Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter, updated from the prior edition, was originally a revised version of the chapter by G.M. Pastores in the sixth edition.
The concept of inborn errors of metabolism (IEM) was introduced by Sir Archibald Garrod in the 1908 Croonian Lectures, and further developed in his Huxley Lecture presented at Charing Cross Hospital in London ( ). IEM constitute a heterogeneous group of disorders resulting from abnormalities in the synthesis, transport, and turnover of dietary and cellular components. Although individually uncommon, IEM collectively represent a significant cause of morbidity and mortality. In aggregate, approximately 1 in 1000 individuals is born with an IEM. Recent advances in expanded newborn screening (NBS) for IEM have allowed earlier identification and decreased sequelae associated with acute decompensation.
Defects of intermediary metabolic pathways induce disease through either accumulation of a toxic metabolite, depletion of a metabolic by-product, or impaired inter- or intracellular transport. When an enzyme deficiency blocks normal catabolic routes, the diversion of metabolism to alternative pathways may disrupt cellular integrity. Deficient enzyme activity may arise from (1) mutations in the primary gene sequence for the protein, with loss of activity; (2) abnormal processing (i.e., defects of post-translational modification); (3) mistaken intracellular localization; (4) mutation resulting in superactivity of enzyme function; and (5) “gain-of-function” mutations. Metabolic defects may also result from defects of a noncatalytic cofactor, structural, or transport protein.
Most IEM are multiorgan disorders that usually impact the nervous system. The clinical course can be acute, subacute, or chronic. Disorders characterized by intoxication or energy depletion usually manifest acutely as altered mental status, often associated with seizures, hypotonia, ataxia, or myopathy. Vomiting and peripheral organ dysfunction (cardiac, hepatic, renal, pancreatic) are other clinical features of acute intoxication. Some IEM follow an insidious course characterized by developmental delay or intellectual deficiency, behavioral problems, sensory–motor impairment, or dementia. In terms of pathophysiology, it is advantageous to categorize IEM into one of three diagnostic groups: (1) disorders involving complex molecules (e.g., lysosomal storage disorders [LSDs]), peroxisomal diseases, congenital defects of glycosylation (CDG), defects of cholesterol synthesis); (2) disorders involving “small molecules” (e.g., amino and organic acidurias, hyperammonemias, lactic acidemias, disorders of vitamins, metal ions); and (3) disorders associated with disruption of cellular energy metabolism (e.g., mitochondrial respiratory-chain defects, disorders of carbohydrate metabolism, disorders of ). Metabolic defects involving complex molecules are generally progressive and unrelated to food intake, whereas those involving small molecules and cellular energy metabolism may be temporally correlated with food intake or metabolic status (e.g., immunization, postsurgical stress, anabolic vs. catabolic status).
Most often, the inheritance of IEM is autosomal recessive, and the heritable trait results in the deficiency of an enzyme or its cofactor. Autosomal-recessive inheritance often accounts for the absence of a family history when the sibship size is small. A smaller number of IEM are transmitted as autosomal-dominant traits (e.g., acute intermittent porphyria [AIP], familial hypercholesterolemia), as X-linked traits (e.g., Fabry disease, Hunter syndrome, Lesch-Nyhan syndrome, ornithine transcarbamylase deficiency), or segregate in a matrilineal fashion (e.g., mitochondrial DNA defects), the latter requiring a sufficient heteroplasmic burden of mutant mitochondria to enable disease expression.
Early diagnosis is important for treatment intervention, genetic counseling, and family support. A major goal of NBS is to reduce the functional impairment of affected probands, and this has been the greatest advance made through the expansion of NBS for the ∼30 disorders on the current list of screened IEM. In addition, most families are unaware of their a priori risk, and early diagnosis of an affected proband facilitates the potential for prenatal diagnosis in future pregnancies. Therapeutic advances in recent years are considerable, especially in the realm of LSDs and ERT (enzyme-replacement therapy) and early diagnosis provides for optimal outcomes. Furthermore, treatment of secondary disabilities (e.g., seizures, sensory impairments, behavioral problems) positively impacts quality of life.
This chapter provides an overview of the major IEM ( Fig. 91.1 ), except for the mitochondrial disorders. Most neurologists are neither metabolic specialists nor biochemical geneticists and are not expected to be knowledgeable concerning the details of all biochemical pathways. This chapter describes those IEM that prominently associate with nervous system dysfunction. A partial listing of IEM associated with key clinical presentations is shown in ![]() eTable 91.1 . For more comprehensive coverage, the reader may consult several recently published books ( ).
eTable 91.1 . For more comprehensive coverage, the reader may consult several recently published books ( ).
| Clinical Finding | Associated Inborn Errors of Metabolism |
|---|---|
| Developmental delay | 3-Hydroxyisobutyrate dehydrogenase deficiency; methylmalonate semialdehyde dehydrogenase deficiency; malonic aciduria; combined methylmalonic and malonic aciduria; d -2-hydroxyglutaric aciduria; succinic semialdehyde dehydrogenase (SSADH) deficiency; pyridoxine-dependent epilepsy; pyridoxamine-5’-phosphate oxidase deficiency; cobalamin disorders; biotinidase deficiency; holocarboxylase synthetase deficiency; short-chain acyl-CoA dehydrogenase deficiency; pyruvate carboxylase deficiency; pyruvate dehydrogenase deficiency (subunits E1, E2, and E3); Leigh syndrome (Leigh subacute necrotizing encephalopathy); complex III deficiency; mitochondrial depletion syndromes; Zellweger syndrome; rhizomelic chondrodysplasia punctate (RCDP); Schindler disease; congenital disorders of glycosylation; aromatic l -amino acid decarboxylase deficiency; dopamine-serotonin vesicular transporter defect; guanidinoacetate methyltransferase (GAMT) deficiency; arginine: glycine amidinotransferase (AGAT) deficiency; creatine transporter deficiency; sterol 27-hydroxylase deficiency; 2-methylacyl-CoA racemase deficiency; Smith-Lemli-Opitz syndrome; chondrodysplasia punctate 2 (male); sterol-C4 methyloxidase deficiency; lanosterol demethylase deficiency; phosphoribosyl pyrophosphate synthetase superactivity; purine nucleoside phosphorylase deficiency; pyrimidine 5’-nucleotidase deficiency; tyrosinemia type II; methionine adenosyl transferase (MAT) type I/II deficiencies; S-adenosylmethionine (SAM) hydrolase deficiency; homocystinuria; adenosine kinase deficiency; N -acetylglutamate synthetase (NAGS) deficiency; carbamyl phosphate synthetase (CPS) 1 deficiency; ornithine transcarbamoylase (OTC) deficiency; citrullinemia types I, II; argininosuccinic aciduria; argininemia; HHH (hyperornithinemia; hyperammonemia; homocitrillinuria) syndrome; glutamine synthetase deficiency; pyrroline-5’-carboxyic acid (P5C) synthase deficiency; isovaleric acidemia; 3-hydroxyisobutyrl-CoA deacylase deficiency |
| Ataxia | Citrullinemia types I, II; HHH syndrome (hyperornithinemia; hyperammonemia; homocitrullinuria); argininemia; argininosuccinic aciduria; OTC deficiency; non-ketotic hyperglycinemia; phosphoglycerate dehydrogenase deficiency; SSADH deficiency; Hartnup disease; EAAT1 glutamate transporter defect; maple syrup urine disease; isovaleric acidemia; 3-methylglutaconyl-CoA hydratase deficiency; Costeff syndrome; 3-oxothiolase deficiency (β-ketothiolase deficiency); propionic acidemia; methylmalonic acidemia; glutaric aciduria type I; l -2-hydroxyglutaric aciduria; folate receptor α deficiency; cerebral folate deficiency; cobalamin disorders; biotinidase deficiency; coenzyme Q 6 deficiency; combined CABC1/ADCK3 deficiency (coenzyme Q 10 deficiency); AOA1 (ataxia oculomotor ataxia 1); GLUT-1 deficiency (glucose transporter deficiency); NARP (neuropathy, ataxia, and retinitis pigmentosa); Leigh syndrome; MERRF (myoclonic epilepsy with ragged-red fibers); mitochondrial myopathy with diabetes mellitus; Kearns-Sayre syndrome; PEO (progressive external ophthalmoplegia); optic atrophy 1 and deafness; LHON (Leber hereditary optic neuropathy); combined oxidative-phosphorylation defect 7; mitochondrial depletion syndromes; leukoencephalopathy with brainstem and spinal cord involvement and elevated lactate; Refsum disease; galactosialidosis; Sandhoff disease; Tay-Sachs disease; Krabbe disease; metachromatic leukodystrophy; Niemann-Pick disease, types 1 and 2; neuronal ceroid lipofuscinosis; mannosidosis; Schindler disease; Salla disease; congenital disorders of glycosylation; dopamine-serotonin vesicular transport defect; sterol 27-hydroxylase deficiency; ribose-5’-phosphate isomerase deficiency; Wilson disease; phosphoribosyl pyrophosphate (PRPP) synthetase deficiency; γ-glutamyl synthetase deficiency; glutathione synthetase deficiency; abetalipoproteinemia; hypobetalipoproteinemia; SANDO (sensory ataxia, neuropathy, dysarthria, and ophthalmoparesis) syndrome |
| Muscle weakness | Sepiapterin reductase (SR) deficiency; SAM hydrolase deficiency; 3-methylcrotonyl-CoA carboxylase deficiency; methylene tetrahydrofolate reductase deficiency; Brown-Vialetto-Van Laere syndrome; Fazio-Londe syndrome; prenyldiphosphate synthase, subunit 2 (PBSS2) deficiency; coenzyme Q 2 deficiency; CABC1/ADCK3 deficiency; myopathic form of CoQ10 deficiency; riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency; glycogen storage disorders; Leigh syndrome; complex IV deficiency; combined oxidative phosphorylation defect 3; ACAD 9 deficiency; Sandhoff disease; Tay-Sachs disease; metachromatic leukodystrophy; cystinosis; congenital disorders of glycosylation; ornithine aminotransferase deficiency; X-linked distal spinal muscular atrophy; thymidine kinase 2 deficiency; mitochondrial ribonucleotide reductase subunit 2 deficiency |
| Behavior abnormalities (aggression, psychosis, OCD, ADD, ADHD) | SSADH deficiency; SAM hydrolase deficiency; folate receptor α deficiency; neuronal ceroid lipofuscinoses (NCL); San Filippo syndromes; dopamine β-hydroxylase deficiency; monoamine oxidase (MAO) deficiency; Smith-Lemli-Opitz (SLO) syndrome; hyperprolinemia; MEGDEL syndrome; X-linked adrenoleukodystrophy and adrenomyeloneuropathy; SR deficiency; dicarboxylic aminoaciduria; CHILD/CK syndrome; short-chain acyl-CoA dehydrogenase (SCAD) deficiency; Niemann-Pick disease, types 1 and 2; mucopolysaccharidoses types I, II (Hurler, Hurler-Schie, and Hunter syndromes); non-ketotic hyperglycinemia (milder variants) |
| Seizures | Neuronal ceroid lipofuscinoses (Batten) diseases; 3-methylglutaconyl-CoA hydratase deficiency; pyridoxine dependent epilepsy; phosphoserine phosphatase deficiency; GABA-transaminase deficiency; SSADH deficiency; early infantile epileptic encephalopathy; guanosine triphosphate cyclohydrolase (GTPCH) deficiency; 6-pyruvoyl-tetrahydropterin (6-PTPS) deficiency; dihydropteridine reductase deficiency; Farber disease; Niemann-Pick disorders (types A, B, and C); methylenetetrahydrofolate reductase (MTHFR) deficiency; Gaucher disease; saposin deficiency (combined); multiple sulfatase deficiency; neuronal ceroid lipofuscinoses (Batten disease); hereditary folate malabsorption; folate receptor α deficiency; homocystinuria; molybdenum cofactor disorders |
| Movement disorder | Costeff syndrome; 2-methyl-3-hydroxybutyryl-CoA dehydrogenase (MHBD) deficiency; 3-oxothiolase deficiency; Canavan disease; hereditary folate malabsorption; folate receptor α deficiency; holocarboxylase synthetase deficiency; GLUT-1 deficiency (glucose transporter deficiency); mitochondrial depletion syndromes; neuronal ceroid lipofuscinoses (Batten disease); dopamine transporter deficiency; GAMT deficiency; AGAT deficiency; SSADH deficiency |
| Autism and autism-spectrum disorders | Phenylketonuria (PKU); cerebral folate deficiency; SSADH deficiency; fumarase deficiency; GAMT deficiency; creatine transporter deficiency; dihydropyrimidine dehydrogenase deficiency; 2-methylbutyrylglycinuria; combined methylmalonic and malonic acidurias; folate receptor (FOLR1) deficiency |
| Headache | Methionine adenosyltransferase (MAT) deficiency I and II; glutaric aciduria type I; oxidative-phosphorylation (OXPHOS) defects (numerous); MAO A deficiency; 2-methylacyl-CoA racemase deficiency; 17α-hydroxylase deficiency; 11β-hydroxylase type I deficiency; MELAS (mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes) syndrome |
| Language difficulties | SR deficiency; SSADH deficiency; MAT deficiency I and III; Niemann-Pick disease, types I and II; neuronal ceroid lipofuscinoses (Batten disease) |
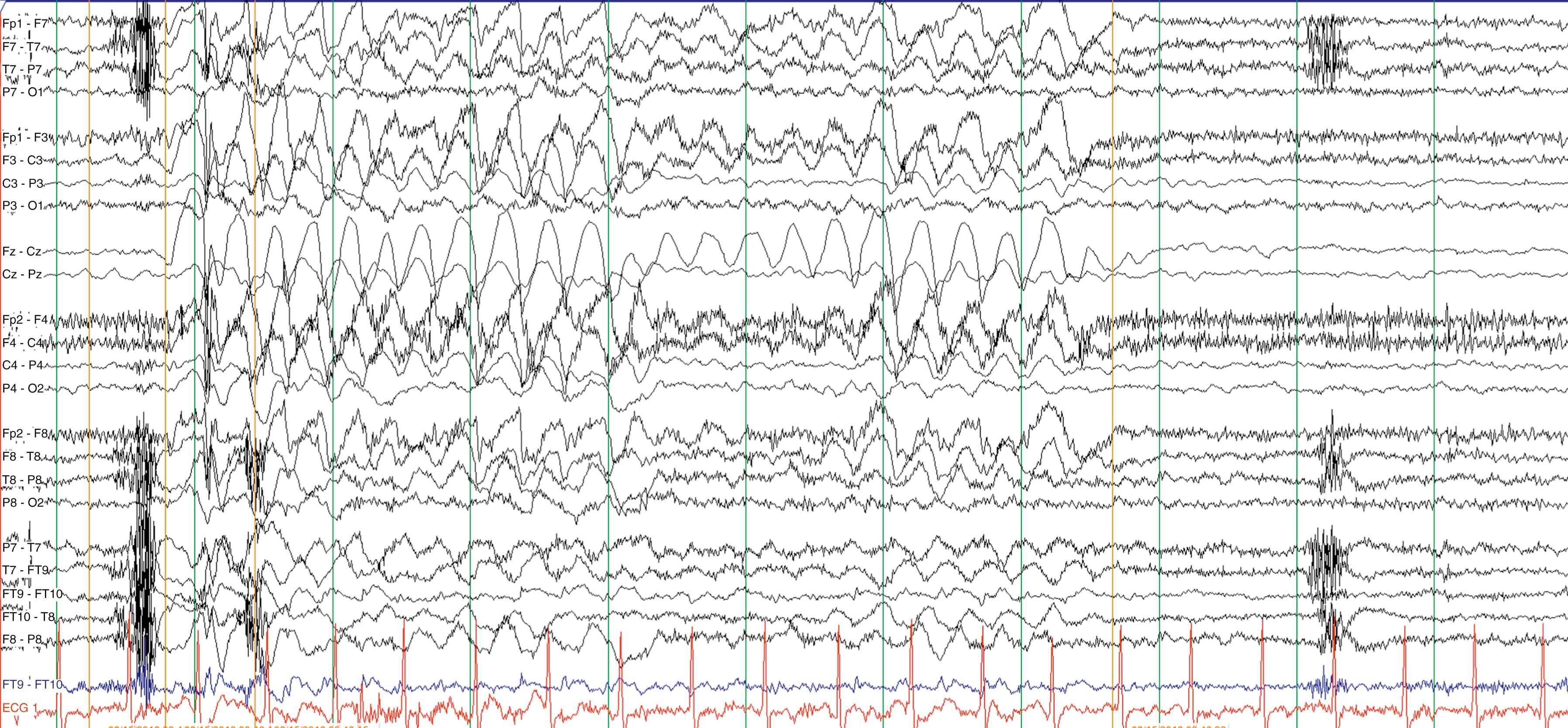
Clinical features, family history, and baseline testing help focus the diagnostic approach. In the setting of acute illness with neurological signs and symptoms, an IEM should be considered, in parallel with more common disorders, even in the face of a noninformative family history. Examine the blood and urine of patients with acute neurological deterioration or even static encephalopathy for signs of acidosis, ketosis, hypoglycemia, and hyperammonemia ( Table 91.2 and ![]() eTable 91.3 ). The caveat is that absence of abnormalities of blood pH, base deficit, blood sugar or ammonia do not necessarily rule out an IEM (e.g., succinic semialdehyde dehydrogenase [SSADH] deficiency) ( ). Moreover, consider screening tests for abnormalities of amino or organic acid metabolism, and be aware that abnormal metabolites may not be present during stable periods or in samples obtained after the acute illness has resolved. Certain static or progressive encephalopathies require analysis of cerebrospinal fluid (CSF; ) ( Table 91.4 ). While CSF examination is time-honored and standard in neurological evaluations, it bears emphasis that hypoglycorrhachia for the diagnosis of glucose transporter 1 (GLUT1) deficiency requires a lumbar puncture, and diagnosis mandates initiation of the ketogenic diet along with carnitine supplementation in the classic neonatal presentation ( ). There is a differential diagnosis to hypoglycorrhachia, including bacterial and tuberculous meningitis, meningocarcinomatosis, subarachnoid hemorrhage, peripheral hypoglycemia, and, in some cases, status epilepticus and mitochondrial disorders. The phenotypic spectrum of GLUT1 deficiency is considerable, from neonatal seizures to later exertional dyskinesias, as well as myoclonic-atonic seizures and early-onset generalized absence seizures ( Fig. 91.2 ).
eTable 91.3 ). The caveat is that absence of abnormalities of blood pH, base deficit, blood sugar or ammonia do not necessarily rule out an IEM (e.g., succinic semialdehyde dehydrogenase [SSADH] deficiency) ( ). Moreover, consider screening tests for abnormalities of amino or organic acid metabolism, and be aware that abnormal metabolites may not be present during stable periods or in samples obtained after the acute illness has resolved. Certain static or progressive encephalopathies require analysis of cerebrospinal fluid (CSF; ) ( Table 91.4 ). While CSF examination is time-honored and standard in neurological evaluations, it bears emphasis that hypoglycorrhachia for the diagnosis of glucose transporter 1 (GLUT1) deficiency requires a lumbar puncture, and diagnosis mandates initiation of the ketogenic diet along with carnitine supplementation in the classic neonatal presentation ( ). There is a differential diagnosis to hypoglycorrhachia, including bacterial and tuberculous meningitis, meningocarcinomatosis, subarachnoid hemorrhage, peripheral hypoglycemia, and, in some cases, status epilepticus and mitochondrial disorders. The phenotypic spectrum of GLUT1 deficiency is considerable, from neonatal seizures to later exertional dyskinesias, as well as myoclonic-atonic seizures and early-onset generalized absence seizures ( Fig. 91.2 ).
| Tests | Clinical Utility |
|---|---|
| Blood gas, anion gap | Urea cycle defects, organic and amino acidurias, fatty acid oxidation disorders, selected glycogen storage disorders, ketone synthesis/utilization defects, OXPHOS defects |
| Ammonia | Urea cycle defects, organic and amino acidurias, fatty acid oxidation defects, hyperinsulinemia-hyperammonemia (HIHA) syndrome |
| Glucose | Organic and amino acidurias, fatty acid oxidation defects, glycogen storage disorders, ketone synthesis/utilization disorders, OXPHOS defects |
| Lactate † | Selected glycogen storage disorders, defects of gluconeogenesis, fatty acid oxidation (often in conjunction with hypoglycemia); OXPHOS defects |
| Urinary ketones | Organic acidurias, fatty acid oxidation defects, selected glycogen storage disorders, ketone utilization/synthesis disorders, selected amino acidurias (predominantly MSUD); gluconeogenic disorders, OXPHOS defects; ability to distinguish hypo- and hyperketotic disorder (especially pertinent to hypoglycemic disorders) |
| Uric acid | Organic and amino acidurias, fatty acid oxidation defects, glycogen storage disorders, ketone synthesis/utilization disorders, OXPHOS defects; disorders of purine and pyrimidine metabolism |
| Creatine kinase | Disorders of fatty acid oxidation, selected glycogen storage disorders, OXPHOS defects |
| Reducing substances (urine) | Disorders of carbohydrate metabolite (primarily galactosemia) |
| Insulin levels (with ketones and/or free fatty acids) | Useful in the differential diagnosis of the hypoglycemic patient; comparisons between the ratio of free fatty acids/ketone bodies, in conjunction with glucose and ketone bodies, assist in differentiating hypo- and hyperketotic disorders. |
| Amino acid analysis (sera/plasma) | Amino acidurias, selected organic acidurias, defects in gluconeogenesis, urea cycle disorders |
| Organic acid analysis (urine) | Organic and amino acidurias, urea cycle defects, fatty acid oxidation disorders, selected glycogen storage disorders, ketone synthesis/utilization defects, defects in gluconeogenesis, OXPHOS defects (urinary orotic acid can also be beneficial in the differential diagnosis of urea cycle defects) |
| Acylcarnitine profile (sera/plasma/bloodspot) | Organic acidurias, defects of fatty acid oxidation, limited utility in differential diagnosis of ketone utilization/synthesis defects |
| Congenital disorders of glycosylation | Isoelectric focusing of serum transferrin (possible to miss selected disorders); isoelectric focusing of serum apolipoprotein C-III; analysis of lipid-linked oligosaccharides; glycan structural analysis (usually MALDI-TOF, or combined with capillary electrophoresis); mutation analyses and/or whole exome sequence analysis |
∗ The above list only represents a brief overview of primary and secondary tests to pursue. Depending upon outcomes (or specific clinical features), more detailed analyses may be warranted, potentially including purine and pyrimidine analyses (urine), neurotransmitter evaluation (cerebrospinal fluid), lipoprotein profile, cholesterol-related metabolites (often in the context of dysmorphia), creatine, polyols, bile acids, very-long chain fatty acids (VLCFA, peroxisomal disease, in conjunction with phytanic acid), and potentially Nuclear magnetic resonance (NMR) spectroscopy.
† Presence or absence of hypoglycemia can be a useful aid to differential diagnosis of disorders that lead to lactic acidemia.
| Sign | Disease |
|---|---|
| Acute Cardiorespiratory Signs | |
| Cardiac failure | FAO, OXPHOS, Fabry disease, GSD III, GSD-II, PA, MMA, MPS-LYSO |
| Arrhythmia | FAO, Kearns-Sayre syndrome, Danon disease, cardiac glycogenosis, triose phosphate isomerase, D-2-hydroxyglutaric aciduria, thiamine deficiency/dependency states |
| Pulmonary hypertension | MTHFR, OXPHOS, NFU-1, LYSO, Cbl-C |
| Stridor | Biotinidase, PDH, OXPHOS, Gaucher disease type II, Krabbe disease |
| Acute Neurological Signs | |
| Coma | UCDs, BCOAs, MSUD, multiple carboxylase, GA-1, MADD, OXPHOS, FAO, ketolysis, hyperinsulinism, gluconeogenesis |
| Seizures | UCDs, BCOAs, hyperinsulinism, NKH, SO-XO def, PEROX, pyridoxine-dependent epilepsy, folinic acid-responsive seizures, AGAT, biotinidase, cerebral folate receptor-α, creatine transporter, GAMT, holocarboxylase synthase, Menkes disease/occipital horn syndrome, PGDH, phosphoserine phosphatase, phosphoserine aminotransferase, PNPO def, OXPHOS, LYSO (Gaucher disease type III, NP disease type C), NCL, GABA transaminase, PDH, ADSL, GS, GLUT1 |
| Oculogyric crisis | Neurotransmitter disorders, Phenylketonuria (PKU) |
| Dystonia | BCOAs and vitamin B 12 metabolism disorders, GA-1, MEGDEL, Wilson disease, aceruloplasminemia, biotin-responsive basal ganglia disease, neurotransmitter disorders, GAMT, homocystinuria, MHBD, HMG-CoA lyase, HMG-CoA synthase, LSD, NKH, SCOT, beta-ketothiolase, Lesch-Nyhan syndrome |
| Stroke-like episode | OXPHOS (MELAS), OTC, CDGs, homocystinuria, OAs, MSUD, Fabry disease, beta-ketothiolase |
| Ataxia | MSUD, PDH, multiple carboxylase, UCDs, BCOAs and inborn errors of cobalamin metabolism, GLUT1, Hartnup, CoQ 10 , CTX, LYSO (NP disease type C), NKH, SSADH, thiamine-responsive encephalopathy |
| Dementia | Aceruloplasminemia, Wilson disease, alpha-mannosidosis, X-ALD, CTX, Gaucher disease type III, MPS I, II, III, IV; NP disease type C, MLD, MHBD, MELAS |
| Psychiatric signs | UCDs, citrin, porphyria, MPS VII, MTHFR, Hartnup disease, creatine transporter, GAMT, homocystinuria, vitamin B 12 metabolism errors, MSUD (variant), MELAS, MLD, BCOAs, NP disease type C, SSADH, Wilson disease |
| Sudden visual loss | MELAS, LEBER, MMA, PA, homocystinurias, X-ALD, porphyria, biotinidase deficiency |
| Sudden hearing loss | MELAS |
| Pain | Fabry disease, Gaucher disease, Krabbe disease, prolidase deficiency |
| Acute peripheral neuropathy | PDH, biotinidase, porphyria, MSUD (variant), MLD, tyrosinemia type I, LCHAD/MTP |
| Basal ganglia lesions | OXPHOS, PA, MMA, MSUD, beta-ketothiolase, GA I, MADD, HMG-CoA lyase, HMG-CoA synthase, MHBD SCOT, biotin-responsive T1 transporter, Wilson disease, aceruloplasminemia, GAMT |
| Hydrocephalus | MPS I, MPS II, MPS VI, Cbl-C, Cbl-D, MTHFR, Gaucher disease, NKH, mannosidosis |
| Myoglobinuria | FAO, muscle GSD, OXPHOS, LIPIN1, porphyria, Wilson disease |
| Cramps | FAO, muscle GSD |
| Acute Hematological and Vascular Signs | |
| Anemia (hemolytic) | GSH synthase, gamma glutamylcysteine synthase, Wilson disease |
| Anemia (nonhemolytic) | Orotic aciduria, inborn errors of cobalamin metabolism, methionine synthase, inborn errors of folate metabolism, MK, Pearson syndrome, OXPHOS, thiamine-responsive megaloblastic anemia |
| Anemia (nonmacrocytic, hemolytic, or due to combined mechanisms) | Abetalipoproteinemia, adenylate kinase, adenosine triphosphatase carnitine uptake, porphyria, di-metal transporter 1, porphyria, galactosemia, glycolytic and pentose-phosphate enzyme, hemochromatosis, IRIDA, LCAT, MK, mitochondrial tyrosyl-tRNA synthetase, MLASA syndrome, pyroglutamic aciduria, pyrimidine 5-nucleotidase, red blood cell glycolysis defects, TALDO, Wilson disease, Wolman disease, X-linked sideroblastic anemia, pyridoxine-responsive epilepsy |
| Neutropenia | GSD1b, Barth syndrome, BCOAs |
| Pancytopenia | Pearson syndrome, MMA, PA, Gaucher disease types I and III, NP types A and B, OXPHOS, adenylate kinase 2, TALDO |
| Macrophage activation syndrome, HLH | LPI, Cbl-C, Wolman disease, Gaucher disease, NP disease |
| Coagulopathy | CDGs, HHH |
| Subdural hematoma | HHH, GA-I, Menkes disease |
| Thromboembolism | CDGs, homocystinurias |
| Acute Liver and Digestive Signs | |
| Fetal hydrops | LYSO (GM1 gangliosidosis, sialidosis type II, I-cell disease, Niemann-Pick type A/C, MPS VII, galactosialidosis), TALDO, CDG, mevalonic aciduria |
| Acute liver failure | Tyrosinemia type I, UCDs, citrin, OXPHOS, CDGs, HFI, FAO, galactosemia, TALDO, Wilson disease, Wolman disease |
| Cholestatic jaundice | Arginase, bile acid synthesis, CDG, CTX, cholesterol synthesis defects, citrin, galactosemia, LCHAD/MTP, MK, NP-C, PEROX, TALDO, tyrosinemia type I |
| Pancreatitis | FAO, OAs, GSD1, CBS, OXPHOS (MELAS), LPL |
| Intestinal pseudo-obstruction | MNGIE, MK def |
| Recurrent vomiting | UCDs, OAs, HFI |
| Acute Endocrinological Signs | |
| Adrenal insufficiency | SLO, ALD, OXPHOS |
| Hyperinsulinemic hypoglycemia | SCHAD, CDGs, HIHA |
| Diabetes | Abnormal proinsulin cleavage, diabetes, deafness and TRMA Kir 6.2, glucokinase, DEND, MMA, PA, IVA, ketolytic, OXPHOS, Wolfram syndrome |
| Acute Nephrological Signs | |
| Hemolytic uremic syndrome | Cbl-C, MHTFR |
| Fanconi syndrome and renal tubular acidosis | Tyrosinemia type I, galactosemia, OXPHOS, cystinosis, HFI, Fanconi-Bickel syndrome, Wilson disease, LPI, CPT1 |
| Acute renal failure | Oxalosis, FAO |
| Nephrotic syndrome | CoQ 10 , CDGs |
| Acute Dermatological Signs | |
| Skin rash | Biotinidase, MK, holocarboxylase synthetase, Hartnup disease, porphyria, steroid sulfatase |
| Orthostatic cyanosis, petechiae | ETHE |
| Abnormal Urine and Sweat Odor | |
| Sweaty feet odor | IVA, 3-methylcrotonylglycinuria, MADD |
| Maple syrup odor | MSUD |
| Musty odor | PKU |
| Fish odor | Trimethylaminuria, dimethylglycine dehydrogenase |
| Other | |
| Febrile episodes | MK |
| Maternal disease of pregnancy/HELLP | LCHAD/MTP |
Neurological deterioration is a characteristic feature of acute intoxication disorders (e.g., certain aminoacidopathies, organic acidurias, and the urea cycle disorders). However, with the revolution of NBS, many patients are identified prior to a potential acute decompensation episode. Abnormal urine odor may be present in diseases associated with the excretion of volatile metabolites (keto-acids [maple syrup] in maple syrup urine disease [MSUD]; sweaty feet in isovaleric acidemia [IVA]; and glutaric acidemia type II). Isolated seizures may be the initial features of vitamin-responsive disorders (e.g., defects of pyridoxine [vitamin B 6 ] and folinic acid metabolism, biotinidase deficiency, and biotin-responsive multiple carboxylase deficiency [MCD]), and are a prominent feature in glycine encephalopathy, sulfite oxidase (SO) deficiency, and formation of the molybdenum cofactor complex. Congenital lactic acidosis and central hypotonia are features of abnormalities of pyruvate metabolism and the Krebs cycle ( ). Recurrent hypoglycemia, in the face of pronounced organomegaly, typically occurs in the glycogen storage disorders (GSDs) affecting the liver and in defects of fatty acid oxidation (FAO; consequent to overutilization) ( ); these diseases are also frequently associated with signs of cardiac involvement (cardiomyopathy, arrhythmias). In the mitochondrial FAO defects, urinary ketone concentrations are pathologically low (associated with the inability to metabolize fatty acids of variable chain length). Analysis of plasma acylcarnitines, and the profile of urinary acylglycines, is helpful in the differential diagnoses of the FAOs ( ). Renal tubular acidosis associated with bicarbonate wasting can be encountered in pyruvate carboxylase (PC) deficiency, methylmalonic acidemia (MMA), carnitine palmitoyltransferase I (CPT-I) deficiency, and cystinosis.
| Levels in Cerebrospinal Fluid | ||||||||
|---|---|---|---|---|---|---|---|---|
| Disorder | Neopterin | Sepiapterin | Biopterin | 5-HTP | HVA | 5-HIAA | 3-OMD | MHPG |
| Dopamine β-hydroxylase deficiency | N | ↑ | ↓↓ | |||||
| Tyrosine hydroxylase deficiency | ↓↓ | N | n | ↓ | ||||
| Aromatic- l -amino acid decarboxylase deficiency | ↑↑ | ↓↓ | ↓↓ | ↑↑↑ | ||||
| Monoamine oxidase A deficiency | ↓ | ↓ | n | ↓ | ||||
| Dopamine transporter deficiency | ↑ | N | ||||||
| Dopamine-serotonin vesicular transport defect | n | N | ||||||
| Guanosine triphosphate (GTP) cyclohydrolase deficiency | ↓↓ | ↓↓ | ↓↓ | ↓ | ||||
| 6-Pyruvoyl-tetrahydropterin synthase (6-PTPS) deficiency | ↑↑↑ | ↓↓↓ | ↓↓ | ↓↓ | ||||
| Dihydropteridine reductase deficiency | n | n-↑ | ↓↓ | ↓↓ | ||||
| Pterin-4-α-carbinolamine dehydratase deficiency (primapterinuria) | ↑-↑↑ | |||||||
| Dopa-responsive dystonia (Segawa disease) | ↓ | ↓ | ↓ | ↓-n | ||||
| Sepiapterin reductase deficiency | n | ↑↑ | ↑ | ↓↓↓ | ↓↓↓ | |||
Ophthalmological examination often provides important insight into the diagnosis of IEM. Vertical supranuclear ophthalmoplegia occurs in Niemann-Pick type C disease, and saccadic initiation failure and defective optokinetic nystagmus may be observed in Gaucher disease type III. Kayser-Fleisher rings (orange or greenish deposits around the limbus of the cornea due to copper deposition within the Descemet basement membrane of the cornea) are strongly suggestive of Wilson disease ( ![]() eTable 91.5 ). Hepatosplenomegaly and other signs of storage (e.g., coarse facies, nonimmune hydrops fetalis, dysostosis multiplex, kyphoscoliosis) occur within the broad spectrum of lysosomal disorders, especially the mucopolysaccharidoses. Liver dysfunction or hepatomegaly (or both) usually manifests in disorders of carbohydrate metabolism (e.g., galactosemia, hereditary fructose intolerance [HFI], GSD [particularly Pompe disease, or GSD type II]), bile acid synthesis defects, tyrosinemia, and CDG. Inability to synthesize and mobilize bile acids (and conjugates) can also result in extensive cholestatic disease ( ). Unconjugated hyperbilirubinemia associated with liver dysfunction or hemolysis in infancy may lead to permanent brain damage due to kernicterus, a major concern in the Crigler-Najjar disorder ( ).
eTable 91.5 ). Hepatosplenomegaly and other signs of storage (e.g., coarse facies, nonimmune hydrops fetalis, dysostosis multiplex, kyphoscoliosis) occur within the broad spectrum of lysosomal disorders, especially the mucopolysaccharidoses. Liver dysfunction or hepatomegaly (or both) usually manifests in disorders of carbohydrate metabolism (e.g., galactosemia, hereditary fructose intolerance [HFI], GSD [particularly Pompe disease, or GSD type II]), bile acid synthesis defects, tyrosinemia, and CDG. Inability to synthesize and mobilize bile acids (and conjugates) can also result in extensive cholestatic disease ( ). Unconjugated hyperbilirubinemia associated with liver dysfunction or hemolysis in infancy may lead to permanent brain damage due to kernicterus, a major concern in the Crigler-Najjar disorder ( ).
| Ophthalmological Abnormality | Associated Inborn Errors of Metabolism |
|---|---|
| Cataracts | Pyrroline-5′-carboxylic acid synthetase deficiency; 3-phosphoglycerate dehydrogenase deficiency; hyperprolinemia; neonatal mitoencephalocardiomyopathy; Fanconi-Bickel syndrome; galactokinase deficiency; galactosemia; disorders of glucose and fructose metabolism; progressive external ophthalmoplegia (PEO); Zellweger syndrome; rhizomelic chondrodysplasia punctate (RCDP); Refsum disease; congenital disorders of glycosylation; disorders of bile acid metabolism; cholesterol biosynthetic disorders, mevalonate kinase deficiency; Wilson disease; disordered ceruloplasmin transport |
| Optic atrophy | Adrenoleukodystrophy; non-ketotic hyperglycinemia; Costeff syndrome; 3-methylglutaconyl-CoA hydratase deficiency (type I 3-methylglutaconic aciduria); propionic acidemia; Canavan disease; biotinidase deficiency; disorders of coenzyme Q 10 metabolism; fumarase deficiency; juvenile optic atrophy; maternally inherited mitochondrial dystonia; Leber hereditary optic neuropathy (LHON); oxidative-phosphorylation (OXPHOS) defects; GM1 gangliosidosis; neuronal ceroid lipofuscinosis (Batten disease); Schindler disease; primary hyperoxaluria, types II and III; congenital disorders of glycosylation; bile acid synthesis defects; ribose-5-phosphate isomerase deficiency; neurodegeneration with brain iron accumulation; 5’-phosphoribosyl pyrophosphate (PRPP) synthetase deficiency; dihydropyrimidine dehydrogenase deficiency |
| Retinopathy | Cobalamin disorders; mitochondrial trifunctional protein (MTP) deficiency; long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency; neuropathy ataxia, and retinitis pigmentosa; Kearns-Sayre syndrome; neuronal ceroid lipofuscinosis (Batten disease); primary hyperoxaluria types I and II; cystinosis; congenital disorders of glycosylation; 2-methylacyl-CoA racemase deficiency; neurodegeneration with brain iron accumulation 1; inosine monophosphate dehydrogenase deficiency; glutathione synthetase deficiency; abetalipoproteinemia |
| Corneal clouding | Hyperprolinemia; GM1 gangliosidosis; mucolipidosis II; fucosidosis; sialidosis; Hurler syndrome (and other mucopolysaccharidoses); lathosterolosis; glutathione synthetase deficiency; familial lecithin cholesterol acyl transferase (LCAT) deficiency |
| Impaired vision, vision loss | Ornithine transcarbamylase deficiency; hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome; cerebral folate deficiency; cobalamin disorders; fumarase deficiency; Mohr-Tranebjaerg syndrome; mitochondrial depletion syndromes; galactosialidosis; Sandhoff disease; Tay-Sachs disease; cystinosis; congenital disorders of glycosylation; neurodegeneration with brain iron accumulation 1 |
| Pigmentary retinopathy | MTP deficiency; neuropathy ataxia and retinitis pigmentosa; Kearns-Sayre syndrome; neuronal ceroid lipofuscinosis (Batten disease); primary hyperoxaluria, all types (I–III); congenital disorders of glycosylation; inosine monophosphate dehydrogenase deficiency; glutathione synthetase deficiency; abetalipoproteinemia |
| Ptosis (eyelid) | Maternally inherited deafness and diabetes; optic atrophy type 1 and deafness; mitochondrial depletion syndromes; tyrosine hydroxylase deficiency; aromatic l-amino acid decarboxylase deficiency; dopamine β-hydroxylase deficiency; dopamine-serotonin vesicular transport defect; Smith-Lemli-Opitz syndrome; chondrodysplasia punctate type 2 (male); lathosterolosis; mitochondrial ribonucleotide reductase subunit 2 deficiency |
| Blindness | Canavan disease; neuropathy ataxia and retinitis pigmentosa; mitochondrial encephalomyopathy, lactic acidosis and stroke (MELAS) syndrome; peroxisomal disorders; neuronal ceroid lipofuscinosis (Batten disease); Krabbe disease; congenital disorders of glycosylation; ornithine aminotransferase (OAT) deficiency; AICAR (aminoimidazole carboxamide ribonucleotide) transformylase deficiency |
| Ophthalmoplegia | OXPHOS defects (mitochondrial); PEO; Leigh syndrome; mitochondrial myopathy with diabetes mellitus; Kearns-Sayre syndrome; optic atrophy type 1 and deafness; mitochondrial depletion syndromes |
| Arcus cornealis | Familial defective apolipoprotein B (APOB); autosomal-recessive hypercholesterolemia (ARH); familial combined hyperlipidemia; familial LCAT (lecithin cholesterol acyltransferase) deficiency (partial) |
| Amblyopia, anterior eye chamber anomalies, buphthalmos, exophthalmia (prooptosis) | Congenital disorders of glycosylation |
| Cortical blindness, tunnel vision, vision loss | Molybdenum cofactor deficiency; OAT deficiency; X-linked adrenoleukodystrophy (ALD); adrenomyeloneuropathy (AMN); Niemann-Pick disease, types A and B; neuronal ceroid lipofuscinosis |
| Coloboma | Pyrimidine biosynthesis disorders; congenital disorders of glycosylation |
| Abnormal eye movements | Sepiapterin reductase deficiency; dihydrofolate reductase (DHFR) deficiency; Gaucher disease; aromatic l -amino acid decarboxylase deficiency; dihydropyrimidine dehydrogenase deficiency; deoxyguanosine kinase deficiency |
| Photodermatitis, photophobia, photosensitivity | Hartnup disease; tyrosinemia type II; glutamate transporter defect; cystinosis; porphyric syndromes |
| Retinal detachment, dystrophy | OAT deficiency; congenital disorders of glycosylation; muscle phosphoglycerate kinase deficiency; Zellweger syndrome; Hunter disease (mucopolysaccharidosis type II); neuronal ceroid lipofuscinosis (Batten disease) |
| Retinitis pigmentosa, chorioretinal degeneration | Zellweger syndrome; Refsum disease; neuropathy ataxia and retinitis pigmentosa; OAT deficiency |
| Cherry red spot | GM1 gangliosidosis; galactosialidosis; Sandhoff disease; Tay-Sachs disease; sialidosis |
| Ectopia lentis | Homocystinuria (cystathionine-β-synthetase deficiency) |
| Lipemia retinalis | Lipoprotein lipase deficiency |
| Lens changes, dislocation | Molybdenum cofactor deficiency, types A and B; glycogen storage disease IIb |
| Myopia | Congenital disorders of glycosylation; glycogen storage disease IIb; homocystinuria (cystathionine-β-synthetase deficiency); OAT deficiency |
| Loss of central vision, night vision, or peripheral retinal pigment | Leber hereditary optic neuropathy (LHON); maternally inherited mitochondrial dystonia; Refsum disease; glycogen storage disease IIb |
| Ocular flutter, ocular hypertelorism, oculogyric crises, oculomotor apraxia | Dopamine transporter deficiency; prolidase deficiency; tyrosine hydroxylase deficiency; dopamine-serotonin vesicular transporter defect, ataxia with oculomotor apraxia 1 (AOA1) |
| Microphthalmia | Rhizomelic chondrodysplasia punctata 2 (female); congenital disorders of glycosylation |
| Poor visual fixation | Congenital disorders of glycosylation; aromatic l -amino acid decarboxylase deficiency; peroxisomal and mitochondrial fission defect |
Cardiomyopathy, both dilated and hypertrophic, may develop in IEM associated with infiltrative (storage) disorders and deficits of energy metabolism. The presence of hepatomegaly and other signs of systemic involvement (e.g., cataracts, coarse facies, renal disease, dysostosis multiplex) may suggest storage disorders of mucopolysaccharide mobilization.
Some disorders may have both a young and a later age of onset, or follow an atypical course. Allelic mutations with partial enzyme activity, or organ-specific expression, may underlie these presentations. Examples include acid maltase deficiency (muscle weakness and respiratory problems without cardiomyopathy), FAO deficits (myoglobinuria and rhabdomyolysis post-exercise) ( ), X-linked adrenomyeloneuropathy (spastic paraparesis associated with spinal cord and peripheral nerve demyelination), glycogen brancher enzyme deficiency (adult polyglucosan body disease with progressive motor neuron disease, sensory loss, neurogenic bladder, and dementia), and AIP (abdominal pain, psychosis). The myopathy associated with statin use may suggest that the use of these drugs in disorders of FAO is contraindicated ( ).
Assessment of the l -carnitine profile (total and esterified carnitine levels in plasma) may be useful when suspecting a primary or secondary carnitine deficiency, as well as defects of fat oxidation and organic acidurias. Carnitine plays an essential role in the transfer of long-chain fatty acids across the inner mitochondrial membrane, in the detoxification of acyl moieties, and in the maintenance of free coenzyme A (CoA) levels. Primary carnitine deficiency (due to defects in the OCTN2 gene associated with defective carnitine transport) leads to cardiac and skeletal muscle disease. Secondary carnitine deficiency occurs in several IEM and is often responsive to oral l -carnitine supplementation that enhances detoxification of accumulated acyl-CoA species and excretion of toxic organic acid species as acylcarnitine analogues.
Tandem mass spectrometry (TMS) analysis of blood spots on filter paper is an effective means of screening for some defects of amino and organic acid metabolism, and FAO defects, among others ( ) ( ![]() eTable 91.6 ). The massive expansion of NBS using TMS has taken advantage of the numerous acylcarnitine analogues associated with multiple disorders, which affords specific identification of disease patterns ( ). Methods for screening of LSDs by TMS have also been recently introduced ( ).
eTable 91.6 ). The massive expansion of NBS using TMS has taken advantage of the numerous acylcarnitine analogues associated with multiple disorders, which affords specific identification of disease patterns ( ). Methods for screening of LSDs by TMS have also been recently introduced ( ).
| Core Condition | Organic AcidDisorder | Fatty Acid Oxidation Disorder | Amino Acid Disorder | Carbohydrate, Endocrine, or Other Disorders |
|---|---|---|---|---|
| Propionic acidemia | X | |||
| Methylmalonic acidemia (methylmalonyl-CoA mutase) | X | |||
| Methylmalonic acidemia (cobalamin disorders) | X | |||
| Isovaleric acidemia | X | |||
| 3-Methylcrotonyl-CoA carboxylase deficiency | X | |||
| 3-Hydroxy-3-methyglutaric aciduria | X | |||
| Holocarboxylase synthase deficiency | X | |||
| β-Ketothiolase deficiency | X | |||
| Glutaric acidemia type I | X | |||
| Carnitine uptake defect/carnitine transport defect | X | |||
| Medium-chain acyl-CoA dehydrogenase deficiency | X | |||
| Very long-chain acyl-CoA dehydrogenase deficiency | X | |||
| Long-chain l -3 hydroxyacyl-CoA dehydrogenase deficiency | X | |||
| Trifunctional protein deficiency | X | |||
| Argininosuccinic aciduria | X | |||
| Citrullinemia, type I | X | |||
| Maple syrup urine disease | X | |||
| Homocystinuria | X | |||
| Classic phenylketonuria | X | |||
| Tyrosinemia, type I | X | |||
| Congenital adrenal hyperplasia | X | |||
| Biotinidase deficiency | X | |||
| Classic galactosemia | X |
∗ United States of America: IEM that are core conditions on the recommended uniform screening panel, as of April 2013.
Histological examination of appropriate tissue samples can provide important insights into the nature of the storage materials found in certain IEM (e.g., lysosomal disorders) ( ). When performing a skin biopsy, it is advisable to obtain samples for microscopic examination in addition to tissue culture. These cultured cells are useful as source material for subsequent biochemical or molecular (genetic) testing. For disorders of amino and organic acids, skin fibroblasts allow confirmatory diagnosis by enzymatic assays. Many laboratories certified to provide clinical biochemical genetic testing are moving away from complex enzymology for diagnostic confirmation, primarily related to expense, complexity, and poor cost recovery issues with insurance. The gold standard for diagnoses has more recently turned to molecular genetic testing ( ), and the latter has been considerably expanded to deep-genomic and whole exome sequencing (WES) ( ). Biopsy of various tissues (liver, heart, and muscle) is necessary for diagnosis of selected diseases (e.g., glycogen storage, disorders of oxidative phosphorylation). Lastly, microscopic examination of scalp hair can provide important diagnostic information, such as Menkes syndrome (pili torti) ( ).
Careful attention to sample requirements and shipping and handling considerations is key for optimal testing outcomes. Clinical information is required to receive guidance in both testing interpretation and patient evaluation. In cases with an established diagnosis, detailed information may be obtained from several websites, including Gene Reviews ( https://www.ncbi/nlm/nih/gov ), Online Mendelian Inheritance in Man (OMIM, < http://www.ncbi.nlm.nih.gov/OMIM >), GeneClinics (< http://www.geneclinics.org >), the National Organization for Rare Disorders (NORD, < http://www.rarediseases.org >), the Online Metabolic and Molecular Bases of Inherited Disease (< ommbid.mhmedical.com >), the Society for Inherited Metabolic Disorders (< http://www.simd.org >), and the Society for the Study of Inborn Errors of Metabolism (< http://www.ssiem.org >). Several patient advocacy support groups provide information about community resources and ongoing clinical trials (e.g., the National PKU Alliance [< http://www.npkua.org >], the Organic Acidemia Association [< http://www.oaanews.org >], the MSUD Family Support Group [< http://www.msud-support.org >], the SSADH Foundation [< http://www.ssadh.net >], and many others).
Molecular genetic techniques offer an alternative means for the diagnostic confirmation of IEM, especially in those instances in which a biochemical or enzymatic diagnostic test is unavailable or requires invasive approaches. This is particularly true for diseases with a founder effect —common mutations in which one or a few alleles account for a significant proportion of cases (e.g., Finnish and Jewish “heritage” diseases). In diseases with a known causal mutation, testing of other family members permits accurate carrier identification, but significant ethical considerations arise and certified genetic counselors should be involved. DNA testing for prenatal diagnosis provides rapid diagnosis in relation to the use of chorionic villi or amniocytes which do not require cell culture. Molecular diagnostic analyses are not completely foolproof, and several caveats exist. For example, when two mutant alleles are identified in an autosomal-recessive disorder, are they on the same chromosome or different chromosomes (e.g., cis or trans )? Studies in parental samples usually help to clarify this issue. Additionally, is the disease predicted from the identified alleles 100% penetrant (e.g., the penetrance of a disease-causing mutation is the proportion of individuals with the mutation who exhibit clinical symptoms)? Caution should always be applied in assignment of causality, particularly for novel mutations or sequence alterations for which functional impacts have not been established ( ).
Examples of disorders for which DNA testing has proven useful include medium-chain acyl-CoA dehydrogenase (MCAD) deficiency ( ), Gaucher disease ( ), and classical MSUD in the old-order Amish ( ). Among patients with MCAD deficiency of northwestern European descent, ∼80% are homozygous for a single missense mutation (A985G), and 17% carry this mutation in combination with another less common allelic alteration. This finding has improved the reliability of MCAD carrier identification and diagnosis, particularly for siblings who may be affected but asymptomatic at the time of family screening. Certain metabolic disorders (e.g., Tay-Sachs disease, Gaucher disease, Niemann-Pick disease type A, and Canavan disease) have an increased prevalence among individuals of Ashkenazi Jewish ancestry (i.e., of Central and Eastern European descent). A limited number of “common” mutations in this population cause the disease, which facilitates targeted screening for appropriate genetic counseling prior to marriage and conception. A number of scanning and screening methodologies exist for molecular genetic analyses in DNA, including polymerase chain reaction (PCR) amplification, discontinuous gradient gel electrophoresis (DGGE), restriction digestion analyses (in which a putative allelic alteration either induces a new restriction site or removes a preexisting site), and dot-blot analyses (using radiolabeled or chemiluminescent-labeled oligonucleotides to bind to a DNA region harboring the mutation). Nonetheless, direct sequencing remains the gold standard ( ). More recent DNA sequencing methods have expanded the molecular diagnostic menu, including multiplex ligation-dependent probe amplification (MLPA) and WES ( ).
Generally, mutations in IEM associate with loss of function, but there are instances in which mutations can result in gain of function, as well as superactivity of enzyme function that can be deleterious. For example, heterozygous somatic mutations in the isocitrate dehydrogenase isozyme 2 that disable the enzyme to catalyze its normal reaction (isocitrate conversion to 2-oxoglutarate) confer a new function: namely, conversion of 2-oxoglutarate to d -2-hydroxyglutarate ( ). Alternatively, specific mutations in the phosphoribosyl pyrophosphate synthetase isoform 1 (PRPP synthetase 1) result in superactivity of the enzyme and the induction of a severe form of hyperuricemia and gout ( ).
A number of IEM manifest with hearing dysfunction ( ![]() eTable 91.7 ). For example, sensorineural deafness is a prominent finding in the LSDs, peroxisomal and mitochondrial diseases, and the congenital disorders of glycosylation (CDG) ( ). Recurrent otitis media in the mucopolysaccharidoses reflects the storage pathophysiology associated with glycosaminoglycan accumulation ( ), while conductive deafness may be prominently associated with CDG and the pyrimidine biosynthetic disorders, the latter including dihydroorotate dehydrogenase deficiency ( ). It may be prudent, therefore, for the clinician confronted with idiopathic deafness or hearing loss to consider an IEM in the differential diagnosis.
eTable 91.7 ). For example, sensorineural deafness is a prominent finding in the LSDs, peroxisomal and mitochondrial diseases, and the congenital disorders of glycosylation (CDG) ( ). Recurrent otitis media in the mucopolysaccharidoses reflects the storage pathophysiology associated with glycosaminoglycan accumulation ( ), while conductive deafness may be prominently associated with CDG and the pyrimidine biosynthetic disorders, the latter including dihydroorotate dehydrogenase deficiency ( ). It may be prudent, therefore, for the clinician confronted with idiopathic deafness or hearing loss to consider an IEM in the differential diagnosis.
| Hearing Abnormality | Associated Inborn Error of Metabolism |
|---|---|
| Sensorineural deafness | Adenosine kinase deficiency; adrenomyeloneuropathy (AMN); biotinidase deficiency; Brown-Vialetto-van Laere syndrome; cerebral folate deficiency; coenzyme Q 6 deficiency; congenital disorders of glycosylation (CDG); 2-oxoglutaric aciduria; succinate-CoA ligase α-subunit deficiency; Gracille syndrome; MEGDEL syndrome; MELAS (mitochondrial encephalomyopathy, lactic aciduria, stroke) syndrome; maternally inherited deafness and diabetes; 2-methyl-3-hydroxybutyryl-CoA dehydrogenase (MHBD) deficiency; Mohr-Tranebjaerg syndrome; optic atrophy type 1 and deafness; mitochondrial depletion syndromes; aminoglycoside-induced deafness; Zellweger syndrome; rhizomelic chondrodysplasia punctate (RCDP) 1; mannosidosis; mucopolysaccharidoses; propionic acidemia; progressive familial intrahepatic cholestasis type 1 (ATP8B1 deficiency); Smith-Lemli-Opitz syndrome; prenyl diphosphate synthase, subunit 1 (PDSS1) deficiency; PRPP (phosphoribosyl 5’-pyrophosphate) 1 synthetase superactivity; Rogers syndrome (thiamine-responsive megaloblastic anemia, TRMA); X-linked adrenoleukodystrophy (X-ALD) |
| Recurrent otitis media | Prolidase deficiency; mucolipidosis II, III; Hurler syndrome, Hurler-Scheie syndrome (mucopolysaccharidosis type 1); Hunter syndrome (mucopolysaccharidosis type 2); Maroteaux-Lamy syndrome (mucopolysaccharidosis type 6). |
| Conductive deafness | Dihydroorotate dehydrogenase deficiency; congenital disorders of glycosylation (CDG) |
Several IEM disrupt the normal sequence of brain development and lead to multiple anomalies, including agenesis or dysgenesis of the corpus callosum, neuronal migration defects, and dysmyelination ( ) ( Table 91.8 ; eTables 91.9–91.13 ![]() ). Cystic necrosis of white matter, with or without basal ganglia involvement, occurs in deficiencies of pyruvate dehydrogenase (PDH), PC, and molybdenum cofactor. Nonsyndromic congenital microcephaly has been associated with maternal PKU, phosphoglycerate dehydrogenase deficiency (a serine biosynthetic disorder), and 2-ketoglutaric aciduria (associated with Amish lethal microcephaly). Multiple mechanisms have been proposed to explain abnormal brain development and encephaloclastic lesions (such as porencephalic cysts) in IEM, including production of a toxic or energy-deficient intrauterine milieu, modification of the content and function of membranes, and disturbance of the normal expression of intrauterine genes responsible for neurulation and neuronal migration ( ).
). Cystic necrosis of white matter, with or without basal ganglia involvement, occurs in deficiencies of pyruvate dehydrogenase (PDH), PC, and molybdenum cofactor. Nonsyndromic congenital microcephaly has been associated with maternal PKU, phosphoglycerate dehydrogenase deficiency (a serine biosynthetic disorder), and 2-ketoglutaric aciduria (associated with Amish lethal microcephaly). Multiple mechanisms have been proposed to explain abnormal brain development and encephaloclastic lesions (such as porencephalic cysts) in IEM, including production of a toxic or energy-deficient intrauterine milieu, modification of the content and function of membranes, and disturbance of the normal expression of intrauterine genes responsible for neurulation and neuronal migration ( ).
| Abnormality | Associated Inborn Errors of Metabolism |
|---|---|
| Agenesis, corpus callosum | CHILD syndrome; glycine encephalopathy; pyridoxine-dependent epilepsy; pyruvate dehydrogenase complex (PDH) E1α, E1β, and E3 deficiencies; combined oxidative phosphorylation defect 2 (combined with dysmorphism and fatal lactic acidosis); S-adenosylmethionine (SAM) hydrolase deficiency; Smith-Lemli-Optiz (SLO) syndrome; chondrodysplasia punctate 2 (male); desmosterolosis; 3-hydroxyisobutyryl-CoA deacylase deficiency; congenital disorders of glycosylation (CDG) |
| Atrophy, corpus callosum | Hyperprolinemia; mucolipidosis II; pyrroline-5˝-carboxylate synthetase deficiency |
| Atrophy, cerebellum | CDG; succinic semialdehyde dehydrogenase (SSADH) deficiency |
| Atrophy, cerebrum | CDG; cerebral folate deficiency; maple syrup urine disease; 3-methylcrotonyl-CoA carboxylase deficiency; 3-methylglutaconyl-CoA hydratase deficiency (type I methylglutaconic aciduria); Costeff syndrome; 3-methylglutaconic acidurias (idiopathic forms); 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) lyase deficiency; 3-phosphoglycerate dehydrogenase deficiency; propionic acidemia; methylmalonic acidemia; molybdenum cofactor deficiency; cobalamin disorders; coenzyme Q 10 deficiency; CDG; SSADH deficiency; dihydropyrimidine dehydrogenase deficiency; deoxyguanosine kinase deficiency; mucolipidosis II, III |
| Atrophy, combined cerebrum and cerebellum | CDG; sulfite oxidase deficiency; MEGDEL syndrome; folate receptor (FOLRI) deficiency; combined saposin deficiency; neuronal ceroid lipofuscinosis (Batten disease); SLC33A1 deficiency with low plasma copper and ceruloplasmin; SSADH |
| Atrophy, frontotemporal | 2-Methyl-3-hydroxybutyryl-CoA dehydrogenase (MHBD) deficiency; malonic aciduria |
| Atrophy, striatal | Glutaric aciduria type I (GA-I) |
| Cerebral infarction | Fabry disease; 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) lyase deficiency; oxidative-phosphorylation (OXPHOS) disorders (e.g., MELAS) |
| Cerebral white matter lesions | 3-Phosphoglycerate dehydrogenase deficiency; l-2-hydroxyglutaric aciduria; Zellweger syndrome; ribose-5’-phosphate isomerase deficiency; SSADH deficiency |
| Dysplasia, cerebellum | CDG; glutaric acidemia II; Menkes disease; Refsum (infantile) disease; Pyruvate dehydrogenase complex (PDHC) deficiency; peroxisomal diseases (neonatal pseudo-ALD, bifunctional enzyme deficiency); respiratory chain enzyme deficiency; SLO syndrome; Zellweger syndrome |
| Holoprosencephaly | SLO syndrome |
| Hypopmyelination | SAM hydrolase deficiency; serine deficiency disorders; AGC1 deficiency (global cerebral hypomyelination); folate receptor-α deficiency; mucolipidosis II; multiple sulfatase deficiency; SLC33A1 deficiency with low plasma copper and ceruloplasmin |
| Hypoplasia, pons | SAM hydrolase deficiency |
| Hypoplasia, unilateral | CHILD syndrome |
| Hypoplasia, temporal | GA-I; |
| Hypoplasia, cerebellar | CDG; cerebral folate deficiency; glutamine synthetase deficiency; neonatal mitochondrial encephalocardiomyopathy; SAM hydrolase deficiency; adenylosuccinate lyase deficiency; |
| Hypoplasia, cerebellar vermis | CDG; phosphoserine aminotransferase deficiency; phosphoserine phosphatase deficiency |
| Lissencephaly/pachygyria | 3-OH-isobutyric aciduria; bifunctional enzyme deficiency, CDG; desmosterolosis; fumarase Pyruvate dehydrogenase complex (PDHC) deficiency; glutaric acidemia 2; glycine encephalopathy; PDHC deficiency; SLO syndrome; Zellweger syndrome |
∗ The listing is necessarily incomplete but meant to demonstrate notable patterns of reported findings in selected IEM.
| Disease | MRI Findings |
|---|---|
| Mucopolysaccharidoses | J-shaped sella, closed meningoceles, enlarged Virchow-Robin spaces, cerebral atrophy/communicating hydrocephalus |
| Peroxisomal disorders | Perisylvian polymicrogyria, germinolytic cysts |
| Leigh syndrome (neonatal form) | Triventricular hydrocephalus, hypoplasic cerebellum |
| Pyruvate carboxylase deficiency | Subependymal cysts |
| Glutaric aciduria type I | Poor opercularization (“batwing” appearance), subdural collections |
| d -2-hydroxyglutaric aciduria | Batwing appearance, subdural collections, incomplete gyration, especially in the posterior regions |
| Menkes disease | Increased vessel tortuosity, subdural collections |
| Methylenetetrahydrofolate reductase deficiency (MTHFR) | Hydrocephalus, microgyria, dilated cerebral vessels |
| Isolated sulfite oxidase deficiency | Cystic-like diffuse white-matter lesions (neonatal hypoxia-like) |
| Disease | MRI Findings |
|---|---|
| GM1 gangliosidosis | T1-weighted hyperintensity (especially in type 1) |
| GM2 gangliosidosis | T1-weighted hyperintensity (especially in Sandhoff disease) |
| Fabry disease | Pulvinar T1-weighted hyperintensity in males (may occur in females, less hyperintense) |
| Fucosidosis | T1-weighted hyperintensity, T2-weighted hypointensity |
| Canavan disease | T2-weighted hyperintensity in the thalamus (and globus pallidus) |
| Wilson disease | T2-weighted hyperintensity |
| Disease | Anatomical Involvement |
|---|---|
| Leigh syndrome (neonatal form) | Cerebellar white matter |
| Cerebrotendinous xanthomatosis | Dentate nucleus calcifications, cerebellar atrophy |
| l-2-Hydroxyglutaric aciduria | Dentate nucleus, cerebellar atrophy, especially in the vermis |
| Succinic semialdehyde dehydrogenase deficiency | T2-weighted hyperintensity of dentate nucleus |
| Disease | MRI Findings |
|---|---|
| GM2 gangliosidoses | T1-weighted hyperintensity, especially in Sandhoff disease |
| Fucosidosis | T1-weighted hyperintensity and T2-weighted hypointensity in the globus pallidus |
| Glutaric aciduria type I | T2-weighted hyperintensity in the basal ganglia |
| Canavan disease | T2-weighted hyperintensity in the globus pallidus (and in the thalamus) |
| l -2-hydroxyglutaric aciduria | T2-weighted hyperintensity in the basal ganglia |
| Isolated sulfite oxidase deficiency | T2-weighted hyperintensity in the globus pallidus |
| Succinic semialdehyde dehydrogenase deficiency | T2-weighted hyperintensity in globus pallidus, subthalamic nucleus |
| Wilson disease | T2-weighted hyperintensity in the basal ganglia |
| Adrenoleukodystrophy |
| Canavan disease |
| Galactosemia |
| Glutaric aciduria type I |
| l -2-hydroxyglutaric aciduria |
| Leigh disease, mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes |
| Maple syrup urine disease |
| Megalencephalic leukoencephalopathy |
| Menkes disease |
| Metachromatic leukodystrophy |
| Pelizaeus-Merzbacher disease |
| Phenylketonuria |
| Rhizomelic chondrodysplasia punctata |
| Succinic semialdehyde dehydrogenase deficiency |
| Tay-Sachs disease |
Magnetic resonance imaging (MRI) is widely employed to obtain brain anatomical data in patients with static and progressive encephalopathy. Advanced imaging techniques such as MR spectroscopy (MRS) and diffusion-weighted imaging (DWI) provide additional insights into brain biochemistry and cell viability, and may highlight diagnostic criteria ( ). For example, MRI shows great utility in the diagnosis of pantothenate kinase-associated neurodegeneration (PKAN) associated with brain iron accumulation, in relation to the characteristic “eye-of-the-tiger” pattern that is observed ( ). MRS and DWI represent quantitative techniques that may prove useful in assessing disease severity and potential response to treatment. MRS may even afford a diagnostic pattern: that is, increased gamma-aminobutyrate (GABA) peak in GABA-transaminase deficiency ( ).
White-matter signal abnormalities suggestive of leukodystrophy can be found in several IEM, including Krabbe disease, metachromatic leukodystrophy (MLD), X-linked adrenoleukodystrophy (ALD), and Canavan disease. In addition to signal hyperintensities noted on T2-weighted imaging, selected features may provide additional diagnostic clues. For example, enlarged perivascular spaces or small cysts are characteristic of the mucopolysaccharidoses and Lowe syndrome. Gray-matter lesions may be found in Zellweger syndrome and other peroxisomal disorders. In glutaric aciduria type 1, imaging may show selective frontotemporal atrophy, especially involving subcortical white matter, accompanied by prominent extra-axial CSF collections and widening of the sylvian fissure with poor opercularization (“bat wing” appearance) ( ). The presence of hyperintense lesions in the subcortical cerebral white matter, basal ganglia, and dentate nuclei, together with cerebellar atrophy, is strongly suggestive of l -2-hydroxyglutaric aciduria ( ). The latter is of interest in comparison to patients with combined d / l -2-hydroxyglutaric aciduria and isolated d -2-hydroxyglutaric aciduria, in which the phenotype features developmental delay, hypotonia, epilepsy, and cardiomyopathy without extensive imaging abnormalities ( ).
The creatine deficiency syndromes, featuring significant developmental delays, hypotonia, and extrapyramidal movement abnormalities, include guanidinoacetate methyltransferase (GAMT), arginine: glycineamidinotransferase (AGAT), and creatine transporter deficiencies. All feature cerebral creatine deficiency detected through MRS ( ). A secondary disruption in creatine levels is observed in ornithine aminotransferase (OAT) deficiency, in which the brain creatine:phosphocreatine ratio is decreased. Brain MRS reveals elevated levels of N -acetylaspartate (NAA) in patients with Canavan disease, whereas patients with mitochondrial defects and defects of gluconeogenesis may show elevated brain lactate. In MLD, MRS reveals decreased NAA and increased choline and myoinositol, compatible with axonal loss, dysmyelination, and gliosis.
A metabolic autopsy is recommended when a patient develops acute fatal metabolic decompensation, and in cases of sudden and unexpected death ( ). A correct diagnosis facilitates appropriate counseling and prenatal diagnosis for subsequent pregnancies. Blood and bile (obtained by direct puncture of the gall bladder) specimens should be collected, spotted on filter paper (two circles for each, about 25 μL), and dried prior to storage. Plasma should be separated from whole blood and frozen, as should urine. A portion of liver tissue should be obtained, rinsed with phosphate buffered saline (PBS), and deep frozen without preservative at −80°C. A skin sample should be obtained under sterile technique (use alcohol and not iodine, which interferes with cell growth), and stored at room temperature in tissue culture medium. When suspecting a storage disorder, obtain a small snip of skin and place it in glutaraldehyde for subsequent electron microscopic studies. Excellent guidelines exist for the metabolic autopsy (< http://www.sudc.org/Portals/0/medical/Mayo_Metabolic_Protocol_2009.pdf >).
The appropriate management of IEM depends upon the particular metabolic and/or molecular derangement. Therapeutic strategies may include one or more of the following approaches: (1) substrate reduction by dietary manipulation or precursor synthesis inhibition; (2) removal (or enhanced clearance) of the toxic metabolite(s); (3) replenishment of depleted metabolites or cofactor supplementation (or both), (4) enzyme (replacement or enhancement via chaperone intervention) therapy; and (5) cell or organ replacement (e.g., bone marrow, liver, heart, or kidney transplantation, hepatocyte transplantation) ( ). Gene therapy is rapidly progressing, with trials recruiting or completed in aromatic amino acid decarboxylase (AADC) deficiency, MLD, Tay-Sachs, Pompe, and Fabry diseases (< http://www.clinicaltrials.gov >). Gene therapeutic approaches are accepted interventions in severe combined immunodeficiency (SCID) and adenosine deaminase deficiency. Numerous gene therapy protocols are active in a number of murine models of IEM, including PKU, Canavan disease, l -AADC deficiency, and others ( ). Liver repopulation (hepatocyte transplant) is feasible, but challenges with early engraftment and long-term expansion remain, and this approach is predominantly useful for instances of liver failure and bridging to eventual liver transplant ( ). Chaperone-mediated therapy for IEM associated with residual enzyme activity is under active examination, especially the utility of cofactors such as sapropterin (tetrahydrobiopterin) in helping to rescue misfolded proteins and enhance enzyme activity in phenylalanine hydroxylase and other proteins ( ). Drug repurposing is gaining increasing interest, such as use of the compound NTBC (see later; employed in patients with tyrosinemia type I) for possible intervention in PKU to increase brain dopamine ( ), or the utility of phenylbutyrate (employed in urea cycle disorders to regulate blood ammonia) to alter the regulation of branched-chain ketoacid dehydrogenase in MSUD (NCT01529060; < http://www.clinicaltrials.gov >). The potential to transplant stem cells with directed differentiation potential remains a very active avenue of research ( ).
Each patient requires an individualized approach, and some disorders require more than one management option. For patients with IEM, generally the “team” approach is necessary, with collaboration between medical geneticist, the biochemical genetics laboratory, genetic counselor, and other specialties as needed. The clinical response to most treatment plans may vary, and residual disturbances are common. Patients may remain at risk for metabolic decompensation when stressed (infection, trauma, surgery, vaccination). Reducing energy expenditure and promoting anabolism are immediate management goals in almost all instances. Emergency measures may prevent further deterioration, but most options are nutritionally incomplete, and extension beyond 48 hours without dietary review is often not prudent. In most situations, provision of symptomatic treatment may necessitate the need for specialized care units with expertise in the specific disease.
Special diets require attention to caloric requirements and balanced nutrition; this is particularly true for minerals and supplements, trace metals, and other parameters. Diseases managed with dietary restriction include phenylketonuria (PKU), MSUD, and homocystinuria. Nonetheless, despite excellent metabolic control, patients with PKU and MSUD may still manifest long-term neurocognitive deficits ( ). Other disorders, such as serine, glutamine, and proline biosynthesis disorders, benefit from recognition and supplementation ( ). Multiple clinical studies suggest that treatment of PKU patients with sapropterin (a cofactor of phenylalanine hydroxylase) provides better disease control and increases dietary phenylalanine tolerance for selected patients, allowing relaxation of dietary phenylalanine restriction ( ). In classic Refsum disease, reduction in dietary phytanate results in significant attenuation of the biochemical and clinical phenotype.
Selected IEM require alternative dietary sources. Medium-chain triglycerides may be administered as a lipid source to patients with very-long-chain acyl-CoA dehydrogenase (VLCAD) and long-chain acyl-CoA dehydrogenase (LCHAD) deficiencies. In Smith-Lemli-Opitz syndrome (SLOS) (7-dehydrocholesterol reductase deficiency), the use of cholesterol supplementation improves somatic parameters and may benefit neurodevelopmental status, although clinical response is variable. In the GSDs, carbohydrate supplementation (usually in the form of cornstarch) prevents hypoglycemia and suppresses secondary metabolic derangement, such as hyperlipidemia and hyperuricemia. In the disorders of the urea cycle, arginine or citrulline supplementation can prime the cycle via replenishment of intermediates not synthesized secondarily to the metabolic block.
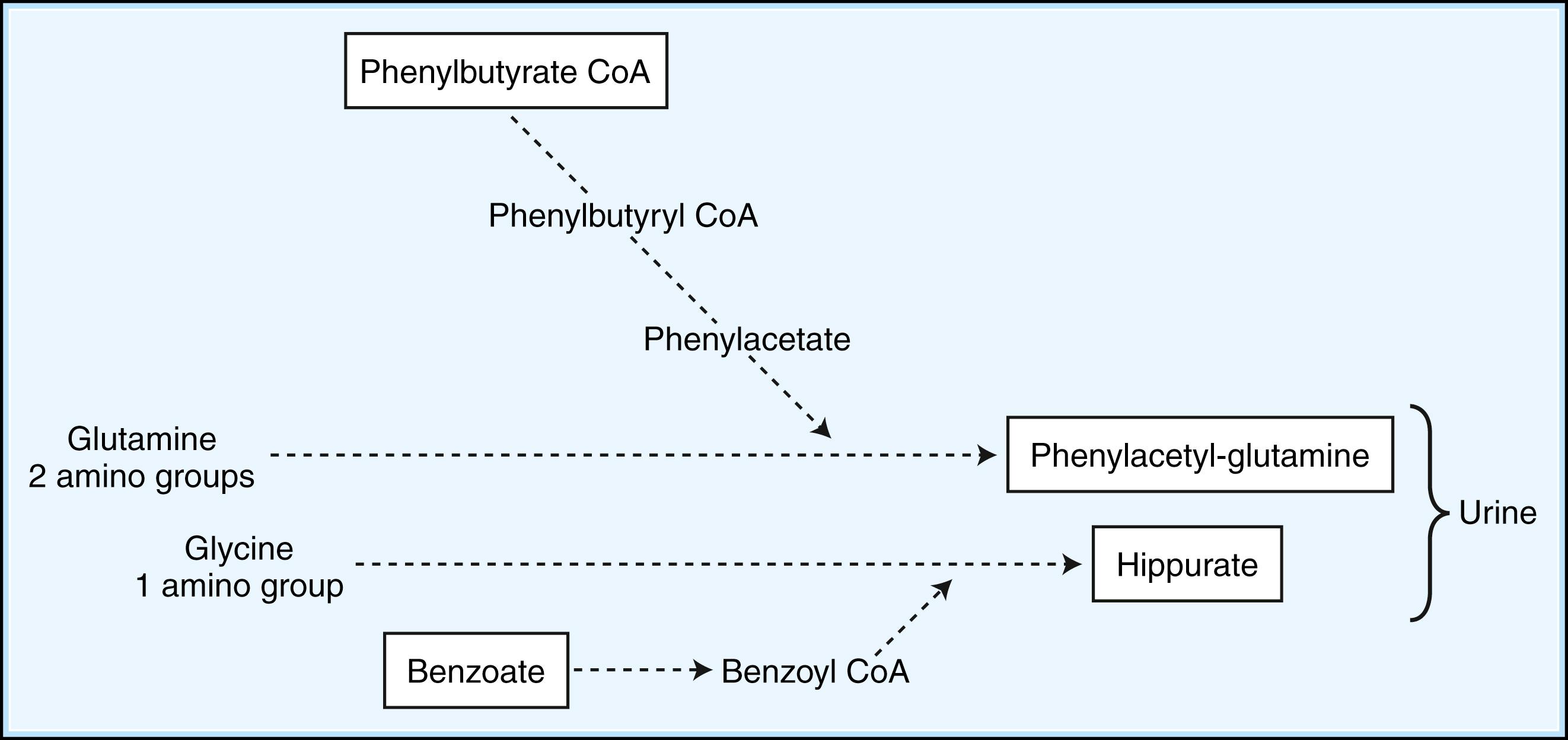
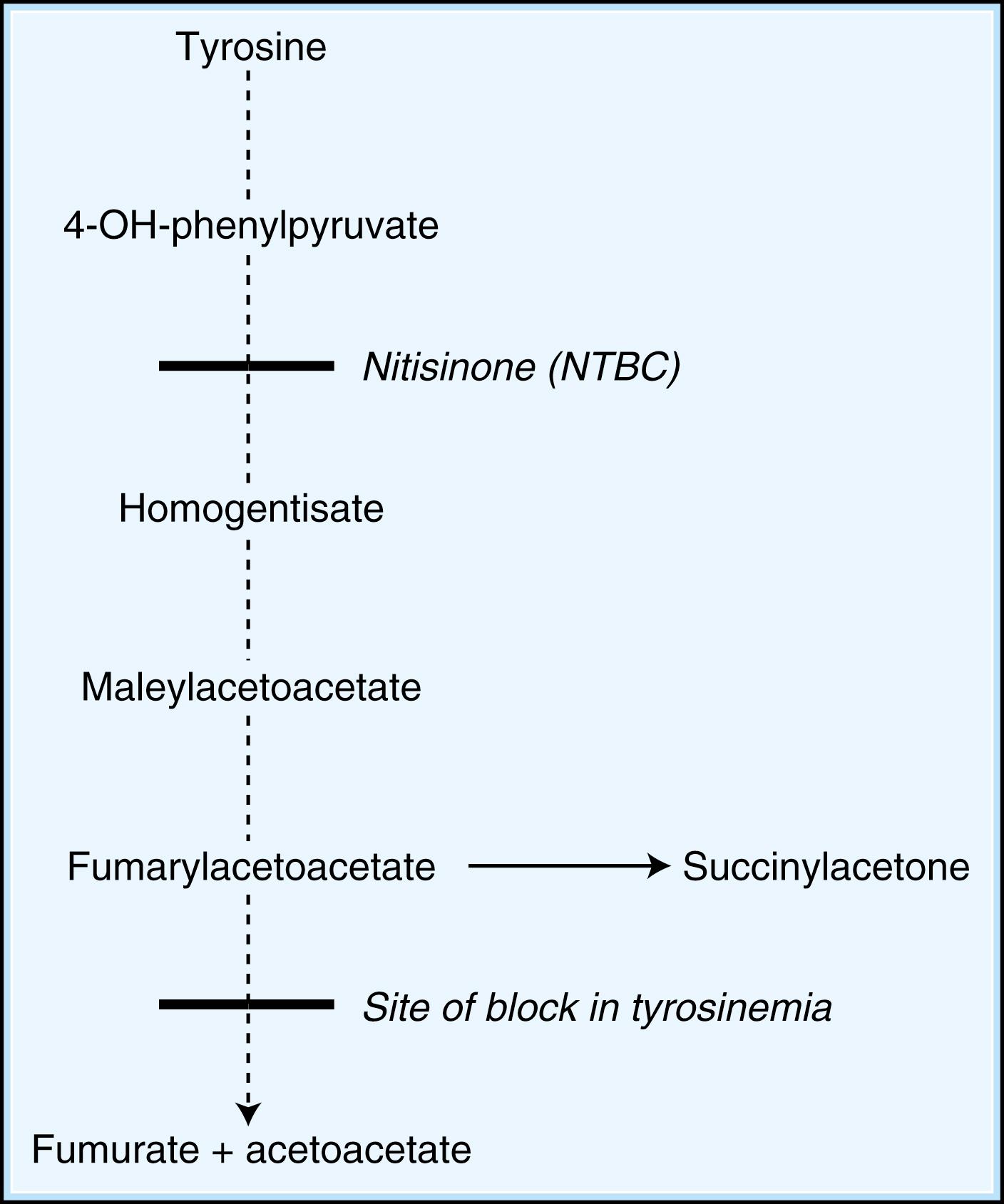
When dietary manipulation fails to correct the accumulation of toxic metabolites, the primary clinical approach is to utilize methods to enhance their excretion or detoxification. For example, in patients with hyperammonemia, sodium benzoate and sodium phenylbutyrate may be orally supplemented, which conjugate with glycine and glutamine, respectively, to facilitate nitrogen excretion ( Fig. 91.3 ). Clinical care must be taken to monitor sodium intake with these supplements, however. In IVA, treatment with oral glycine results in enhanced conjugation and excretion of isovalerylglycine. Administration of cysteamine to patients with cystinosis promotes the formation of cysteine, which is subsequently excreted. Cysteamine has also been piloted in the treatment of neuronal ceroid lipofuscinosis ( ). Carnitine supplementation given to patients with organic acidemia prevents carnitine deficiency secondary to the excretion of acylcarnitine species, while simultaneously repleting intracellular concentrations of CoA. During acute metabolic decompensation, dialysis and hemofiltration facilitate the rapid clearance of toxic metabolites. These techniques are already a part of the treatment of multiple IEM, especially the urea cycle disorders and the ensuant hyperammonemia encountered with decompensation ( ). In X-linked ALD, administration of glycerol trioleate and trierucate (Lorenzo’s oil) to asymptomatic patients may modify the disease course and progression ( ). A large number of novel therapeutic strategies for IEM employ substrate synthesis inhibitors to block the production of toxic metabolites ( Fig. 91.4 ). An excellent example is the use of NTBC (2-nitro-4-trifluoro-methylbenzoyl-1,3-cyclohexanedione) in tyrosinemia type I (fumarylacetoacetate hydrolase deficiency), a defect in the distal tyrosine metabolic pathway. Via blockade of an early step of tyrosine metabolism, NTBC administration prohibits the production of cytotoxic succinylacetone and fumarylacetoacetate, which are associated with the development of hepatocellular carcinoma. For tyrosinemia type I, NTBC is a life-saving intervention ( ). Miglustat, an iminosugar that inhibits glycosphingolipid biosynthesis in a reversible fashion, has shown clinical utility (improved peripheral organ and hematological parameters) in a variety of glycosphingolipid storage disorders, including Gaucher disease and Niemann-Pick C disease ( ). It has also been piloted in Tay-Sachs and Sandhoff diseases, cystic fibrosis, and GM2 gangliosidosis.
Replenishing depleted substrates may significantly correct the underlying defect in some IEM. In carnitine transport defects, l -carnitine supplementation can resolve cardiomyopathy and prevent further episodes of hypoketotic hypoglycemia. In other disorders, the production or binding affinity of a cofactor required for enzyme function is impaired. Administration of pharmacological doses of the required supplement corrects the defect. This is most evident in the vitamin B 6 -dependent disorders ( ) ( ![]() eTable 91.14 ), thiamine- and riboflavin-responsive defects, as well as the vitamin B 12 - and biotin-dependent disorders ( ). In selected PKU patients, administration of pharmacological doses of tetrahydrobiopterin (BH4) may be beneficial in reducing the associated hyperphenylalaninemia ( ). Pharmacological doses of tetrahydrofolate may be therapeutic in patients with folate deficiency, selected disorders of methionine, glycine, and homocysteine metabolism, and dihydropteridine reductase deficiency ( ).
eTable 91.14 ), thiamine- and riboflavin-responsive defects, as well as the vitamin B 12 - and biotin-dependent disorders ( ). In selected PKU patients, administration of pharmacological doses of tetrahydrobiopterin (BH4) may be beneficial in reducing the associated hyperphenylalaninemia ( ). Pharmacological doses of tetrahydrofolate may be therapeutic in patients with folate deficiency, selected disorders of methionine, glycine, and homocysteine metabolism, and dihydropteridine reductase deficiency ( ).
| Disorder | Alternative Name | Abbreviation | Gene Symbol | Chromosomal Localization | Affected Protein | OMIM No. |
|---|---|---|---|---|---|---|
| Vitamin B 6 -Dependent and Responsive Disorders | ||||||
| Pyridoxine-dependent epilepsy (PDE) | Alpha-amino adipic semialdehyde (AASA) dehydrogenase deficiency | AASADHD | ALDH7A1 | 5q31 | Alpha-amino adipic semialdehyde dehydrogenase | 266100 |
| Pyridox(am)ine 5′-phosphate oxidase deficiency | Pyridoxal 5′-phosphate (PLP)-dependent seizures | PNPO deficiency | PNPO | 17q21.32 | Pyridox(am)ine 5’-phosphate oxidase | 610090 |
| Hyperprolinemia type II | Pyrroline-5-carboxylate dehydrogenase deficiency | P5CDH | ALDH4A1 | 1p36.13 | Pyrroline-5-carboxylate dehydrogenase | 239510 |
| Congenital hypophosphatasia | Phosphoethanolaminuria | HOPS | ALPL | 1p36.12 | Alkaline phosphatase | 241500 |
| Creatine Disorders | ||||||
| Guanidinoacetate methyltransferase deficiency | GAMT | GAMT | 19p13.3 | Guanidinoacetate methyltransferase | 612736 | |
| Arginine: glycine amidinotransferase deficiency | AGAT | GATM | 15q15.3 | Arginine: glycine amidinotransferase | 612718 | |
| Creatine transporter deficiency | X-linked creatine deficiency syndrome | CrT, SLC6A8 | SLC6A8 | Xq28 | SLC6A8 transporter (CrT, creatine transporter) | 300352 |
| Ornithine aminotransferase deficiency | Gyrate atrophy of the choroid and retina | OAT, GACR | OAT | 10q26.13 | Ornithine aminotransferase | 258870 |
Enzyme replacement therapy (ERT) has rapidly expanded as a treatment option in the past decade. ERT has been piloted in the majority of the mucopolysaccharidoses, Gaucher, Fabry, and Niemann-Pick diseases, and others ( ). The utility of ERT relies on the presence of the appropriate sugar residues on the exogenously administered enzyme that can target it to, and facilitate the intake into, the appropriate cell organelle (e.g., usually the lysosome). Enzymes produced in culture or in batch processes are purified and administered via regular intravenous infusions of the recombinant protein. While benefits are substantial in relation to peripheral organ function and hematological parameters, neurological complications (if present) are generally nonresponsive due to the inability of recombinant proteins to cross the blood-brain barrier (BBB). Moreover, the annual cost of replacement therapy is substantial. A valuable adjunct to ERT, bone marrow transplant (BMT) and hematopoietic stem cell transplantation (HSCT) have been performed in patients with LSD for more than 25 years ( ). The primary goal of these approaches is to provide enzyme-replete cells. The advantage of BMT/HSCT is the capacity to impact brain function, and to potentially slow neurodegenerative outcomes. Challenges with both approaches remain, however, including donor limitation issues, procedural risks, long-term immunosuppression, and graft-versus-host disease (GVHD) ( ). Storage disorders characterized by rapid neurodegeneration, such as Hunter and Sanfilippo syndromes, were previously felt to be poor candidates for either protocol, but emerging data indicate that neurodegeneration may be slowed in these disorders employing HSCT ( ).
Organ transplantation is a concomitant therapy for several IEM, including those with a significant hepatic phenotype. The latter include Crigler-Najjar syndrome, the hyperoxalurias, ornithine transcarbamylase deficiency, and classical MSUD (primarily for patients carrying the classical Amish allele) ( ). Concomitant single-organ failure is seen in many IEM, including end-stage renal insufficiency in Fabry disease, cystinosis, and methylmalonic aciduria. Domino liver transplantation continues to gain traction in the clinic. In the latter, the diseased organ (e.g., MSUD liver) is transplanted to an individual with end-stage hepatic failure who is a very low candidate on the transplant list ( ), generating a chimeric individual with regard to enzyme function.
Symptomatic treatment remains a vital component of patient care. Indeed, several palliative measures improve quality of life and reduce the incidence and severity of disease-related complications. For instance, corticosteroid and mineralocorticoid replacement are essential in patients with ALD and adrenal insufficiency, while supplementation with l -dopa improves motor function in patients with tyrosine hydroxylase (TH) deficiency. d -Deamino-arginine-vasopressin (DDAVP) reduces the tendency for abnormal bleeding during surgery of patients with GSD type 1A (von Gierke disease), while granulocyte colony-stimulating factor (G-CSF) administered to patients with GSD IB and neutropenia minimizes the risk of recurrent bacterial infection and gastrointestinal tract ulceration. Additionally, patients with certain metabolic disorders necessitate special considerations for anesthesia and surgery. For instance, upper airway obstructive disease in patients with mucopolysaccharidosis may lead to problems during induction and extubation. Hypoglycemia must be avoided in patients with FAO defects, GSD, and disorders of gluconeogenesis, which may require careful planning in terms of the time the procedure is undertaken and maintenance of euglycemia throughout, employing intravenous administration of 10% glucose.
Finally, the importance of effective genetic counseling must be reinforced, for both the patient and the family coping with the affected patient. Since the majority of IEM are autosomal-recessive disorders, prenatal diagnosis is available for most disorders, employing either metabolite, enzymatic, or, more commonly, molecular-based diagnostics. Parents of children with IEM display higher scores on a stress index, lower scores on adaptive behavior scales, and greater anxiety concerning their child’s future and that of their composite family ( ).
Because of earlier diagnosis and intervention, many affected children with IEM have achieved longer survival. This is in large part due to the evaluation of NBS noted earlier, which has often provided diagnoses within the first week, thereby obviating major episodes of acute decompensation in the neonatal period due to an absence of diagnosis. In most instances, the provision of early patient care has been by pediatricians and metabolic specialists. Most physicians caring for adults are not prepared to assume the care of these patients as they grow older, and the services of well-trained medical geneticists are needed in these instances. Familiarity with the natural history of the disease, offered by the medical geneticist and the biochemical genetics laboratory, may lead to anticipatory guidance and appropriate monitoring, with early intervention at the first sign of trouble ( ) (see Chapter 56 for further discussion of care transition). Several examples illustrate this. Hepatic adenomas, which may become malignant, develop in the second and third decade of life in patients with GSD IA, while patients with tyrosinemia are also at risk for hepatocellular carcinoma. Other late complications include acute and chronic recurrent pancreatitis, which occurs in association with the hyperlipidemias, porphyrias, and organic acid disorders ( ). Cholelithiasis is a common occurrence in patients with MLD and Gaucher disease, and in disorders of bile formation and biliary transport. Thromboembolism causes morbidity in homocystinuria and methylenetetrahydrofolate reductase (MTHFR) deficiency. Cardiomyopathy and retinopathy may complicate LCHAD and other selected disorders of fat oxidation. Renal insufficiency or failure may develop in patients with cystinosis, Fabry disease, GSD I, and MMA. Metabolic stroke with bilateral globus pallidus abnormalities and extrapyramidal signs may be associated with metabolic decompensation in MMA.
As most IEM affect multiple systems, the involvement of a multidisciplinary team with central coordination by a primary physician is essential, and the roles of the medical geneticist and counselor remain critical. Patients and family members usually appreciate being included in the decision-making process. These moments of interaction provide an opportunity to assess the family’s understanding of the disease and its management as well as their coping mechanisms, and provide a guideline for the need of additional counseling and guidance. Efforts must be directed to ensuring that the patient reaches his or her maximum potential, and educational programs must be adapted to the appropriate developmental level and cognitive capacity to minimize frustration and associated behavioral problems. It is important to prepare for increasing handicap, and to take appropriate steps to facilitate individual performance during activities of daily living, since self-care, communication skills, and mobility issues will likely require special and individualized attention. Quality-of-life surveys are emerging in the rare-disease community for both the patient and the caregiver ( ).
All individuals become increasingly self-conscious of their body image during puberty. It is critical that caregivers enhance self-esteem in adolescents who feel stigmatized by their physical appearance, especially in instances in which facial dysmorphic features and skeletal deformities are involved. Dysarthria impedes communication, and disturbances of bowel and bladder continence undermine self-confidence and social interaction. Some disorders are associated with delayed puberty (GSD IA, galactosemia, and CDG), or premature ovarian failure (hypergonadotropic hypogonadism). Osteoporosis is often underrecognized as an associated condition of IEM. This most often occurs in disorders causing poor mobility due to cognitive or neuromuscular impairment, or those IEM characterized by chronic acidosis and renal insufficiency. When dietary regimens are required for disease control, be aware that the nature of adolescence may lead to nonconformity and a state of “rebellion.” Peer pressure may lead to noncompliance, and the patient must understand the potential implications of the disease, his or her role in management, and long-term outcomes. An engaged family support group is fundamental to this process. Pregnancy is a critical time, requiring measures to ensure a good maternal-fetal outcome ( ). This is especially reflected in the maternal PKU syndrome, for which pregnant PKU patients who maintain inadequate control of their phenylalanine levels are at heightened risk of delivering a fetus with microcephaly, developmental delays, and heart defects ( ). Nonetheless, early diagnosis and excellent long-term management have led to successful pregnancies in many IEM, including MMA, homocystinuria, propionic acidemia, and glutaric aciduria type I, among others ( ). Clearly, the role of pre-pregnancy counseling and interim management of risks pertinent to each disorder require close attention ( ).
Selected IEM have particular requirements and concerns. For example, women with homocystinuria may have an increased risk for spontaneous abortion and preeclampsia. Pregnancy may exacerbate the cutaneous lesions of porphyria cutanea tarda during the first trimester. Women who are carriers of ornithine transcarbamoylase (OTC) deficiency may develop a hyperammonemic encephalopathy during the postpartum period, and postpartum metabolic decompensation also occurs in MSUD. Lysinuric protein intolerance (LPI) is associated with increased risk of anemia, toxemia, and intrauterine growth retardation during pregnancy, and bleeding complications during delivery. Attention to maternal protein intake and control of hyperammonemia and other problems during pregnancy are essential ( ).
Psychiatric symptoms are primary features of some disorders or may develop secondary to metabolic decompensation ( ). For example, there is a growing association between the presentation of autism spectrum and the neurological sequelae of many IEM ( ). Behavioral changes (e.g., agitation, delirium) occur in individuals with X-linked ALD, late-onset GM2 gangliosidosis, MLD, urea cycle defects, and porphyria. Cognitive and behavioral problems also occur in children with PKU, especially in those who are not compliant with dietary restriction. Neuropsychiatric findings, such as OCD, PDD, ADD, and attention-deficit/hyperactivity disorder (ADHD), are prominent in numerous IEM, especially in SSADH deficiency and disorders of monoamine metabolism, involving the metabolism of dopamine and serotonin. Children subjected to BMT are at risk for neuropsychological complications secondary to chemotherapy and irradiation.
Several spontaneous animal models of human IEM exist, but successful breeding is uncommon. For example, Sly syndrome has been found to occur in a breed of beagle, while the Watanabe heritable hyperlipidemic rabbit (WHHL), the Gunn rat, and the Long Evans Cinnamon (LEC) rat models have been instrumental in defining the pathophysiology of hypercholesterolemia, Crigler-Najjar syndrome, and Wilson disease ( ). The literature is abundant with IEM occurring in selected canine strains, particularly relevant to the dog due to the tendency for inbreeding and drift to enhanced expression of rare, autosomal-recessive variants ( ). The seminal work by Capecchi and colleagues on homologous recombination in the mouse led to the genesis of knockout mice for the characterization of disease, especially the IEM ( ). Valuable murine knockouts have been developed in a plethora of IEM, including MSUD, PKU, tyrosinemia type I, SSADH deficiency, and many of the mucopolysaccharidoses and LSDs ( ). Currently available animal models have proven useful in defining the pathophysiology of many IEM and in the preclinical testing of various drugs. In some cases, these investigations have provided the rationale for further clinical trials in humans ( ). Although similarities may exist between knockout mice and the corresponding human IEM, species-specific differences in disease expression result from alternative pathways of substrate processing. These considerations highlight the need for caution in carrying over observations made in animal models to humans.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here