Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Accurate annular sizing is critical for valve selection and minimizing complications during the performance of transcatheter aortic valve replacement (TAVR).
Electrocardiogram (ECG)-gated computed tomographic (CT) angiography before TAVR is an integral part of the preprocedural evaluation of the aortoannular complex for valve sizing and mitigation of complications.
CT angiography before TAVR plays an important role in the evaluation of vascular access, potential vascular access complications, and nonstandard implantation routes.
When CT angiography is not possible, alternative imaging options exist but should be performed at imaging centers of excellence that have an understanding of the specific needs of the structural heart disease proceduralist.
Periprocedural guidance of TAVR replies primarily on fluoroscopy and echocardiography to assist with device delivery for the assessment of immediate catastrophic complications.
Longitudinal postprocedural monitoring of TAVR focuses on an echocardiographic evaluation of paravalvular aortic regurgitation, valve migration, and valve degeneration, although increasingly ECG-gated CT angiography is used for evaluation of valve thrombosis.
The unknown long-term durability of current-generation TAVR valves is an area of interest as implantation expands to lower-risk cohorts, and the ideal frequency of imaging surveillance is unclear.
Dedicated imagers with expertise and training in structural heart disease interventions should perform the preprocedural imaging assessment of TAVR.
Since its inception, transcatheter aortic valve replacement (TAVR) has been at the forefront of technologic innovation. Its development and widespread adoption has relied on advances in interventional cardiology and cardiac imaging. From the first clinical TAVR implantation in humans by Criber and colleagues in 2002 as a bailout procedure for a patient with a bicuspid aortic valve and low-flow, low-gradient (LFLG) severe aortic stenosis (AS), TAVR has pushed the limits of cardiology and its imaging techniques. Although TAVR is still performed on an urgent or emergent basis, the field has moved dramatically forward, and at many centers, it is widely accepted and routinely performed in the outpatient setting with a minimal hospital stay. Currently, less than 10% of cases are done on an urgent or emergent basis, and registry data suggest outcomes are worse when TAVR is performed under duress.
The successful move toward scheduled outpatient procedures has been facilitated by thoughtful and deliberate preprocedural planning. Central to this process is good-quality imaging, and what initially began as a small cottage industry of imaging specialists has now blossomed into the new field of multimodality structural and interventional imaging. This has been made possible by refinements in transcatheter technologies and the maturation of modern imaging techniques such as three-dimensional (3D) echocardiography, ECG-gated multidetector computed tomography (MDCT), and cardiac magnetic resonance imaging (CMR). The integration of these specialists into the multidisciplinary heart team has been critical for the growth and success of transcatheter therapies such as TAVR and has highlighted the importance of a collaborative approach to the complex decision making and problem solving often required for these cases.
Many large, randomized clinical trials have been performed for TAVR therapy, and their outcomes are discussed in detail in Chapter 12 . Relevant highlights of some of the major trials and their imaging implications are discussed in this chapter.
The first major multicenter trial for TAVR with a surgical comparison was the Placement of Aortic Transcatheter Valves (PARTNER I) trial, which studied an early-generation balloon-expandable transcatheter bioprosthetic valve. At the time of the study, valve sizing was based on the transthoracic echocardiography (TTE) assessment of the aortic annulus. In this trial, although TAVR compared favorably with surgical aortic valve replacement (SAVR), the overall mortality rate was high, with many of the adverse events driven by paravalvular aortic regurgitation and vascular access complications. As seen in Fig. 13.1 , at 2 years after the procedure, paravalvular aortic regurgitation was proportionally related to increased mortality. These adverse outcomes identified specific targets for improvement and have become a major focus of innovation for new TAVR related technologies. It is notable then, that the parallel CoreValve US Pivotal trial, which evaluated a self-expanding nitinol bioprosthetic TAVR valve, incorporated the routine use of MDCT to visualize the aortic annulus, thoracic vasculature, and iliofemoral vasculature. Perhaps due to large early-generation delivery sheaths, vascular access complications were still high. However, rates of moderate or severe paravalvular regurgitation were only 6.1% compared with the 12.2% seen PARTNER I, which may potentially be attributed to the integrative use of MDCT for annular measurements. Due to clinical trial data and increasing evidence of the value of accurate annular sizing, all modern TAVR trials primarily use routine aortic annulus sizing performed with MDCT, although transesophageal echocardiography (TEE) or CMR is sometimes used in selected cases.
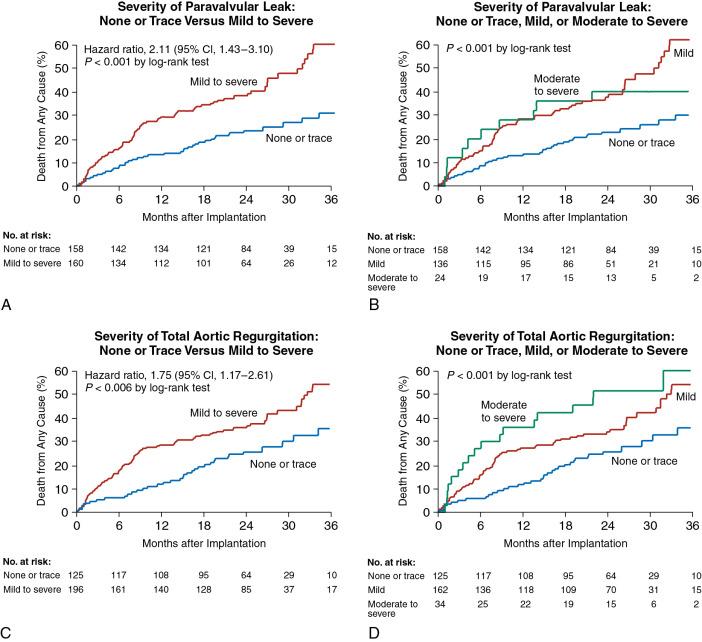
Despite improvements in TAVR, the importance of addressing paravalvular aortic regurgitation continues to be important. Data from an intermediate-risk TAVR cohort ( Fig. 13.2 ) showed that although rates of moderate to severe paravalvular regurgitation were overall lower at only 3.7%, mortality rates continue to be driven by larger amounts of aortic regurgitation. Given the continued importance of reducing paravalvular aortic regurgitation, technology continues to advance, with some of the newest-generation TAVR valves under investigation reporting less than 1% rates of moderate or greater paravalvular regurgitation. , Although outcomes from TAVR are improving due to better operator experience and better imaging and technology, complications arising during TAVR are swift and frequently catastrophic. This underlies the need for comprehensive preprocedural planning for TAVR using an integrative, multimodality imaging approach that uses the unique advantages of each imaging modality ( Table 13.1 ).
| Imaging Technique | Advantages | Limitations |
|---|---|---|
|
|
|
|
|
|
|
|
|
Echocardiography is the foundation of imaging for valvular heart disease. It is widely available, is cost-effective, and has years of validation regarding its use for diagnosis, staging, and prognostication. , Its high temporal resolution and tightly integrated use of Doppler echocardiography often makes echocardiography the ideal tool to evaluate valvular pathology. For the complex patients who are often referred for TAVR, the optimal use of echocardiography requires a nuanced understanding of the guideline-based evaluation of AS. Echocardiography also provides interrogation of physiologic parameters such as diastolic function and right ventricular function, which may be helpful for patient selection and prognostication. , Beyond a standard evaluation, echocardiography continues to drive innovation with continually improving image quality and innovative technologies such as strain and 3D echocardiography, which continue to improve our understanding of valve physiology and pathology.
However, the minimally invasive approach of TAVR lacks the exposure and visualization of a traditional operative field; and echocardiography by itself often is insufficient for comprehensive preprocedural planning. , As clinical experience with TAVR has increased and transcatheter procedures have become more complex, it has become clear that inadequate planning before TAVR can increase complications such as suboptimal valve deployment, coronary obstruction, aortic injury, heart block, and embolization of the valve prosthesis. Many of these complications ( Table 13.2 ) can be predicted and mitigated by the use of supplemental imaging for patient selection, preprocedural planning, and periprocedural decision making. ,
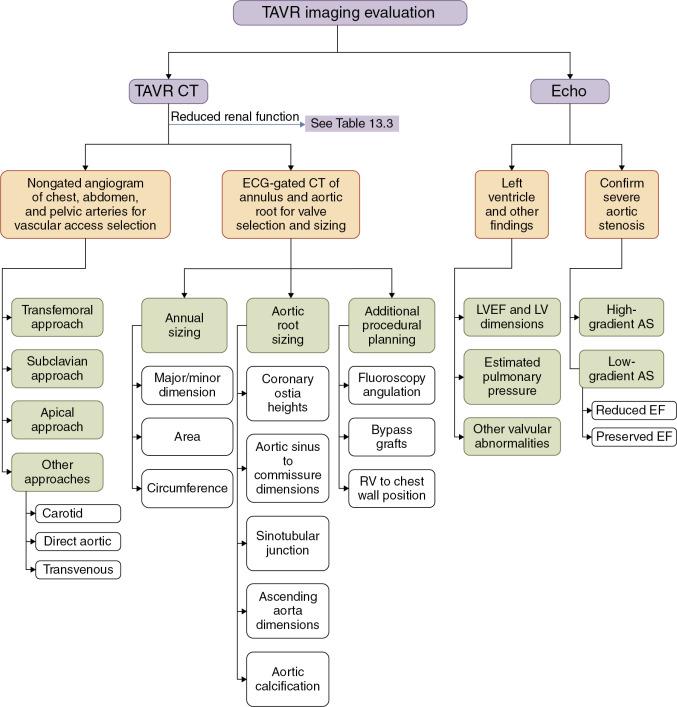
A multimodality approach to imaging is used for TAVR ( Fig. 13.3 ). Integration of MDCT into TAVR clinical trials vastly increased understanding of the aortoannular complex, specific preprocedural planning needs, and potential surgical complications. Table 13.3 shows the checklist of imaging components that are routinely evaluated before TAVR. The standardized imaging pathway, which highlights integration of MDCT, was made possible by technologic advances in image acquisition and postprocessing that were initially developed for coronary computed tomographic angiography but then adapted to the planning of transcatheter therapies for structural heart disease therapies.
| Region of Interest | Recommended Approach and Key Measurements | Additional Comments | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preprocedure | |||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
| Renal Function | Recommended Approach | Key Parameters | |||||||||||||||||||||||||
| Vascular Access (Imaging Depends on Renal Function) | |||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
| Imaging Goals | Recommended Approach | Additional Details | |||||||||||||||||||||||||
| Periprocedure | |||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
| Long-Term Postprocedure | |||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
a Given the use of CT, the role in annular sizing before TAVR with TEE is limited. Periprocedural use of TEE is limited to cases performed with general anesthesia.
b Unless otherwise noted, TAVR CTA refers to a CT angiogram of the chest, abdomen, and pelvis. Typically, the thorax is acquired using ECG-gated multiphase acquisition. At a minimum, acquisition and reconstruction should include end-systole, usually between 30% and 40% of the R-R window.
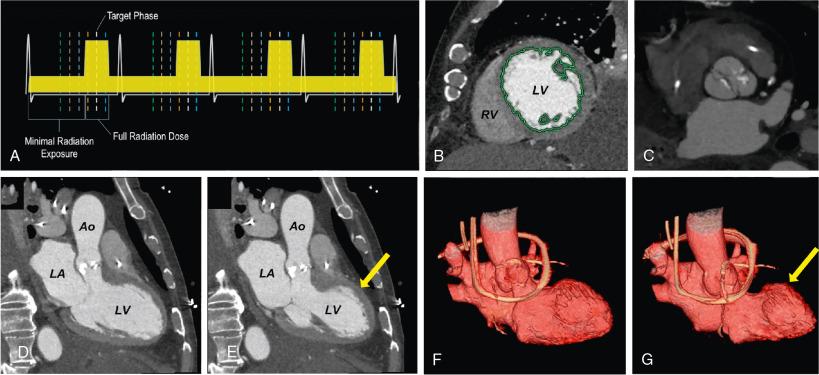
Most modern MDCT scanners have the required capabilities of ECG-synchronized acquisition and high spatial resolution that allow images to be reconstructed in any plane with sharp delineation of various cardiac structures throughout the cardiac cycle. Fig. 13.4 demonstrates the technique of ECG gating and some of the anatomic and functional information that can extracted from the data sets, such as assessments of wall motion, ejection fraction, and valve motion. Additional MDCT tools include unique postprocessing or rendering techniques that can be used to improve understanding of complex valve disease. Fig. 13.5 highlights how these tools are valuable for making an accurate diagnosis and assist in quickly and effectively communicating unusual aspects of cardiac and valve pathology to the heart team to aid in a successful procedure.
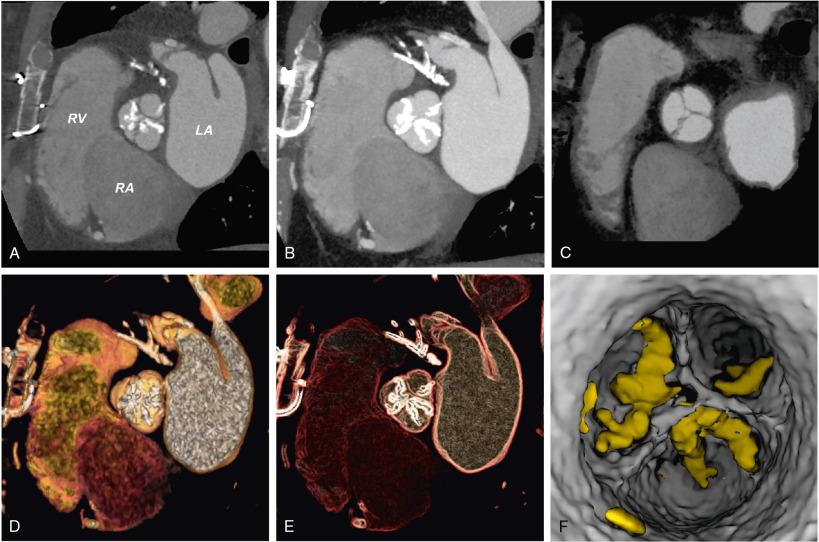
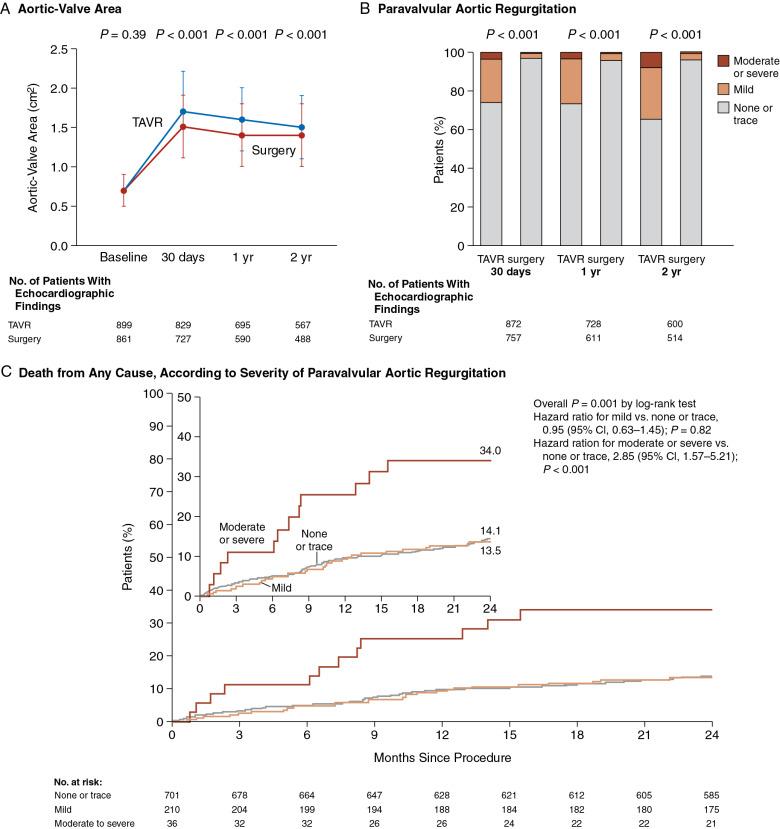
Application of these new technologies and techniques to the preprocedural planning of TAVR have given a new appreciation of the complex and dynamic nature of the aortoannular complex, vascular atherosclerotic burden, and characteristics of the thoracoabdominal aorta and iliofemoral branches. All of the measurements routinely made with MDCT are shown in Table 13.4 . Integration of MDCT improves the accuracy of TAVR prosthesis sizing, and paravalvular aortic regurgitation has decreased from approximately 75.3% to 55%, and mild or more severe paravalvular regurgitation has decreased from approximately 20.5% to 7.5%.
| Region of Interest | Specific Measurements | Measurement Technique | Additional Comments |
|---|---|---|---|
| Valve Size and Type | |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Vascular Access Planning | |||
|
|
Major/minor diameters of the following:
|
|
|
|
|
|
|
Stenosis of the following:
|
|
|
|
Distance from inferior margin of femoral head to femoral bifurcation |
|
|
The accumulating body of evidence for the use of MDCT in the planning of TAVR is compelling. In most large-volume centers, MDCT is the foundation of the standard imaging pathway for TAVR preprocedural planning. ,
CMR and magnetic resonance angiography (MRA) can provide an alternative comprehensive assessment of the aortic valve, annulus, aortic root, thoracoabdominal aorta, and luminal caliber of the iliofemoral branches with good correlation with MDCT. CMR also provides for interrogation of myocardial function and mechanisms of valve pathology. If quantification of hemodynamics is desired, a stack of cine images in a short-axis orientation can be used to quantify left ventricular (LV) volumes. This information can be used to calculate cardiac stroke volumes, which can be internally confirmed using velocity-encoded flow imaging. These techniques are useful for establishing the diagnosis of valve disease when the results of echocardiography are unclear.
The unique tissue characterization abilities of CMR are likely to play a growing role in patient selection. Evidence indicates that cardiac amyloid may be more prevalent in patients with AS, and identification of cardiac amyloid or other coexisting cardiomyopathies may constrain post-TAVR outcomes and thereby inform patient-centered discussions about undergoing the procedure.
A checklist of the anatomic structures that can be evaluated with these techniques is shown in Table 13.5 . However, image quality with CMR and MRA greatly depends on image pulse sequence selection, skill of the operator in proper image acquisition, and patient cooperation, including the ability to lie flat and perform breath holds. For valvular and structural heart disease, successful planning with CMR and MRA requires attention to detail and meticulous care in image acquisition and postprocessing of the images. CMR and MRA for valvular heart disease should be performed only at an imaging center of excellence, where dedicated and experienced imagers are facile with the nuances of CMR and understand the specific needs of the structural heart team.
| Region of Interest | Specific Measurement | Purpose | Measurement Technique |
|---|---|---|---|
| Valve Size and Type | |||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Height from annulus to inferior margin of left main coronary artery and the inferior margin of the right coronary artery |
| Vascular Access Planning | |||
|
|
|
|
|
|
|
|
|
|
|
|
Correct assessment and sizing of the aortic annulus can be challenging because it is an elliptical, virtual ring formed by the basal attachments of the aortic valve leaflets. It is a dynamic structure that undergoes conformational pulsatile changes throughout the cardiac cycle, with an average relative difference between the maximum and minimum cross-sectional areas of 18.2 ± 6.1%. Fig. 13.6 illustrates this change and shows that the predominance of the conformational change is driven by an increase in size of the minimum cross-sectional dimension, leading to a larger and more circular shape during systole (typically 30%–40% of the RR interval of the cardiac cycle). The aortic annulus is typically measured at peak systole to avoid undersizing of the TAVR prosthesis , because valve undersizing can lead to increased rates of paravalvular aortic regurgitation, valve migration, or valve embolization. ,
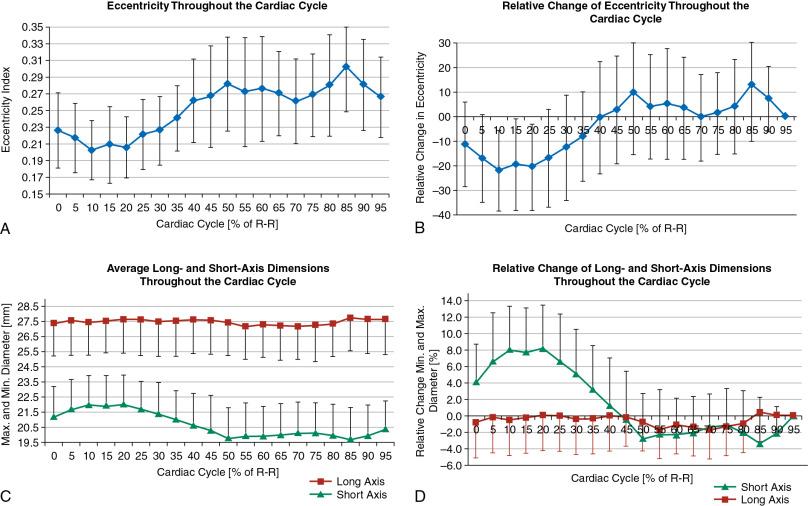
Recommendations for valve selection and sizing vary by vendor, and these tasks have historically been performed using multiple measurement techniques, including minimum and maximum cross-sectional dimensions, circumference-derived area, and direct planimetry of the annular area. To account for distortion and compliance of the annular tissue caused by valve deployment, oversizing of the valve (10%–15%) has traditionally been recommended, although specifics depend on manufacture recommendations. Greater degrees of oversizing should be avoided because they increase the risk of complications such as coronary obstruction, heart block, , and annular rupture. , , Complications such as heart block may be variable between vendors. Whether these variations reflect differences in valve sizing methodology or intrinsic differences in risk between valve technologies is unknown, but the accuracy of valve sizing is likely to play a role because left bundle branch block (LBBB) prevalence during or after TAVR is high, and preexisting right bundle branch block (RBBB) is associated with sudden cardiac death and progression to complete heart block.
Due to the importance of the aortic annulus for TAVR, annular sizing should be performed carefully, particularly when using multiple imaging modalities. It is essential that the imaging provider understand the bias and comparative effectiveness and measurement biases for each imaging modality.
3D MDCT data sets are easily manipulated using dedicated workstations to visualize cardiac structures in any plane. The freedom to fully manipulate data allows ideal imaging of the aortic annulus ( Fig. 13.7 ).
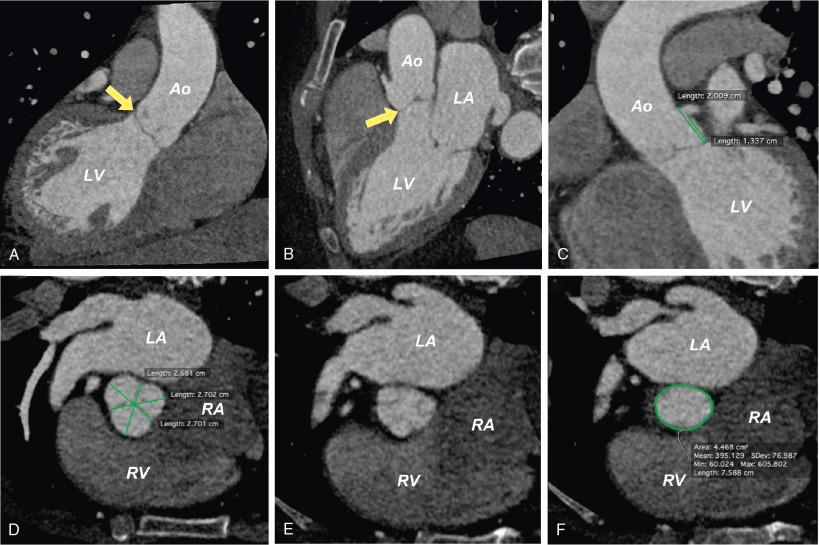
Because the aortic annulus is not a physical structure, but rather the virtual plane prescribed by the insertion points of each of the three coronary cusps, measurement reproducibility can be a challenge for an inexperienced operator. When performed by experienced operators, aortic annulus measurements have an excellent correlation ( r values between 0.94 and 0.96). However, even experienced operators using a single method of annulus measurement for valve sizing (e.g., dimensions, perimeter, area) can yield a difference in prosthesis sizing of 6% to 11% between observers. If multiple measurement parameters are used for internal validation of prosthesis selection, differences in TAVR valve size selection occur in only 3% to 4% of patients. This small but clinically significant variation highlights the critical importance of experience, training, and continual quality assessment to achieve accurate and reproducible valve sizing.
Beyond annular sizing, MDCT enables analysis of the degree and complexity of calcification of the aortic annulus and LV outflow tract. Extensive or eccentric calcifications may increase the risk of annular rupture, paravalvular aortic regurgitation, or coronary obstruction.
Coronary obstruction is a catastrophic complication of TAVR that can happen at the time of TAVR valve deployment or in a delayed fashion, in which case it most frequently occurs in the first 7 days after implantation. Most data for this are provided by retrospective registry analysis, and the rarity of occurrence due to early identification of the potential problem has limited the data for the specific metrics provided by MDCT to prevent immediate or delayed coronary obstruction. Practically, if coronary heights are greater than the valve struts at the predicted landing zone of the TAVR valve, the risk of coronary obstruction should be minimized. This risk is theoretically reduced if there is sufficient distance between predicted implantation distance of the TAVR valve and the coronary ostia.
The risk of coronary obstruction during TAVR increases with the placement of a valve-in-valve (VIV) TAVR, in which a transcatheter aortic valve is placed in a preexisting surgical aortic valve. VIV TAVR is feasible and increasing in frequency, and understanding the imaging nuances of the procedure is important. In addition to a higher risk of coronary obstruction, patients undergoing VIV TAVR are at higher risk for patient-prosthesis mismatch, which is associated with worse outcomes. To reduce the rate of patient-prosthesis mismatch, some groups have demonstrated that valve fracture with a balloon valvuloplasty before TAVR is feasible and can provide improved hemodynamics. , Preprocedural analysis with CTA is likely to improve the safety of VIV TAVR, particularly when facilitated by valve fracture. If preprocedural imaging suggests that a patient may be at high risk for coronary obstruction, transcatheter electrocautery for the intentional perforation and laceration of the high-risk leaflets before TAVR is technically feasible.
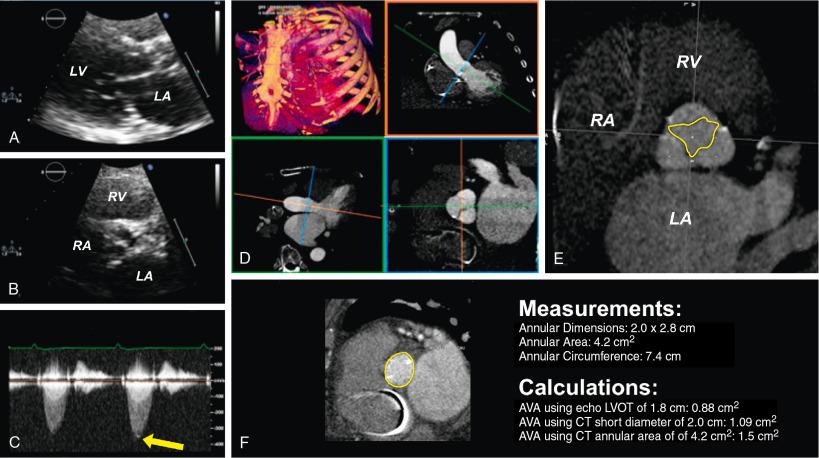
MDCT holds promise for improving the diagnosis of AS in patients when echocardiographic parameters may be unclear. In diseases such as LFLG severe AS, aortic valve calcium scoring can improve the diagnosis. MDCT cine imaging of the stenotic valve with direct planimetry of the anatomic aortic valve area can help establish the severity of AS and determine whether the patient may benefit from treatment with TAVR. Other applications of MDCT for LFLG severe AS include using the improved visualization of the aortic annulus as part of the continuity equation for calculation of the aortic valve area. Compared with echocardiography, the MDCT-derived annulus is systematically larger, and it has been suggested an aortic valve area determined by MDCT of less than 1.2 cm 2 calculated with the continuity equation is comparable to the aortic valve area calculated with echocardiography of less than 1.0 cm 2 . Fig. 13.8 shows a case in which these principles were helpful in clinical decision making.
MDCT should be avoided for some TAVR patients, most commonly those with acute kidney injury or significant chronic kidney disease not yet requiring dialysis. In these cases, iodinated contrast should be avoided if possible. Iodinated contrast is also best avoided for patients with severe anaphylaxis in response to iodinated contrast, even with prophylactic premedication protocols.
As the experience with transcatheter therapies increases, lower risk and younger patients previously excluded from clinical trials, such as those with bicuspid aortic valves and complex congenital heart diseases, are undergoing TAVR. This means that patients will begin undergoing serial evaluations with MDCT and exposure to fluoroscopy at younger ages. Although the effects of ionizing radiation at the doses administered for medical imaging are likely negligible for many of the high-risk elderly patients with severe AS being treated with TAVR, cumulative radiation exposure is an important consideration for the younger patients being considered in the future for transcatheter therapies. These patients will create a need for using alternative imaging techniques such as CMR, leveraging advances in 3D TEE, and creating novel fusion imaging techniques.
Although echocardiography will continue to play a role in TAVR, its use in annular assessment can be problematic because standard two-dimensional (2D) TTE imaging most easily visualizes the short axis of an oval annulus. , Increasing availability of 3D TTE probes allow integration of newer techniques such as biplane guidance, and use of full-volume 3D acquisitions may offer improved delineation of valve and annular morphology. Full-volume 3D TTE data sets are similar to MDCT in that they allow for post hoc image processing. However, 3D TTE data sets are often limited in spatial and temporal resolution and require specific experience and training for efficient and reproducible postprocessing. Despite the technical hurdles, image quality of 3D imaging probes continues to improve and may become more reliable for routine use for direct preprocedural planning of TAVR.
Echocardiographic imaging with TEE plays a role in the preprocedural and periprocedural guidance of structural heart disease interventions. , , , TEE, especially with 3D imaging techniques, can provide reasonable anatomic delineation of the aortic annulus ( Fig. 13.9 ). The annulus can be measured during an ongoing TEE study, but more commonly, it is acquired as a 3D data set to be manipulated and measured later.
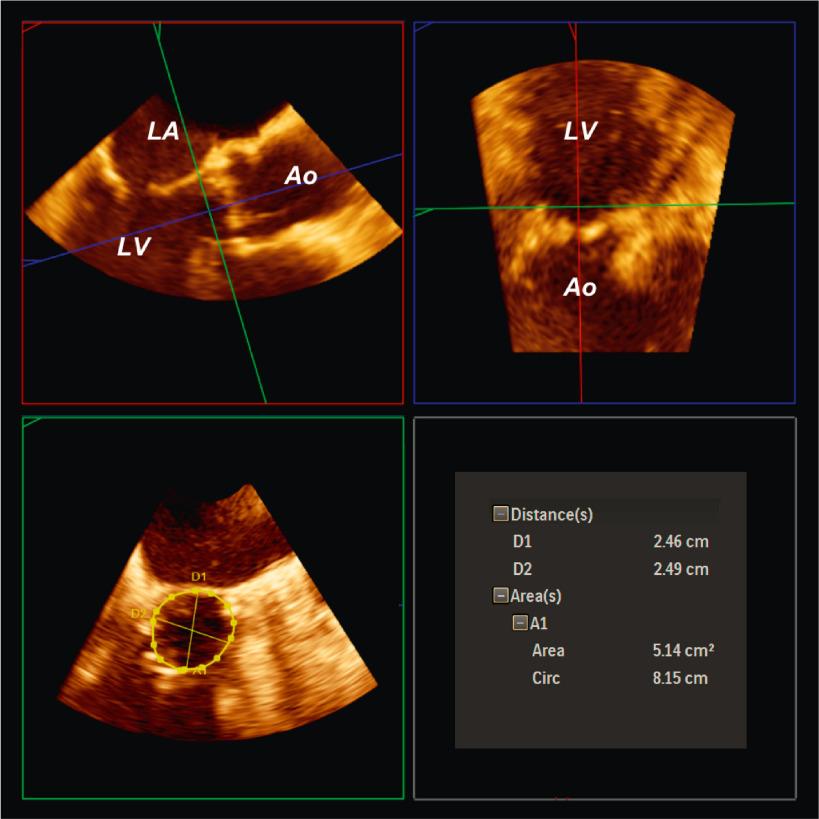
TEE studies performed before the procedure are helpful in that multiple acquisitions can be made without concern for nephrotoxic iodinated contrast administration or ionizing radiation exposure. Annular measurements are a prime example of the importance of comparative imaging studies because direct planimetry of the aortic annulus on 3D TEE compared with MDCT shows systemic underestimation of the echocardiographically derived annular sizes. This has important clinical implications and may lead to discrepant TAVR valve sizes in up to 50% of patients. Fig. 13.10 shows that these types of sizing discrepancies can lead to increases in paravalvular aortic regurgitation.
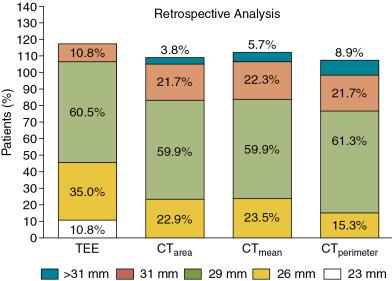
Another major drawback of performing preprocedural TEE for TAVR planning is that it adds a second procedure with incremental risks for a sick and frail population. These risks are somewhat mitigated if the TEE is performed immediately before TAVR as part of the procedure itself. However, when done in this fashion, there are often time constraints that do not allow thoughtful integration of the data a priori. Unusual situations, such as very small or large annuli, may also halt the procedure if an appropriately sized prosthesis is not on hand.
Large calcifications, which may predispose to paravalvular aortic regurgitation, are easily seen on MDCT but may be more difficult to assess by TEE due to acoustic shadowing. Many aspects of the MDCT planning process, such as coronary heights, are not as easily or reliably identified with TEE. If a hard stop is identified only by TEE at the time of the planned intervention, unnecessary procedures such as central vascular access may have been obtained, and the overall laboratory workflow may be disrupted.
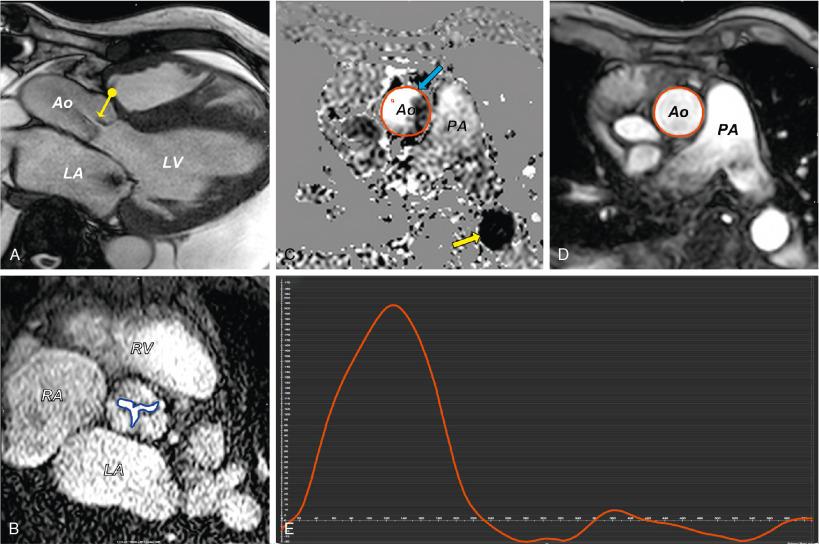
CMR is most frequently used for physiologic assessment and clarification of many cardiovascular diagnoses ( Fig. 13.11 A,B). Particularly in highly calcified valves, it can be helpful in establishing the presence of a bicuspid or dysplastic aortic valve. Occasionally when MDCT or 3D echocardiography is not feasible, CMR with intrinsic bright blood cine imaging can be used to provide a detailed assessment of the aortic valve, aortic root, aortic annulus, and coronary ostia.
When available, a free-breathing, noncontrast, navigator-gated, whole-heart 3D acquisition can be helpful for annular measurements ( Fig. 13.12 ). Unlike standard 2D bright blood cine images, these fully 3D data sets allow accurate measurements and reconstructions of the aortic annulus and vasculature structures after the primary acquisition period. However, unlike MDCT, the gated-MRA images typically are obtained only at a single phase of the cardiac cycle and are not adept at detecting the conformational changes of the aortic annulus.
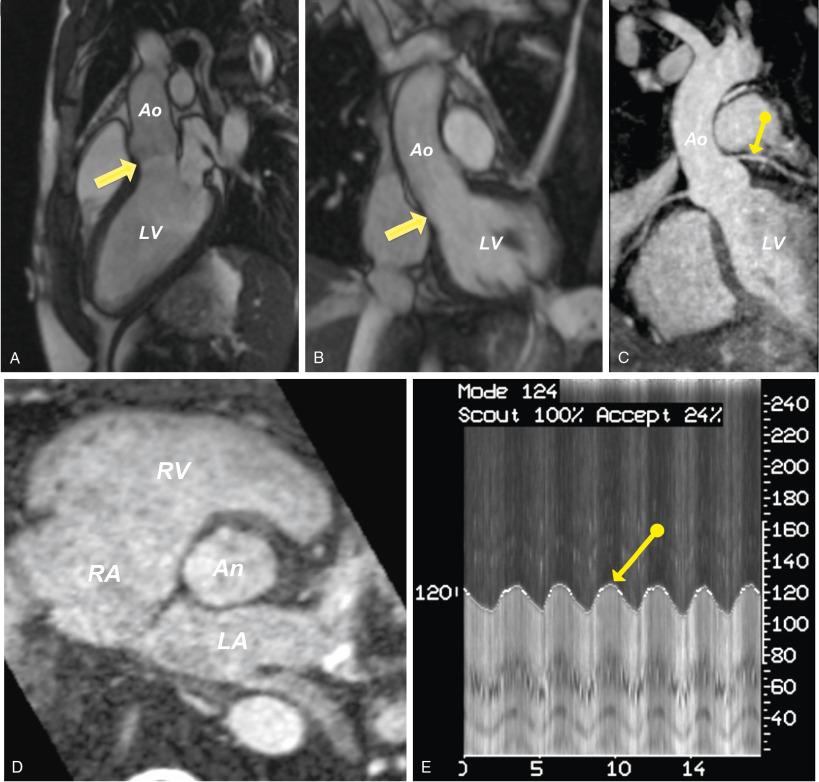
The dual ECG and respiratory gating required for these studies can be fairly prolonged, proportionate to the spatial resolution desired, which may be problematic for elderly, frail patients. In some centers, gadolinium contrast–based MRA images can be used for the aortic annulus, but the ECG-gated sequences required for annular sizing are not widely available and probably offers little benefit over MDCT.
If all other avenues are exhausted and annular sizing remains unclear, confirmation of aortic annulus size can be performed invasively with a balloon at the time of the procedure. As procedures become more complex, novel techniques such as 3D printing have garnered significant interest and may be helpful in planning these challenging cases. Beyond actual printing of 3D models, computer-aided modeling techniques with virtual valve implantation may also provide insights and planning information before TAVR.
When alternatives to CT are used, many studies have demonstrated differences exist in measurements between techniques. Caution and experience are important to understand the strengths and limits of each technology.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here