Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In 1850/1851, the German physiologist and physicist Hermann Helmholtz (1821–1894, ennobled 1883) designed the direct ophthalmoscope, enabling the founders of modern ophthalmology to view the living ocular fundus for the first time. New disorders were described, making this instrument essential in establishing the young specialty of ophthalmology. Until ophthalmic photography became available, ophthalmic images were recorded by artists who meticulously drew and painted detailed renditions of the ocular diseases they had observed. Later, and for decades, ophthalmic photography using 35 mm film became the gold standard for external, anterior segment and fundus photography. This has now been replaced by digital photography. Camera systems and optics have remained relatively unchanged, with the exception of the development of non-mydriatic fundus cameras, which allow imaging of the fundus without pupil dilation.
Several new techniques to examine and image the eye have been established over the past few decades, shifting ophthalmic imaging from mere documentation to structural analysis of the eye, expanding our understanding of many diseases. The advent of scanning laser ophthalmoscopy (SLO) in 1981 and of optical coherence tomography (OCT) in 1991 dramatically enhanced the diagnostic value of ophthalmic imaging. The applications of OCT are continuing to expand rapidly with new imaging modalities.
Nevertheless, a careful clinical examination of the patient is essential to decide which type of imaging is needed. Using imaging techniques indiscriminately can result in confusion, waste of resources, anxiety, and potentially, the provision of unnecessary treatment. Accurate documentation of clinical findings also has a medicolegal dimension. In this chapter, we attempt to provide a framework for the physician to facilitate decisions regarding which imaging technique to use. Specific findings in certain entities are discussed in their respective chapters.
In many settings, external ophthalmological findings can be documented. For example, lid fissure height and margin reflex distance are important parameters in patients with upper lid ptosis; ocular motility in patients with strabismus and eyelid swelling and erythema in cellulitis can be photographed. Other periocular and adnexal findings may warrant documentation, such as anterior orbital masses, lid lesion and anterior segment pathologies, which often can be imaged well with regular photography. Children might not tolerate instruments close to their face, which makes external photography helpful ( Fig. 8.1 ).
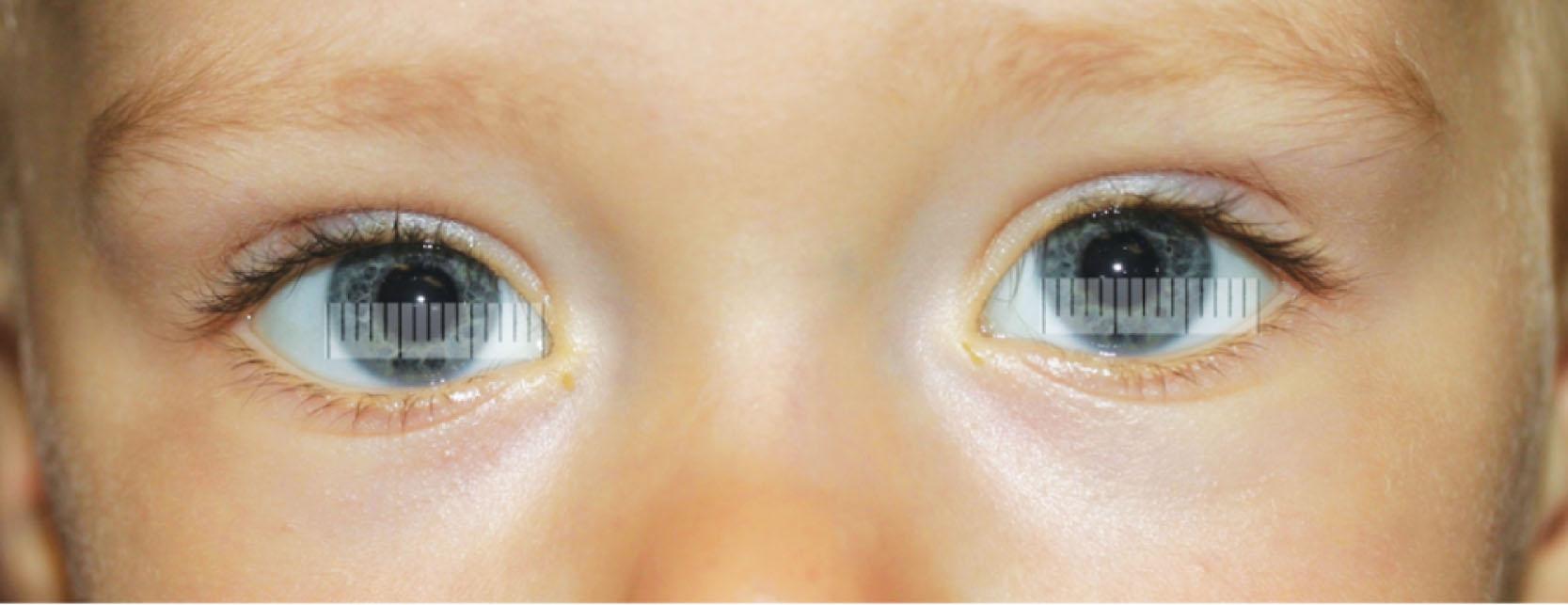
In young children, slit-lamp examination is sometimes impossible and photography may allow the clinician to document findings. Measuring the corneal diameter helps to establish the diagnosis in some patients (microphthalmos, infantile glaucoma) and is best done photographically. Judging and comparing lens opacities in pediatric cataract or the extent of subluxation of the lens (e.g. in Marfan syndrome) is facilitated by photographic documentation.
The RetCam (Clarity Medical Systems, Inc., Pleasanton, CA) is a contact digital photography system. With special lenses, it can be used for anterior segment photography. The anterior chamber angle may be visualized and good agreement with slit-lamp gonioscopy in detecting angle closure has been reported.
When evaluating newborns for retinopathy of prematurity, sequential photographic documentation of retinal findings is helpful. The use of digital fundus photos for remote diagnosis (telemedicine), which holds promise in areas with limited medical care, is another possibility. Retinal lesions can be documented, which is essential in managing tumors such as retinoblastoma.
Optic disc characteristics such as color, sharpness of its borders, as well as size and shape of the optic cup can be assessed on a photograph (using magnification if necessary) and comparison with subsequent photographs is possible, helping to identify change.
With the remarkable advancement of fundus imaging technologies, wide-field imaging (WFI, 50–100 degrees field of view, to the posterior edge of vortex vein ampulla when centered on the fovea) and ultra wide-field imaging (UWFI – 110-220 degrees) have become increasingly popular, and have made imaging of the peripheral fundus easier. Different systems are commercially available. Because it is portable, the RetCam is used widely in infants and children ( Fig. 8.2 ). Scanning laser ophthalmoscopy (SLO) may be used for WFI and UWFI (see below, Fig. 8.14 ).
In red-free fundus photography, the imaging light illuminating the fundus is filtered to remove red colors, allowing only light with wavelength 495–570 µm to pass. Since hemoglobin absorbs light of this wavelength, retinal vessels and hemorrhages appear dark and are more easily seen than on color photographs. Visualization of retinal holes (darker than the surrounding retina) is also facilitated. Drusen (macular and optic disc), exudates, cotton-wool spots, and defects in the retinal pigment epithelium (RPE) are lighter on red-free photography. The retinal nerve fiber layer (RNFL) is lighter and defects appear as darker areas with arcuate borders of RNFL bundles.
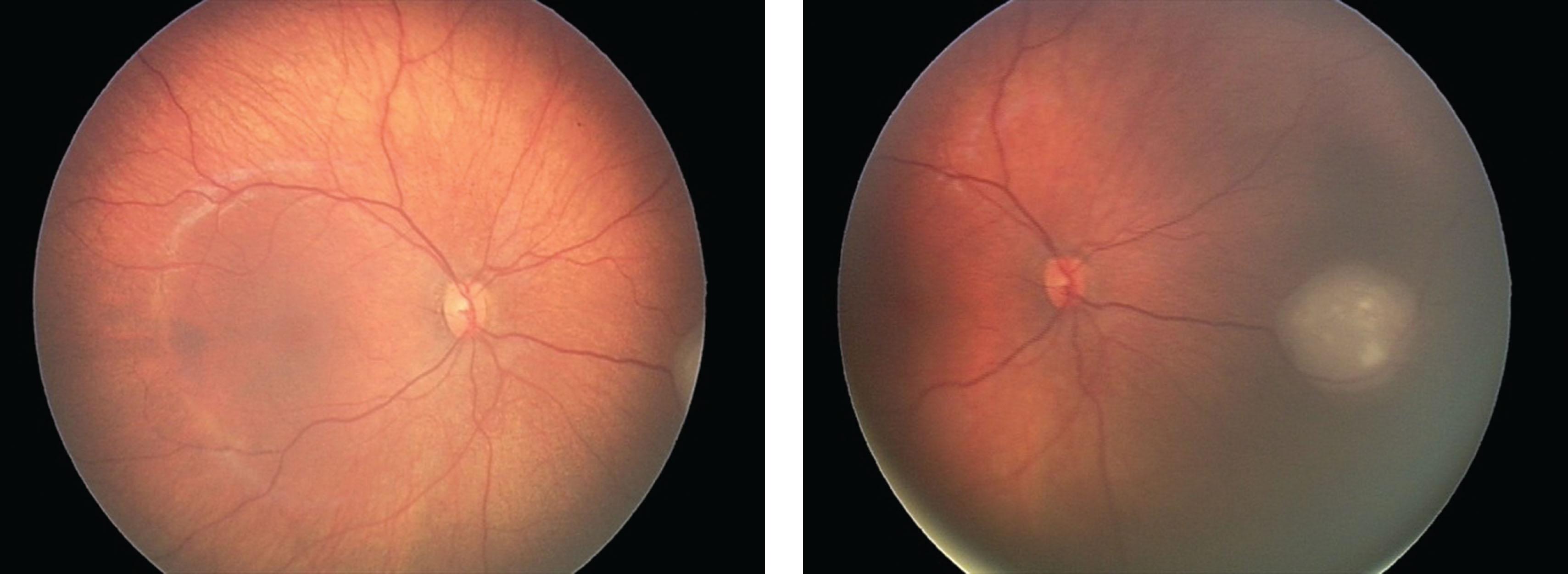
Infrared fundus photography is usually obtained using scanning laser ophthalmoscopy (SLO). Compared with regular white flash fundus photography or photography using a laser with a shorter wavelength (blue, green), offers better visualization of lesions located in the outer retina or under the retina ( Fig. 8.3 ).

Confocal microscopy (CM) has been used in other tissues since 1955, and was first used to study the cornea in 1990. Like specular microscopy, CM can image the cornea with very high resolution, at a cellular level. Unlike light microscopy, which is limited by scattered light and reflections outside the focal plane, in CM the illumination and detection paths have the same focal plane. CM is particularly helpful in diagnosing acanthamoeba keratitis (amoebic cysts and swollen corneal nerves can be detected) and visualizing pathology in individual layers of the cornea (e.g. guttae in endothelium in Fuchs endothelial dystrophy).
The first keratoscope was described by Henry Goode in 1847 and used the reflection of a square object on the corneal surface. The Placido disc (described by Antonio Placido in 1880) consists of concentric light and dark rings with a central opening to observe the reflections of these rings on the cornea. Any corneal irregularity (astigmatism, scars) will distort the reflection, so that the reflected rings do not appear circular. Videokeratoscopy became available in the 1980s and made analysis of several thousands of sampling points possible. With Placido disc-based topography, a corneal map can be created and color-coded based on corneal curvature or elevation. Advantages of this technology include noninvasiveness and intuitive interpretation. Regular and irregular astigmatism are easily distinguished and corneal disorders such as keratoconus can be identified based on their characteristic appearance. Disadvantages of this technology include the absence of information about the posterior corneal surface and limited corneal coverage (60% of the entire surface).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here