Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Inborn errors of metabolism manifest as a variety of metabolic defects that may complicate the management of anesthesia ( Table 18.1 ). In some instances, these defects are clinically asymptomatic and become manifest only in response to specific triggering events, such as ingestion of certain foods or administration of certain drugs, including some anesthetic drugs.
| Porphyria Purine metabolism disorders Carbohydrate metabolism disorders Hemochromatosis Wilson disease |
Porphyria is a group of metabolic disorders, each of which results from a specific enzyme deficiency in the heme synthetic pathway that leads to the accumulation of the preceding form of porphyrin. Physiologically, heme is the most important porphyrin and is bound to proteins to form hemoproteins such as hemoglobin and cytochrome P450 isoenzymes. Production of heme is regulated by the activity of aminolevulinic acid (ALA) synthase present in mitochondria. ALA synthase formation is controlled by endogenous concentrations of heme in a feedback loop that ensures the level of heme production parallels heme requirement. ALA synthase is readily inducible, and therefore its supply can respond rapidly to increase in heme requirements. However, in porphyria, any increase in heme requirements results in accumulation of pathway intermediates ( Fig. 18.1 ).
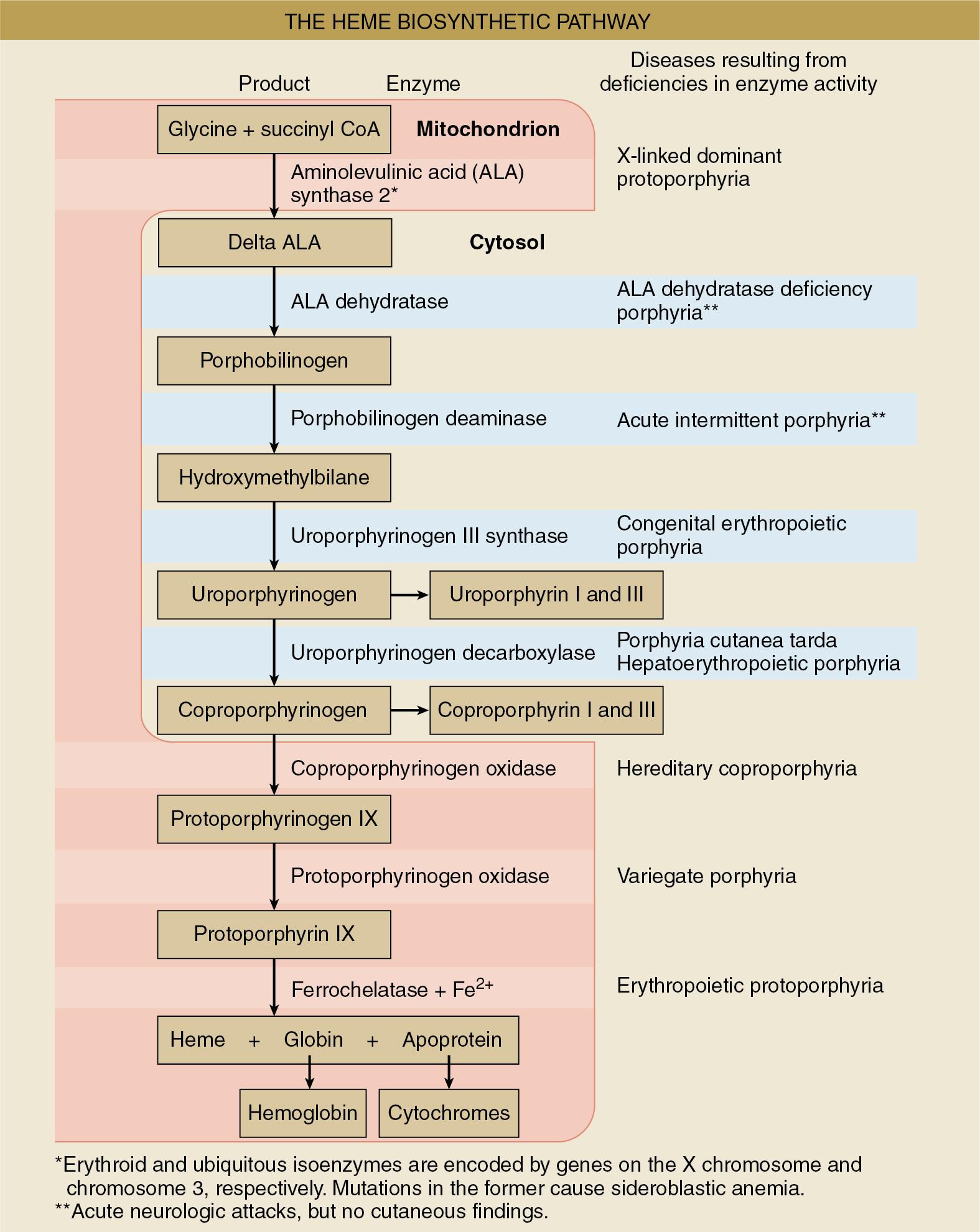
Porphyria is classified as either hepatic or erythropoietic depending on the primary site of overproduction or accumulation of the precursor porphyrin ( Table 18.2 ). However, for anesthesiologists, the more functional classification of acute versus nonacute porphyria may be more important since only acute forms of porphyria are relevant to the management of anesthesia ( Table 18.3 ). They are the only forms of porphyria that can result in life-threatening reactions in response to drugs often used in the perioperative period.
| Hepatic Acute intermittent porphyria Variegate porphyria Hereditary coproporphyria Aminolevulinic acid dehydratase porphyria Porphyria cutanea tarda |
| Erythropoietic Congenital erythropoietic protoporphyria Erythropoietic protoporphyria X-linked protoporphyria |
| Acute Porphyrias Acute intermittent porphyria Variegate porphyria Hereditary coproporphyria Aminolevulinic acid dehydratase porphyria |
| Nonacute Porphyrias Porphyria cutanea tarda Congenital erythropoietic protoporphyria Erythropoietic protoporphyria X-linked protoporphyria |
Acute porphyria is a group of inherited autosomal dominant disorders with variable expression. The enzyme defects in these forms of porphyria are deficiencies rather than absolute deficits of heme pathway enzymes. Although there is no direct influence of gender on the pattern of inheritance, attacks occur more frequently in women and are most frequent during the third and fourth decades of life. Furthermore, attacks are rare before puberty or following the onset of menopause. In the presence of deficient enzymes in the heme synthesis pathway, any event that induces ALA synthase activity will precipitate porphyrinogen buildup that eventually leads to porphyria attacks. Drugs, fasting (e.g., before elective surgery), dehydration, stress (e.g., associated with anesthesia and surgery), infection, and hormonal fluctuations such as those during menstruation can all trigger acute attacks. Pregnancy in patients with acute porphyria is often associated with spontaneous abortion, systemic hypertension, and an increased incidence of low-birthweight infants.
Of all the acute porphyria, acute intermittent porphyria is the most common subtype and the one most likely to be life threatening. Acute intermittent porphyria mainly affects the gastrointestinal and nervous systems. The defective enzyme is porphobilinogen deaminase, and the gene encoding this enzyme is located on chromosome 11. Since the enzyme deficiency occurs early in the heme synthetic pathway, an excess of these heme precursors does not cause skin disease.
Variegate porphyria is characterized by neurotoxicity and cutaneous photosensitivity. Skin lesions are usually bullous eruptions and occur on exposure to sunlight. This photosensitivity can be attributed to increases in light-absorbing porphyrin intermediates and their metabolites. The enzyme defect is late in the heme synthetic pathway at the level of protoporphyrinogen oxidase. The gene encoding this enzyme is on chromosome 1.
Hereditary coproporphyria is a rare form of acute porphyria. Patients with coproporphyria typically experience neurotoxicity and cutaneous hypersensitivity, although these signs tend to be less severe than is seen in variegate porphyria. The defective enzyme is coproporphyrinogen oxidase, encoded on chromosome 9.
ALA dehydratase (ALAD) porphyria is a rare autosomal recessive disorder. The gene encoding ALAD is on chromosome 9.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here