Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hydrops fetalis (HF) is defined as the presence of excessive fetal fluid in two or more of the following spaces: abdominal ascites, pleural effusion, pericardial effusion, skin edema, polyhydramnios, or placentomegaly. The diagnosis is made by ultrasound imaging evaluating body cavities, the placenta, and the amniotic fluid volume. Classically, HF has been classified into two categories based on etiology: Immune HF accounts for an estimated 10%-24%, with nonimmune HF accounting for 76%-90% of cases and becoming increasingly the main cause of HF ( Table 23.1 ).
| Diagnostic Criteria | Definition |
|---|---|
| Presence or absence of red blood cell alloimmunization (immune vs. nonimmune HF) | Rh-D status Antibody screen |
| Plus Two or More Abnormal Fluid Collections | |
| Ascites | Echolucent rim encompassing entire fetal abdomen in the transverse view |
| Pleural effusions | Unilateral or bilateral visualization of lung border |
| Pericardial effusion | Nonphysiologic fluid ≥2 mm |
| Skin edema | Subcutaneous edema ≥5 mm |
| Polyhydramnios | Single deepest fluid pocket ≥8 mm AFI ≥25 cm |
| Placentomegaly | Placenta ≥6 cm thickness |
Immune hydrops fetalis (IHF) was initially recognized as the combination of neonatal hydrops, anemia, and jaundice along with erythroblastosis and red blood cell hemolysis by Diamond and coinvestigators in 1932. The pathophysiology of the IHF was explained by the work of Landsteiner and associates, who discovered the rhesus (Rh) antigen in 1940. In 1941, Levine and coworkers observed that if an Rh-negative woman is exposed to red blood cells that are Rh positive, she will form antibodies that may result in transplacental passage of the antibodies to fetal red blood cells and may result in fetal hemolysis. The resultant maternal alloimmunization was initially documented by Chown and coworkers.
Anti-D antibodies were proposed as a method to prevent the nonimmunized Rh-negative mother from becoming sensitized after birth. Freda and coworkers studied the efficacy of specific anti-D IgG in Rh-negative male prison volunteers who were given Rh-D-positive blood and observed that sensitization could be prevented. A subsequent investigation confirmed that if prisoners were given Rh-D-positive blood and received anti-D IgG within 72 hours that alloimmunization did not occur.
Blood group antigens were among the first discoveries of genetic polymorphisms in human proteins. Of these, the Rhesus factor (the largest of all of the blood groups) has more than 50 different described antigens, with additional discoveries likely to continue. Most of these are quantitative antigens (affecting the amounts of Rh factors expressed), although some are qualitative in nature. Not all antigens have clinical significance in pregnancy.
Three of the most concerning antigens in pregnancy, the Rh(D) and Rh(CcEe) antigens, are encoded at two human loci, found on chromosome #1 (1p36.1). They can cause antibody formation in pregnancy and lead to significant hemolytic disease of the newborn (HDN) in future pregnancies. Although there are multiple nomenclature systems for maternal rhesus (DCE) status, the Fisher and Race method has clinical advantages, such as ease of use and remembrance, and was also proved genetically correct. In this system, each parent contributes an allele from the Rh(D), Rh(C), and Rh(E) gene loci. In broad terms, these alleles are called D or d, C or c, and E or e. A person can be homozygous or heterozygous for each of these alleles. The genes are so closely linked on chromosome #1 that crossover between the alleles is not thought to occur.
The D antigen is a transmembrane protein with several extracellular loops located on erythrocytes and their immediate progenitors. Most alleles of the Rh(D) gene (≈170 identified) are caused by nonsense or missense mutations, although frameshift and splice-site mutations have also been reported. Alleles that cause strongly reduced or complete absence of the Rh-D antigen are often called DEL or negative alleles, respectively. These include the most common European d (Rh-negative phenotype). The Rh-negative phenotype is most common in individuals of European and North American descent (15%-17%), then followed by African and Indian (3%-8%), with the rarest in Asia (0.1%-0.3%). Thirty percent of Rh-negative Asians actually carry a DEL allele, whereas this phenotype is rare in Europeans. Other alleles may result in “partial D” antigens (qualitative). There are also alleles that produce a “weak” Rh(D) antigen. DEL, negative, partial D, and weak D alleles can be clinically significant in pregnancy and increase the risk for HDN.
The D alleles are dominant over the d alleles. It is important to note that d is simply the absence of D antigen; there has been no identification of an actual d antigen. Thus, it is the presence or absence (and the extent) of the D antigen that will determine if an individual is Rh positive or Rh negative. There are significantly fewer alleles identified at the Rh(CcEe) gene. Unlike the Rh(D) alleles, the Cc and Ee alleles are codominant; both are expressed in a heterozygote state. The mutations/changes between the alleles are limited and result in amino acid changes at only five locations. Partial C, c, E, and e antigens can be present, but the clinical significance of these in pregnancy is undetermined.
The Kell blood group gene, located on chromosome #7 (7q33), has many polymorphisms, and its alleles encode for approximately 20-25 antigens. Behind the Rh and ABO blood group antigens, Kell antigens carry the most risk for HDN in human pregnancy. The two most common alleles are K and k. The Kell protein transverses the red blood cell (RBC) membrane only once, unlike D and CcEe. Kell antibodies work differently in the lysing of fetal RBCs than Rh and CcEe antibodies; Kell antibodies actually destroy early precursors of erythrocytes in the fetal liver and effect early proliferation of RBCs, instead of only attacking mature RBCs in fetal circulation.
Because each of these antigens can lead to hemolytic disease of the newborn, knowing ethnicity may be helpful in predicting risks for fetal status in a broad sense or if the father of a fetus is unavailable. Of course, when possible, it is advisable to obtain paternal DCE antigen and/or Kell antigen genotyping in at-risk pregnancies. Certainly other antigens have been linked to HDN, but genotyping is not available for all of these blood groups. These results are of great importance in delineating the best courses of action during gestation for monitoring an at-risk fetus.
Typical methods of monitoring at-risk fetuses have included monitoring of maternal antibody titers; targeted ultrasounds to obtain serial middle cerebral artery peak systolic velocity (MCA-PSV) values and exclude the presence of hydrops, amniocentesis, or chorionic villus sampling (CVS) for fetal antigen genotyping; and/or amniocentesis to determine ΔOD450, whereas the latter is rarely used clinically anymore. Traditionally, fetal genotyping has been performed using polymerase chain reaction (PCR) on amniocytes or chorionic villi. New technology now allows for noninvasive determination of fetal Rh-D antigen status through the use of cell-free fetal DNA collection from a maternal blood sample. Although this technology is relatively new, this method of testing has proved to be very accurate (94%-97%), with high sensitivity and specificity. Because fetal DNA is directly tested from maternal serum, it reduces the need for invasive testing, Rh-D prophylaxis, and paternal antigen genotyping in most cases.
Alloimmunization, which was formerly known as isoimmunization, occurs when a mother develops antibodies to a paternally derived red blood cell antigen that is inherited by the fetus or from a transfusion of unmatched or mismatched blood. If this occurs, hemolytic disease of the fetus or neonate in subsequent pregnancies can occur.
In Rh alloimmunization, an Rh-D-negative mother is exposed to Rh-D-positive blood. A quantifiable amount of hemorrhage occurs between the fetus and the mother in up to 75% of all pregnancies. This may range from less than 0.1 mL of blood in 60% of pregnancies to an excess of 5 mL in less than 1% of pregnancies. In an analysis of maternal fetal hemorrhage, 0.01 mL of fetal blood was detected in the maternal circulation in 3%, 12%, and 46% in the first, second, and third trimesters of pregnancy, respectively. The risk of sensitization is related to the amount of blood that passes between the fetus and mother. For 0.1 mL of blood, the risk of sensitization is 3% and for 0.4 mL, the risk rises to 22%. Rh sensitization has been reported as early as 38 days, and fetal maternal hemorrhage has been demonstrated as early as 5-6 weeks (mean maternal fetal transfusion at 8 weeks is 0.3 mL). The minimal amount of fetal blood needed to cause alloimmunization is estimated to be 0.25 mL. The greatest risk occurs at the time of delivery when that risk is approximately 17%. ABO incompatibility of the mother and fetus in pregnancy with Rh sensitization (mother O; father A, B, or AB) will reduce the sensitization risk to 2%. Additionally, 30% of Rh-negative individuals are nonresponders and will not become sensitized. Box 23.1 summarizes the numerous risk factors for maternal fetal hemorrhage during pregnancy.
Spontaneous abortion
Elective abortion
Amniocentesis
Chorionic villus sampling
Antepartum hemorrhage
Fetal blood sampling
Ectopic pregnancy
Blunt abdominal trauma
Fetal death in utero
Abruption
Placenta previa with bleeding
Cesarean delivery
Manual removal of the placenta
Immune hydrops fetalis (IHF) is the end result of progressive red blood cell destruction, in which even with the recruitment of extramedullary tissue (liver and spleen), the severity of the hemolysis results in severe fetal anemia, HF, and often fetal death. Although the fetus is able to compensate for progressive anemia, one study determined that once the hemoglobin deficit has exceeded 7 g/dL, this compensatory ability of the fetus may become exhausted and IHF will ensue. The concept of a fixed hemoglobin level for IHF likely needs to be adjusted for gestational age, because with advancing gestational age the mean hemoglobin value increases, going from 10.6 g/dL at 18 weeks to 13.8 g/dL at 40 weeks. It has been suggested that a hemoglobin value of less than 0.5 times the median for gestational age might be a more appropriate predictor for fetuses at significant risk for HF.
Progressive ultrasound findings of IHF include the early findings of polyhydramnios and placentomegaly, followed by hepatomegaly and pericardial effusions and late findings of ascites, scalp edema, pleural effusions, and finally, oligohydramnios. Severe fetal anemia (fetal hemoglobin of ≤5 g/dL) associated with the development of HF has implications on fetal survival. In a study with intravascular transfusions, the survival rate for nonhydropic fetuses was 94% compared with 74% in fetuses with hydrops.
The exact mechanism that causes IHF is unknown. Proposed mechanisms include high output cardiac failure, liver dysfunction, and fetal hypoxemia from fetal anemia. It is likely that a combination of all three is responsible for fetal hydrops.
Prevention of Rh alloimmunization begins at the first prenatal visit. A careful history should be taken of any problems associated with previous pregnancies or deliveries, prior blood transfusions, or blood disorders. Initial laboratory work should include ABO typing, Rh-D status, and an antibody screen. If the mother is Rh-D negative, this should be carefully documented in the chart. If the antibody screen is positive, the antibody test and a titer of the antibody should be repeated and a determination should be made for antibodies, Rh-D, and others that are not Rh-D, to ascertain if the antibody is known to cause hemolytic disease of the newborn ( Fig. 23.1 ). If the antibody is positive Rh-D, then the patient should be questioned about a prior injection of anti-D immune globulin, as this could be the reason for the positive antibody titer.
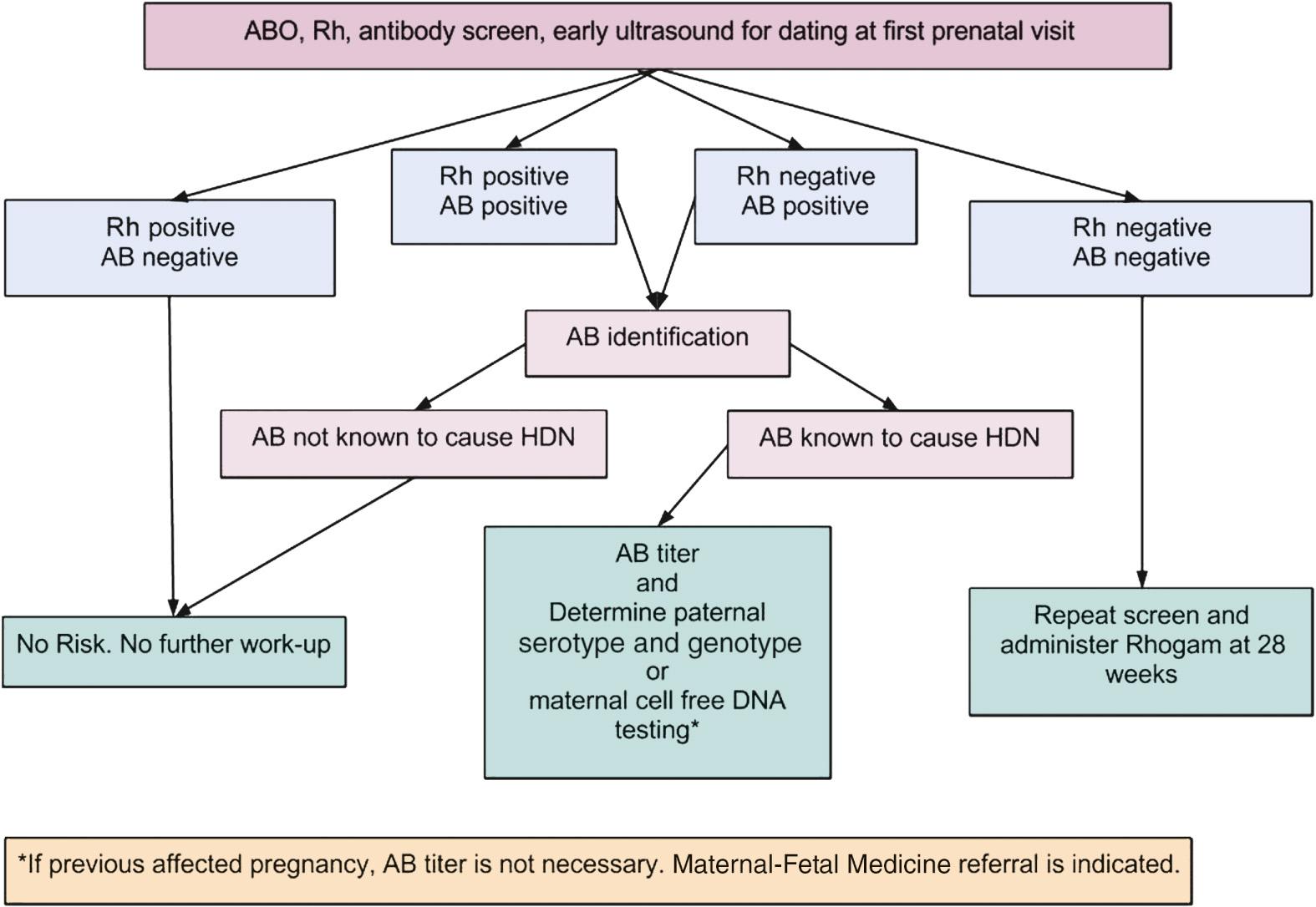
Anti-D immune globulin is a passive immunoglobulin that when given to Rh-D-negative women will prevent the vast majority of cases of alloimmunization. Unfortunately, there are no other immune globulins specific for the other red blood cell antigens. After the initial screen at the first prenatal visit, a repeat Rh-D antibody screen is done at 24-28 weeks. If the antibody screen is negative, 300 µg of anti-D immune globulin is administered at that gestational age and then again within 72 hours of delivery if the neonate is Rh-D positive. Other accepted regimens are 100 µg at 28 and 34 weeks and following delivery if the neonate is Rh-D positive. Suggested doses of anti-D immune globulin for a first-trimester induced or spontaneous abortion, amniocentesis, or chorionic villus sampling, and first-trimester ectopic pregnancy, are 50–120 µg of anti-D immune globulin up to 12 weeks’ gestation. After 12 weeks’ gestation it is recommended to use 300 µg of anti-D immune globulin. Three hundred micrograms of anti-D immune globulin will suppress the immune response of 30 mL of fetal blood or 15 mL of packed D-positive red blood cells. Women should be screened at delivery and when maternal fetal hemorrhage is suspected, to prevent an inadequate dose of anti-D immune globulin being administered and subsequent alloimmunization ( Table 23.2 ).
| Indication | Justification | Dosage (µg) | |
|---|---|---|---|
| First Trimester | Second or Third Trimester | ||
| Spontaneous abortion/IUFD | 2%-3% sensitization | 50 | 300 |
| Therapeutic abortion | 4%-5% sensitization | 50 | 300 |
| Ectopic pregnancy | 2%-5% sensitization | 50 | 300 |
| Chorionic villus sampling | 50% FMH | 50 | 300 |
| Amniocentesis | 10% FMH | 300 | 300 |
| Percutaneous umbilical blood sampling | 40% FMH | 300 | 300 |
| Abruptio placenta/placenta previa | Variable | 300 | 300 |
| Antepartum vaginal bleeding | Variable | 300 | 300 |
| External cephalic version | Variable | N/A | 300 |
| Trauma | Variable | 300 | 300 |
| Pregnancy (28 weeks' gestation and ≤72 h postpartum) | 7%-8% sensitization * 15% sensitization † |
N/A | 300 |
| Delivery | 50% FMH | N/A | 300 |
Newer techniques using molecular genetics have permitted the preimplantation diagnosis of the Rh-D status. This technology has the potential to prevent Rh isoimmunization through selective transfer of Rh-D-negative embryos. In the case of severe maternal alloimmunization, the approach can prevent fetal hemolytic disease with its associated morbidity and mortality. In the first use of this technology in 2005, an unaffected Rh-negative neonate was born to an Rh-D alloimmunized mother. Following intracytoplasmic sperm injection, 12 embryos were obtained, and on day 3, biopsies were performed followed by PCR. Two Rh-D-negative embryos that were subsequently transferred resulted in the delivery of a healthy Rh-D-negative infant at 39 weeks of gestation. Notwithstanding the emotional, psychological, and financial burdens of in vitro fertilization, this represents a potentially lifesaving approach for selected couples with maternal Rh alloimmunization.
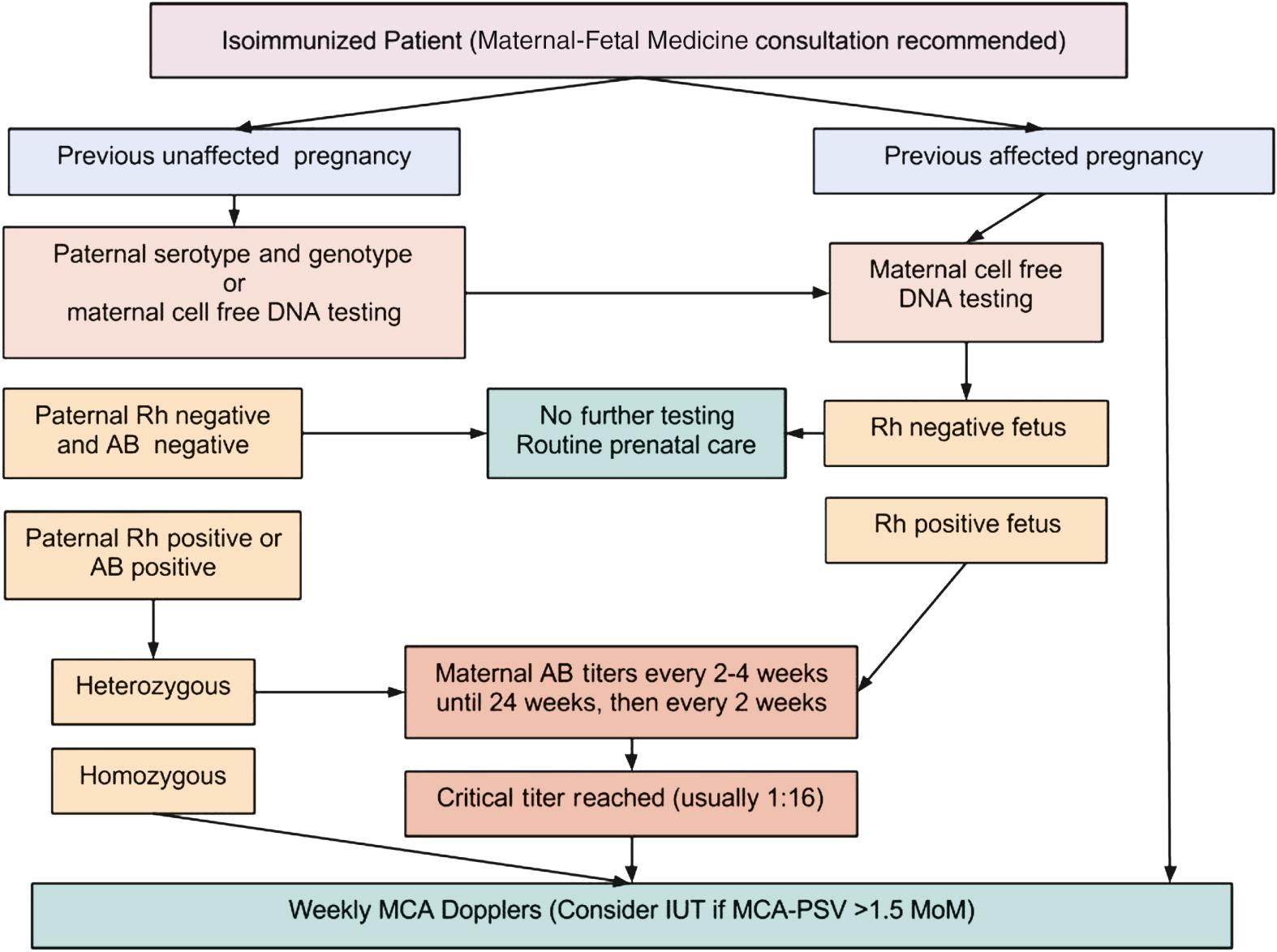
Detection of Rh alloimmunization begins with the initial laboratory work. Pregnant women who are Rh negative are screened for antibodies by the indirect Coombs test. This allows detection of Rh-D antibodies as well as antibodies to other atypical antigens known to cause alloimmunization. If Rh-negative women are alloimmunized to Rh-D, they need to have their anti-D titer determined, because that titer is known to roughly correlate with the severity of the disease and the need for Doppler flow studies. Although each lab sets its own standards based on experience, anti-D titers of 1:16 or greater require further assessment. If the titer is less than 1:16, the patient can be followed with monthly titers.
In sensitized Rh-D–negative women where the father is Rh-D positive, paternal genotyping may help clarify the fetal risk. PCR determination of paternal Rh-D status can facilitate counseling and dictate the need for intervention, because paternal heterozygosity is associated with a 50% risk of fetal Rh-D negativity, whereas homozygosity always produces an Rh-D-positive fetus, assuming correct paternity. Fetal Rh-D typing can also be done with amniocytes in the second trimester or with villi in the first trimester ( Fig. 23.2 ). More recently, fetal Rh-D genotyping has been achieved through isolation of cell-free fetal DNA from the maternal circulation and provides a noninvasive way to directly assess fetal blood type and antibody status.
Cordocentesis and sampling of fetal blood is the most accurate method to determine if fetal anemia is present. However, this technique is invasive and carries a fetal loss risk of up to 3%. This has led to the use of other techniques to evaluate when the risk for fetal anemia is present so that fetal blood sampling can be undertaken. Fetal anemia had been determined previously by amniocentesis and determination of risk for fetal anemia using optical density of amniotic fluid (ΔOD450). This method used an assessment of the bilirubin content of amniotic fluid to determine the estimated level of fetal anemia. Often, a series of amniocenteses was necessary to monitor the pregnancy and determine when a more invasive cordocentesis was undertaken. With improvements in ultrasound technology, this method has been replaced by serial assessments of the peak systolic velocity (PSV) of the middle cerebral artery (MCA) in the detection of fetal anemia.
The objective of Doppler flow studies is to detect physiologic compensatory changes in the fetal circulation that would be associated with mild to moderate anemia. As anemia progresses, both right and left ventricular outputs increase up to 45% with the heart rate remaining unchanged. This increases stroke volume. Progressive anemia leads to decreased blood viscosity. If the given fetal blood vessel cross-sectional area also remains unchanged, and applying Poiseuille's law (blood velocity is directly proportional to flow), increased stroke volume results in increased flow (flow being the product of velocity and the cross-sectional area).
A number of fetal vessels were evaluated by Doppler flow studies to determine which vessel would be the most predictive of fetal anemia. The MCA was selected because it is well known that the cerebral arteries respond rapidly to hypoxemia by increasing blood flow velocity to maintain cerebral perfusion. Additionally, the MCA is easy to identify with low interobserver and intraobserver variability. Mari and the Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses measured the hemoglobin concentrations in blood obtained by cordocentesis and correlated those concentrations with MCA-PSV in 111 fetuses at risk for anemia secondary to red blood cell alloimmunization. The group used a hemoglobin concentration of 5 g/dL as a threshold to calculate degrees of anemia, because fetal hydrops is rare with a hemoglobin concentration greater than 5 g/dL. Strict cutoffs using receiver-operating–characteristic curves were determined to detect 100% of those fetuses with moderate to severe anemia. They found the optimal threshold was 1.29 times the median for mild anemia, 1.5 times the median for moderate anemia, and 1.55 times the median for severe anemia, with a false-positive rate of 12%. These thresholds were chosen so as to not miss any fetus with moderate or severe anemia that might result in either fetal hydrops or death ( Table 23.3 ). Measurements of the MCA-PSV can be started as early as 16-18 weeks and should be repeated at 1- to 2-week intervals.
| Peak Systolic Velocity of Middle Cerebral Artery | |||||
|---|---|---|---|---|---|
| Gestational Age (weeks) | Multiples of the Median | ||||
| 1 | 1.3 | 1.5 | 1.7 | 2 | |
| 15 | 20 | 26 | 30 | 34 | 40 |
| 16 | 21 | 27 | 32 | 36 | 42 |
| 17 | 22 | 29 | 33 | 37 | 44 |
| 18 | 23 | 30 | 35 | 39 | 46 |
| 19 | 24 | 31 | 36 | 41 | 48 |
| 20 | 25 | 33 | 38 | 43 | 50 |
| 21 | 26 | 34 | 39 | 44 | 52 |
| 22 | 28 | 36 | 42 | 48 | 56 |
| 23 | 29 | 38 | 44 | 49 | 58 |
| 24 | 30 | 39 | 45 | 51 | 60 |
| 25 | 32 | 42 | 48 | 54 | 64 |
| 26 | 33 | 43 | 50 | 56 | 66 |
| 27 | 35 | 46 | 53 | 60 | 70 |
| 28 | 37 | 48 | 56 | 63 | 74 |
| 29 | 38 | 49 | 57 | 65 | 76 |
| 30 | 40 | 52 | 60 | 68 | 80 |
| 31 | 42 | 55 | 63 | 71 | 84 |
| 32 | 44 | 57 | 66 | 75 | 88 |
| 33 | 46 | 60 | 69 | 78 | 92 |
| 34 | 48 | 62 | 72 | 82 | 96 |
| 35 | 50 | 65 | 75 | 85 | 100 |
| 36 | 53 | 69 | 80 | 90 | 106 |
| 37 | 55 | 72 | 83 | 94 | 110 |
| 38 | 58 | 75 | 87 | 99 | 116 |
| 39 | 61 | 79 | 92 | 104 | 122 |
| 40 | 63 | 82 | 95 | 107 | 126 |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here