Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Visualizing the genitourinary system was one of the first goals of the nascent radiological sciences at the turn of the 20th century. It was a fortuitous discovery that the sodium iodide patients were taking for syphilis made their bladder denser (“opacified”) on x-ray imaging. The development of organic iodine agents that could be administered intravenously and would be excreted in the urine advanced the field of kidney imaging. This created an image of the kidneys, ureter, and bladder that could be captured on x-ray film. Although intravenous contrast opacifies every organ receiving blood flow, the development of contrast agents for genitourinary radiology and the fact that these agents were excreted through the kidneys have linked the two ever since.
Kidney imaging has evolved at a tremendous rate, and evaluating kidney disease in the modern era often relies on gathering information from multiple different imaging techniques.
Conventional radiography (x-rays) of the kidneys has limited value for most kidney disease processes. Radiography has excellent contrast resolution for calcium and gas, so kidney stones or gas-containing infections can be seen. However, it has poor resolution for soft tissues, and at best the outline of the kidney appears like a “ghost” on a radiograph.
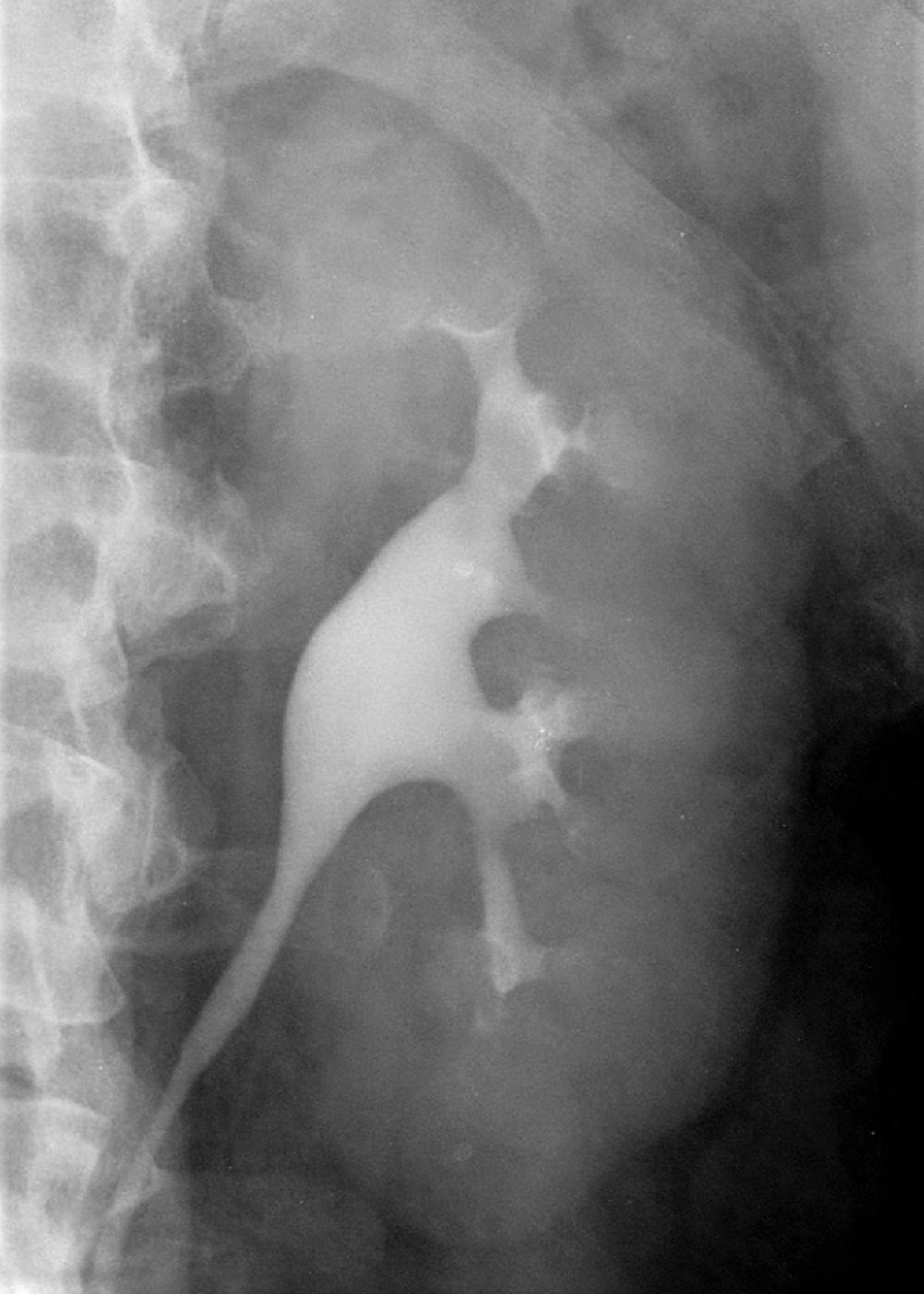
Intravenous urography (or intravenous pyelography) addresses the visibility problem when an injected iodine “contrast” collects in the kidney parenchyma and is then excreted ( Fig. 6.1 ). The technique is close to a hundred years old and has been almost completely supplanted by CT urography, which is faster and more versatile, with much better ability to visualize the kidney parenchyma (in addition to other abdominal organs).
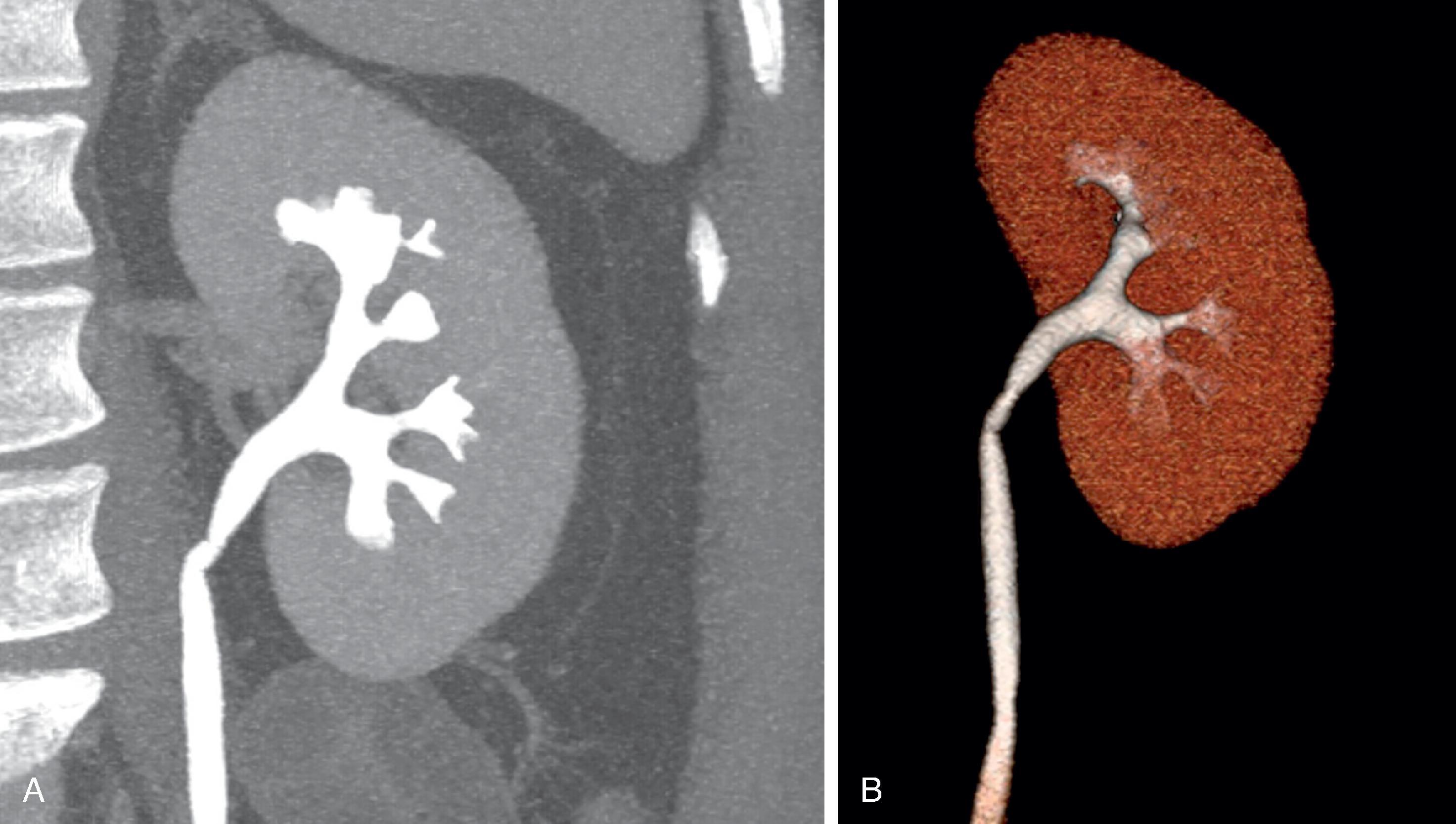
Computed tomography administers x-rays at different angles around an arc (tomography) to build a sinogram of different attenuation values. This sinogram is then deconvoluted by a computer to provide two-dimensional images of a patient ( Fig. 6.2 B); these can be reconstructed into three-dimensional images, if desired ( Fig. 6.2 C).
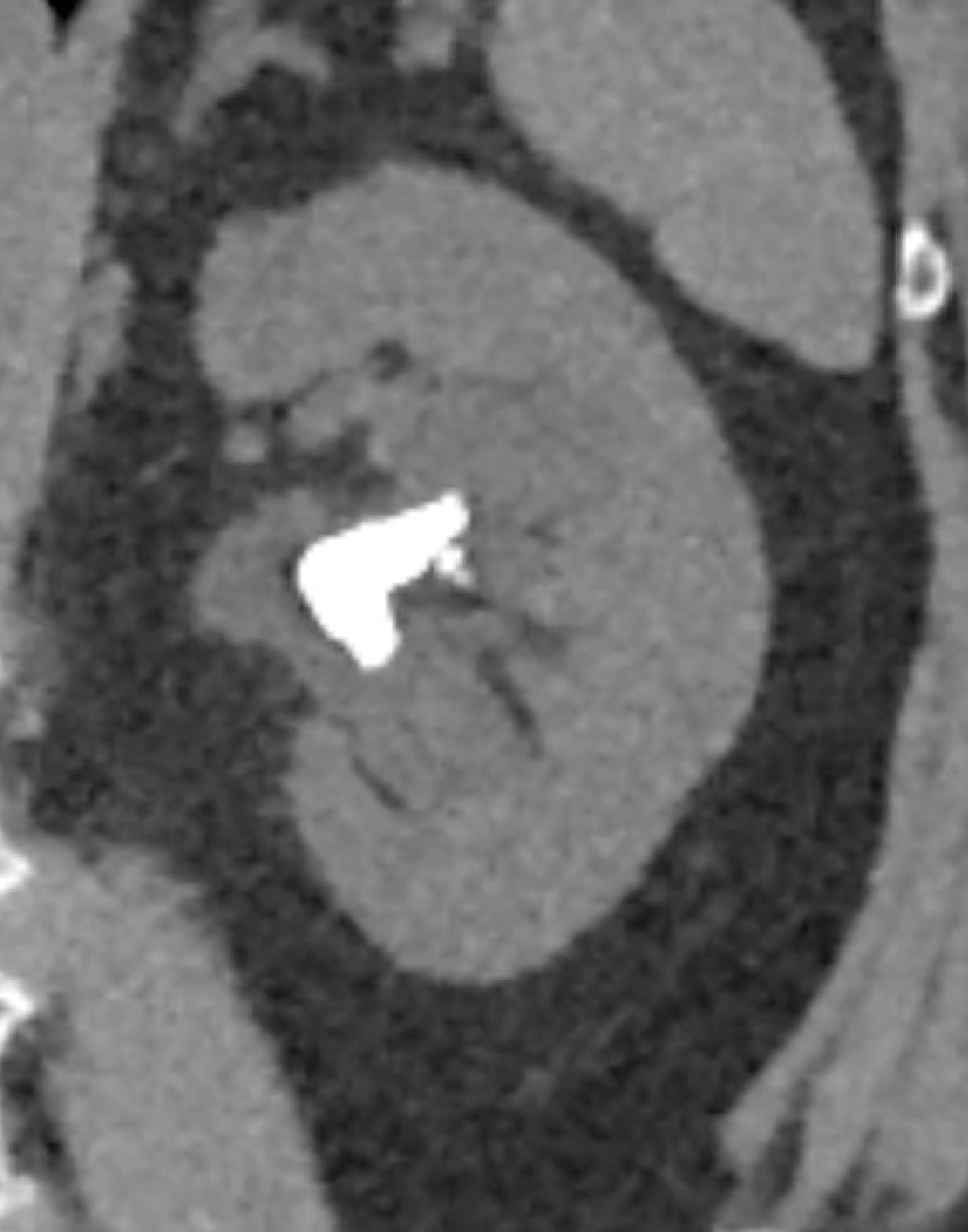
CT scans can be performed without contrast, particularly if evaluating for stones ( Fig. 6.3 ), but they may also employ the same iodinated contrast used in intravenous urography. CT scans with contrast can also be protocoled to scan at multiple different time points to gather more information about the kidney parenchyma or the enhancement pattern of kidney masses ( Fig. 6.4 ).
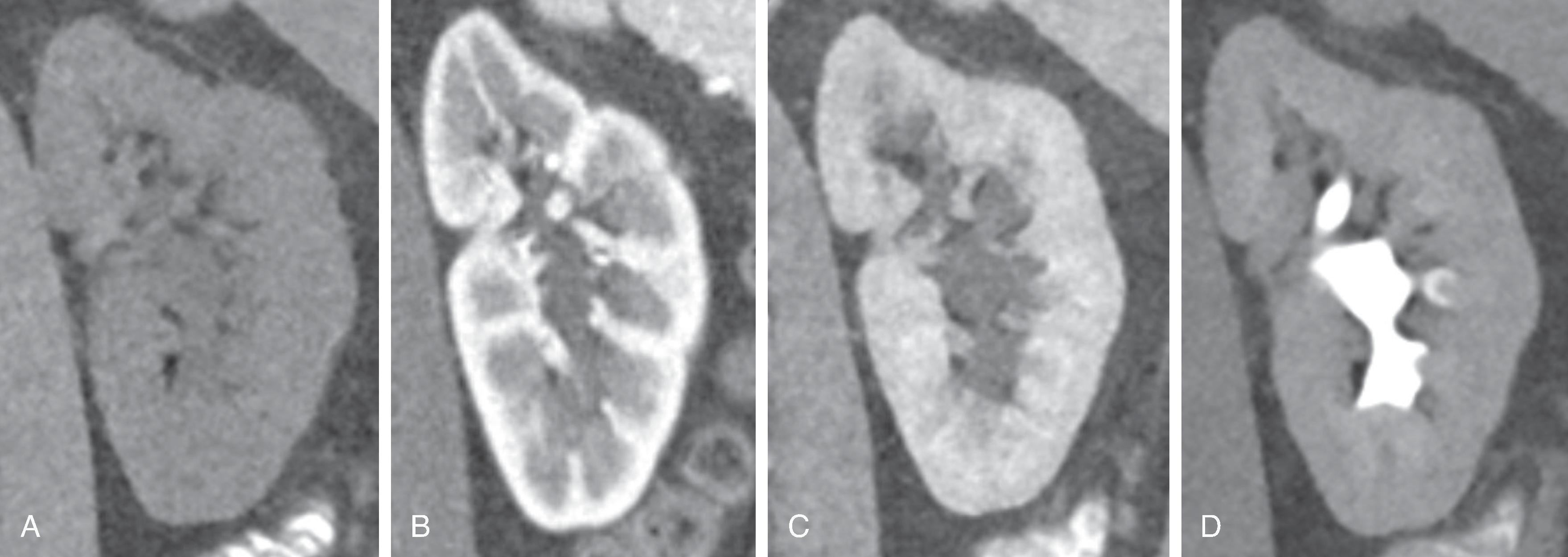
Although far more powerful than traditional radiographic techniques, CT administers a higher radiation dose. Whether the absolute value of the radiation dose is significant in a patient’s lifetime is debated and depends on the patient’s age and the number of studies performed.
Ultrasound is conceptually similar to sonar and uses non-ionizing sound waves to construct an image ( Fig. 6.5 A). It has a number of qualities that stand out as an imaging technique. It is portable, creates images quickly, and can display them in real time. Its ability to image continuously also allows it to detect Doppler shift in soundwaves and provides valuable information about movement, such as the flow of blood through vessels ( Fig. 6.5 B).
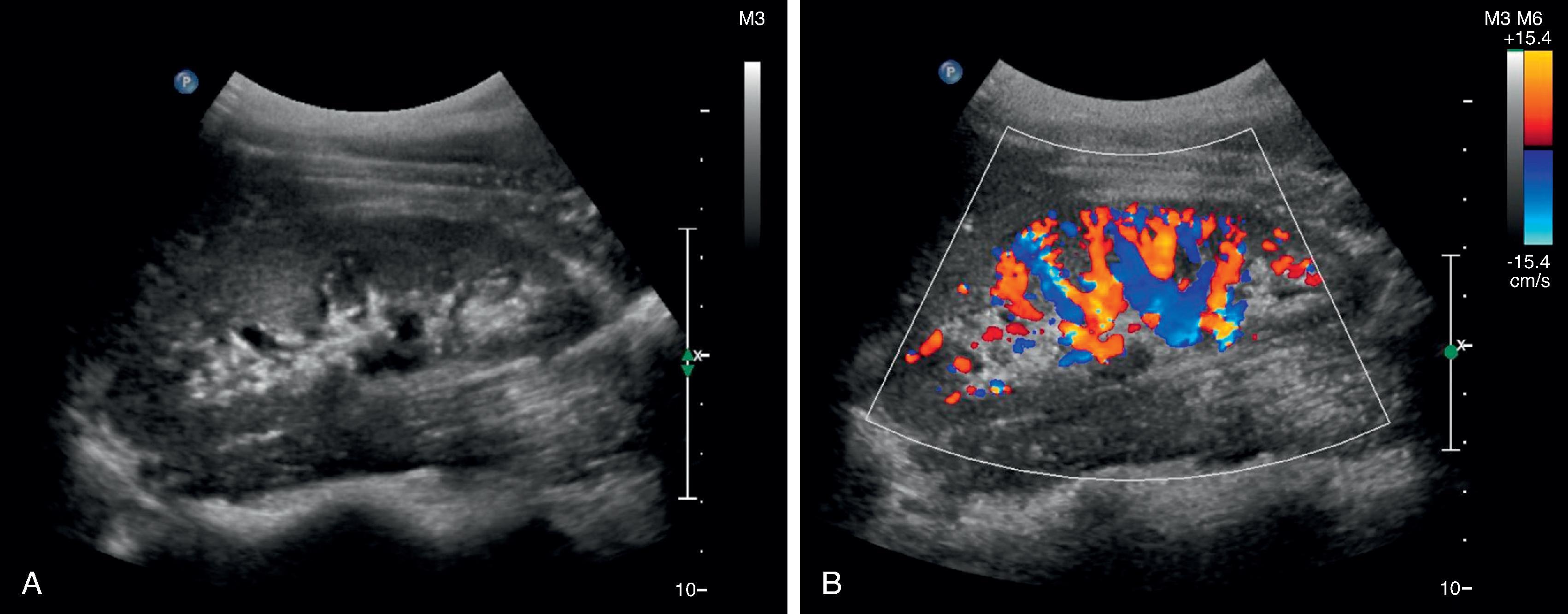
Ultrasound also has its limitations. Unlike traditional radiographic techniques, calcium and gas are poorly imaged and anything behind them is blocked from view. Large patient habitus also limits ultrasound visualization.
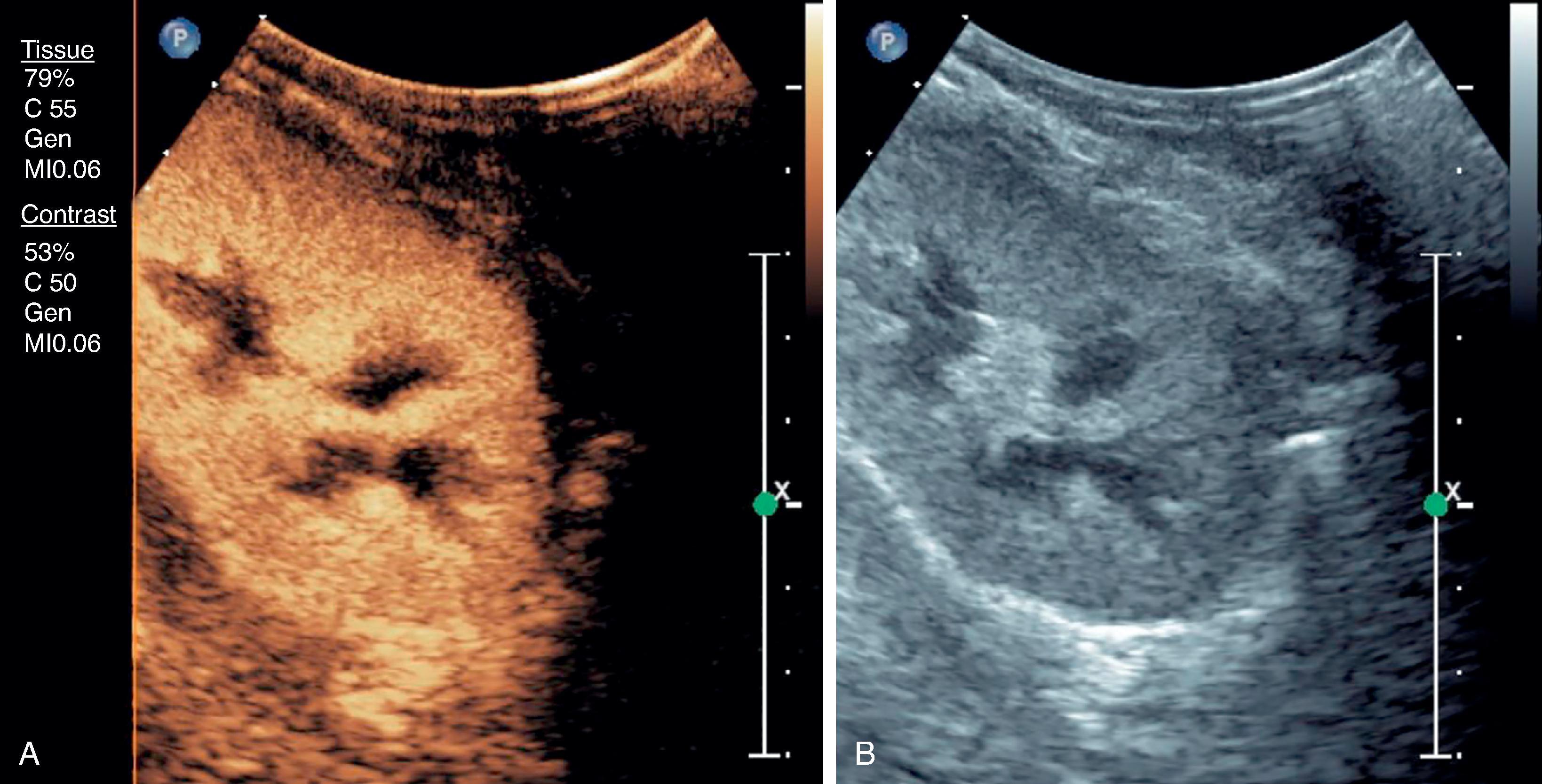
Development of contrast microbubbles for ultrasound (contrast-enhanced ultrasound, CEUS) now permits evaluation of perfusion of the kidney parenchyma and kidney masses. The enhancement is analogous to iodinated contrast media for CT ( Fig. 6.6 ), but the limitations of body habitus, gas, and calcifications remain.
Magnetic resonance imaging (MRI) takes advantage of the spin of protons (usually hydrogen ions) in different microenvironments in order to generate image contrast. MRI employs time-varying magnetic fields and radio frequency electromagnetic radiation, but, unlike CT, ionizing radiation is not needed. A set of magnetic field gradients and radio wave pulses is called a sequence , and these sequences can be modified to take advantage of different types of tissue contrast ( Fig. 6.7 ). Sequences can range from less than a minute in length to several minutes long. These sequences are arranged in protocols and the type of protocol is tailored to answer a specific clinical question.
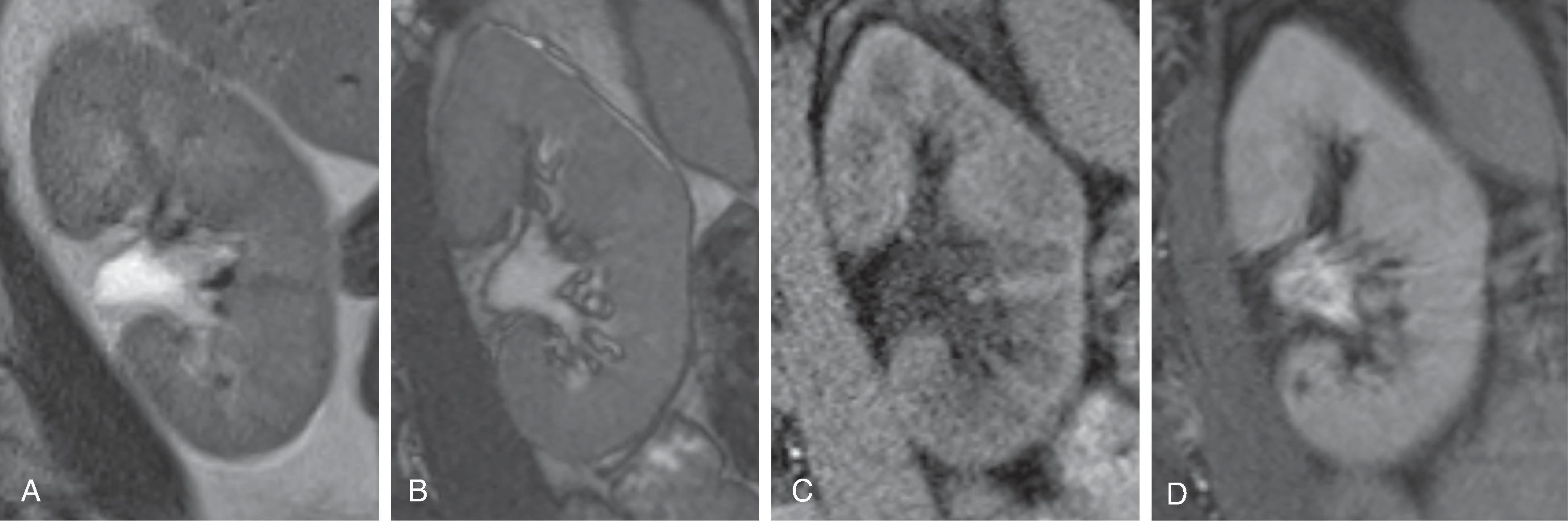
MRI is a very powerful imaging tool for illustrating different kinds of tissue contrast within the kidneys. Spatial resolution is usually quite good if the patient can participate in a breath hold, but it is usually not as good as CT. This is why CT is preferred for evaluation of the collecting system, which often requires fine detail for detecting small urothelial lesions.
Besides the excellent tissue contrast, another advantage of MRI is that it does not require iodinated contrast, so hypersensitivity to iodine-containing compounds is not an issue. MRI can often answer clinical questions without contrast, but, if contrast is needed, a gadolinium-containing contrast can be used (discussed later in chapter).
The drawbacks to MRI include the longer duration of the exam, which can be difficult for patients to tolerate, especially since the bore of the MRI is narrow to improve the magnetic field strength and the machine can be loud as the magnetic gradients are shifting. Open design MRI machines are available, but these often yield suboptimal images, limiting their utility. Patients must also be capable of holding their breath, since motion artifact from breathing will degrade MRI images. Finally, cost and availability can be issues: MRI machines are expensive and require a great deal of maintenance, so the cost of these exams is greater than with other imaging modalities.
Kidney scintigraphy was developed early in the history of kidney imaging and remains a powerful technique for quantifying excretory function of the kidneys, particularly differential function between kidneys and assessing how much obstruction is occurring in a dilated collecting system.
The method of imaging is different than the techniques previously described. Whereas other techniques pass radiation through a patient to generate an image, in kidney scintigraphy a radiotracer – a compound labeled with a radionuclide – emits radiation from within the patient and images are generated by a special camera that detects the radiation ( Fig. 6.8 A). These emissions can be tracked over time to indicate a tracer’s passage within and through the kidney ( Fig. 6.8 B).
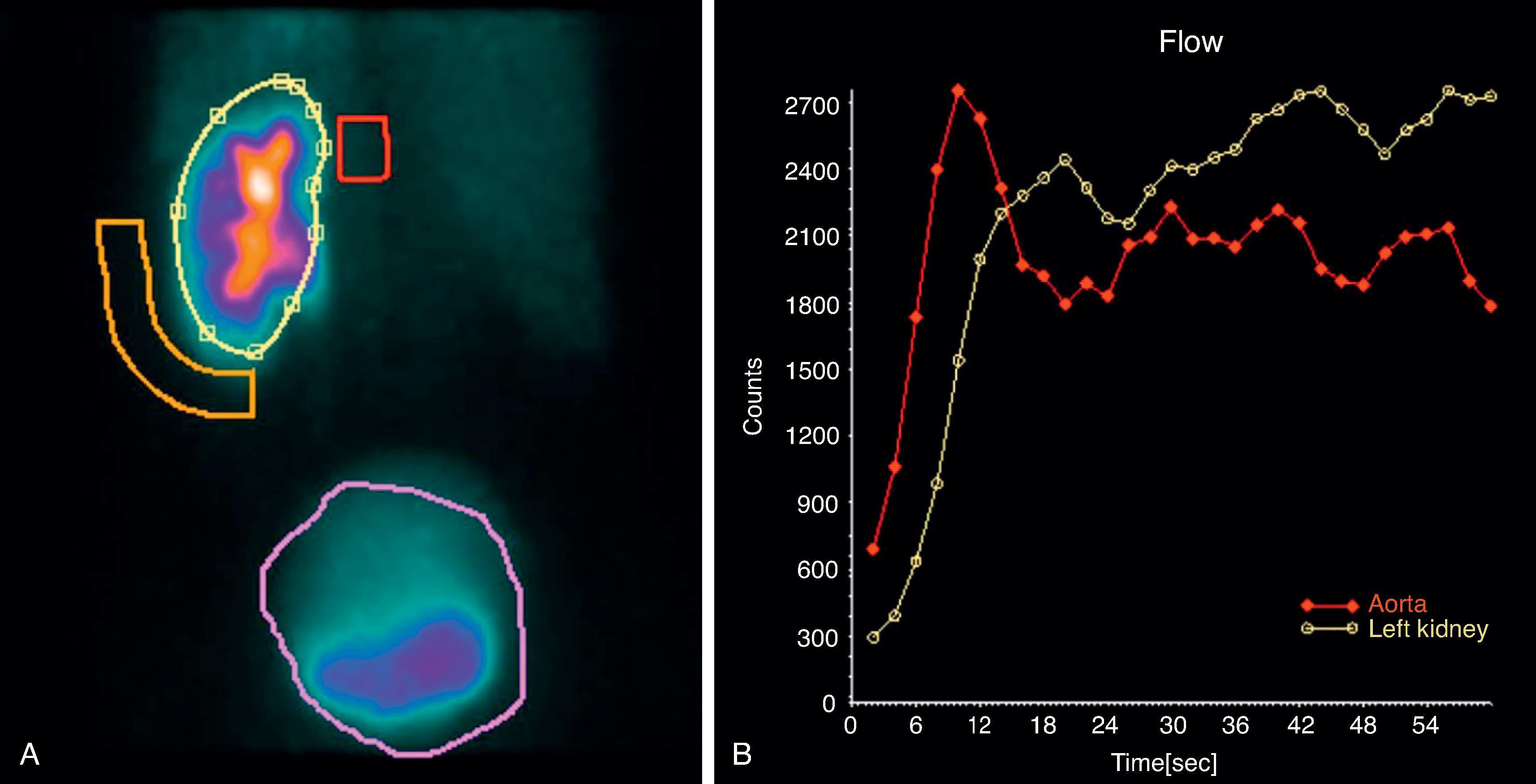
The biology of the radiotracer should inform the selection of the agent for specific clinical applications. For instance, a commonly used radiotracer, Technetium 99m-mercaptoacetyltriglycine (Tc-99m MAG3), is secreted by the proximal tubules, improving imaging in patients with reduced kidney function compared to other agents.
Although the quantitative abilities of kidney scintigraphy give it a special place within kidney imaging, scintigraphy also has some drawbacks. The radiotracers emit ionizing radiation, similar to CT, but usually at a lower dose. The spatial resolution of the imaging is also poor relative to other imaging techniques, so kidney scintigraphy is often combined with ultrasound, CT, or MRI to appreciate both structure and function of the kidneys.
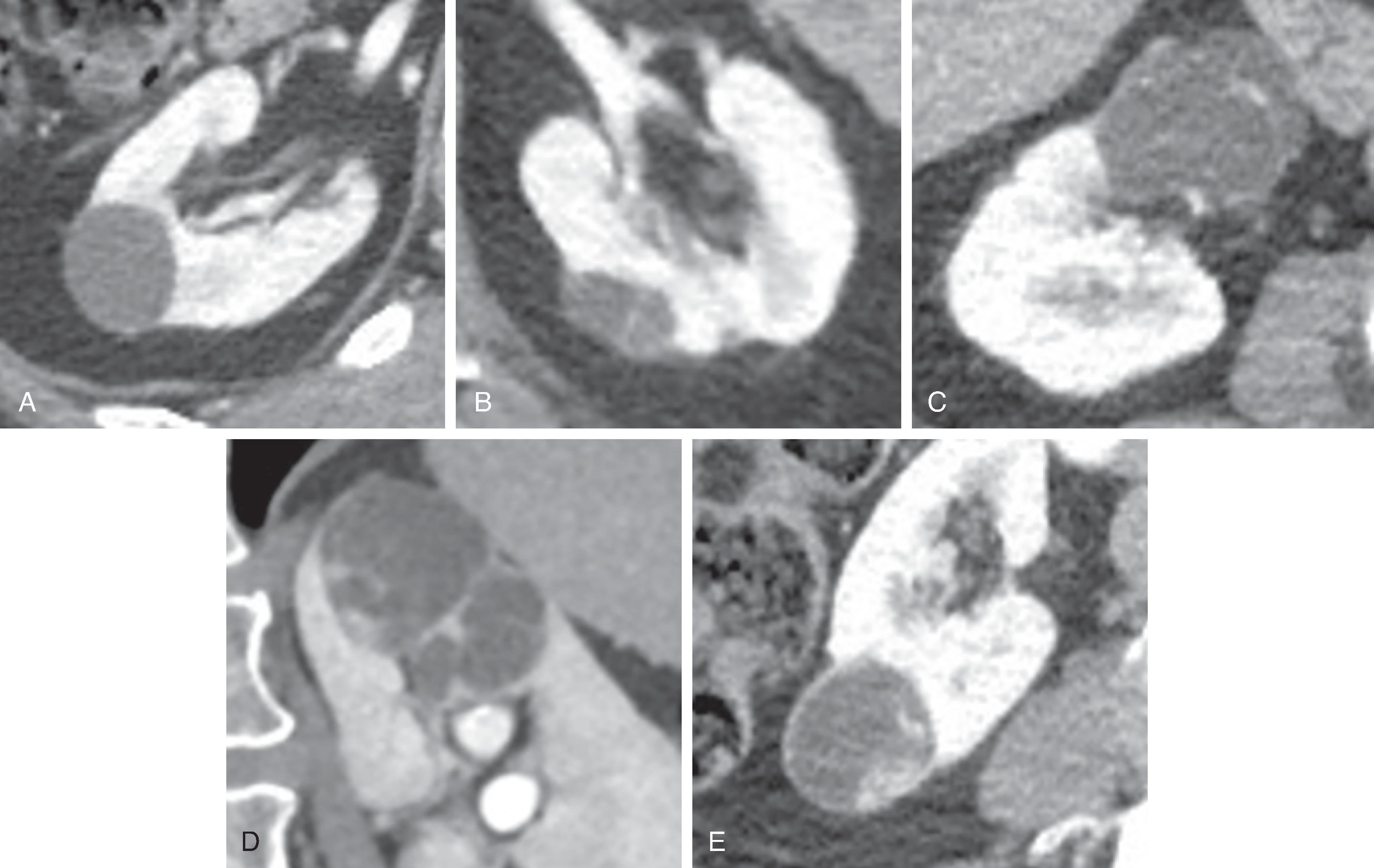
Kidney cysts are commonly encountered lesions in general radiology practice. The term “cyst” encompasses epithelial cysts, peri/parapelvic cysts, and caliceal diverticula, which are benign and of no clinical consequence. On ultrasound, simple cysts are well-marginated, anechoic lesions with a thin wall. Simple cysts measure near water attenuation (range of 0-20 Hounsfield units) on CT, are hyperintense on T2-weighted images (similar to CSF or other fluid intensity structures) on MRI, and do not enhance with contrast.
The Bosniak classification, first described in 1986 and refined over time, has been widely used by radiologists and urologists to stratify the risk of cystic kidney lesions for malignancy based on contrast-enhanced CT characteristics ( Fig. 6.9 ). proposed an update to the Bosniak classification to better define imaging terms, formally incorporate MRI into the classification system, and improve sensitivity and specificity for malignancy. A simple cyst is categorized as Bosniak I. A Bosniak II lesion is a minimally complicated but benign cyst with few thin septa. A Bosniak IIF lesion is a complicated cyst with multiple thin septa or minimally thickened septa or wall. The “F” refers to “follow-up,” as these lesions are usually benign, but the risk of malignancy is non-zero; therefore imaging surveillance with ultrasound, CT, or MRI is recommended, reasonably at 6 months. A Bosniak III lesion has thick or irregular septa or wall. A Bosniak IV lesion has one or more enhancing nodules. Bosniak III and IV lesions are typically resected. In the 2019 update to the Bosniak classification, “few” has been defined as one to three and “multiple” as four or more, with regard to number of septa. The terms “thin,” “minimally thickened,” and “thick” have been assigned measurements of ≤2 mm, 3 mm, and ≥4 mm, respectively. The terms “irregular” and “nodule” also have defined imaging features and measurements. Calcifications of any morphology along septa or wall are now placed in the Bosniak II category, as these are not, as an isolated feature, predictive of malignancy.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary cystic kidney disorder and typically leads to chronic kidney disease (CKD) in adulthood. Kidney cysts are both cortical and medullary in location and increase in size and number over time. The kidneys eventually become massively enlarged and replaced by innumerable cysts of varying sizes, with little identifiable normal kidney parenchyma ( Fig. 6.10 ). Since kidney cysts are ubiquitous in practice, thresholds for considering a diagnosis of ADPKD have been devised that depend on age and whether or not the patient has a family history of the disease. If the patient has a family history of ADPKD, and if the patient is less than 40 years of age, then three cysts bilaterally suggest the diagnosis; if 40-60 years old, then four cysts bilaterally; and if greater than 60 years old, then eight cysts bilaterally. If the patient has no family history of ADPKD, then ≥20 cysts distributed bilaterally is suggestive of the diagnosis.
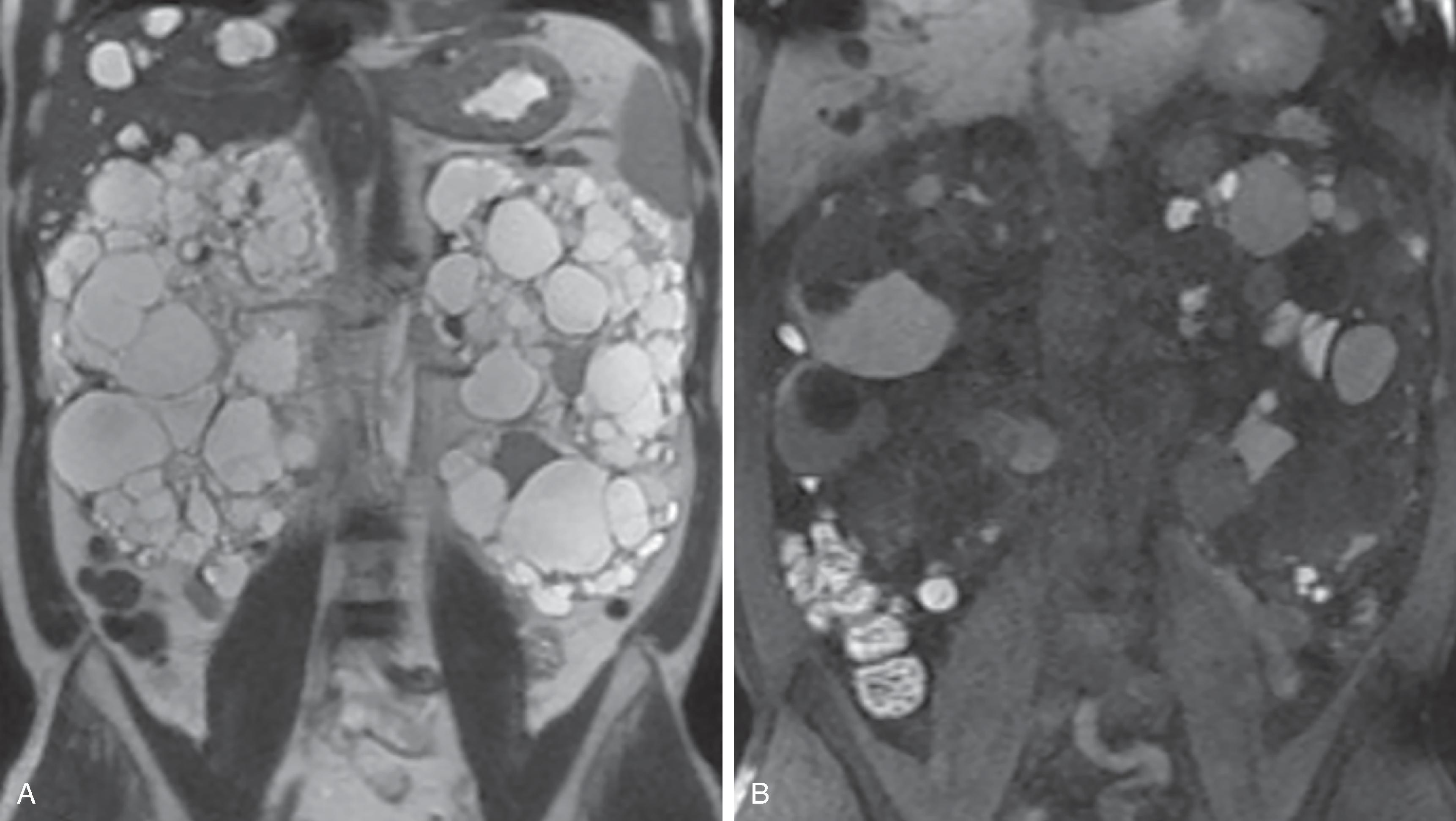
While cysts are well seen by ultrasound, evaluation is challenging due to the large size of the kidneys, and evaluation is also limited by patient body habitus and operator experience. The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) has shown that total kidney volume (TKV), measured by MRI or CT, is a prognostic biomarker in assessing disease progression and treatment response. The gold standard for measuring TKV is manual segmentation, which is labor-intensive and requires radiological expertise and specialized computer software. The Mayo Clinic imaging classification (MCIC) risk assessment uses an ellipsoid formula, requiring only measurement of the three orthogonal axes of each kidney, which is more practical and still accurate. CT is a good modality to assess for complications such as hemorrhage, infection, or malignancy. The risk of renal cell carcinoma is not increased in ADPKD. However, MRI remains the preferred modality for identifying a solid mass among numerous cysts, due to superior soft tissue contrast, even without intravenous gadolinium.
Acquired cystic disease refers to the development of kidney cysts in patients with end-stage kidney disease, in the absence of a hereditary cystic kidney disorder. Cysts can be cortical or medullary in location, and the incidence increases with length of time on dialysis. Kidneys are small and echogenic on ultrasound. CT and MRI better demonstrate cysts of varying size and complexity, typically less than <3 cm in diameter. There is an increased risk of developing renal cell carcinoma, which occurs in approximately 7% of patients. Kidney cysts regress after kidney transplant, but the increased risk of malignancy in the native kidneys persists. Screening of asymptomatic patients for malignancy is controversial.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here