaped lamellar keratoplasty Partial ring lamellar keratoplasty “Donut” or annular lamellar keratoplasty Ring lamellar keratoplasty |
Epikeratophakia is an older surgical procedure initially invented to correct aphakia, firstly described by Werblin and Kaufman in 1981. Other indications later included high myopia, hyperopia, and keratoconus. The technique consisted of placing a lamellar donor graft on top of a deepithelialized host cornea and suturing it into a prepared groove. The procedure has been abandoned due to poorly predictable visual outcomes, long visual recovery, the risk of postoperative irregular astigmatism, progressive myopia, reduced contrast sensitivity, and interface opacity.
This group of LKs includes different techniques in which the graft is harvested in various shapes and depths, in accordance with the shape and the depth of the affected cornea (lamellar crescentic keratoplasty, “banana” graft, partial ring LK, D-shaped LK, C-shaped LK, “donut” LK, annular LK, ring LK).
Initially, a limited conjunctival peritomy adjacent to the area of the corneal thinning or ectasia may be necessary. The pathologic cornea to be excised should be marked including a rim of normal corneal tissue to facilitate suturing the graft. Different trephines (corneal and dermatologic trephines) or sharp blades can be used to delineate the borders and the shape of the area to be excised. A free hand manual dissection of the designated area is performed with a crescent blade, or similar instruments, trying to create a square hedge profile, to improve graft-host edge matching and to facilitate suturing. After lamellar excision of the diseased corneal tissue, a lamellar graft is harvested in similar fashion, matching shape and depth of the recipient area, and secured to the host with 10-0 nylon. These techniques have been reported even in cases of perforations. ,
Current indications include a variety of corneal disorders characterized by peripheral circumferential thinning or ectasia, with the aim of providing tectonic stability and/or improvement in corneal surface regularity: advanced Terrien marginal degeneration, pellucid marginal degeneration (PMD), Mooren ulcer, Fuchs marginal keratitis, infectious disorders with peripheral melting, and peripheral ulcerative keratitis (PUK).
Initially, the expression “lamellar keratoplasty” was used to refer only to ALK and only to manual dissection techniques. These procedures were considered technically demanding, time consuming, lacking standardization and reproducibility, and providing variable visual outcomes due to interface scarring and irregularities.
To overcome these issues, in 1998, some surgeons began using an excimer laser to remove the recipient corneal stroma, attempting to obtain a good-quality stromal bed surface as an alternative of the manual dissection. The procedure was named excimer laser lamellar keratoplasty (ELLK) . The residual stromal bed thickness was usually set at 200 μm to avoid endothelial damage and to preserve thickness and curvature. A donor button approximately 350 μm thick was also prepared with a laser ablation from the endothelial side. Despite relatively encouraging results in small series, owing to the different curvature of the anterior and posterior surface of the cornea in keratoconic eyes, this procedure could create a pachymetrically dishomogeneous host bed, limiting the achievable visual outcomes. This was especially true in cases of advanced and decentered ectasia. ,
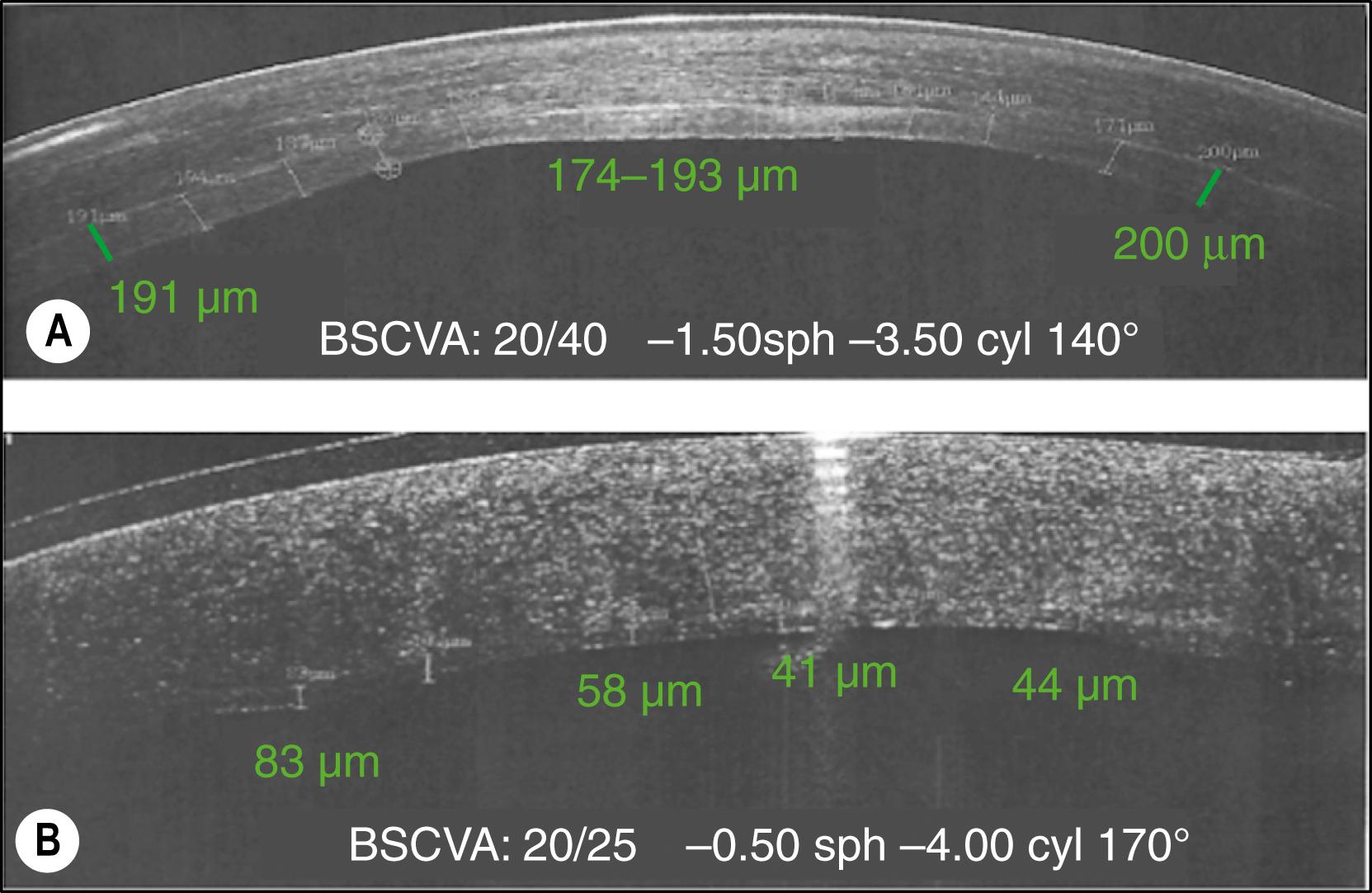
The most recent laser devices and custom ablation algorithms have been used to try to improve the results and broaden the indications for excimer laser technology in ALK: corneal lamellar ablation for transplantation (CLAT) . Despite these efforts and relatively acceptable visual outcomes, the mean estimated residual bed thickness was 200 μm. Today we know that the residual host bed in ALK has to be thinner than 80 μm, smooth, and uniform in thickness to provide visual outcomes comparable with PK ( Fig. 118.1 ; ![]() ).
).
Video 118.1 Deep Anterior Lamellar Keratoplasty (DALK) in a Previous Corneal Lamellar Ablation for Transplantation (CLAT).
The video shows a case of DALK performed in a previous CLAT with poor visual recovery related to a thick residual corneal bed. Previous graft is marked and removed. Surgeon tries to perform a big bubble (BB) in the residual recipient stroma. BB fails creating corneal emphysema. Some air goes into the anterior chamber though the trabecular meshwork. A layer-by-layer manual dissection is then performed, starting from the opening of the corneal deep tunnel that was created to attempt the BB. The surgeon keeps dissecting the stroma using a blunt 27 G spatula. The video ends showing the beautiful deep, smooth and regular achieved stromal bed and the visual outcome. Vincenzo Sarnicola, Caterina Sarnicola, Enrica Sarnicola.
Microkeratome-assisted ALK has been proposed with the aim of attaining a smooth stromal dissection. This technique is now predominantly known as automated therapeutic lamellar keratoplasty (ATLK) thanks to the advent of newer automated microkeratomes for refractive surgery. Despite initial acceptance, the technique has been progressively abandoned owing to some major limitations. The use of microkeratome may, in fact, reproduce the surface irregularities in the lamellar cut when creating the recipient flap. The procedure may not be successful in performing lamellar dissection in eccentrically steep or thin corneas, especially with areas of elevation. Additionally, inherent inaccuracies in the depth of the lamellar dissection preclude its use in deeper stromal lesions. Furthermore, ATLK did not gain popularity because of the elaborate instrumentation required and the degree of attention to details that, if overlooked, may lead to significant complications.
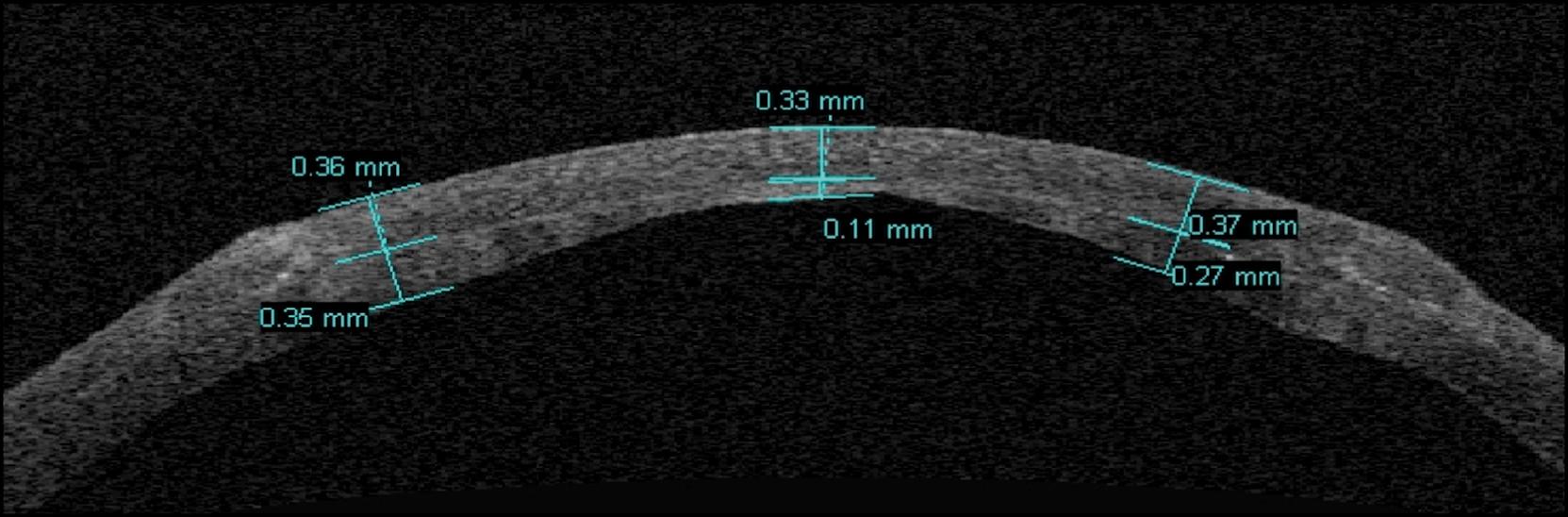
The use of femtosecond lasers has been proposed as an alternative to the excimer laser and microkeratome. Despite the use of femtosecond laser to create the trephination groove seems to be helpful in reducing astigmatism, there are a lot of concerns about its efficacy and safeness when used to prepare the residual stromal bed. , , Studies have shown that deep femtosecond laser ablation, using high energy, creates irregularities and bridges that give poor optical quality, caused by different biomechanics between the posterior corneal lamellae and the anterior stroma. Newer femtosecond laser settings are the object of current studies to create a cut that resembles the optimum cut achieved in the anterior cornea during refractive surgery; however, as for the excimer laser, there are also limitations in creating a residual host bed thinner than 150 μm without damaging the endothelial cells and producing a pachymetrically homogeneous host bed given the different curvature between anterior and posterior surface, especially in advanced keratoconus patients ( Fig. 118.2 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here